Clostridium difficile infection and colonisation in children under 3 years of age: prospective comparative study
Infekce a kolonizace Clostridium difficile u dětí do 3 let věku: prospektivní srovnávací studie
Cíl: Navzdory narůstajícímu trendu klostridiové kolitidy a vysoké míře kolonizace Clostridium difficile mezi mladšími dětskými pacienty, malé dětí zůstávají k nemoci poměrně rezistentní. Cílem studie bylo rozlišit, zda existuje rozdíl v klostridiové kolitidě mezi dětmi s průjmem a bez průjmu, určit počet záchytů klostridiové kolitidy ve sledované skupině, zhodnotit závažnost klostridiové kolitidy u dětí do 3 let věku s průjmem.
Metody: Prospektivní hodnocení bylo vedeno od května 2015 do června 2016. Děti mladší 3 let byly zapsány do dvou skupin. Každý vzorek stolice byl testován dvoustupňovým algoritmem zahrnující imunochromatografický test a polymerázovou řetězovou reakci.
Výsledky: Zapsáno 147 pacientů s průjmem a 75 kontrol. Počet záchytů klostridiové kolitidy u dětí s průjmem byl 2 % (3/147), počet záchytů toxigenních kmenů ve skupině s průjmem ve srovnání s kontrolní skupinou byl 11,6 % (3/147) vs. 10,6 % (8/75) (p < 0,9999).
Závěr: Nebyl pozorován žádný významný rozdíl mezi dětmi s průjmem a bez průjmu. Nedoporučujeme cíleně vyšetřovat děti na Clostridium difficile při absenci rizikových faktorů.
Klíčová slova:
deti – novorozenci – průjem – pediatrie – kolonizace – Clostridium difficile
Authors:
V. Musil 1; L. Homola 1; M. Vrba 2; A. Braunová 1; T. Kravalová 1; M. Malá 1; L. Krbková 1
Authors‘ workplace:
Department of Pediatric Infectious Diseases, Faculty of Medicine, Masaryk University, University Hospital, Brno, Czech Republic
1; Department of Clinical Microbiology, University Hospital, Brno, Czech Republic
2
Published in:
Epidemiol. Mikrobiol. Imunol. 68, 2019, č. 2, s. 59-64
Category:
Original Papers
Overview
Aims: Despite an increasing trend in Clostridium difficile infections (CDI) and high C. difficile colonization rate especially among younger children, infants remain quite resistant to the disease. The goals of this study were to distinguish whether there exists a difference in CDI between children with or without diarrhoea, ascertain the prevalence of CDI, and assess CDI severity in children under 3 years with diarrhoea in our institution.
Methods: A prospective study was conducted from May 2015 to June 2016. Children 3 years of age or younger were enrolled and into two groups. Every faecal sample was tested using a diagnostic two-step screening algorithm including an immunochromatographic test and polymerase chain reaction.
Results: The study enrolled 147 children with diarrhoea and 75 control patients. The prevalence of CDI in children with diarrhoea was 2% (3/147), the prevalence of toxigenic C. difficile in the diarrhoeal group compared to the control group was 11.6 % (17/147) vs. 10.6% (8/75) (p < 0.9999).
Conclusions: No significant difference was observed between infants with diarrhoea and the control group. We recommend not examining for C. difficile children not exhibiting specific risk factors.
Keywords:
body mass index – diarrhoea – Clostridium difficile – Neonates – paediatrics – colonisation
INTRODUCTION
Clostridium difficile is a non-invasive bacteria, the pathogenesis of which is based upon the action of enterotoxin A and cytotoxin B binding to receptors of intestinal epithelial cells. The toxins mediate intestinal disease with a broad spectrum of clinical manifestations ranging from mild diarrhoea to life-threatening disease. The development CDI requires disruptions in the human intestinal microbiota [1–3].
Epidemiology and severity of C. difficile infections have been widely studied in adults. Some reports assume that CDI in the child population is on the rise, but recent study dispute that there is an upward trend [4–7]. A considerable role in C. difficile’s changing epidemiology has been attributed to the discovery of an epidemic hypervirulent strain, North American Pulsed Field Type 1, ribotype 027 (NAP1), which has been responsible for outbreaks [8–10]. Other ribotypes have been revealed in subsequent years. In relation to the hypervirulent strains, a third toxin, the so-called binary toxin, has been uncovered [1, 11].
Despite the high colonization rate, infants remain quite resistant to the disease. The mechanism of that resistance, however, is obscure. Some hypotheses suppose that the cellular components essential for attachment of the toxin are absent or that infants lack appropriate toxin receptors on the bowel mucous membrane, as some studies have demonstrated on animal models [12–14].
The aims of the present study were to determine whether or not there exist differences between children with and without diarrhoea, ascertain the prevalence of CDI, and assess the severity of CDI within our institution in children under 3 years of age with diarrhoea.
