An updated perspective on diagnostics and treatment of idiopathic granulomatous mastitis
Nový pohľad na diagnostiku a liečbu idiopatickej granulomatóznej mastitídy
Cíl: Prezentovať naše skúsenosti s protizápalovou liečbou idiopatickej granulomatóznej mastitídy (IGM – idiopathic granulomatous mastitis) a poukázať na špecifiká jej diagnostiky. Metodika: Pacientky s akútnym zápalovým ochorením prsníka po ultrasonografickom vyšetrení s odobratím anamnestických údajov podstúpili histologizáciu procesu core cut bio psiou, ktorá potvrdila IGM. Následne bola aplikovaná protizápalová liečba v kombinácii kolchicín, vitamín E a lokálne obklady z odvaru zo skorocelu kopijovitého. U každej pacientky sme zaznamenali i prípadnú extra muros aplikovanú liečbu pred histologizáciou (antibio tiká, chirurgická intervencia, čas od nástupu príznakov k potvrdeniu dia gnózy). Analyzovali sme efekt nami podávanej protizápalovej liečby (nástup účinku, nežiadúce účinky, recidívy, dĺžku liečby nutnej k vymiznutiu ťažkostí). Výsledky: V období rokov 2016 až 2022 sme diagnostikovali a bio psiou verifikovali IGM u 53 pacientok, 45 (84,9 %) podstúpilo nami navrhovanú protizápalovú liečbu, osem (15,1 %) sa rozhodlo pre iný druh terapie. Aktuálne je bez liečby a bez klinických prejavov 27 pacientok (60 %): priemerná dĺžka liečby bola 34 mesiacov, zlepšenie klinického stavu sa prejavilo za 2–8 týždňov (priemer 3 týždne). Nežiadúce účinky v podobe dyspepsie hlásili štyri pacientky (14,81 %). U piatich (18,52 %) došlo k recidíve po 1–36 mesiacoch (priemer 7 mesiacov). Pacientky po ukončení liečby 22 (81,48 %) sú aktuálne 3–70 mesiacov (priemer 34 mesiacov) bez ťažkostí. U zvyšných 18 (40 %) pacientok aktuálne prebieha liečba, v trvaní 3–41 mesiacov (priemer 19 mesiacov). Závěr: Protizápalová liečba kolchicínom, za súčasnej podpornej liečby (obklady z odvaru skorocelu kopijovitého a vitamín E), predstavuje pri minimálnych nežiaducich účinkoch sľubný trend v terapii IGM.
Authors:
L. Vanovčanová 1
; E. Minariková 2
Authors‘ workplace:
II. Radiology Department, Faculty of Medicine, Comenius University and St. Elisabeth Cancer Institute, Bratislava, Slovak Republic
1; Department of Breast Diseases, National Oncological Institute, Bratislava, Slovak Republic
2
Published in:
Ceska Gynekol 2023; 88(6): 435-441
Category:
Original Article
Overview
Aim: To present our experiences with the anti-inflammatory treatment of idiopathic granulomatous mastitis (IGM) and highlight the imaging and anamnestic specifics of its diagnosis. Methods: Patients with acute inflammatory breast disease underwent ultrasound examination followed by a collection of anamnestic data, and histological analysis of the process was performed using core-cut bio psy, confirming IGM. Subsequently, anti-inflammatory treatment was administered, consisting of a combination of colchicine, vitamin E, and local compresses made from an infusion of Plantago lanceolata. We also recorded any additional treatments administered extra muros prior to histological analysis (such as antibio tics, surgical intervention, and time from onset of symptoms to confirmation of diagnosis). We analyzed the effect of the anti-inflammatory treatment administered, including the onset of improvement, adverse effects, recurrences, and duration of treatment required for symptom resolution. Results: Between 2016 and 2022, we diagnosed and histologically confirmed IGM in 53 patients through bio psy. Of these, 45 (84.9%) underwent the anti-inflammatory treatment we proposed, while eight (15.1%) opted for a different form of therapy. Currently, 27 patients (60%) are without treatment and clinical manifestations. The average duration of treatment was 34 months, and improvement in the clinical condition was observed within 2–8 weeks (average of 3 months). Four patients (14.81%) reported dyspepsia as an adverse effect. Recurrence occurred in five patients (18.52%) after 1–36 months (average of 7 months). Patients (22, 81.48%) who completed the treatment are without difficulties for 3–70 months (average of 34 months). The remaining 18 patients (40%) are currently undergoing treatment, lasting 3–41 months (average of 19 months). Conclusion: Anti-inflammatory treatment with colchicine, along with supportive therapy (compresses made from an infusion of Plantago lanceolata and vitamin E), represents a promising trend in the therapy of IGM, with minimal adverse effects.
