Immunolocalization of protein-bound 3-nitrotyrosine in inflammatory myopathies
Imunohistochemická detekce 3-nitrotyrosinu vázaného na proteiny u zánětlivých myopatií
3-nitrotyrosin (3NT) je považován za jeden z indikátorů tvorby oxidu dusnatého (NO). Cílem studie bylo zdokumentovat přítomnost a distribuci 3NT ve svalových biopsiích pacientů s idiopatickými zánětlivými myopatiemi (IIM) i nezánětlivými myopatiemi a posoudit, zda polymyozitida (PM) a dermatomyozitida (DM) mohou být odlišeny na základě imunohistochemického průkazu 3NT ve svalové biopsii. Imunohistochemická analýza pomocí monoklonální protilátky proti 3NT byla provedena na zmražených řezech připravených ze svalových biopsii 54 pacientů s IIM (tj. PM a DM) a s různými nezánětlivými myopatiemi.
3NT imunopozitivita byla přítomna v endoteliálních buňkách a jejich blízkém okolí v biopsiích pacientů s DM a PM, ale pouze v těch oblastech tkáňových řezů, kde byly současně přítomny zánětlivé infiltráty. Reakce na průkazu 3NT byly zcela negativní ve tkáňových řezech pacientů s IIM v oblastech vzorků bez zánětlivých infiltrátů a také ve vzorcích svalové tkáně pacientů s nezánětlivými myopatiemi. Endoteliální buňky byly ale překvapivě pozitivní i u případů prokázaných nezánětlivých myopatií se sekundární lymfocytární infiltrací (myodystrofie, myasthenia gravis).
I přes patogenetický význam tohoto pozorování lze konstatovat, že imunohistochemický průkaz 3NT nemá využitelný diagnostický potenciál v diferenciální diagnostice IIM ve svalové biopsii.
Klíčová slova:
oxid dusnatý – 3-nitrotyrosin – polymyositida – dermatomyositida – idiopatické zánětlivé myopatie – myodystrofie – myasthenia gravis
Authors:
J. Zámečník 1; R. Vytášek 2; J. Vencovský 2; V. Vilím 2
Authors‘ workplace:
Department of Pathology and Molecular Medicine, Charles University, nd Medical Faculty and University Hospital Motol, Prague, Czech Republic
1; Institute of Rheumatology, Prague, Czech Republic
2
Published in:
Čes.-slov. Patol., 47, 2011, No. 2, p. 62-65
Category:
Original Article
Overview
3-nitrotyrosine (3NT) is regarded as a “footprint” of nitric oxide generation. The study aimed at documenting the presence and distribution of 3-nitrotyrosine (3NT) in muscle tissue samples from patients with idiopathic inflammatory myopathies (IIM) as well as from those with non-inflammatory myopathies to consider whether polymyositis (PM) and dermatomyositis (DM) could be distinguished based on 3NT immunohistochemistry in muscle biopsy. Cryosections prepared from muscle biopsies of 54 patients with either IIM, i.e. PM and DM, or various non-inflammatory myopathies were immunostained using monoclonal antibody against 3NT.
The 3NT immunostaining was localized to endothelial cells and their close surroundings in muscle biopsies of DM and PM patients but only in those areas of tissue sections where inflammatory cell infiltrates were present. No 3NT positivity was found in tissue sections of IIM patients without inflammatory infiltrates in the studied sample as well as in muscle tissue sections of patients with non-inflammatory myopathies. However, the endothelial cells were also positive in cases of confirmed non-inflammatory myopathies with secondary lymphocytic infiltration (myodystrophies, myasthenia gravis).
Despite the pathogenetic significance, the 3NT immunohistochemistry is of low diagnostic value for the differential diagnosis of IIM in muscle biopsy.
