Histologic Findings after Sodium Phosphate Bowel Preparation for ColonoscopyDiagnostic Pitfalls of Colonoscopic Biopsies
Nálezy ve sliznici tlustého střeva po orálním podání fosforečnanu sodného v přípravě před kolonoskopií. Morfologické změny napodobující kolitidu
Orální aplikace fosforečnanu sodného – NaPO4 (NaP) je stále častěji používána k přípravě před kolonoskopií pro dobrou snášenlivost pacienty a účinnou očistu tlustého střeva. V poslední době bylo ale prokázáno, že roztoky NaP vyvolávají poškození sliznice tlustého střeva. V této studii uvádíme nálezy v segmentálních biopsiích sliznice tlustého střeva u 42 nemocných po aplikaci NaP. Soubor tvořilo 25 mužů a 17 žen ve věku 19–81 let (průměrný věk 46,7 let). U 37 nemocných byly klinicky uváděny průjmy, nadýmání a bolesti břicha; nejčastější indikací k endoskopickému vyšetření bylo klinické podezření na mikroskopickou kolitidu. V 5 případech bylo endoskopické vyšetření provedeno k vyloučení nádorového onemocnění. Žádný nemocný neužíval před vyšetřením léky. Histologicky byl v biopsiích sliznice tlustého střeva u všech nemocných nalezen mírný ložiskový edém, překrvení a hemoragie, u 26 z nich (61,9 %) byla kromě hemoragií v povrchové části lamina propria ložisková kulatobuněčná zánětlivá infiltrace, ale struktura sliznice zůstala zachovaná. Povrchový epitel sliznice byl částečně stržený a v zachovaných úsecích normální nebo oploštělý. U 5 nemocných (11,9 %) se v jednom nebo v několika bioptických vzorcích nacházely v lamina propria a/nebo v povrchovém epitelu nehojné polynukleáry, ojedinělá bazální kryptitida a zvýšená proliferace a apoptóza epitelu krypt. U dvou nemocných s fokální kryptitidou (4,8 %) byly ve sliznici zachyceny malé povrchové eroze. Mírná bazální kryptitida a nápadná apoptóza epitelu na bázi krypt se vyskytovaly ve dvou zánětlivých pseudopolypech (u dvou nemocných). V malých solitárních tubulárních adenomech u 4 nemocných nebyly nalezeny žádné reaktivní změny. Dále bylo vyšetřeno 300 náhodně vybraných hyperplastických polypů tlustého střeva odstraněných endoskopicky po aplikaci NaP. Ve dvou z těchto polypů (0,75 %) se nacházela mírná kryptitida a v povrchové části krypt ojedinělé mnohojaderné epitelie. Ve srovnání s výsledky ostatních studií, které sledovaly abnormality ve sliznici tlustého střeva po aplikaci NaP, se patologické změny v naší sestavě vyskytovaly přibližně ve stejném rozsahu, což pravděpodobně souvisí s podobným složením užívaných roztoků NaP.