Keywords:
Mastitis – idiopathic granulomatous mastitis – breast ultrasound – core cut biopsy – anti-inflamatory treatment
Introduction
Idiopathic granulomatous mastitis (IGM) is a chronic breast disease of unknown etiology that mimics malignant conditions of the mammary gland. The relatively dramatic onset of symptoms, the rapid development of clinical manifestations, and significant subjective discomfort are characteristics of this dia g nosis. The broad differential diagnosis, resulting from the variable clinical pre sentation and diverse findings in imaging methods, leads to prolonged dia g – nostic processes, incorrect treatment choices, and subsequent prolonged stress and difficulties for patients. As demonstrated by several years of experience in our institution, IGM is not a rare diagnosis. However, it often remains unrecognized for a long time, is treated incorrectly, and is prone to recurrence. Since it primarily affects young premenopausal patients, it is evident that this chronic and lingering disease significantly impairs their quality of life.
In this study, we present the experiences from our institution and offer a comprehensive diagnostic perspective on IGM, along with new therapeutic approaches and the monitoring of treatment effects.
Wolloch and Kessler [1] first described IGM in a small sample of five patients in 1972 as a benign inflammatory process of the breast, mimicking breast carcinoma and differing from other mastitis diagnoses at the time.
From a pathological perspective, after excluding infectious agents through Ziehl-Nielsen and Gram staining, IGM is characterized as a non-specific granulomatous inflammatory process localized in the lobules. The granulomas consist of epithelioid histiocytes, Langhans giant cells, and lymphocytic infiltrates. With disease progression and merging of granulomas, fatty necrosis, abscess formation, and fibrosis occur, leading to the loss of lobular distribution [2].
The etiology of IGM remains an unanswered question, and opinions on it vary greatly. Published studies have considered infectious factors (such as the influence of Corynebacterium), autoimmune causes, smoking, associations with pregnancy, breastfeeding [3,4], hyperpro lactinemia [5], and even genetic factors as the increased incidence of the disease in certain ethnic groups suggests their influence [6]. However, none of the above factors have been reliably identified as triggers for the disease.
Methodology and patient cohort
Patients
From 2016 to 2022, we enrolled 53 patients aged 29 to 68 years (median age of 37 years) who were histologically confirmed to have IGM through core-cut bio psy. For all patients, we recorded the onset of initial symptoms, previous extramural treatment (antibio tic therapy, surgical intervention), and the time elapsed since the last breastfeeding.
Diagnostics and Treatment Monitoring
All patients underwent ultrasound examination (Acuson Juniper, Siemens, linear transducer of 12.5 and 17 MHz), microbial culture examination, and subsequent core-cut bio psy with a 14G needle, collecting 4–6 samples. After confirming the diagnosis, they underwent an magnetic resonance imaging (MRI) examination to assess the extent of the disease (Siemens Verio 3T). Subsequently, regular ultrasound examinations (every three months) were performed to monitor the effect of anti-inflammatory treatment, and breast MRI examinations were conducted before the planned treatment completion.
Treatment
Patients with a confirmed diagnosis of IGM were recommended anti-inflammatory treatment, which included colchicine (Colchicum-dispert) at a dose of 0.5 mg 3-times a day (with gradual dose reduction when symptoms regress), vitamin E at a dose of 400 mg once a day (reducing to 200 mg once a day after first month of treatment), and local treatment with compresses made from an infusion of Plantago lanceolata leaves, applied 3-times a day.
Statistical analysis
Retrospectively, we evaluated the following data:
Anamnestic information from the period before the correct diagnosis of IGM was established: time (in months) from the onset of initial symptoms to the confirmation of the correct diagnosis, the time elapsed since the last breastfeeding (in months), administration of antibio tics, and surgical intervention.
