Characterization of residual coronary sinus‑related tachycardia during ablation of longstanding persistent atrial fibrillation
Charakteristika reziduálních tachykardií spojených s koronárním sinem během ablace dlouhodobé perzistentní fibrilace síní
Cíl:
Cílem práce je charakteristika tachykardií spojených s koronárním sinem (CS), které se u pacientů s primární dlouhodobou perzistentní fibrilací síní (FS) objevily jako poslední reziduální arytmie a které k obnovení sinusového rytmu (SR) vyžadovaly ablaci v CS nebo ve velké srdeční žíle.
Metodika:
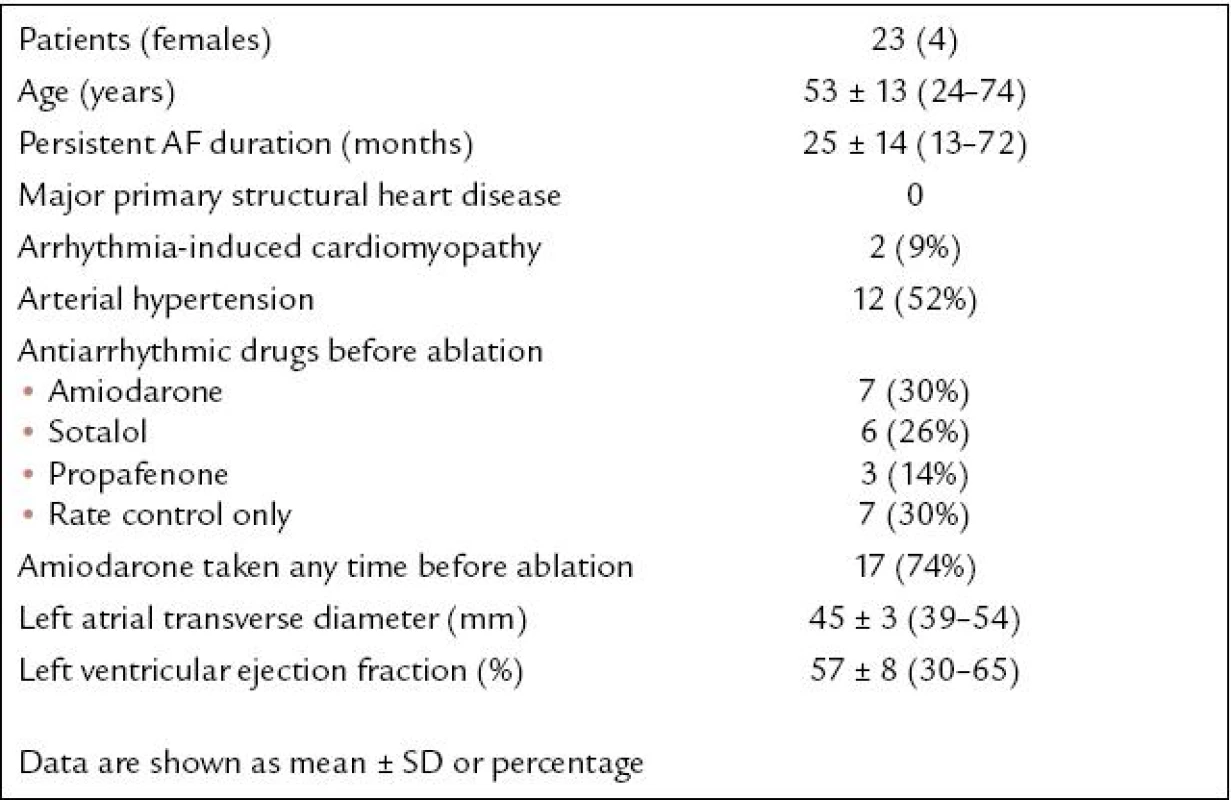
Práce zahrnula 23 pacientů, kteří představovali 23 % z 99 pacientů, u nichž byl sinusový rytmus obnoven vlastní ablací. Lokalizovaná reentry tachykardie omezená na muskulaturu CS byla pozorována u 8 (35%) pacientů, zatímco perimitrální reentry tachykardie byla zaznamenána u 14 (61%) pacientů. Dvacet (87%) pacientů zůstávalo bez recidiv arytmie bez antirytmik I. nebo III. třídy po dobu 33 ± 10 (12 – 53) měsíců sledování.
Závěr:
Většina reziduálních tachykardií souvisejících s CS jeví vlastnosti reentry, přičemž jedna třetina využívá myokard CS jako substrát pro reentry nezávislý na síňovém myokardu.