MATERIALS AND METHODS
Patients and samples
We performed a prospective case-control study on hospitalized children (≤ 3 years of age) with or without diarrhoea. The study was conducted from 11 May 2015 to 11 June 2016 in the Department of Paediatric Infectious Diseases, University Hospital Brno. This 60-bed hospital facility provides paediatric outpatient and inpatient care, including intensive care, for children younger than 19 years of age with a spectrum of paediatric diagnoses. Children ≤ 3 years of age were enrolled and separated into two groups and several subgroups. The first group was composed of patients investigated and admitted due to diarrhoeal disease regardless of the final diagnosis (diarrhoea as a sign of intestinal or extra-intestinal cause). Inpatients without diarrhoea were enrolled into the control group. Inpatients with varying final diagnoses (respiratory infection, infectious mononucleosis, tonsillitis, scarlet fever, cervical lymphadenitis, staphylococcal scalded skin syndrome, herpetic stomatitis, chickenpox, urinary infections, aseptic meningitis, influenza, toxoallergic rash) were recruited at our institution. The subgroups were divided according to age: neonates (defined as infants aged 28 days or younger), infants ≤ 1 year old, 1–2 years old, and 2–3 years old. Only one faecal specimen was obtained from each patient. In the first group, the faecal samples were submitted at the beginning of hospitalization (1st or 2nd day). In the second group, the samples were collected whenever possible. Two infants required therapy in the intensive care unit.
Inclusion and exclusion criteria
The criteria for inclusion into the diarrhoea patients group was defined as a presence of liquid stools with defecation frequency of 3 or more stools within 24 hours or more frequently than is normal for the individual on the basis of a personal history. The stool frequency was ascertained from the patient’s parent. In neonates, diarrhoea was evaluated as a change in stool calibre. The control group was determined by absence of diarrhoea before admission to the hospital. CDI was defined as presence of diarrhoea and a positive stool test result for toxigenic C. difficile (IT toxin/PCR gene or toxin +) and with exclusion of non-C. difficile enteropathogenic organisms. Severe CDI was defined as a patient with CDI who fulfilled the following criteria within 30 days of symptom onset: history of admission to the intensive care unit (ICU), history of surgery for toxic megacolon, motility disorder, perforation or refractory colitis, or death caused by CDI (because of their usefulness, the criteria from Sathyendran et al. were used) [15]. None of the enrolled children required hospitalization for CDI before stool sample collection. Community-acquired CDI was determined by the absence of hospitalization in the previous 3 months and a hospital stay not exceeding 48 h at the time of sampling. Hospital-acquired CDI was determined by a history of hospitalization (less than 3 months) and/or duration of hospitalization lasting more than 48 h at the time of sampling. Children with previous CDI history were excluded.
Data collection
The electronic clinical record was reviewed on the day of stool sampling. Patient data included age, gender, duration of hospitalization, risk factors (hematologic and solid malignancies, hematologic diseases, immunosuppression, solid organ transplantation, inflammatory bowel disease or other gastrointestinal disorders, repeated hospitalizations, previous antibiotic and cytotoxic drug exposure, and gastric acid suppression within 10 weeks of the day of the stool specimen), and details of treatment. Laboratory data including blood test results and additional microbiological testing were recorded. The information was obtained from the medical history record in the hospital’s database.
Detection and identification of C. difficile
Faecal specimens were stored at 4 °C and transported in sterile containers. Stool analysis for C. difficile was conducted within 24 h after stool sampling. Diagnosis of CDI was based on a diagnostic two-step screening algorithm. Each faecal sample was tested by IT for the presence of the GDH and toxins A and B (using TECHLAB C. DIFF QUICK CHEK COMPLETE). Faecal samples that were GDH negative and toxin A/B nega-
tive were evaluated as negative. Stools which were GDH positive and toxin A/B positive were interpreted as positive. Faecal samples that were GDH positive but toxin A/B negative were confirmed by PCR (GeneXpert Clostridium difficile Cepheid) for presence of a gene for toxin B, the binary toxin, and/or tcdC deletion in nucleotide 117. Stools which were GDH positive and PCR toxin B gene positive were interpreted as “suspected CDI” (Toxigenic strains of C. difficile) [16]. Patients with this result are included in a cluster of CDI. None of the stool samples for C. difficile were cultured. Each faecal sample in the first group was also tested for non-C. difficile diarrhoeal pathogens using stool culture and immunochromatographic tests for detection of rotavirus, norovirus, and adenovirus (Immunoquick NoRotAdeno, BIOSYNEX). Recommendations for CDI were followed [16].
Informed consent
Ethical approval was obtained from the University Hospital Ethics Committee. Written informed consent for the University Hospital to sample stools was received from parents or legal guardians at the time of hospital admissions.