The course and effect of treatment in patients who underwent anti-inflammatory therapy, explicitly considering: improvement of symptoms after initiating therapy or the speed of response (in months), the symptom recurrence during or after treatment (in months) and its possible causes, adverse effects of treatment, and duration of therapy (in months).
Pretreatment data analysis
In the group of diagnosed patients (53), 36 (67.92%) women in the premenopausal or perimenopausal age range reported a time interval of 1–8 years (average of 4.4 years) since their last breastfeeding, six patients (11.32%) did not breastfeed at all, and for 11 patients (20.75%), this information was irrelevant due to their age. The time required to establish the diagnosis was 1–42 months (average of 8.6 months). All patients had received repeated antibio tic treatment, with 40 (75.47%) receiving more than three different types of antibio tics despite negative cultures. Thirty-six patients (67.92%) underwent surgical intervention.
Treatment results
Out of 53 patients aged 29–68 years (median 37 years) with a confirmed dia g – nosis of IGM, 45 (84.9%) underwent anti-inflammatory treatment consisting of a combination of colchicine + vitamin E + compresses of Plantago lanceolate. The remaining eight patients (15.1%) opted for a different type of treatment, and we do not have information about the progression of their condition.
Currently, 27 out of 45 patients (60%) have completed the treatment, with an average duration of 21.3 months. In these women, we observed improvement in clinical symptoms within 2–8 weeks (average of 3 weeks). Transient adverse effects in the form of dyspepsia were reported by only four patients (14.81%). Elevation of hepatic enzymes was observed in two women (7.4%), and one patient complained of hair loss. Four patients (14.81%) experienced worsening of their clinical condition upon reducing the colchicine dosage, which subsequently improved upon returning to the original dosage.
Disease relapse occurred in five patients (18.52%) within 1–36 months (average of 7 months) after completing the treatment. Patients attributed excessive stress 3 (60%) and a more severe course of viral infection 2 (40%) as the causes. In all cases, symptoms subsided upon resuming the treatment. One patient with complex therapy for advanced multiple sclerosis experienced disease recurrence after seven months of treatment. The remaining patients 22 (81.48%) who completed the treatment have been without clinical difficulties for 3–70 months (average of 34 months).
Currently, 18 patients are continuing therapy. The duration of treatment ranges from 3 to 41 months (average of 19 months), and improvement in clinical condition was observed within 2–16 weeks (average of 4 weeks). Transient worsening of symptoms upon reducing the colchicine dosage was observed in four patients (22.82%), and one patient experienced a recurrence after discontinuing the treatment voluntarily. All patients reported regression of problems upon returning to the original dosage of anti-inflammatory treatment. None of the patients reported any adverse effects of the therapy.
Discussion
Clinical presentation
Despite the mentioned variability in the clinical presentation, specific patterns can be observed in the clinical manifestations that should raise suspicion of IGM upon the first contact with the patient.
A typical patient is in the premenopausal age range within 5 years of the last breastfeeding. They present a history of suddenly appearing, palpably painful resistance that gradually loses its rigidity and leads to fluctuation. There is focal or diffuse reddening of the breast skin, while the nipple-areolar complex usually remains primarily unaffected. The patient is usually afebrile, with occasional transient subfebrile episodes. In cases of prolonged difficulties, a spontaneous secretion of non-foul-smelling purulent content from the nipple or through a cutaneous fistula is reported. The fistula can occur spontaneously or at the site of previous interventions. A typical characteristic is the improvement of subjective discomfort and relief from pain after the evacuation of purulent content. In untreated women, there is a gradual progression to skin hyperpigmentation, deformity, scarring of the breast, and repeated exacerbations of inflammation, with the duration of each phase being individual. Patients report repeated and long-term antibio tic treatment (three or more times) despite repeatedly negative culture results of abscess contents, with this therapy being ineffective. Those who undergo surgical resection of affected tissue often experience recurrences in the previously healthy tissue of the mammary gland.
Changes in laboratory parameters are nonspecific, with transiently elevated CRP occasionally observed without leukocytosis. Tumor markers are negative, and the culture of purulent contents excludes the presence of aerobic, anaerobic, and specific bacteria and fungi.