Klíčová slova:
tlusté střevo – příprava ke kolonoskopii – fosforečnan sodný – biopsie tlustého střeva – kolitida – zánětlivý pseudopolyp – hyperplastický polyp
Authors:
A. Chlumská 1,2; Z. Beneš 3; P. Mukenšnabl 1; M. Zámečník 4
Authors‘ workplace:
Sikl`s Department of Pathology, Faculty Hospital of Charles University, Pilsen, Czech Republic
1; Laboratory of Surgical Pathology, Pilsen, Czech Republic
2; Department of Hepatogastroenterology, Thomayer Faculty Hospital of Charles University, Prague, Czech Republic
3; Histamed, s. r. o., Trencin, Slovak Republic
4
Published in:
Čes.-slov. Patol., 46, 2010, No. 2, p. 37-41
Category:
Original Article
Overview
Oral sodium phosphate (NaP) has been increasingly used for bowel preparation before the colonoscopy because it shows good patients tolerance and effective bowel cleansing ability. However, new studies describe that NaP can induce colonic mucosal damage. For better characterization of these changes, we examined histologically segmental colonic biopsies from 42 patients receiving NaP bowel solution before the colonoscopy. The series includes 25 male and 17 female patients in age from 19 to 81 years (average age 46.7 ys). Clinical symptoms in 37 patients included diarrhea, constipation, bleeding and abdominal cramps. The most frequent reason for colonoscopy was suspicion of microscopic colitis. Five patients underwent endoscopy to rule out the presence of neoplasia. None of the patients took drugs before the colonoscopy. Histologically, all specimens showed mild focal edema, hyperemia and hemorrhages. In addition to edema and hemorrhage, in 26 patients (61.9%), patchy mononuclear infiltration in the upper part of lamina propria and increased epithelial cell proliferation of individual crypts were seen. Mucosal structure was normal, with partial sloughing of normal or flattened surface epithelium. In 5 patients (11.9%), some biopsy samples contained scattered neutrophilic leucocytes in the lamina propria/superficial epithelium, isolated basal cryptitis, increased proliferation and apoptosis of the crypt epithelium. In two patients with focal cryptitis (4.8%), small erosions were found. Mild basal cryptitis, increased proliferation and striking apoptosis were present in two inflammatory pseudopolyps (in two patients). In 4 patients, solitary tubular adenomas with low-grade dysplasia without any reactive changes were found. In addition, 300 hyperplastic polyps removed endoscopically after the NaP application, were examined. Two polyps (0.75%) showed cryptitis and isolated multinucleated epithelial cells in the superficial part of the crypts. Our results are similar to those previously described in other studies of colonic changes after the NaP application. It reflects probably a similarity in composition of used NaP solutions.
Key words:
oral sodium phosphate bowel preparation – segmental colonic mucosal biopsies – colitis – inflammatory polyp – hyperplastic polyp
Colonoscopy is an important method for assessing the colonic mucosa. Adequate colonic preparation is essential for bowel visualization and good colonoscopic study. Oral sodium phosphate (NaP) is increasingly used as a colonic cleansing agent before the colonoscopy (1, 3, 4, 19, 22, 26, 28). NaP is a strong osmotic laxative and has been shown to be efficacious and well tolerated. Reported side effects of preparation with NaP are electrolyte disturbances, mainly asymptomatic hyperphosphatemia and hypocalcemia (1, 6, 19, 26). Serious hyperphosphatemia is rare, and it occursusually in patients with severe renal diseases or in older persons (1, 3, 6, 12, 19). In these “risk-bearing” cases, the endoscopists prefer preparation with other solutions, e.g. with polyethylene glycol. Recently, colonic mucosal changes associated with NaP use have been described. They ranged from macroscopically visible colitis-like hyperemia and aphtoid ulcers to “histologic-only“ mucosal abnormalities that mimicked inflammatory diseases, especially in the distal colon and rectum (4, 5, 11, 17, 18, 20, 22, 23, 26-28).
The aim of this study was to assess the entire spectrum and frequency of the colonic mucosal abnormalities induced by NaP bowel preparation. The knowledge of these often misleading features should be considered by biopsy interpretation and differential diagnosis of bowel lesions.
Material and Methods
Forty two patients underwent colonoscopy after bowel preparation with NaP (solution containing monosodium phosphate and disodium phosphate). NaP was administered in two doses containing 21.0 g disodium phosphate dodecahydrate and 7.8 g sodium dihydrogen phosphate dihydrate. Each dose was diluted in 225.0 g of cold water. The first dose was given in the evening before the examination (18 : 00 p.m.), and the second in the morning before procedure (7 : 00 a.m.). The day before the colonoscopy the patients were encouraged to drink at least 3 litres or more of clear liquids. They all did not show inflammatory bowel disease (IBD) or other colitis morphologically as well as according to subsequent clinical course. The series included 25 men and 17 women, the average age was 46.7 years (range 19 – 81 years). Clinical symptoms included intermittent diarrhea, chronic constipation, alternating diarrhea/constipation, bloating or abdominal cramps. Most often it was necessary to rule out microscopic colitis, and for this reason the colonoscopy was performed. Five patients underwent endoscopy to rule out neoplasia. None of the patients used antibiotics, NSAID or other drugs before the colonoscopy. In each patient, the biopsy specimens from 4 to 10 localizations of the whole colon were examined. In addition, 300 randomly selected hyperplastic colonic polyps, all removed after the NaP application, were examined.