Statistical analysis
Children with diarrhoea were compared to control patients with respect to a number of variables. Fisher’s exact test was used as appropriate for comparing categorical variables. Significance level for statistical hypothesis testing was α = 0.05.
RESULTS
Patient demographic data, clinical characteristics, and laboratory results of our samples are presented in Table 1. One hundred and forty-seven patients with diarrhoea and seventy-five control patients were enrolled in the study. The median age was 1.2 years (range from 3 days to 34 months) and 1.5 years (range from 3 days to 36 months) in the first and control groups, respectively. The stool samples were tested for C. difficile over a 13-month period. The results according to sex and age subgroups are not major and are presented in Table 1 and Figure 1.
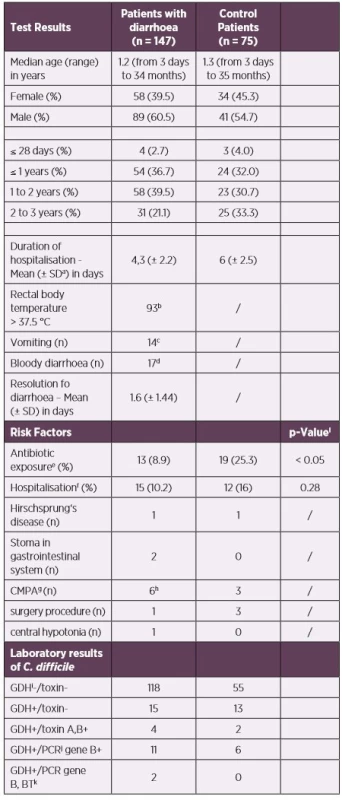
b93/139, missing data for 8 children
c14/137, missing data for 10 children
d17/136, missing data for 11 children
eantibiotic exposure 12 weeks prior to stool collection
fhospitalisation 60 days prior to stool collection
gcow’s milk protein allergy (CMPA)
h1x suspected CMPI
iGDH – Glutamate-dehydrogenase
jPCR – Polymerase Chain Reaction
kbinary toxin
lsignificance level of α = 0.05 for statistical hypothesis testing
Diagnosis of CDI is based on the diagnostic two step-screening
algorithm, which was described in “materials and methods “
(combination of IT and PCR).
Abbreviations: CDI – Clostridium difficile infection, IT –
immunochromatographic test, PCR – polymerase chain reaction