Klíčová slova:
fibrilace síní – perzistentní – ablace – koronární sinus – tachykardie
Authors:
M. Fiala J. Chovančík 1,2 1; D. Wojnarová 1; V. Bulková 1; J. Pindor 1; H. Szymeczek 1; R. Lábrová 2; O. Toman 2; J. Januška 1; J. Špinar 2
Authors‘ workplace:
Department of Cardiology, Heart Center, Hospital Podlesí, Třinec, head prim. Marian Branny, M. D.
1; Department of Cardiology Medical Faculty Masaryk University and University Hospital, Brno, head prof. Jindřich Špinar, M. D., Ph. D., F. E. S. C.
2
Published in:
Vnitř Lék 2011; 57(1): 33-42
Category:
Original Contributions
Overview
Purpose:
The aim was to characterize the coronary sinus (CS)‑related tachycardia that occurred as the last residual arrhythmia and required ablation within the CS or great cardiac vein to restore sinus rhythm (SR) in patients with primary longstanding persistent AF.
Methods:
The study included 23 patients in whom stable SR was restored by ablation inside the vein during the first or repeat ablation.
Results:
The 23 subjects represented 23% of the 99 patients in whom SR was restored by ablation. A reentry tachycardia confined to the CS musculature was suggested in 8 (35%) patients, and a peri ‑ mitral reentry circuit was present in 14 (61%) patients. Twenty (87%) patients have remained free from arrhythmia and class I or III antiarrhythmic drugs for 33 ± 10 (12 – 53) months.
Conclusion:
A majority of the residual CS‑related tachycardias exhibit properties of reentry, one third utilizing the CS musculature as a reentry substrate independent of the atrial myocardium.
Key words:
atrial fibrillation – persistent – ablation – coronary sinus – tachycardia
Introduction
Restoration of sinus rhythm (SR) by ablation of longstanding persistent atrial fibrillation (AF) appears to be an important predictor of better clinical outcome [1,2]. Employing a staged left atrial (LA) ablation strategy, SR restoration is preceded by AF conversion into a residual monomorphic LA tachycardia in a majority of the patients [1,2]. Having commenced ablation at the LA posterior wall around the pulmonary veins (PVs), successful ablation sites are mainly clustered at the LA anterior wall around the LA appendage (LAA), Bachmann’s bundle region (BB), and the inter - atrial septum [1,2]. In some instances, this residual tachycardia requires ablation within the coronary sinus (CS) or great cardiac vein (GCV) [1 – 5].
The purpose of this study is to characterize tachycardias related to the CS musculature that occurred during the first or repeat ablation as a residual arrhythmia resistant to LA endocardial ablation in patients who were referred for catheter ablation of primary longstanding persistent AF.
Methods
Study population
The study population consisted of 23 patients (representing 16% of 140 consecutive patients referred to catheter ablation of primary longstanding persistent AF between January 2005 and June 2008) in whom SR was restored by ablation of the residual tachycardia within the CS or GCV (baseline characteristics prior to the first ablation are listed in Tab. 1). The patients did not have major structural cardiac disease, except for two cases of proved arrhythmia-induced cardiomyopathy. If the tachycardia was affected by ablation within the CS - GCV, however, and the final radiofrequency (RF) energy application restoring SR permanently was deployed endocardially, the patient was excluded. The patients were informed in detail about all aspects of the procedure and gave written consent.
Longstanding persistent AF
Longstanding persistent AF included AF lasting for > 12 months without intervening SR, resistant to maximum antiarrhythmic therapy including amio-darone, unless it was contraindicated, and resistant to or recurring within 7 days after electric cardioversion.
Definition of CS or GCV ablation
Angiography of the CS - GCV was not routinely performed. Ablation within the GCV was considered if applied beyond the usual position of the valve of Vieussens. In practice, in the left anterior oblique fluoroscopy projection, it was beyond the 4 o’clock position on an imaginary clock face.
Anticoagulation protocol
Warfarin was stopped five days prior to the ablation and low - molecular - weight (LMW) heparin was given until the procedure. Transesophageal echocardiography (TEE) was performed the morning before the ablation procedure to exclude intracardiac thrombus. Following transseptal puncture, a bolus of heparin followed by continuous heparin infusion was administered to maintain activated clotting time (ACT) ideally between 300 – 400 s. Heparin was stopped at the end of the procedure and LMW heparin was started immediately and continued until warfarin, re-administered one day after the procedure, reached therapeutic levels. Treatment with warfarin was maintained individually for at least 3 – 6 months and then discontinued in cases of stable SR and satisfactory LAA flow velocity at the control TEE.
