-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
Free-flap monitoring: review and clinical approach
Authors: V. Lovětínská 1,2,3,4; A. Sukop 3,4; L. Klein 5,6; Anna Brandejsová 3
Authors place of work: Charles University, First Faculty of Medicine, and Central Military University Hospital, Department of Orthopaedic Trauma, Prague, Czech Republic 1; San Antonio Military Medical Centre, Texas, U. S. A. 2; Charles University, Third Faculty of Medicine, and Faculty Hospital Královské Vinohrady, Department of Plastic Surgery, Prague, Czech Republic 3; Charles University, Third Faculty of Medicine, Prague, Czech Republic 4; Charles University Teaching Hospital, Department of Surgery, Division of Plastic Surgery and Burns Treatment, Hradec Králové, Czech Republic 5; University of Defence, Faculty of Military Health Sciences, Department of Military Surgery, Hradec Králové, Czech Republic 6
Published in the journal: ACTA CHIRURGIAE PLASTICAE, 61, 1-4, 2019, pp. 16-23
INTRODUCTION
Free tissue transfer is on the top of the reconstructive ladder in plastic surgery. The first introduction of the free flap was in 1973 by Daniel and Taylor as composite tissue transfer by venous anastomosis of a compound leg injury1. Since then, this approach takes place in routine practice in nearly every department of plastic surgery. Microvascular free flap reconstruction is a versatile option for coverage of multitude wounds all over the body. In order to maintain the best results, close flap monitoring by a skilled surgeon as well as trained nurses is mandatory following the completion of a successful operation2. In order to maintain the best results in free flap reconstructive surgery, multiple usage of a variety of alternative techniques for free flap monitoring was introduced. Because not only the procedure alone, but also the postoperative monitoring and prospective flap failure pose significant financial cost, some of the most common or latest known techniques for free flap monitoring are reviewed and mutually compared in this article.
FREE FLAP FAILURE
The overall different free flap tissue transfer complication rate has been reported in around 30% with complete flap loss of less than 4%. The main reasons for free flap failure are vascular compromise, such as thrombosis (venous and arterial), arterial insufficiency and vasospasm, active bleeding, hematoma, venous congestion or technical anastomotic problems4. Six percent to 14% of free flaps are re-explored due to vascular complications with 95% of cases presenting within 72 hours following the surgery. Without prompt recognition and surgical intervention, vascular compromise will eventually progress to irreversible and total loss of the free flap due to the “non-reflow” phenomena6,9,10. The average critical ischemia time is 11 hours. It depends on the type of tissue in a particular flap. For instance, the myocutaneus flap is more sensitive to ischemia and the average primary critical ischemia time is 9 hours, whereas fasciocutaneus flaps are more resistant to the effect of ischemia, and therefore, can tolerate a longer time of ischemia3. This is documented by Cusano and Fernandes that non-buried flaps with vascular compromise clinically detected within 48 hours after surgery have a 77% flap salvage rate, whereas failure in buried flaps went unrecognized until a wound complication manifested (generally more than 7 days postoperatively)7. This resulted in later re-exploration and flap salvage rate of 0%7. Nonvascular complications are usually not so severe and they are mainly wound related including infection, necrosis, dehiscence, seroma or hematoma with presence later in the postoperative course. There are many factors that predict possible vascular compromise. Absolute prognostic factors are the age of the patient, presence of infection, surgery time, pedicle characteristics, tight closure and vein graft usage. Relative factors include comorbidities, type of vessel selection, location of the defect, hydration, hypothermia, buried or osseous flaps. One of the contributing factors of late free flap failure seems to be the application of high-dose heparin. Nowadays, there is a trend away from complex pharmacotherapy regimens supported by many authors. Some optimal medical regimens are mentioned in Table 1. Several previous studies have shown high risks of hematoma with the use of high-dose heparin treatment in comparison with other anticoagulation regimens. Despite the fact that the early direct re-exploration and anastomosis revision is advised in the event of vascular based issues with the flap, it is also necessary to evaluate and control systematic factors prior to the surgery. In some cases, it is sufficient to correct hypovolemia, hypotension or mechanical factors such as flap area position and external compression causing kinking of the vascular pedicle. In specific situations thrombolysis, thrombectomy or leech therapy can be an option to surgery, considering situations when the patient’s vital functions are not stable, successful flap revision is too small or only a part of the flap is affected.