The specimens were fixed in 10% formaldehyde and embedded in paraffin. Sections were cut at 5 μm and stained with hematoxylin and eosin (HE). Immunohistochemistry was performed using the MIB-1 antibody (Dako Cytomation, Glostrup, Denmark). Cell proliferation and apoptosis were calculated in 10 well oriented crypts in each case.
Results
None of the patients had any gastrointestinal or extra-gastrointestinal complication after the NaP application. By colonoscopy, the gross findings ranged from normal appearance to minimal patchy erythema of the colonic mucosa, more often in the rectosigmoideal part. In two patients, scattered ulcers on the erythematous mucosa were seen.
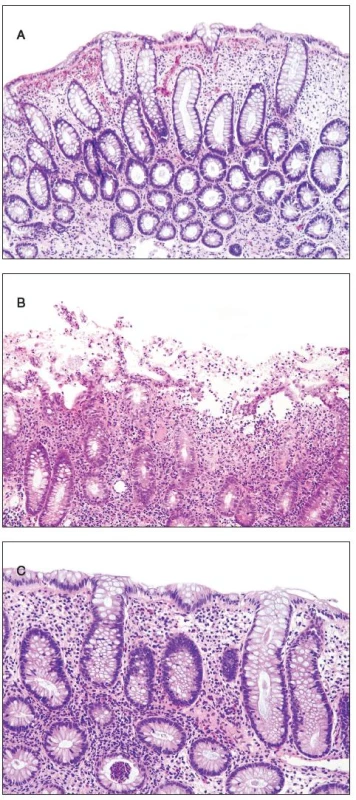
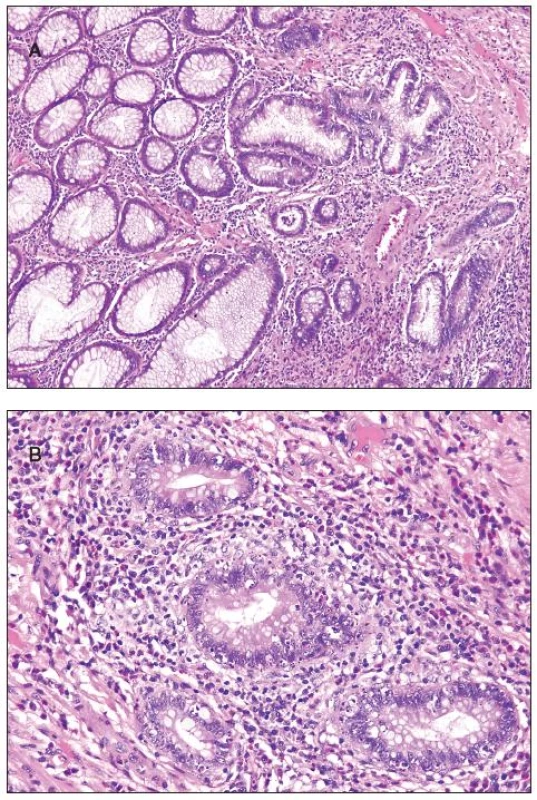
Histologically, at least one biopsy specimen exhibited mild focal edema and superficial hyperemia/fresh hemorrhages in every of the 42 patients (figure 1A). In addition, focal and variable increase in lymphocytes and plasma cells in the upper portion of the lamina propria was found in 26 cases (61.9%). In these biopsies, the crypt epithelium showed mildly increased cell proliferation in individual crypts and no increased apoptosis. The mucosal structure was normal, and the surface epithelium was partially sloughed (figure 1B). The preserved epithelium was normal or at most slightly and focally flattened. In 5 patients (11.9%) (two 59-yr-old men and 3 women aged 30, 39 and 42 ys, respectively), some biopsy fragments contained scattered neutrophilic leucocytes in the lamina propria and/or in the superficial epithelium, and in addition they showed single crypt injury to discrete foci of focal cryptitis restricted mostly to the lower half of the crypts (figure 1C). Crypt distortion was not found. Foci of cryptitis showed reactive epithelial changes with increased epithelial proliferation characterized by extension of MIB-1 positive nuclei into the middle third of the mucosal crypts and mildly enhanced apoptosis. The number of apoptotic bodies was 1 to 2 per 10 crypts. In the superficial epithelium the apoptosis was not enhanced. In two female patients (4.8%), isolated erosions in the focal cryptitis were found. In two inflammatory pseudopolyps from two patients, we have found basal cryptitis, remarkably increased proliferation of the epithelium and frequent apoptotic bodies (figure 2). Solitary tubular adenomas with low-grade dysplasia were found in 4 cases. These adenomas showed none of above mentioned changes after the NaP application.