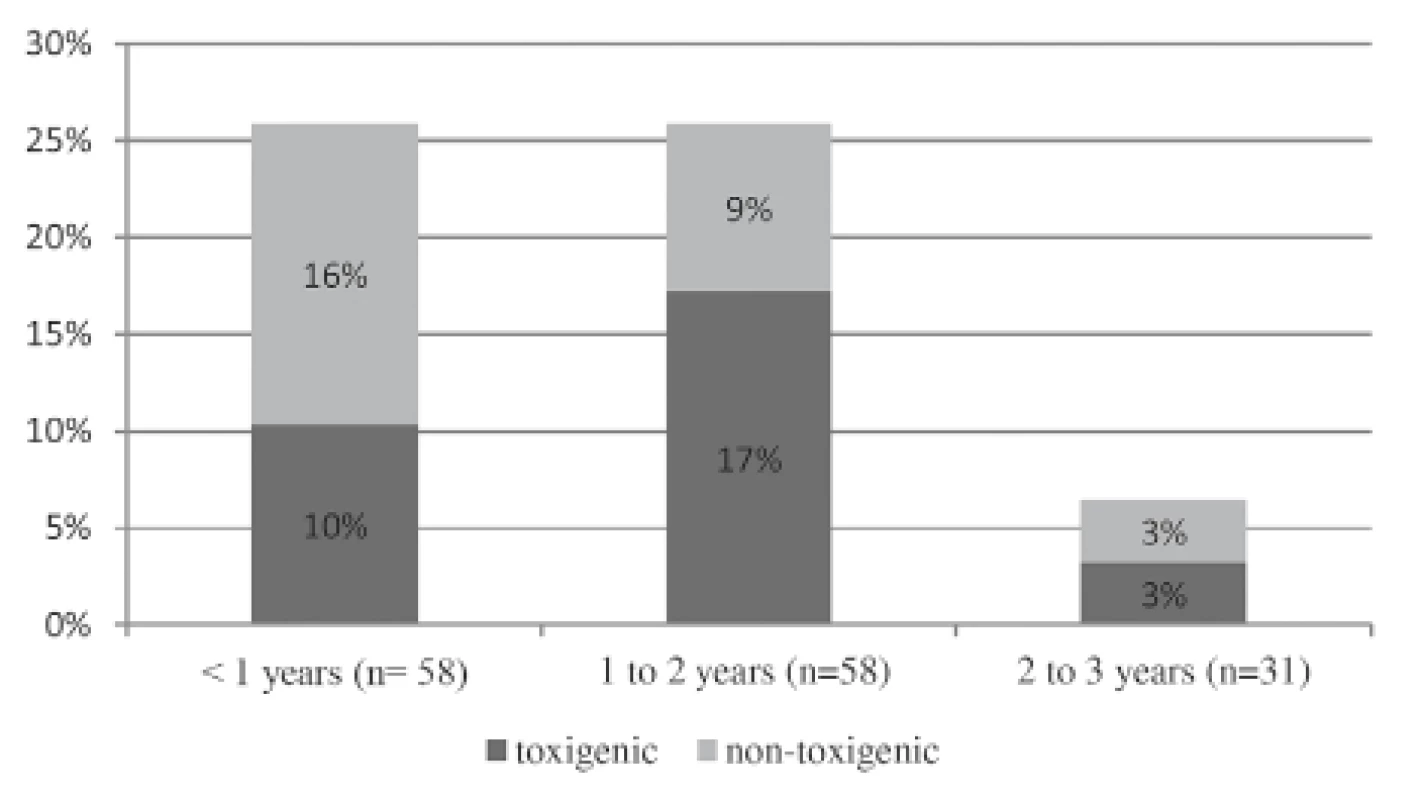
Among symptomatic children, 11.6% (n = 17) were toxigenic C. difficile izolates (see Table 1), including 2.7% (n = 4) of samples detected by IT and 7.5% (n = 11) confirmed by PCR, while in 1.4% (n = 2) of cases the binary toxin was revealed but no deletion in nucleotide 117 was presented. The CDI definition was fulfilled in 2% (n = 3) of patients. One child might be categorized under hospital-acquired C. difficile. By comparison, in the control group, of the total 10.7% (n = 8) toxigenic C. difficile isolates, 2.7% (n = 2) were toxin A/B positive while gene for toxin B was detected in 8% (n = 6) of stools. Binary toxin and deletion in nucleotide 117 were not recorded in any samples.
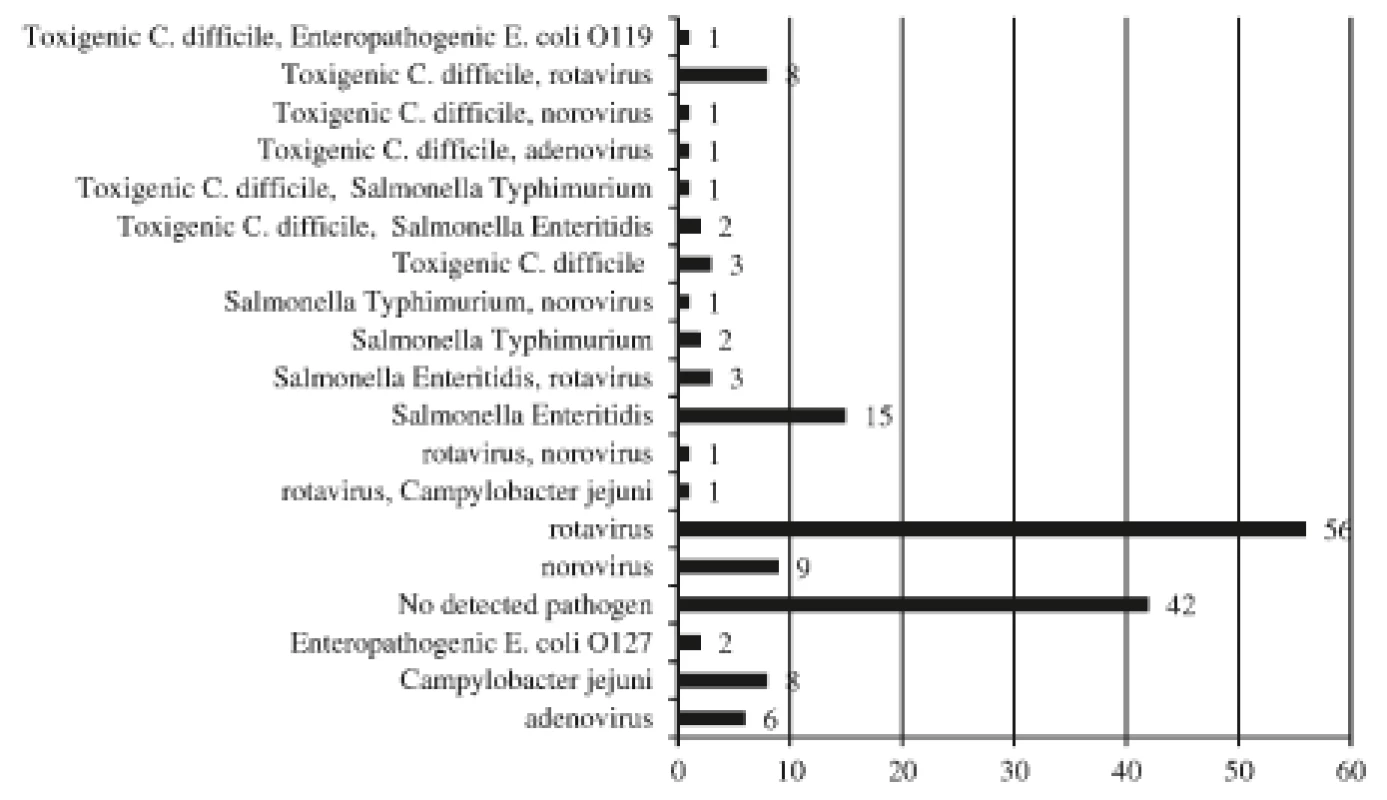
Altogether non C. difficile intestinal pathogens were discovered in 106 stool samples (data are summarized in Figure 2) 72% (76/106) of which were viral agents. Of 17 positive C. difficile toxin specimens, a parallel bowel pathogen was detected in 14 stools (rotavirus, n = 8; norovirus, n = 1; adenovirus, n = 1; Salmonella enteritidis, n = 2; Salmonella typhimurium, n = 1; enteropathogenic Escherichia coli, n = 1) while no pathogen was revealed in the 3 remaining stool samples. Salmonella enteritidis was cultivated from 1 infant with diarrhoeal disease (GDH+/PCR gene B+), but the same bacterium had been detected during a previous hospitalization. The infection could have been due to intestinal carriage, the patient was excluded from the CDI group.