Characteristics of IGM in imaging modalities
Since IGM typically affects patients younger than 40 years, the initial dia g nostic method of choice is an ultrasound examination. It should be emphasized that this examination provides an opportunity for targeted questions, clarification of medical history, and clinical examination.
In the sonographic image, we find edema of the skin cover at the site of resistance and erythema. Targeted ultrasound at the palpable resistance reveals a more or less well-defined hypoechoic formation, usually with a non-homogeneous structure and irregular contours. In some cases, there may be evidence of fluid overflow or sedimentation, which becomes more pronounced when the patient changes position. A characteristic feature of IGM is the presence of multiple collections that communicate with each other through tubular-shaped formations. In the retro-areolar space, dilated lactiferous ducts filled with thickened hypoechoic contents can be observed. In the ipsilateral axillary area, reactive and occasionally accentuated lymph nodes (LNs) of usually sub-limited size are found. In contrast, pathological enlargement or morphological changes in lymph nodes are not typical features of IGM [4,7].
Mammography is part of the dia g nostic protocol for patients over 40 years old. In younger women, mammography is usually not indicated unless there is a strong suspicion of malignancy. The goal is to exclude the presence of pathological clusters of microcalcifications, which do not correspond to the imaging characteristics of IGM and should raise suspicion of a malignant process.
The mammographic image of IGM is nonspecific [8], similar to mastitis of other etiology: the breast is edematous, poorly compressible, and the skin cover is thickened. The breast tissue may have increased density and a reticular structure, with the possibility of opacities. In the displayed axillary region, there may be reactive lymph nodes, but the findings are often negative.
Sonographically-guided core-cut bio psy plays a crucial role in the diagnosis. It is usually performed under local anesthesia, typically using a 14 G needle. Its primary purpose is to exclude malignancy. Samples must be taken from multiple locations within the lesion to avoid sampling only necrotic parts and potential diagnostic challenges. To obtain specific and relevant results, it is necessary to complete the histological request form thoroughly and provide the pathologist with all available information and the working diagnosis. Specific questions are essential to obtain specific answers.
The role of MRI in the diagnostic process is, according to some authors, controversial [9,10]. From our experience, breast MRI is valuable in determining the extent of the disease and monitoring the effectiveness of therapy since ultrasound tends to underestimate the extent of the disease [11,12]. We recommend MRI at the beginning of treatment and when planning the end of therapy. Prematurely terminated treatment leads to early disease recurrence. Discrete residual abscess formations, especially in the depth of the glandular tissue, are often indistinguishable in sonographic images; therefore, we consider MRI the method of choice before completion of the treatment.
IGM in MRI imaging has relatively nonspecific manifestations; nevertheless, their comprehensive interpretation, together with ultrasound (USG) and clinical presentation, enhances the specificity of this imaging method. Typically, we find edematous infiltration of the breast tissue and thickening of the skin. Dilated ducts often present content with higher amounts of proteins. Abscess formations typically appear as T2 hyperintense collections that communicate with each other, showing peripheral rim enhancement. The enhancement pattern depends on the stage of their development. It is common to identify fistulas – T2 hyperintense fine tubular structures extending from the abscess collection to the skin surface. In the findings, infiltrates and nodular enhancement of the gland can imitate breast carcinoma or, in combination with other characteristics, suggest carcinomatous mastitis.
Our experience indicates that in the differential diagnosis, the absence of infiltrative behaviour in the lesions and their predominantly expansive character argues against carcinomatous mastitis. Another characteristic is the lack of pathologically altered axillary lymph nodes, which, in the presence of a usually large local lesion in the breast, supports the diagnosis of IGM.