Keywords:
nitric oxide – 3-nitrotyrosine – polymyositis – dermatomyositis – idiopathic inflammatory myopathy – myodystrophy – myasthenia gravis
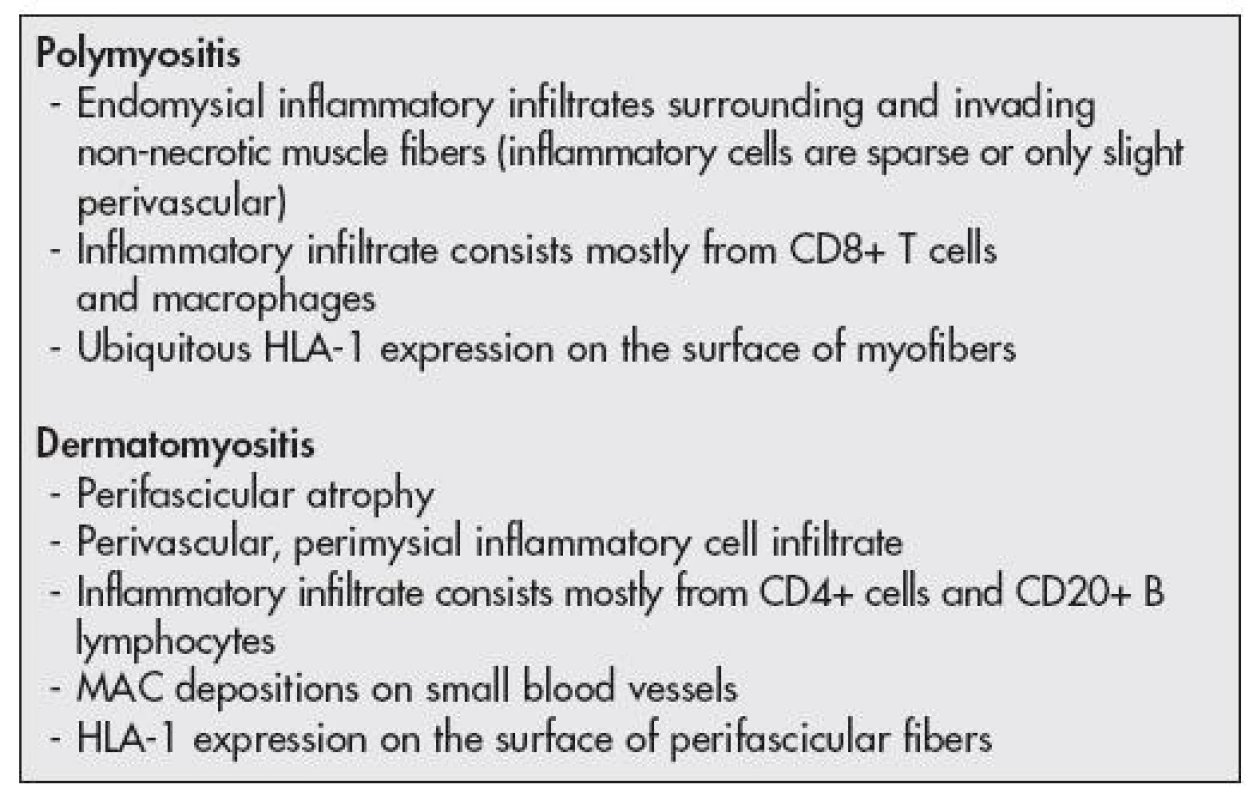
Polymyositis (PM) and dermatomyositis (DM) represent the subtypes of the idiopathic inflammatory myopathies (IIM). The main attack appears to be directed against the microvasculature in DM, where activation and deposition of complement targets the endothelium of capillaries of muscle tissue, and the proper muscle damage is supposed to be a consequence of the resulting ischemia. On the other hand, the muscle fiber damage seems to be caused directly by cytotoxic CD8+ T cells and macrophages in PM (1). Although immunohistochemistry is a helpful aid to diagnosis of IIM by enabling the immunophenotypic analysis of the inflammatory infiltrate as well as by revealing some of the underlying immune mechanisms (2), the discrimination between the subtypes of IIM by muscle biopsy remains difficult in a proportion of cases. But, it is of upmost importance for the therapeutical decisions.