(n = 147)
The numbers of agents, some children had multiple aetiologies.
Among clinical symptoms in children with toxigenic C. difficile isolates (n = 17) during hospitalisation (the results are presented in Table 2), the majority (n = 13) had fever, vomiting occurred in 6 patients, and bloody diarrhoea was observed in 2 children. Considering the age group concerned, abdominal pain was not assessed. Seven patients had common complications of dehydration – hypoglycaemia, mineral disturbances, high levels of nitrogen-containing compounds (urea, creatinine). In one child with underlying comorbidities (Down syndrome, Hirschsprung’s disease), transversostomy, repeated hospitalizations, and antibiotic treatment in last month (cefotaxime, metronidazole, gentamicin, co-trimoxazole), intensive care unit therapy and specific CDI treatment (colectomy + fidaxomicin) of the child was required. A colonoscopy was conducted, whereby inflammatory changes and superficial ulcers in the intestinal lining were described. In other children, endoscopy, X-ray, and ultrasonography were not required. The patient met the CDI criteria for severe form. The remaining 2 patients (who met the criteria for likely CDI) did not need treatment because their diarrhoeal infections were self-limited. One infant had gastrostomy and VACTERL syndrome. No risk factor was revealed in the remaining case (other risk factors are listed in Table 1 and Table 2).
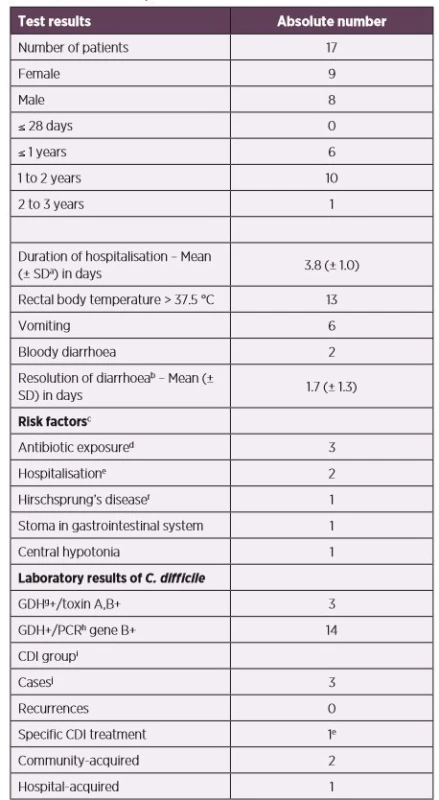
bself-limited resolution without specific CDI treatment
csome patients had more risk factors
dantibiotic exposure 12 weeks prior to stool collection
ehospitalisation 60 days prior to stool collection
fthe combination of partial colectomy (with a colostomy) and fidaxomicin 200 mg
twice daily for 10 days
gGDH – Glutamate-dehydrogenase
hPCR – Polymerase Chain Reaction
iCDI (Clostridium difficile infection) definition is determined in the „Materials and
Methods“
jone boy (age: 2.1 years), two girls (age: 1.3 and 1.5 years, respectively)
DISCUSSION
In the chosen age group, the colonization rate has been shown to be high despite low morbidity [6, 7, 12, 13, 15]. Although earlier national guidelines had discouraged stool testing for C. difficile in younger infants, the updated recommendations have considered association with CDI in every child group. Unfortunatelly the global guidelines are typically based on adult patients, not children [6, 7, 10, 15, 17–19].