Electrophysiological study
Antiarrhythmic drugs were discontinued for five half-life periods before the procedure with the exception of amiodarone. For the ablation procedure, a 10 - pole electrode catheter (Daig, St. Jude Medical, Minnetonka, MN, USA) was positioned in the CS, and a 10 - pole electrode ring catheter (LASSO, Biosense Webster, Diamond Bar, CA, USA) and a 3.5-mm irrigated - tip (NaviStar ThermoCool, Biosense Webster, Diamond Bar, CA, USA) mapping/ ablation catheter were inserted in the LA and PVs. Surface ECG and bipolar endocardial electrograms filtered from 30 to 500 Hz were monitored and stored on the Cardiolab System (Prucka Engineering, Sugar Land, TX, USA). Bipolar atrial pacing was performed using the UHS 20 stimulator (Biotronik GmbH & Co, Berlin, Germany). RF energy was applied with a Stockert generator (Biosense Webster, Diamond Bar, CA, USA). Three - dimensional mapping was performed using the CARTO system (Biosense Webster, Diamond Bar, CA, USA). For irrigated power - controlled ablation in the LA, irrigation of 17 – 30 ml/ min and temperature and power limits of 42 °C and 35 W, respectively, were used. Irrigation and power were limited to 20 ml/ min and 15 – 20 W within the GCV and the CS.
First ablation strategy
The ablation strategy employed during the first ablation procedure has been described in detail previously [2]. Briefly, the ablation strategy consisted of PV antra encircling, LA linear lesions, possible additional focal LA lesions, and CS - GCV ablation aimed at successive elimination of PV and extra - venous focal sources or reentry circuits. LA linear lesions were performed following full PV antra isolation in the following order: (postero)lateral mitral isthmus, roof, and posteroseptal mitral isthmus. Then focal RF applications targeting the posterior endocardial aspect of the CS and/ or the inter - atrial septum were deployed. Subsequently, a linear lesion was drawn from the anterior aspect of the mitral annulus (MA) towards the LA roof along the septal rim of the LAA. This last linear lesion was intentionally left incomplete when crossing BB to prevent significant LAA conduction delay during SR. Ablation within the LAA and aggressive ablation along the BB was avoided. Regions exhibiting complex fractionated atrial electrograms (CFAEs) seen during the mapping were not specifically targeted by primary focal ablation but were crossed by the linear lesions and, if CFAEs and short cycles were still present elsewhere after linear ablation, they could be targeted for subsequent focal RF applications.
Converting residual LA tachycardias were not re-mapped electroanatomically for reasons such as alternating multiple LA tachycardia forms, edema along the ablation lines, and conduction block into the freshly dissociated LA segments [2]. Therefore, ablation of the residual LA tachycardia was mainly guided by entrainment.
The procedure endpoints were, successively, full PV isolation and SR restoration by ablation together with an effort to preserve early LAA activation. If the arrhythmia could not be terminated by ablation, electric cardioversion was performed. In addition to local electric silence along the ablation lesions as mapped endocardially, the functional lesion continuity was ascertained as follows: Full conduction block across the circumferential lesions was validated by the ring catheter within the PV antra. Full conduction block across the LA roof lesion and mitral isthmus was tested by activation change during LAA pacing and differential pacing if capture of the adjacent regions by pacing was possible. Full CS - GCV (and simultaneous mitral isthmus) conduction block proved by differential pacing was an additional endpoint in case of converting peri - mitral reentry tachycardia resistant to endocardial ablation. Full or partial (equal to slow CS activity dissociated from the faster LAA rates) CS isolation was also completed in case of continuing AF that could not be converted into tachycardia or SR. CS ablation and conduction block were not pursued when the converting tachycardia from which SR was restored was not related to the CS.
Repeat ablation strategy
Recurrent persistent AF or multiple alternating LA tachycardias were targeted by repeating the aforementioned ablation strategy. Stable persistent LA tachycardia was mapped and ablated selectively using a combination of 3 - D mapping and entrainment. SR restoration and noninducibility of any arrhythmia as assessed by incremental atrial pacing up to a minimum of 300 bpm and repeated in long sequences 5 times were the procedure endpoints. In cases of PV - LA conduction recovery, PV antra re-isolation was the integral endpoint of the repeat ablation. Additional endpoints related to the linear and CS ablation were identical to the first ablation; therefore if SR was restored from AF or tachycardia not related to the mitral isthmus or CS, and noninducibility of any arrhythmia was accomplished, epicardial CS - GCV ablation was not pursued to achieve full local block.
CS-related tachycardia mapping and ablation
Once participation of the CS musculature in the tachycardia mechanism was suggested, peri - mitral reentry was confirmed or ruled out by pacing for entrainment. CS - GCV ablation was started after endocardial ablation had failed to affect the tachycardia. In cases of peri - mitral reentry tachycardia, ablation was usually started within the distal CS or GCV and could be gradually extended towards the proximal CS. In cases of tachycardia circumscribed to the CS musculature, ablation was started at the site of matching post-pacing interval (PPI) corresponding usually to the site of multi-component fractionated potential. Full CS - GCV conduction block, presenting during SR as a split of the nearby CS potentials and reversal of the CS - GCV activation sequence behind the ablation site, was confirmed by the LAA pacing and differential pacing.