The “reactive oxygen and nitrogen species” seem to be involved in the pathogenesis of autoimmune inflammatory myopathies; however, their importance has not been well understood (3–5). Formation of nitric oxide (NO), a gaseous free radical, is catalyzed by different isoforms of nitric oxide synthase (NOS). All the major isoforms of constitutively expressed NOS (i.e., neuronal and endothelial isoforms) are detectable in the skeletal muscle and they enable NO to fulfill many regulatory functions (6). One isoform of NOS is inducible (iNOS) and it can be expressed by a plethora of cells following a stimulation with diverse stimuli e.g., proinflammatory cytokines (7). The detection of differing mechanisms and consequences of the oxidative stress was suggested to be helpful for distinguishing between PM and DM – whereas constant and increased expression of iNOS was found in endomysial infiltrates of PM, cells in perimysial infiltrates of DM were shown to be largely negative (3).
Half-life of NO is less than 15 seconds; it is rapidly metabolized to nitrate and nitrite in the presence of oxygen. However, in tissues NO can react by many other pathways (7), one of them being the reaction with superoxide free radical (O2) that produces peroxynitrite (ONOO ) (8). There are many possible reactions of peroxynitrite in vivo; one of them is a reaction with free or protein-bound tyrosine that produces 3-nitrotyrosine (3NT), relatively stable end product that can be determined in biological samples (9).
Regarding the reported difference in expression of iNOS in PM and DM (3) we tested the hypothesis postulating that these two diagnoses could be distinguished based on 3NT immunolocalization. We also searched for the expression of 3NT in non-inflammatory myopathies with special interest in those, where secondary lymphocytic infiltrates can occur, since they represent an important differential diagnosis of IIM (10,11).
MATERIAL AND METHODS
Patients
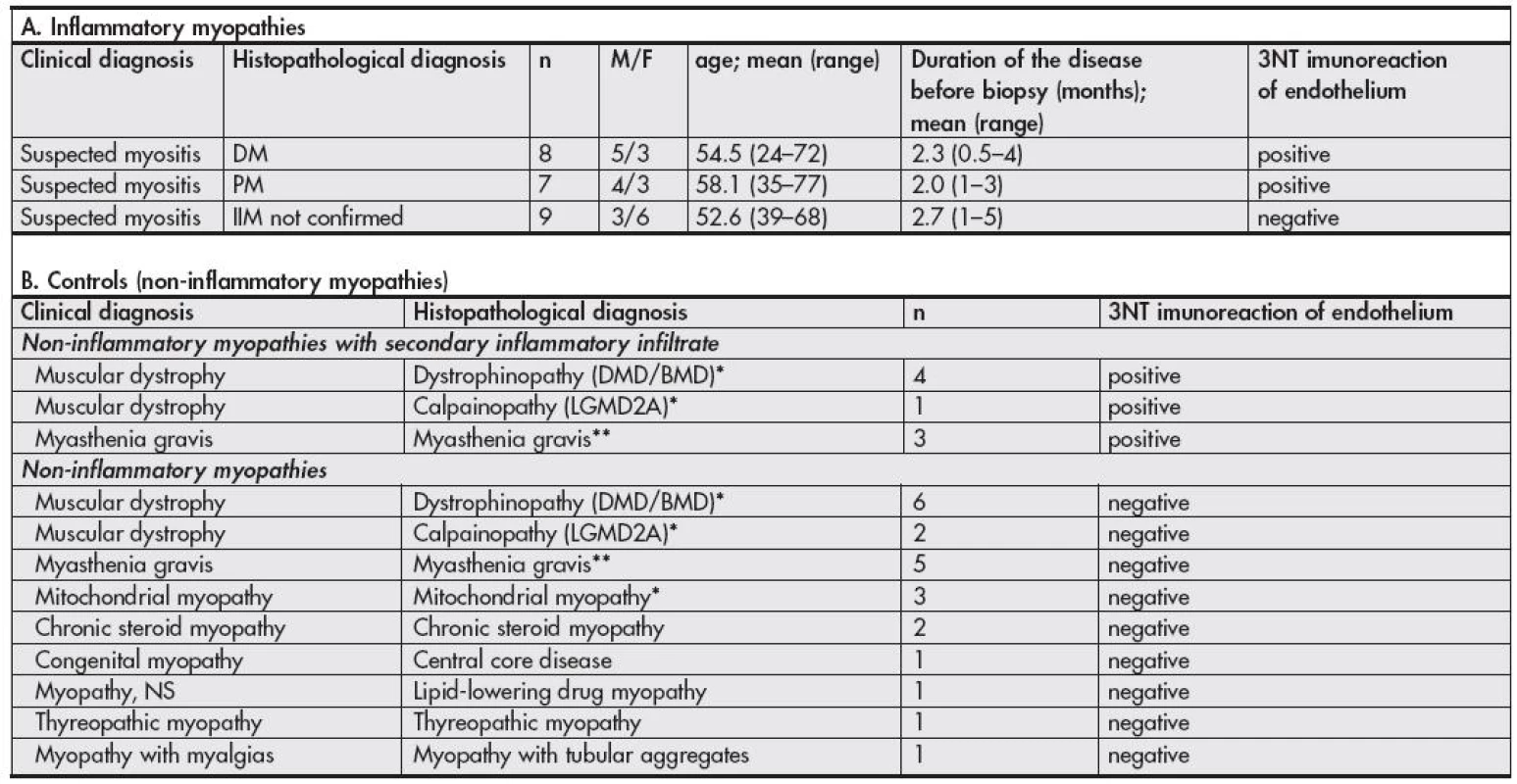
Muscle biopsies were obtained from 54 patients (see Table 1). 24 patients were referred to the muscle biopsy for the clinical suspicion from IIM and it was verified by muscle biopsy in 15 cases (8 DM, 7 PM). Only patients that were not treated before the biopsy were included into the study. The control group consisted of 30 muscle biopsies of cases with confirmed non-inflammatory myopathies, from which in 8 cases secondary lymphocytic infiltrates were found in the muscle biopsy – in five myodystrophies and in three cases of myasthenia gravis.