The human intestine is sterile at birth. By 1 year of age, child and adult intestinal microbiota become similar [20]. Studies reported an average C. difficile colonization rate of 37% of stools in neonates (in the first 28 days after birth) with wide variations [20,21] Between 1 and 12 months of age, colonization declines to an approximate rate of 10% of children. After the first year of life, the asymptomatic carriage appears to approximate the colonization rate in adults (3–4%) [2, 20, 22]. The high colonization rate in infancy has been attributed to lower capacity of bowel microflora to inhibit C. difficile growth [23]. It is also affected by infant nutrition [20, 24]. In our study, the total prevalence of toxigenic C. difficile strains in the diarrhoeal group compared to that of the control group was 11.6% (17/147) vs. 10.7% (8/75), which was statistically insignificant (p > 0.9999). We suggest there is a minimal causal relationship between the presence of toxin and diarrhoea. Our findings could be influenced is fact that the majority of those individuals enrolled were otherwise healthy infants with an absence of risk factors. In other study, Sathyendran et al. had reported 15.6% positive stool specimens among patients with median age of 1.2 years, of which 28% were toxin-positive and 72% were PCR positive [15]. The toxigenic C. difficile rate had been influenced by the recruited sample of children. The detection of toxigenic C. difficile seems to occur fairly frequently, and positive results must be interpreted while viewing each case in a comprehensive manner.
It is generally presumed that the majority of CDI cases in children are attributable to colonization. No clear definition of CDI in this age group yet exists [7]. Detection of CDI has been complicated by an absence of reliable faecal biomarkers for CDI [25]. In our study, the overall prevalence of CDI in children with diarrhoea was 2% (3/147), while 17.6% (3/17) of children (with positive toxin/gene for toxin B in faeces) met the criteria for CDI. Most studies have included epidemiology of toxigenic C. difficile strains without evaluating the individual CDI cases or have been conducted among children of different age groups (different research design). Sathyendran et al. published outcomes using the same molecular methods and algorithm, determining that 14% (46/320) of children (median age of 1.2 years) met criteria for CDI [15]. This distinction could be due to a higher proportion of children within risk groups. Sixty-three percent of those patients were treated as compared to 33% (1/3) in our study. The main contributing reason can be seen in the elected sample of patients [15].
Although older studies had reported a high colonization rate of toxigenic strains in neonates [20], our study found no neonates testing positive for the presence of toxigenic C. difficile strains. Unfortunately, the number of enrolled neonates was low (n = 7). Similar findings had been presented by Rousseau et al. [21], with only 4.8% of positive samples, and by Sathyendran et al. [15], where the prevalence remained at 2.6% (1/39) despite a longer hospitalization period. Those authors had hypothesized prevention practices (e.g. hand hygiene) to be a contributing factor [15]. Moreover, the current diagnostic algorithm should be more sensitive. We would also point out the possibility of transient carriage [20] and emphasize the low number of published cases of pseudomembranous colitis in neonates [5].
According to age subgrous in both groups, detection of toxigenic strains was around 10% in infants (≤ 1 year of age), which was comparable to the conclusions from other studies [15, 20, 21]. None of the aforementioned children with toxigenic C. difficile strains fulfilled the CDI definition. The American Academy of Pediatrics recommends that children younger than 1 year of age not be tested unless a child has bowel motility problems or is situated in an outbreak situation [10]. We could not agree more with this statement, because our records did not identify these factors in the enrolled children. In the older age population (1–2 years of age), high toxigenic C. difficile prevalence in the diarrhoeal case group compared to the control group (16.9% and 8.7%, respectively) was surprisingly revealed, but only 20% of those (2/10) in fact met the CDI criteria. In view of the fact that most of the recruited children were otherwise healthy and with an absence of risk factors, the high rate remains a mystery in comparison to other reported data [20, 21]. This conclusion could be partially influenced by the small number of children in the subgroups. The oldest age group exhibited the presumptive low toxigenic rate [20]. Statistical analysis was not beneficial for comparison of the subgroups.
A majority of reported studies highlight previous antibiotic exposure and repeated hospitalization as significant risk factors [6, 7, 15, 17]. An absence of risk factors in the diarrhoeal group can be explained by the method of patient selection, as there is a predominance of viral infections in the selected age groups and low morbidity in the general child population. The significantly higher rate of antibiotic exposure among controls (p < 0.05) might be due to the high level of antibiotic prescriptions among children with respiratory infections during winter and spring months (data are not included in the text). Meanwhile, cow’s milk protein allergy is a controversial risk factor in relation to CDI [6].