Follow-up
History and standard 12 - lead ECGs were taken, and 24 - hour Holter ECG recordings, health records, and multiple ECG recordings obtained from the local cardiologist were reviewed at outpatient visits after 6 weeks and at 3, 6, 9 and 12 months during the first year and then every 6 months, and also in the meantime, whenever required because of symptoms. Three-week transtelephonic ECG monitoring was employed after 6 months and when needed because of unclear symptoms. Arrhythmia recurrence was diagnosed in the presence of ECG documentation or history of irregular palpitations suggestive of an arrhythmia lasting more than 30 s.
Statistical analysis
Continuous variables were expressed as mean ± standard deviation (SD). Categorical variables were expressed as proportions.
Results
Prevalence of CS-related tachycardia
Including one and two repeat ablations in 58 and 16 patients, SR was restored during at least one of the ablation procedures in 99 (71%) out of the 140 patients undergoing ablation of primary longstanding persistent AF. The 23 subjects with successful ablation within the CS - GCV represented 23% of the 99 patients in whom SR was restored by ablation. CS - GCV ablation was required to restore SR in 7 (12%), 12 (25%) (1 repeat ablation for identical CS-related tachycardia in patient #1), and 5 (31%) patients out of 59, 48, and 16 patients, respectively, in whom SR was restored by the first, second, or third ablation procedure.
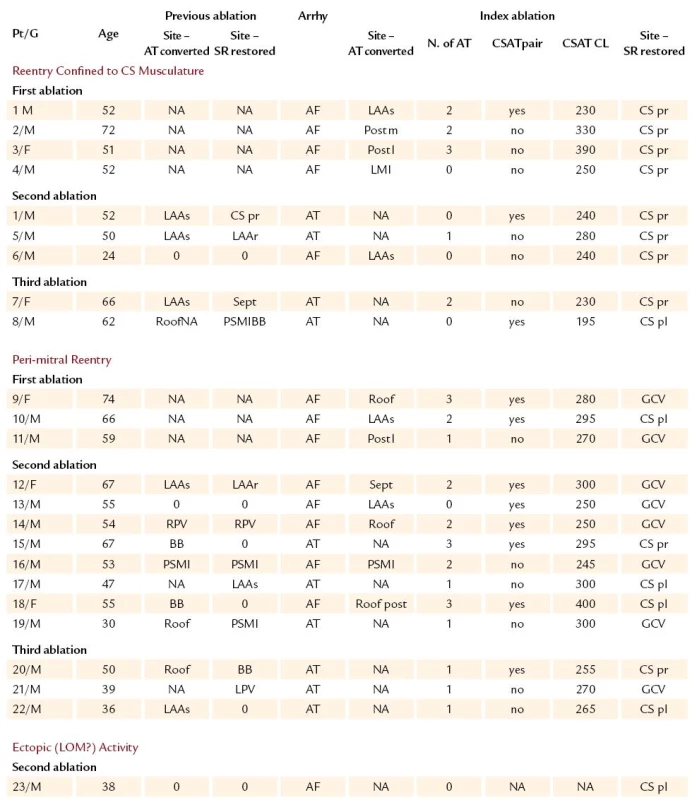
Results of previous ablation
Of the patients with CS-related tachycardia present during their second ablation, SR was restored during their first ablation via LA tachycardia in 6 patients (#1, 5, 12, 14, 16, 19), and directly from AF in 1 patient (#17). Of the patients with CS-related tachycardia present during their third ablation, SR was restored by the first ablation in only 1 patient (#8). During their second ablation, SR was restored by ablation of recurrent LA tachycardia in 2 patients (#8, 21), and from recurrent persistent AF via converting LA tachycardia in 2 patients (#7, 20). In these 4 patients, atrial pacing up 300 bpm did not induce any arrhythmia at the end of the second ablation (details in Tab. 2).
Procedure, fluoroscopy, and RF energy application time
The mean procedure time of the 24 ablation procedures was 287 ± 66 (180 – 400) minutes, the mean fluoroscopy time was 23 ± 7 (13 – 37) minutes, and the mean RF energy application time was 93 ± 45 (31 – 191) minutes.
Complications
There was one femoral bleeding cured surgically, followed by subsequent femoral venous thrombosis that was completely dissolved by heparin without consequences. CS angiography was not routinely performed; however, no clinical signs of cardiac ischemia or symptoms related to CS occlusion occurred. Temporary chest discomfort after ablation that could suggest pericarditis was observed in 2 patients, and routine postablation transthoracic echocardiography revealed transient pericardial effusion not exceeding 4 mm in 3 patients.