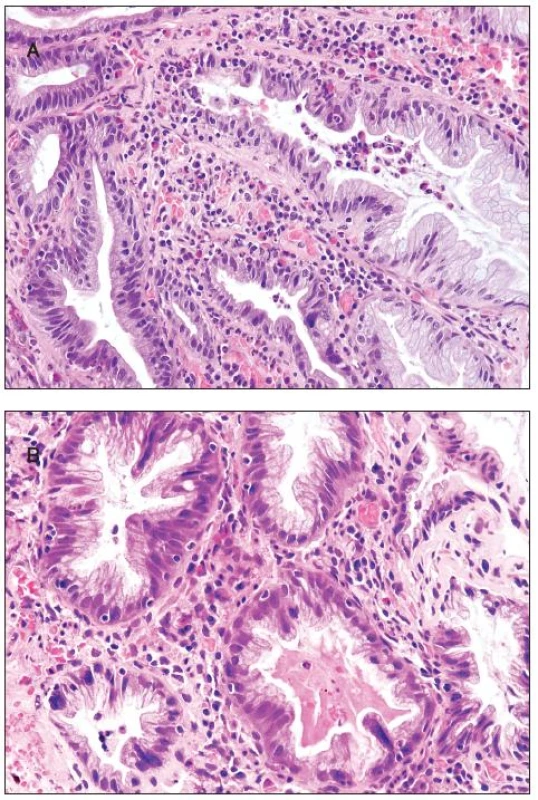
All 300 hyperplastic polyps were located in the left colon, and they all were of goblet cell type with classical serrated architecture. Two polyps (0.75%) showed active inflammation with cryptitis and with numerous eosinophils, increased epithelial cell proliferation and apoptosis (figure 3A). In addition, multinucleated epithelial giant cells were seen in the upper half of the crypts. The multinucleated epithelial giant cells contained four or more nuclei which were enlarged, slightly irregular and hyperchromatic (figure 3B). Some of these nuclei protruded into the crypt lumen.
Discussion
Orally administered NaP has increasingly found favor in the preparation of the bowel for colonoscopy because of effective bowel cleansing and good tolerance by the patients (1, 3, 4, 19, 22, 26, 28). However, hypertonic phosphate solution can cause a variety of mild nonspecific pathological changes in the colorectal mucosa, such as mild edema, focal hemorrhage and patchy increased mononuclear infiltration (5, 17, 18, 24). More pronounced abnormalities that were described include following: minimal mixed inflammation with neutrophils, focal basal cryptitis, erosions and aphtous ulcers without evidence of chronic inflammatory changes, expansion of the crypt proliferative zone and increased apoptotic bodies in the crypts (4, 5, 11, 17, 18, 20, 22, 26, 28). Similar findings, mainly minimal to mild edema, hyperemia, superficial fresh hemorrhage in the upper portion of the lamina propria, and focal mild superficial epithelial damage exhibited some colonic biopsies (especially from the sigmoid and rectum) in all of our patients. In 26 cases (61.9%), increased patchy mononuclear infiltration beneath the surface epithelium was present in addition to edema, hyperemia and hemorrhage. However, it should be stressed that such inflammation is of limited value. In the colorectal mucosa, especially in the caecum and ascending colon, the lamina propria is inhabited normally by round inflammatory cells (7, 8, 21, 24). This physiologically increased infiltration is distributed equally in the whole thickness of the mucosa. In contrast, the round cell infiltration after NaP preparation is higher in the superficial part of the mucosa and it tapers off toward the base of the mucosa (7, 8). The NaP associated infiltration is visible better in the rectal mucosa where the physiological infiltration is sparest.