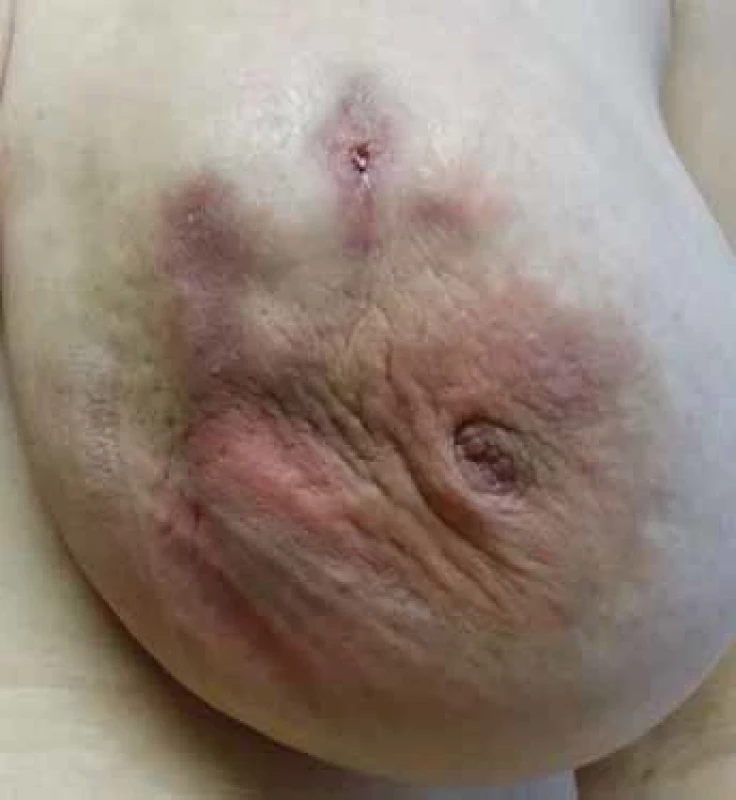



A new perspective on IGM therapy
The treatment of IGM has always been a subject of extensive discussion and need for more consensus, just like the question of its etiology. Multidisciplinary approaches and combined therapies dominate in published foreign studies [13]. These often involve the use of broad-spectrum antibio tics or their combination, although to establish a diagnosis of IGM, it is necessary to exclude infectious agents through special staining in histopathological analysis (Gram, Ziehl-Nielsen, PAS). In our practice, we have observed antibio tic ineffectiveness in all patients, with the majority of them (75.47%) having a history of repeated treatments (more than three times) upon arrival at our facility.
Similarly, we frequently encounter an approach based on immune system suppression. However, the authors of studies differ in dosage and duration of therapy. Freeman et al. [14] propose a low-dose prednisone treatment, specifically 16 mg twice daily for 2 weeks, with a gradual dose reduction over 2 months. Their published data shows treatment fails in two-thirds of patients (66%), leading to significant adverse effects. Another approach involves the long-term administration of high-dose prednisone. DeHerthogh et al. [15] already recommended this treatment in 1980, suggesting a 30 mg/day dose for 2 months. Although it resulted in a reduction of inflammatory changes, it also led to adverse effects such as hyperglycemia, weight gain, and even Cushing‘s syndrome. Despite known risks, this approach has become the stan dard and still persists. Current studies describe IGM treatment with doses up to 1 mg/kg/day for 2–6 months, with authors reporting recurrence rates of up to 15% [16]. According to recent review papers, corticosteroid therapy is responsible for the highest proportion of early locoregional recurrences [17].
Another option is methotrexate treatment. Postolova et al. reported a dosage of 20–25 mg per week for an average duration of 15 months. The response to this therapy is high (94%), but disease remission typically lasts only 3 years on average. The question arises as to whether methotrexate can be considered a suitable treatment for IGM, especially since it mainly affects women of reproductive age [18].
Surgical treatment in the form of wide excision to the level of healthy tissue is an alternative. Due to the extent of the disease, adherence to this approach often requires radical intervention. However, recurrence occurs in up to 25% of cases, along with scarring of residual tissue and the formation of chronic fistulas [19]. This approach is unnecessarily mutilating, given the uncertain prognosis.
Our institution has utilized a combined therapy based on long-term anti-inflammatory treatment and support of healing processes since 2016. Colchicine therapy has proven effective; its therapeutic effects have been observed in cases of rheumatoid arthritis, gout, and familial Mediterranean fever [20]. Colchicine blocks the activation of the inflammatory cascade, formation of leukotrienes, cytokines, and phagocytosis. It has only mild side effects (nausea, stomach pain), which rarely occur or are transient at the beginning of therapy. During treatment, we regularly monitor liver and kidney parameters. We initiate therapy with a full dose of Colchicum-Dispert 0.5mg 3-times a day and gradually reduce the dose (1.5-1.0 - -0.5 mg/day) based on the response to treatment.