Characteristics of the tachycardia confined to CS musculature
In 8 patients, a peri - mitral reentry was excluded by endocardial and GCV entrainment mapping. A single regular CS-related tachycardia, exhibiting septal - to - lateral (S - L) CS activation sequence, was found in 6 patients. This tachycardia resulted directly from AF ablation (#4, 6) or appeared after elimination of other converting LA tachycardias (#2, 3, 5, 7). None of the tachycardias was specified electroanatomically. Ablation within the proximal CS was guided by entrainment with matching post-pacing interval (PPI), and the ablation site typically displayed local multi-component potential.
In another 2 patients, a pair of tachycardias was observed. In patient #1, the first ablation resulted in a pair of alternating tachycardias sharing a complex fractionated potential within the proximal CS irrespective of the tachycardia activation sequence. Ablation at this site with matching PPI terminated the tachycardia (Fig. 1). The same tachycardia recurred and full proximal CS conduction block was required to eliminate the tachycardia permanently at repeat ablation (Fig. 2). In patient #8, CS-related tachycardia form exhibited a double activation wave - front within one tachycardia CL as recorded by the 10 - pole CS catheter. Matching or near - matching PPIs were found along the CS. Posterolateral CS ablation at the site of complex multi-component potential eliminated the tachycardia (Fig. 3) (Tab. 2). Noninducibility of any tachyarrhythmia was achieved in all patients undergoing repeat ablation and full CS conduction block was finally evident in all 8 patients.



Characteristics of the peri - mitral reentry tachycardia
Peri - mitral reentry tachycardia was proved in 14 patients. In 6 patients, tachycardia with a single CS activation sequence was present. Lateral - to - septal (L - S) activation sequence was observed in patients #11, 16, 21, 22; and S - L activation sequence in patients #17, 19. In patients #11, 16, this tachycardia occurred after AF conversion and was not mapped electroanatomically. In patients #17, 19, 21, 22, a recurrent peri - mitral reentry tachycardia was proved by combination of electroanatomic and entrainment mapping.
In 8 patients (#9, 10, 12, 13, 14, 15, 18, 20) a pair of tachycardias exhibiting S - L and L - S activation sequences was observed (Fig. 4). Epicardial ablation within the venous system restored SR after endocardial ablation at both the mitral isthmuses and along the posterior CS failed to affect the tachycardia. Full CS - GCV conduction block (or isolation) was achieved in all patients except patients #9 and 20, in whom advanced partial block was accepted on account of the tachycardia noninducibility. Noninducibility of any atrial tachyarrhythmia was achieved in all 11 patients undergoing repeat ablation.

Characteristics of the focal CS-related tachycardia
In 1 patient (#23), an irregular tachycardia was terminated by posterolateral CS ablation at the presumed connection of the ligament of Marshall (LOM); ectopic activity from the LOM was its likely mechanism.
Long-term results
During the follow-up of 33 ± 10 (12 – 53) months since the last ablation procedure, 19 (83%) patients remained free from any documented arrhythmia and class I or III antiarrhythmic drugs. In an additional patient #4, atrial tachycardia recurred within the first 3 months but than disappeared spontaneously without need to reinstate class I or III antiarrhythmic drug. Another 3 patients (#9, 20, 21), including the two in whom partial CS conduction block was tolerated, experienced AT recurrences that required intervention. In patients #9 and 21, a presumably recurrent peri - mitral reentry tachycardia was suppressed by restoring previously ineffective amiodarone; patient #20 underwent additional successful ablation of a right atrial ectopic focus.
Discussion
Main findings
We describe a spectrum of tachycardias related to CS musculature that occurred during the first or repeat ablation procedure in patients with primary longstanding persistent AF who required ablation within the CS or GCV to restore SR permanently. These tachycardias resulted from a complex staged LA ablation strategy as the last residual arrhythmias, which allowed proper characterization of the arrhythmia mechanisms and the ablation endpoints.
The major findings are the following:
- CS-related tachycardia as the last arrhythmia preceding restoration of stable SR is found in at least 16% of the patients undergoing ablation for longstanding persistent AF, and it occurs in increasing proportions with a growing number of repeat ablations;
- the majority of these tachycardias exhibit properties of reentry;
- two thirds of these tachycardias represent a peri - mitral reentry;
- one third of these tachycardias seem to be confined to the CS musculature independent of the atrial myocardium;
- full local CS conduction block appears to be necessary to prevent recurrences of the tachycardia.
Incidence of CS-related tachycardia
In this cohort of patients, SR was permanently restored by the CS - GCV ablation in 16% of patients referred for primary longstanding persistent AF. However, a higher prevalence of this residual tachycardia can be expected, as it reached 23% of the patients in whom ablation successfully restored SR. This tachycardia was increasingly found during repeat ablations, possibly also due to higher probability of its unmasking.