The presence of small number of neutrophils and occasional eosinophils in the lamina propria/superficial epithelium, focal crypt injury (cryptitis) or focal active colitis (FAC) were originally regarded as signs of inflammatory bowel disease (IBD), especially of Crohn’s disease. However, long-term follow-up of the patients usually excluded the possibility of IBD (4, 9, 11). Recently, FAC was described in association with resolving acute infections, self limited colitis or ischemic colitis (7-11,16, 20, 27). Similar changes were seen after NSAID or steroids, immunosuppressive agents, and in antibiotic colitis (9-11, 16, 20, 23, 27). For these reasons, the presence of FAC in an asymptomatic patient should be regarded as a clinically insignificant finding (11). An important cause of FAC is the use of oral NaP bowel preparation (4, 11, 18, 20, 26, 28). The reported frequency of focal cryptitis and/or aphtoid ulcerations after NaP application ranges from 2.6 to 26% (4, 11, 21, 22, 28). Inflammatory changes were restricted to a portion of the crypts and tended to be in lower half of the crypts, together with increased epithelial proliferation and enhanced number of apoptotic bodies (4, 11, 16, 20, 26). In our series, FAC was found in one to two of segmental colonic mucosal samples of 5 patients (11. 9%), and in two of them (4. 8%), single erosions were present. The erosions/ulcers reported in some studies were associated, like in our cases, with mild epithelial crypt proliferation and with extension of MIB-1 positive nuclei into the middle third of the crypts (4, 5, 18).
Regarding apoptosis in the crypts, it was increased in our series (1-2/10 crypts), and similar findings were reported by others (2, 4, 11, 16, 20, 26). Thus, NaP bowel preparation produces mildly increased crypt epithelial apoptosis in inflammed crypts with epithelial proliferation. The reported number of apoptotic bodies was 1.3 Ī 0. 4 per 10 crypts, whereas the physiological number was observed to be 0.4 Ī 0. 1 per 10 crypts (4). In these studies, the apoptosis was counted only in the crypt epithelium whereas the superficial epithelium was not mentioned. In our series, we have not seen an increase in apoptosis in the superficial epithelium, i.e., the increased apoptosis appears to be limited to the crypts. Apoptosis was always associated with inflammation in our series as well as in previous studies, except of Jacob et al. study (13). In their study of “apoptotic colopathy“ after the oral NaP bowel preparation, an increased number of apoptotic bodies was accompanied only sometimes with cryptitis. Interestingly, in some of these patients with “apoptotic colopathy”, subsequent biopsy performed after a use of solution different from NaP, did not show the mentioned pathologic changes. Other common causes for mild to moderate increase of apoptosis in the crypts are use of laxatives, chronic constipation, diarrhea, NSAID, steroids, and chemotherapeutic agents (2, 16, 20, 23). In these instances, the alteration lasts usually longer, and, therefore, the apoptosis is often associated with melanosis coli (2). This contrasts with “one-shot“ and short-time alteration after the NaP application. Of interest, we have seen remarkably increased apoptosis also in two inflammatory pseudopolyps. We think that this finding is related to enhanced inflammatory irritation in the polyp (in addition to the effect of NaP).
Among 300 hyperplastic polyps removed after the NaP preparation, we have found in 2 cases (0.75%) multinucleated epithelial cells in the upper parts of the polyps. These cells contained four or more nuclei which had been enlarged, slightly irregular and hyperchromatic. Some nuclei protruded into the lumen of the crypt. Recently, Lambie and Brown (15) described these cells in a series of hyperplastic polyps. In contrast to our results, the cells were found in lower parts of the hyperplastic polyps. Like in our cases, the polyps with multinucleated epithelial cells showed always active inflammation, increased epithelial cell proliferation, enhanced apoptosis within crypts and they were associated with NaP bowel preparation. These features could suggest viral etiology or dysplastic nature of these cells. However, Kambhan et al. (14) examined multinucleated epithelial cells immunohistochemically and ultrastructurally, and they did not find any prove for viral etiology or for premalignant nature. Their findings support the previously suggested view that these cells represent a nonspecific, possibly degenerative response to inflammation and injury, and that they should be distinguished from dysplasia (25). We think that epithelial alteration after NaP application could contribute to multinucleation and pseudoatypia in our cases. From a practical point of view, this change must not be misinterpreted as dysplasia. Helpful features are absence of mitoses, restriction to the polyp, lack of “common“ mononuclear cells with atypia, and visible inflammation.