Supportive treatment includes vitamin E at 400 mg/day for its antioxidant effects. Along with the topical application of compresses made from Plantago lanceolate decoction, it accelerates the healing of skin ulcerations and fistulas, suppresses scarring, and prevents pigmentation.
Based on our results, anti-inflammatory colchicine treatment, combined with local therapy and vitamin E, represents a promising trend in IGM therapy. Although our results are relatively short-term, it can already be stated that, with minimal side effects, the effect of this treatment surpasses that of any previously applied therapy (Fig. 1–4). A comparison of the results of various treatment approaches is presented in Tab. 1. Based on our experience, we emphasise the necessity of long-term and systematic treatment, even in cases where skin changes and subjective difficulties have disappeared. Changes in the breast tissue regress more slowly, and their complete regression needs to be confirmed by breast MRI examination before the treatment termination. This prevents premature termination of therapy with the risk of disease recurrence.

Conclusion
In conclusion, it is necessary to highlight the contribution of a multidisciplinary approach involving the mammologist, radiologist, and pathologist. The goal of this approach is not only to establish an accurate diagnosis promptly but also to provide effective treatment with minimal side effects and a low risk of disease relapse.
ORCID author
L. Vanovčanová 0000-0003-2363-1238
Submitted/Doručené: 20. 3. 2023
Accepted/Prijaté: 12. 5. 2023
Lucia Vanovčanová, MD, PhD
II. Radiology Department
Faculty of Medicine
Comenius University
St. Elisabeth Cancer Institute
Heydukova 10
812 50 Bratislava
Slovak Republic
lucia.vanovcan@gmail.com
Sources
1. Kessler E, Wolloch Y. Granulomatous mastitis: a lesion clinically simulating carcinoma. Am J Clin Pathol 1972; 58 (6): 642–646. doi: 10.1093/ajcp/58.6.642.
2. Hoda S. Inflammatory and Reactive Tumors. In: Hoda SA, Brogi E, Koerner FC et al (eds). Rosen’s Breast Pathology. 4th editon. Philadelphia: Lippincott Williams & Wilkins 2014.
3. Brown KL, Tang PH. Postlactational tumoral granulomatous mastitis: a localized immune phenomenon. Am J Surg 1979; 138 (2): 326–329. doi: 10.1016/0002-9610 (79) 90397-0.
4. Fazzio RT, Shah SS, Sandhu NP et al. Idiopathic granulomatous mastitis: imaging update and review. Insights Imaging 2016; 7 (4): 531–539. doi: 10.1007/s13244-016-0499-0.
5. Yukawa M, Watatani M, Isono S et al. Management of granulomatous mastitis: a series of 13 patients who were evaluated for treatment without corticosteroids. Int J Surg 2015; 100 (5): 774–782. doi: 10.9738/INTSURG-D-14-00 231.1.
6. Altinoprak F, Kivilcim T, Ozkan OV. Aetiology of idiopathic granulomatous mastitis. World J Clin Cases 2014; 2 (12): 852–858. doi: 10.12998/wjcc.v2.i12.852.
7. Omranipour R, Mohammadi SF, Samimi P. Idiopathic granulomatous lobular mastitis – report of 43 cases from iran; introducing a preliminary clinical practice guideline. Breast Care (Basel) 2013; 8 (6): 439–443. doi: 10.1159/000357 320.
8. Sripathi S, Ayachit A, Bala A et al. Idiopathic granulomatous mastitis: a diagnostic dilemma for the breast radiologist. Insights Imaging 2016; 7 (4): 523–529. doi: 10.1007/s13244-016-0497-2.
9. Graziano L, Bitencourt AV, da Silva CB et al. Imaging features of idiopathic granulomatous mastitis – case report. Rev Assoc Med Bras 2016; 62 (4): 303–306. doi: 10.1590/1806-9282.62.04. 303.
10. Poyraz N, Emlik GD, Batur A et al. Magnetic resonance imaging features of idiopathic granulomatous mastitis: a retrospective analysis. Iran J Radiol 2016; 13 (3): e20873. doi: 10.5812/iranjradiol.20873.