Previously, Chugh et al demonstrated atrial tachycardia arising within the CS in 9 (27%) of 33 patients who developed this arrhythmia after previous AF ablation, and also in an additional 5 patients in whom a macroreentry tachycardia involving the CS developed during the first AF ablation procedure. Unlike in our study, more than half of these patients presented with primary paroxysmal AF, and the incidence of CS-related tachycardia among patients with longstanding persistent AF ablation was not stated [4]. In 60 patients with long-lasting persistent AF, Haïssaguerre et al reported SR restoration by ablation of the converting tachycardia at the mid or ostial CS in 8 patients, which accounted for 13% of all patients, and for 18% of the patients with converting AT [1]. Recently, Haïssaguerre et al reported the impact of the CS ablation in 30 patients with persistent AF. Following full PV isolation, they included CS ablation at various stages of their ablation protocol and terminated AF (i.e., converted into SR or into a monomorphic tachycardia) by the CS ablation in 9 (30%) patients [5]. However, the exact proportion of conversions into SR by epicardial CS ablation was not specified, as the term CS ablation was used for epicardial as well as endocardial approach.
Mechanism of CS-related tachycardia
CS musculature and LOM have been described as a harbor of ectopic foci initiating and maintaining atrial tachycardia or AF [3,6 – 11]. CS myocardium has been also implicated as a source of primary left atrial flutter [12] or the source of sustained macroreentry tachycardia after ablation of paroxysmal or persistent AF [4].
In this study, reentry mechanism was present in 22 (96%) of the 23 patients with CS-related tachycardia. It was supported by stable tachycardia CL, tachycardia entrainment, occurrence of the tachycardia in pairs with reverse activation sequences, ability to eliminate the tachycardia pair by ablation at a single site, and the need for local conduction block to eliminate the arrhythmia permanently. This finding is in agreement with the experience of Chugh et al, who designated the tachycardia as macroreentry in 14 (88%) of 16 patients [4]. On the other hand, Haïssaguerre et al found a substantial proportion of the tachycardias arising from the CS as being of focal origin [1,5]. Among our patients, only one irregular tachycardia was found and terminated in the posterolateral CS, which implicated possible interruption of the LOM - CS connection. Similar findings were described by Chugh et al, who ablated two foci in the middle and distal CS position and suspected the LOM as the possible arrhythmia source [4].
Most of the CS-related tachycardias were peri - mitral in which a substantial portion of the circuit constituted by the venous musculature was inaccessible for effective endocardial ablation. Ablation of the peri - mitral circuit at other LA sites may be effective; however, complete anterior lesions and aggressive ablation along the BB and the high septum were restrained in order to avoid the LAA conduction delay. Finally, septal ablation close to the MA may carry a risk of inadvertent AV conduction damage.
On the other hand, one third of tachycardias implied existence of reentry confined to the CS musculature without participation of the LA myocardium. First, extensive endocardial ablation failed to affect the tachycardia. Second, the tachycardia promptly responded to the epicardial CS ablation that spatially corresponded to the site of previous ineffective endocardial ablation. Third, pacing for entrainment along the MA and within the GCV failed to evoke matching PPI. Finally, a double-wave activation pattern recorded along the 10 - pole CS catheter within one tachycardia CL (patient #16) most convincingly demonstrated propagation along the CS musculature in both directions (such as, e. g., propagation around the scar) independent of LA myocardium (Fig. 3). Prevalence of this phenomenon is not known, but it might have been missed in some other patients with single S - L tachycardia, in whom we have observed similar double activation limited to a shorter segment of the CS or disparity in timing between potentials recorded by the ablation catheter at the contra - lateral wall within shorter CS segment as compared to the corresponding bipole of the CS catheter. No specific maneuvers were performed to systematically assess double activation wave - fronts within the CS.
Clinical implications
In patients with longstanding persistent AF, the complex CS - GCV structure [13,14] can constitute substrate for tachycardias that directly result from AF conversion without intervening SR, or that evolve as a persistent recurrent tachycardia. Some of these tachycardias seem to represent one of the possible primary sources that initiate and maintain AF; others, usually peri - mitral reentry tachycardias, may survive ablation as one of the residual reentry circuits or may newly evolve from postablation atrial remodeling. The latter is supported by the tachycardia occurrence despite previous achievement of noninducibility of any tachyarrhythmia; however, recurrence of the tachycardia may also be explained by temporary suppression of pre-existing epicardial arrhythmogenic substrate by previous adjacent endocardial ablation.