Our study has a number of limitations. The timing of stool collection differed between the two groups – symptomatic patients (< 48 hours of admission) and asymptomatic patients (at any time during admission). This difference could have impacted positive results in control group (duration of hospitalization could increase C. difficile colonisation). The majority of those individuals enrolled were otherwise healthy children with an absence of risk factors. Statistical analysis was beneficial only for comparison of the two main groups, while the subgroups, genders and numbers of risk factors were too small for meaningful statistical analysis. Moreover, the reliance on clinical data and medical history could be confounding. The criteria used for determining CDI had limitations. Defecation frequency before hospitalization was based on the parents’ perceptions. Identification of viral pathogens was limited to three viruses – an unknown pathogen could not be excluded. Diarrheagenic parasites were not identified because of their presumed low incidence in developed countries [26]. Generally, CDI severity was not appraised due to the low number CDI cases. The research was conducted solely in our department. It could be difficult to follow the study because of its design.
CONCLUSION
Our findings indicate that C. difficile is a significant but infrequent pathogen in hospitalized children with diarrhoea. Essentially healthy children usually exhibit a favourable co urse of infection. Cases should be evaluated individually in the absence of optimal CDI diagnostics in infants. We recommend not to examine the youngest paediatric populations if children do not exhibit the aforementioned risk factors. Stool testing for non C. difficile bowel pathogens should be emphasized for children ≤ 3 years of age in cases of C. difficile-positive stool samples. There is a need for more contemporary works on C difficile colonization/infection in infants and young children.
List of abbreviations
BT – Binary toxin
C. difficile – Clostridium difficile
CDI – Clostridium difficile infection
EPEC – Enteropathogenic Escherichia coli
GDH – Glutamate-dehydrogenase
IBD – Inflammatory bowel disease
ICU – Intensive care unit
IT – Immunochromatographic test
PCR – Polymerase Chain Reaction
VACTERL (acronym) – refers to the nonrandom co-occurrence of birth defects: Vertebral anomalies, Anal atresia, Cardiac defects, Tracheoesophageal fistula and/or Esophageal atresia, Renal & Radial anomalies and Limb defects
Conflict of interest statement
The authors declare that they have no conflict of interest. This work was supported by the Faculty of Medicine, Masaryk University, Brno, Czech Republic (grant number: MUNI/A/1172/2015) and the University Hospital, Brno, Czech Republic.
Author contributions
All of the authors of this manuscript are responsible for the reported findings. VM collected all clinical data of practical approach. All authors participated in the searching of the published literature for this review; analysis and interpretation of the data; drafting and/or revising and/or making intellectual contributions to the content of the manuscript; All authors approved the final version of the manuscript submitted for publication.
Aknowledgements
We thank a physician Michal Kyr from the Departmentof Pediatric Oncology for performing of statistical analysis.
This manuscript has not been previously published and is not under consideration for publication elsewhere.
Do redakce došlo dne 23. 9. 2018.
Adresa pro korespondenci:
MUDr. Václav Musil
Fakultní nemocnice Brno
Jihlavská 20
625 00 Brno
e-mail: Musil.Vaclav@fnbrno.cz
Sources
1. Rineh A, Kelso MJ, Vatansever F, et al. Clostridium difficile infection: molecular pathogenesis and novel therapeutics. Expert Rev Anti Infect Ther, 2014;12 : 131–150.
2. Nicholson MR, Thomsen IP, Edwards KM. Controversies Surrounding Clostridium difficile Infection in Infants and Young. Children, 2014;1 : 40–47.
3. Surawicz C, Brandt LJ, Binion DG, et al. Guidelines for Diagnosis, Treatment, and Prevention of Clostridium difficile Infections. Am J Gastroenterol, 2013;108 : 478–498.
4. Dulęba K, Pawłowska M, Wietlicka-Piszcz M. Clostridium difficile infection in children hospitalized due to diarrhea. Eur J Clin Microbiol Infect, 2014;33 : 201–209.
5. Zilberberg MD, Shorr AF, Kollef MH. Increase in Adult Clostridium difficile–related Hospitalizations and Case-Fatality Rate, United States, 2000–2005. Emerg Infect Dis, 2008;14 : 929–931.
6. Pai S, Aliyu SH, Enoch DA, et al. Five Years Experience of Clostridium difficile Infection in Children at a UK Tertiary Hospital: Proposed Criteria for Diagnosis and Management. PLoS ONE, 2012;7 : 51728.
7. Faust SN, Wilcox MH, Banaszkiewicz A, et al. Lack of evidence for an unmet need to treat Clostridium difficile infection in infants aged <2 years: expert recommendations on how to address this issue. Clin Infect Dis, 2015;60 : 912–918.
8. O’Connor JR, Johnson S, Gerding DN. Clostridium difficile Infection Caused by the Epidemic BI/NAP1/027 Strain. Gastroenterology, 2009;136 : 1913–1924.
9. Warny M, Pepin J, Fang A, et al. Toxin production by an emerging strain of Clostridium difficile associated with outbreaks of severe disease in North America and Europe. The Lancet, 2005;366 : 1079–1084.