11. Vanovcanova L, Lehotska V, Machale kova K et al. Idiopathic granulomatous mastitis – a new approach in diagnostics and treatment. Neoplasma 2019; 66 (4): 661–668. doi: 10.4149/neo_ 2019_190201N100.
12. Laukova T, Bystricka N. Idiopatická granulomatózna mastitída. Onkológia (Bratislava) 2018; 13 (6): 420–424.
13. Ivanička V, Kasčák P. Idiopatická granulomatózna mastitída. Ceska Gynekol 2022; 87 (5): 334–337. doi: 10.48095/cccg2022334.
14. Freeman CM, Xia BT, Lewis JD et al. Idiopathic granulomatous mastitis: a diagnostic and therapeutic challenge. Am J of Surg 2017; 214 (4): 701–706. doi: 10.1016/j.amjsurg.2017.07. 002.
15. DeHertogh DA, Rossof AH, Harris AA et al. Prednisone management of granulomatous mastitis. N Engl J Med 1980; 303 (14): 799––800. doi: 10.1056/NEJM198010023031 406.
16. Keller K, Meisel C, Petzold A et al. Granulomatöse Mastitis – möglicher diagnostischer und therapeutischer Ablauf anhand von Fallbeispielen. Senologie 2018; 15 (02): e23. doi: 10.1055/s-0038-1651735.
17. Akbari M, Negahi A, Dabbagh N et al. Idiopathic Granulomatous Mastitis (IGM): clinical features and non-surgical management. Int J Cancer Manag 2023; 16 (1): e119945. Doi: 10.5812/ijcm-119945.
18. Postolova A, Troxell ML, Wapnir IL et al. Methotrexate in the treatment of idiopathic granulomatous mastitis. J Rheumatol 2019; 47 (6): 924–927. doi: 10.3899/jrheum.181205.
19. Shin YD, Park SS, Song YJ et al. Is surgical excision necessary for the treatment of Granulomatous lobular mastitis? BMC Womens Health 2017; 17 (1): 49. doi: 10.1186/s12905-017-04 12-0.
20. Leung YY, Yao Hui LL, Kraus VB. Colchicine – update on mechanisms of action and therapeutic uses. Semin Arthritis Rheum 2015; 45 (3): 341–350. doi: 10.1016/j.semarthrit.2015.03. 013.
Labels
Paediatric gynaecology Gynaecology and obstetrics Reproduction medicineArticle was published in
Czech Gynaecology

2023 Issue 6
-
All articles in this issue
- Is there a difference between acute appendicitis in pregnant and non-pregnant women?
- Are the serum delta neutrophil index and systemic inflammatory index useful as predictive parameters for preeclampsia and HELLP syndrome?
- Medical Termination of Pregnancy (MToP) in the 1st trimester – the role of human chorionic gonadotropin and ultrasound in pregnancy diagnosis and MToP follow-up
- Pregnancy termination indications and outcomes before 24 weeks of gestation – a case series
- An updated perspective on diagnostics and treatment of idiopathic granulomatous mastitis
- A rare case of a live abdominal pregnancy in a woman with subsequent gestation in utero
- DNA analysis of partial hydatidiform mole revealing triandric monogynic tetraploidy
- Outcome of a patient with Herlyn-Werner-Wunderlich syndrome treated with Balloon septostomy – pre- and postsurgical ultrasound findings
- Methods and techniques of fertility preservation in patients with endometriosis
- Assisted oocyte activation
- Effect of pelvic floor status on the outcome of pelvic organ prolapse surgery
- Preeclampsia and diabetes mellitus
- Diagnosis of complicated gynaecological inflammations by computed tomography – one center experience
- Emergent hysterectomy for placenta accreta spectrum – some additional techniques
- Diagnostika a léčba endometriózy: Doporučený postup Sekce pro léčbu endometriózy ČGPS ČLS JEP
- Czech Gynaecology
- Journal archive
- Current issue
- About the journal
Most read in this issue
- Diagnostika a léčba endometriózy: Doporučený postup Sekce pro léčbu endometriózy ČGPS ČLS JEP
- Preeclampsia and diabetes mellitus
- Assisted oocyte activation
- Is there a difference between acute appendicitis in pregnant and non-pregnant women?