Muscle biopsy
Muscle tissues were snap frozen in isopentane (2-methylbutane, Sigma-Aldrich Co.) cooled in liquid nitrogen. Cryosections were examined by routine hematoxylin-eosin staining and a conventional spectrum of histochemical reactions, including myofibrillary ATPase, nicotinamide adenine dinucleotide-tetrazolium reductase, succinate dehydrogenase and cytochrome C oxidase (12). Immunohistochemistry was used according to standard protocols to phenotype lymphocytes using antibodies against the antigens CD20, CD3, CD8, CD4 and to examine the expression of sarcolemmal and nuclear proteins associated with myodystrophies (including dystrophine, sarcoglycans, merosin, dysferlin, caveolin and emerin; described in detail elsewhere (11)). Expression of HLA-I antigens (clone W6/32, dilution 1 : 900; Dako, Co.) as well as of membrane attack complex of complement (MAC, C5b-9; clone aE11, dilution 1 : 100; Dako, Co.), which were shown to be helpful in the diagnosis of myositis (2), was also examined immunohistochemically. The antigen-antibody complexes were visualized by biotin-streptavidin detection systems (LSAB2 System, Dako Co.).
The histopathological diagnosis of IIM was done based on the criteria outlined by the recommendation of the 119th European Neuro Muscular Centre International Workshop (13) (Table 2, Fig. 1A,B)

3NT immunohistochemistry
The novel anti-3NT monoclonal antibody 60-E3 was developed by immunizing mice with nitrated BSA (bovine serum albumin) as described previously (14). Three tissue sections from each case were incubated with the primary antibody 60-E3 diluted 1 : 100 in the antibody diluent (DakoCytomation, Co., cat. no. S2022, ready to use) for 30 min at 37°C. After repeated washes with phosphate buffered saline the antigen-antibody complexes were visualized using EnVision™+ Single Reagents (DakoCytomation, Co.). We used the system following the long protocol (30 min) that offers extremely high sensitivity. The sections were washed in Tris-buffered saline for 5 min and 3,3’-diaminobenzidine was used as a chromogenic substrate. The sections were counterstained slightly with hematoxylin and mounted into permanent mounting media. Negative controls were provided by omission of the primary antibody and by using primary isotype controls in corresponding concentration.