In conclusion, our study shows that oral application of NaP induces quite wide spectrum of colorectal mucosal damages ranging from edema, hemorrhage and mononuclear infiltration to FAC with erosions, increased epithelial crypt proliferation and apoptosis. Our results are similar to those of previous studies (4, 5, 11, 17, 18, 20, 22, 26-28). This can be explained by similarity in composition of used NaP solutions. As patients with other known causes of colonic diseases were excluded, our findings provide additional evidence indicating adverse mucosal effects of NaP. Because NaP-induced changes can mimic other and more serious inflammatory processes, the pathologists should be familiar with them and consider them when examining colonoscopic biopsy.
Correspondence address:
doc. MUDr. A. Chlumska,
CSc.
Biopticka
lab.
Mikulasske
nam. 4
326
00 Pilsen, Czech Republic
E-mail:
chlumska@medima.cz
Sources
1. Belsey, J., Epstein, O., Heresbach, D.: Systematic review: oral bowel preparation for colonoscopy. Aliment. Pharmacol. Ther., 25, 2007, s. 373–384.
2. Byers, R.J., Marsh, P., Parkinson, D. et al.: Melanosis coli is associated with an increase in colonic epithelial apoptosis and not with laxative use. Histopathology, 30, 1997, s. 160–164.
3. Cyrany, J., Rejchrt, S., Pintér, M. et al.: Očista tračníku před koloskopickým vyšetřením. Folia Gastroenterol. Hepatol. 6, 2008, s. 97–104.
4. Driman, D.K., Preiksaitis, H.G.: Colorectal inflammation and increased cell proliferation associated with oral sodium phosphate bowel preparation solution. Hum. Pathol., 29, 1998, s. 972–978.
5. Fenoglio-Preiser, C.M.: Gastrointestinal Pathology. 1st ed., Philadelphia: Lippincott-Raven Publisher, 1999, s. 786–788.
6. Fine, A., Patterson, J.: Severe hyperphosphatemia following phosphate administration for bowel preparation in patients with renal failure: two cases and a review of the literature. Am. J. Kidney Diseases, 29, 1997, s. 103–105.
7. Geboes, K., Van Eyken, P.: Inflammatory bowel disease unclassified and indeterminate colitis: the role of the pathologist. J. Clin. Pathol., 62, 2009, s. 201–205.
8. Geboes, K., Villanacci, V.: Terminology for the diagnosis of colitis. J. Clin. Pathol., 58, 2005, s. 1133–1134.
9. Goldstein, N.S.: Isolated ileal erosions in patients with mildly altered bowel habits. A follow-up study of 28 patients. Am. J. Clin. Pathol., 125, 2006, s. 838–846.
10. Goldstein, N.S., Cinenza, A.N.: The histopathology of nonsteroidal anti-inflammatory drug-associated colitis. Am. J. Clin. Pathol.,110, 1998, s. 622–628.
11. Greenson, J.K., Stern, R.A., Carpenter, S.L. et al.: The clinical significance of focal active colitis. Hum. Pathol., 28, 1997, s. 729–733.
12. Hoffmanová, I., Janotová, D., Havrda, M. et al.: Komplikace po přípravě k vyšetření tlustého střeva. CS Gastroenterol. Hepatol., 62 (Suppl 3), 2008, s. 14.
13. Jacob, E.K., Kammer, P.P., Pardi, D.S. et al.: ”Apoptotic colopathy“ is an effect of oral sodium phosphate bowel preparation solution. Mod. Pathol., 17 (Suppl 1), 2004, s. 118A.
14. Kambham, N., Troxell, M., Longacre, T.A.: Multinucleated epithelial giant cells in colorectal polyps. A potential mimic of viropathic and/or dysplastic changes. Am. J. Surg. Pathol., 29, 2005, s. 912–919.