10. Schutze GE, Willoughby RE. Committee on Infectious Diseases & American Academy of Pediatrics. Clostridium difficile infection in infants and children. Pediatrics, 2013;131 : 196–200.
11. Gerding DN, Johnson S, Rupnik M, et al. Clostridium difficile binary toxin CDT: mechanism, epidemiology, and potential clinical importance. Gut Microbes, 2014;5 : 15–27.
12. Eglow R, Pothoulakis C, Itzkowitz S, et al. Diminished Clostridium difficile toxin A sensitivity in newborn rabbit ileum is associated with decreased toxin A receptor. J Clin Invest, 1992;90 : 822–829.
13. Keel MK, Songer JG. The distribution and density of Clostridium difficile toxin receptors on the intestinal mucosa of neonatal pigs. Vet Pathol, 2007;44 : 814–822.
14. Antonara S, Leber AL. Diagnosis of Clostridium difficile Infections in Children. J Clin Microbiol 2016;54 : 1425–1433.
15. Sathyendran V, McAuliffe GN, Swager T, et al. Clostridium difficile as a cause of healthcare-associated diarrhoea among children in Auckland, New Zealand: clinical and molecular epidemiology. Eur J Clin Microbiol Infect Dis, 2014;33 : 1741–1747.
16. Beneš J, Husa P, Nyč O, et al. Diagnosis and therapy of Clostridium difficile infection: Czech national guidelines. Klin Mikrobiol Inf Lek, 2014;20 : 56–66.
17. Sandora TJ, Fung M, Flaherty K, et al. Epidemiology and Risk Factors for Clostridium difficile Infection in Children. Pediatr Infect Dis J, 2011;30 : 580–584.
18. Wultańska D, Banaszkiewicz A, Radzikowski A, et al. Clostridium difficile infection in Polish pediatric outpatients with inflammatory bowel disease. Eur J Clin Microbiol Infect Dis, 2010;29 : 1265–1270.
19. Wendt JM, Cohen JA, Mu Y, et al. Clostridium difficile infection among children across diverse US geographic locations. Pediatrics, 2014;133 : 651–658.
20. Jangi S, Lamont JT. Asymptomatic colonization by Clostridium difficile in infants: implications for disease in later life. J Pediatr Gastroenterol Nutr, 2010;51 : 2–7.
21. Rousseau C, Lemée L, Le Monnier A, et al. Prevalence and diversity of Clostridium difficile strains in infants. J Med Microbiol, 2011;60 : 1112–1118.
22. Schäffler H, Breitrück A. Clostridium difficile – From Colonization to Infection. Front Microbiol, 2018;9 : 646.
23. Adlerberth I, Huang H, Lindberg E, et al. Toxin-producing Clostridium difficile strains as long-term gut colonizers in healthy infants. J Clin Microbiol, 2014;52 : 173–179.
24. Penders J, Vink C, Driessen C, et al. Quantification of Bifidobacterium spp., Escherichia coli and Clostridium difficile in faecal samples of breast-fed and formula-fed infants by real-time PCR. FEMS Microbiol Lett, 2005;243 : 141–147.
25. El Feghaly RE, Stauber JL, Tarr PI, et al. Intestinal inflammatory biomarkers and outcome in pediatric Clostridium difficile infections. J Pediatr, 2013;163 : 1697–1704.e2.
26. Klein EJ, Boster DR, Stapp JR, et al. Diarrhea etiology in a Children’s Hospital Emergency Department: a prospective cohort study. Clin Infect Dis, 2006;43 : 807–813.
Labels
Hygiene and epidemiology Medical virology Clinical microbiologyArticle was published in
Epidemiology, Microbiology, Immunology

2019 Issue 2
-
All articles in this issue
- Determination of antimicrobial activity of Achatina reticulata slime
- Surveillance of antibiotic resistance of Streptococcus pneumoniae in the Czech Republic, respiratory study results, 2010–2017
- Tularemia – zoonosis carrying a potential risk of bioterrorism
- Actinomycosis – an umbrella review and three case reports of severe pelvic actinomycosis treated conservatively
- In-vivo interspecies transmission of carbapenemase KPC in a long-term treated female patient
- Diagnosis and treatment of Bartonella endocarditis
- Clostridium difficile infection and colonisation in children under 3 years of age: prospective comparative study
- Reduction of Thymoglobuline from 7.5 mg/kg to 6 mg/kg in conditioning regimen extended time to the first cytomegalovirus detection after allogenic haematopoietic stem cell transplantation
- Epidemiology, Microbiology, Immunology
- Journal archive
- Current issue
- About the journal
Most read in this issue
- Actinomycosis – an umbrella review and three case reports of severe pelvic actinomycosis treated conservatively
- In-vivo interspecies transmission of carbapenemase KPC in a long-term treated female patient
- Tularemia – zoonosis carrying a potential risk of bioterrorism
- Clostridium difficile infection and colonisation in children under 3 years of age: prospective comparative study