RESULTS
The results are summarized in Table 1. We observed strong 3NT immunostaining localized to endothelial cells and their close surrounding (Fig. 1) in muscle biopsies of DM and PM patients, but only in those areas of tissue sections where inflammatory cell infiltrates were present. No 3NT positivity was found in tissue sections of clinically suspected IIM patients without inflammatory changes in the studied tissue sample areas as well as in muscle tissue sections of patients with non-inflammatory myopathies. Surprisingly, the endothelial cells and their close surrounding were also positive in cases of non-inflammatory myopathies with secondary lymphocytic infiltration (Fig. 2).

DISCUSSION
To our knowledge, the immunolocalization of 3NT in inflammatory myopathies was not studied so far. We demonstrated 3NT immunostaining of endothelial cells in the inflamed muscle tissue of both the PM and the DM patients, however, also in the vicinity of secondary lymphocytic infiltrates in some cases of the non-inflammatory myopathies.
Although we did not approach positive identification of NOS isoforms responsible for the generation of the corresponding NO, we can speculate that iNOS represents the particular NOS subtype. While cells containing constitutively expressed NOS produce small amounts of NO (picomoles) in response to receptor stimulation quickly and transiently, cells that contain iNOS produce large amounts of NO for a prolonged period. It is known that induction of iNOS may occur in the activated endothelial cells (15) and we have observed 3NT positivity in endothelia restricted solely to the inflamed areas.
The reaction of peroxynitrite with free or protein-bound tyrosine that produces 3-nitrotyrosine is nonspecific and occurs randomly with the first tyrosine that peroxynitrite encounters (9). The generation of peroxynitrite depends not only on an expression and activity of NOS but also on an expression of many enzyme systems that determine the level of superoxide; furthermore, tyrosine can be nitrated also by several other, peroxynitrite-independent reactions (16). Thus, 3NT cannot be regarded as a simple footprint of NO generation but rather as a potential measure of exposure to oxidative stress, an imbalance between the generation and removal of “reactive oxygen and nitrogen species”. Our results are consistent with an expectation that the strongest 3NT immunoreaction should be observed at the site of generation of reactive nitrating species (i.e., in the endothelium) and that the diffuse and continuously weaker staining of regions surrounding strongly positive endothelial cells corresponds likely to the penetration of reactive nitrating species from the site of their generation to the surrounding tissues limited by their brief biological half-lives.
In summary, the immunolocalization of 3NT was exclusively localized to endothelial cells and their close surrounding in inflamed areas of IIM studied and to endothelial cells near the secondary lymphocytic infiltration in cases of non-inflammatory myopathies. Despite the pathogenetic significance, we conclude that the 3NT immunohistochemistry is of low diagnostic value for the distinction between the subtypes of inflammatory myopathies in muscle biopsy. Moreover, the 3NT immunopositivity of endothelial cells in cases of myositis-mimicking non-inflammatory myopathies disqualifies the 3NT immunohistochemistry as an aid to the differential diagnosis of IIM.
ACKNOWLEDGEMENTS
Supported by the Research Projects of the Ministry of Health of the Czech Republic No. VZ MZOFNM2005 and VZ No. 00023728.
Correspondence
address:
Josef Zámečník, M.D., Ph.D.
Department
of Pathology and Molecular Medicine,
Charles University, 2nd Medical Faculty and University Hospital Motol,
V Uvalu 84, 15006 Prague, Czech Republic
tel.:
+420 224 435 635
e-mail:
josef.zamecnik@lfmotol.cuni.cz
REFERENCES
1. Dalakas MC, Hohlfeld R. Polymyositis and dermatomyositis. Lancet 2003; 362(9388): 971–982.
2. Vogel H, Zámečník J. Diagnostic immunohistology of muscle diseases. J Neuropathol Exp Neurol 2005; 64(3): 181–193.
3. DePaepe B, Racz GZ, Schröder JM, DeBleecker JL. Expression and distribution of the nitric oxide synthases in idiopathic inflammatory myopathies. Acta Neuropathol 2004; 108(1): 37–42.