Peri - mitral reentry can possibly be interrupted at different LA sites; however, aggressive ablation at the anterior LA wall, BB, and the septum may be associated with significant LAA conduction delay and its potential adverse hemodynamic consequences [15]. This study corroborates the necessity for a relatively aggressive CS - GCV ablation that must result in full local conduction block within the venous musculature in order to achieve full (postero)lateral mitral isthmus conduction block [16]. Recurrences of presumably peri - mitral reentry tachycardia in three patients, in two of whom partial CS conduction block was tolerated, further support the need for full local CS conduction block to eliminate such a tachycardia with certainty.
A substantial proportion of the CS-related reentry tachycardias appear to be confined to the CS musculature independent of the LA myocardium. Opposite walls of the vein or different layers of the venous wall seem to constitute separate arms of the local reentry circuit. Ablation within the vein, ideally associated with full local conduction block, represents the only way to eliminate the arrhythmia substrate.
Prophylactic full CS isolation or block may be considered to rule out CS-related tachycardias as observed in this study; however, several factors are involved. First, pure CS ostial ablation may not eliminate the tachycardia utilizing a reentry substrate confined to the CS myocardium and spreading over a deeper segment of the vein. Second, prophylactic CS ablation is futile (let alone free from potential serious complications) in a substantial subset of patients, as many residual or recurrent tachycardias occur independently of the presence or absence of CS isolation or mitral isthmus block [17,18]. Third, relatively aggressive CS ablation may be required, while limited CS ablation that does not lead to full CS conduction block would be ineffective. Therefore, it remains a dilemma whether aggressive prophylactic CS - GCV ablation should be performed routinely, which will prevent the CS-related tachycardia in a very limited proportion of patients, or whether it should not be considered until participation of the venous musculature in the tachycardia mechanism is involved and venous ablation becomes inevitable. Whatever approach is correct, the possibility of subsequent tissue recovery (despite acute demonstration of full local CS conduction block) will make the CS-related tachycardia a clinical reality, posing a challenge to the electrophysiologists dealing with longstanding persistent AF.
Limitations
Without direct visualization, the CS - GCV definition may seem loose. On the other hand, successful RF applications delivered at the 3 o’clock position and beyond on an imaginary clock face strongly suggest ablation within the GCV portion.
The lack of systematically obtaining CS angiograms, routine coronary angiography or of other objective evaluation of potential detrimental effects of ablation on the CS - GCV, coronary arteries or esophagus is another limitation to this report [19,20]. On the other hand, no serious complications such as pericardial effusion requiring intervention, acute coronary syndrome, or esophageal wall damage were observed. These findings are in agreement with previous experience of other authors, who also did not routinely perform CS or coronary angiography and other examinations to detect any potential complications of CS - GCV ablation [4,5].
Funding sources
This study was supported by a grant from the Czech Ministry of Health IGA MZ NR9143 – 3/ 2007, and by a grant from IGA AGEL N. 15/ 2007.
Martin Fiala, M.D., Ph.D.
www.nempodlesi.cz
e-mail: martin.fiala@gmail.com
Doručeno do redakce: 9. 3. 2010
Přijato po recenzi: 11. 8. 2010
Sources
1. Haïsssaguerre M, Sanders P, Hocini M et al. Catheter ablation of long-lasting persistent atrial fibrillation: critical structures for termination. J Cardiovasc Electrophysiol, 2005; 16 : 1125 – 1137.
2. Fiala M, Chovančík J, Nevřalová R et al. Termination of long-lasting persistent versus short - lasting persistent and paroxysmal atrial fibrillation by ablation. Pacing Clin Electrophysiol 2008; 31 : 985 – 997.
3. Sanders P, Jaïs P, Hocini M et al. Electrical disconnection of the coronary sinus by radiofrequency catheter ablation to isolate a trigger of atrial fibrillation. J Cardiovasc Electrophysiol 2004; 15 : 364 – 368.
4. Chugh A, Oral H, Good E et al. Catheter ablation of atypical flutter and atrial tachycardia within the coronary sinus after left atrial ablation for atrial fibrillation. J Am Coll Cardiol 2005; 46 : 83 – 91.
5. Haïssaguerre M, Hocini M, Takahashi Y et al. Impact of catheter ablation of the coronary sinus on paroxysmal or persistent atrial fibrillation. J Cardiovasc Electrophysiol 2007; 18 : 378 – 386.
6. Volkmer M, Antz M, Hebe J et al. Focal atrial tachycardia originating from the musculature of the coronary sinus. J Cardiovasc Electrophysiol 2002; 13 : 68 – 71.
7. Knecht S, O’Neill MD, Matsuo S et al. Focal Arrhythmia confined within the coronary sinus and maintaining atrial fibrillation. J Cardiovasc Electrophysiol 2007; 18 : 1140 – 1146.