15. Lambie, D.L.J., Brown, I.S.: Multinucleate epithelial change in colorectal hyperplastic polyps: a review of 27 cases. J. Clin. Pathol., 61, 2008, s. 611–614.
16. Lee, F.D.: Drug-related intestinal disease. Current Diagnostic. Pathol., 4, 1997, s. 128–134.
17. Ming, Si-Chun, Goldman, H.: Pathology of the Gastrointestinal Tract. Williams and Wilkins, 1998.
18. Noffsinger, A., Fenoglio-Preiser, C.M., Maru, D. et al.: Gastrointestinal Diseases. Atlas of Nontumor Pathology. 1st series, fascicle 5, Washington, DC: Am. Registry of Pathology, 2007, s. 319–320.
19. O’Donovan, A.N., Somers, S., Farrow, R. et al.: A prospective blinded randomized trial comparing oral sodium phosphate and polyethylene glycol solutions for bowel preparation prior to barium enema. Clin. Radiology, 52, 1997, s. 791–793.
20. Parfitt, J.R., Driman, D.K.: Pathological effects of drugs on the gastrointestinal tract: a review. Hum. Pathol., 38, 2007, s. 527–536.
21. Paski, S.C., Wightman, R., Robert, M.E. et al.: The importance of recognizing increased cecal inflammation in health and avoiding the misdiagnosis of nonspecific colitis. Am. J. Gastroenterol., 102, 2007, s. 2294–2299.
22. Rejchrt, S., Bureš, J., Široký, M. et al.: A prospective, observational study of colonic mucosal abnormalities associated with orally administered sodium phosphate for colon cleansing before colonoscopy. Gastrointest. Endosc., 59, 2004, s. 651–654.
23. Selbst, M.K., Ahrens, W.A., Robert, M.E. et al.: Spectrum of histologic changes in colonic biopsies in patients treated with mycophenolate mofetil. Mod. Patol., 22, 2009, s. 737–743.
24. Tsang, P., Rotterdam, H.: Biopsy diagnosis of colitis. Possibilities and pitfalls. Am. J. Surg. Pathol., 23, 1999, s. 423–430.
25. Vakiani, E., Yantiss, R.K.: Pathologic features and biologic importance of colorectal serrated polyps. Adv. Anat. Pathol., 16, 2009, s. 79–91.
26. Watts, D.A., Lessells, A.M., Penman, I.D. et al.: Endoscopic and histologic features of sodium phosphate bowel preparation-induced colonic ulceration: case report and review. Gastrointest. Endosc., 55, 2002, s. 584–587.
27. Wong, N.A.C.S., Penman, I.D., Campbell, S. et al.: Microscopic focal cryptitis associated with oral sodium phosphate bowel preparation. Histopathology, 36, 2000, s. 476–478.
28. Zwas, F.R., Cirillo, N.W., El-Serag, H.B. et al.: Colonic mucosal abnormalities associated with oral sodium phosphate solution. Gastrointest Endosc, 43, 1996, 463–466.
Labels
Anatomical pathology Forensic medical examiner ToxicologyArticle was published in
Czecho-Slovak Pathology

2010 Issue 2
-
All articles in this issue
- Fibroblasts – Known or Unknown Cells
- Blood Vessels and Lymphatics in Calcific Aortic Stenosis –In Support of its Inflammatory Pathogenesis
- Histologic Findings after Sodium Phosphate Bowel Preparation for ColonoscopyDiagnostic Pitfalls of Colonoscopic Biopsies
- Guidelines for autopsy investigation of sudden cardiac death
- Czecho-Slovak Pathology
- Journal archive
- Current issue
- About the journal
Most read in this issue
- Fibroblasts – Known or Unknown Cells
- Histologic Findings after Sodium Phosphate Bowel Preparation for ColonoscopyDiagnostic Pitfalls of Colonoscopic Biopsies
- Blood Vessels and Lymphatics in Calcific Aortic Stenosis –In Support of its Inflammatory Pathogenesis
- Guidelines for autopsy investigation of sudden cardiac death