4. De Paepe B, Creus KK, De Bleecker JL. Role of cytokines and chemokines in idiopathic inflammatory myopathies. Curr Opin Rheumatol 2009; 21(6): 610–616.
5. Creus KK, De Paepe B, De Bleecker JL. Idiopathic inflammatory myopathies and the classical NF-kappaB complex: current insights and implications for therapy. Autoimmun Rev 2009; 8(7): 627–631.
6. Stamler JS, Meissner G. Physiology of nitric oxide in skeletal muscle. Physiol Rev 2001; 81(1): 209–237.
7. Clancy RM, Amin AR, Abramson SB. The role of nitric oxide in inflammation and immunity. Arthritis Rheum 1998; 41(7): 1141–1151.
8. Reiter CD, Teng R-J, Beckman JS. Superoxide reacts with nitric oxide to nitrate tyrosine at physiological pH via peroxynitrite. J Biol Chem 2000; 275(42): 32460–32466.
9. Herce-Pagliali C, Kotecha S, Shuker DEG. Analytical methods for 3-nitrotyrosine as a marker of exposure to reactive nitrogen species. Nitric Oxide 1998; 2(5): 324–336.
10. Tidball JG, Wehling-Henricks M. Damage and inflammation in muscular dystrophy: potential implications and relationships with autoimmune myositis. Curr Opin Rheumatol 2005; 17(6): 707–713.
11. Zámečník J, Veselý D, Jakubička B, et al. Muscle lymphocytic infiltrates in thymoma-associated myasthenia gravis are phenotypically different from those in polymyositis. Neuromuscul Disord 2007; 17(11-12): 935–942.
12. Sheehan DC, Hrapchak BB. Theory and practice of histotechnology. 2nd ed. Columbus, Ohio: Mosby; 1987.
13. Hoogendijk JE, Amato AA, Lecky BR et al. 119th ENMC international workshop: trial design in adult idiopathic inflammatory myopathies, with the exception of inclusion body myositis, 10–12 October 2003, Naarden, The Netherlands. Neuromuscul Disord 2004; 14(5): 337–345.
14. Niederlová J, Kodetová D, Kryštůfková O, Vilím V. Imunolokalizace 3-nitrotyrosinu v synoviální tkáni. Cesk Revmatol 2006; 14(2): 49–52.
15. Middleton J, Americh L, Gayon R, et al. Endothelial cell phenotypes in the rheumatoid synovium: activated, angiogenic, apoptotic and leaky. Arthritis Res Ther 2004; 6(2): 60–72.
16. Gow AJ, Farkouh CR, Munson DA, Posencheg MA, Ischiropoulos H. Biological significance of nitric oxide-mediated protein modifications. Am J Physiol Lung Cell Mol Physiol 2004; 287(2): 262–268.
Labels
Anatomical pathology Forensic medical examiner ToxicologyArticle was published in
Czecho-Slovak Pathology

2011 Issue 2
-
All articles in this issue
- Viral hepatitis at the beginning of the 21th century – value of liver biopsy in the context of development of new non-invasive diagnostic methods and in relation to the modern therapy of chronic viral hepatitis
- Prague Hepatology Meeting 2010 – hepatopathology news
- How to improve the histopathological diagnosis of hepatocellular carcinoma in daily practice?
- Fibrosing cholestatic hepatitis – disease not only of transplanted patients. A report of eight cases
- Pseudoangiomatous stromal hyperplasia with giant cells in the female breast. No association with neurofibromatosis?
- Immunolocalization of protein-bound 3-nitrotyrosine in inflammatory myopathies
- Czecho-Slovak Pathology
- Journal archive
- Current issue
- About the journal
Most read in this issue
- Fibrosing cholestatic hepatitis – disease not only of transplanted patients. A report of eight cases
- Viral hepatitis at the beginning of the 21th century – value of liver biopsy in the context of development of new non-invasive diagnostic methods and in relation to the modern therapy of chronic viral hepatitis
- Pseudoangiomatous stromal hyperplasia with giant cells in the female breast. No association with neurofibromatosis?
- How to improve the histopathological diagnosis of hepatocellular carcinoma in daily practice?