8. Rostock T, Lutomsky B, Steven D et al. The coronary sinus as a focal source of paroxysmal atrial fibrillation: more evidence for the “fifth pulmonary vein”? Pacing Clin Electrophysiol 2007; 30 : 1027 – 1031.
9. Hwang C, Wu TJ, Doshi RN et al. Vein of Marshall cannulation for the analysis of electrical activity in patients with focal atrial fibrillation. Circulation 2000; 101 : 1503 – 1505.
10. Chen PS, Wu TS, Hwang C et al. Thoracic veins and the mechanism of nonparoxysmal atrial fibrillation. Cardiovasc Res 2002; 54 : 295 – 301.
11. Knecht S, Jaïs P, Lim KT et al. Slow conduction of the vein of Marshall in the context of permanent atrial fibrillation. J Cardiovasc Electrophysiol 2007; 18 : 1004 – 1005.
12. Badhar N, Kalman JM, Sparks PB et al. Atrial tachycardia arising from the coronary sinus musculature: electrophysiologic characteristics and long-term outcomes of radiofrequency ablation. J Am Coll Cardiol 2005; 46 : 1921 – 1930.
13. Chauvin M, Shah D, Haïssaguerre M et al. The anatomic basis of connection between the coronary sinus and the left atrium in humans. Circulation 2000; 101 : 647 – 652.
14. Ho Y, Sanchez - Quintana D, Cabrera JA et al. Anatomy of the left atrium: Implications for radiofrequency ablation of atrial fibrillation. J Cardiovasc Electrophysiol 1999; 10 : 1525 – 1533.
15. Kuhne M, Ho SY, Morady F et al. Elimination of left atrial appendage potentials during radiofrequency ablation near the right superior pulmonary vein. Heart Rhythm 2008; 5 : 475 – 478.
16. Jaïs P, Hocini M, Hsu LF et al. Technique and results of linear ablation at the mitral annulus. Circulation 2004; 110 : 2996 – 3002.
17. Jaïs P, Sanders P, Hsu LF et al. Flutter localized to the anterior left atrium after catheter ablation of atrial fibrillation. J Cardiovasc Electrophysiol 2006; 17 : 279 – 285.
18. Takahashi Y, Takahashi A, Miyazaki S et al. Electrophysiologic characteristics of localized reentrant atrial tachycardia occurring after catheter ablation of long-lasting persistent atrial fibrillation. J Cardiovasc Electrophysiol 2009; 20 : 623 – 629.
19. Takahashi Y, Jaïs P, Hocini M et al. Acute occlusion of the left circumflex coronary artery during mitral isthmus linear ablation. J Cardiovasc Electrophysiol 2005, 16 : 1104 – 1107.
20. Ren JF, Lin D, Marchlinski FE et al. Esophageal imaging and strategies for avoiding injury during the left atrial ablation for atrial fibrillation. Heart Rhythm 2006; 3, 1156 – 1161.
Labels
Diabetology Endocrinology Internal medicineArticle was published in
Internal Medicine

2011 Issue 1
-
All articles in this issue
- Obesity, body mass index, waist circumference and mortality – editorial
- Obesity, body mass index, waist circumference and mortality – editorial
- Acute heart failure and early development of left ventricular dysfunction in patients with ST segment elevation acute myocardial infarction managed with primary percutaneous coronary intervention
- Contribution of whole‑ body magnetic resonance in the diagnostics of monoclonal gammopathy of undetermined significance, multiple myeloma, and the assessment of Durie‑ Salmon Plus staging system
- The influence of some factors on presence of varices and variceal bleeding in liver cirrhosis patients
- Therapeutic hypothermia after non‑traumatic cardiac arrest for 12 hours: Hospital Karlovy Vary from 2006 to 2009
- Role of genetics in prediction of osteoporosis risk
- Obesity, body mass index, waist circumference and mortality
- Why there is atrial fibrillation after cardiac operations?
- Schnitzler syndrome: case report, the experience with glucocorticoid and anakinra (KineretTM) therapies and monitoring of systemic cytokine response
- A delay in the HELLP syndrome diagnosis
- A case of a flapping infected thrombus in the internal jugular vein, septic pneumonias and heparin‑induced thrombocytopaenia
- Diagnosis and treatment of acute pulmonary embolism in 2010
- Therapeutic hypothermia after cardiac arrest: why and for how long? – editorial
- Genes and osteoporosis – editorial
- Characterization of residual coronary sinus‑related tachycardia during ablation of longstanding persistent atrial fibrillation
- Internal Medicine
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue
- A delay in the HELLP syndrome diagnosis
- Diagnosis and treatment of acute pulmonary embolism in 2010
- Obesity, body mass index, waist circumference and mortality
- A case of a flapping infected thrombus in the internal jugular vein, septic pneumonias and heparin‑induced thrombocytopaenia