Tab. 1. Possible free flap pharmacotherapy schedule 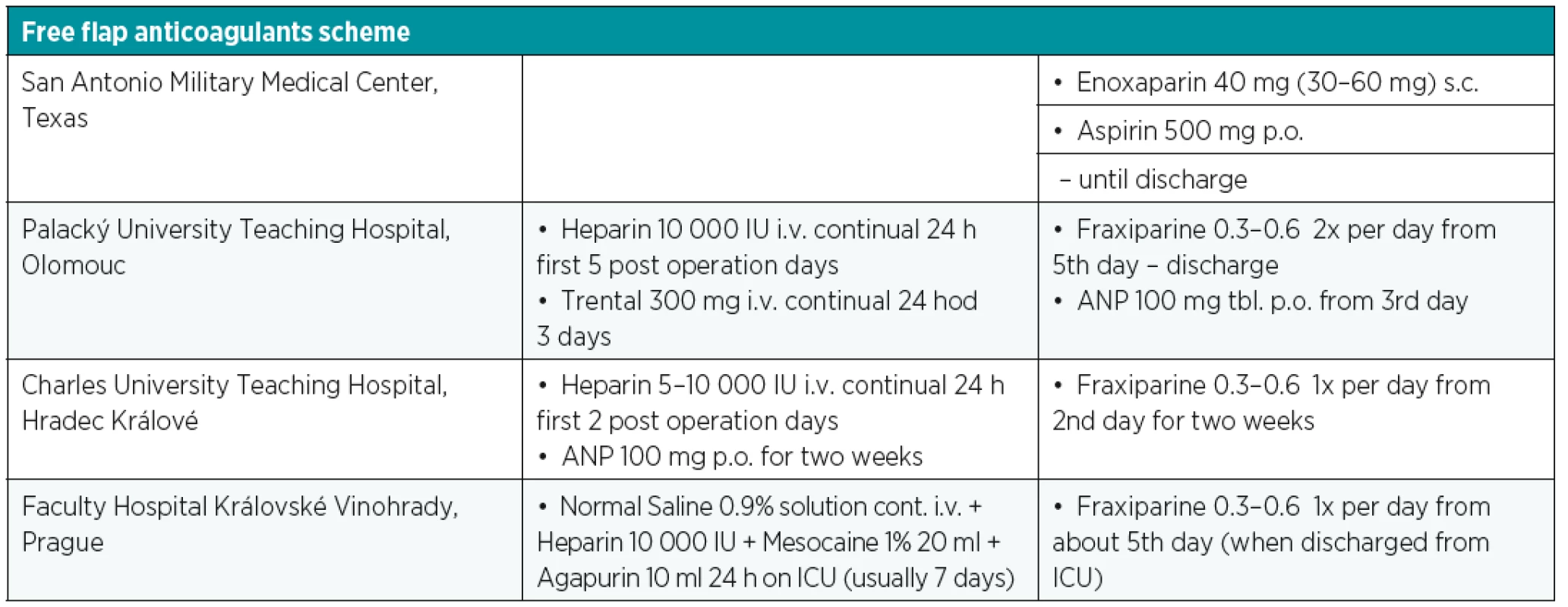
CLINICAL MONITORING AND PROTOCOL
The successful salvage of free flap tissue transfer depends on early problem detection, decision making and, if necessary, rapid exploration. Therefore, following a monitoring protocol is crucial, including various potential flap perfusion patterns such as checking the colour, temperature, turgor, swelling, capillary refill time, bleeding after pin prick test and signal from acoustic Doppler ultrasonography in an intra-operatively marked skin area directly over the pedicle or island flap in buried flaps. This physical examination of the flap is, however, subjective and observer-dependent, sometimes being difficult to implement effectively. Even though a variety of the newest modalities have been developed, none of these have provided a better outcome than clinical examination4. A universal protocol could help standardize the procedures in flap monitoring, because of a high variation in skill level due to the lack of clinical experience needed to assess flap viability by nurses and young resident doctors. The critical postoperative period is usually the first 72 hours. In this period, the patient and flap should be monitored frequently – every 30–60 min. The significant variation exists in frequency depending on each department guidelines, but one of the most common is Q 30 min first 3 hours, Q 1 hour up to 48 hours after surgery, Q 2 hours up to 72 hours after the surgery and then 4–8 hours until hospital discharge. Although there is no consensus on postoperative free flap care, some of the most important regimens should be maintained. In general, intravascular volume status with adequate urine output ensure good flap perfusion. The patient should remain normotensive with regards to the patients with arterial hypertension and relative hypotension after surgery because of opioid use5. Pressure on the flap must be avoided with the patient’s position, attention should be paid to excessively tight dressing and circumferential bandages. Elevation of the extremity or trunk is advised and activity restriction including weight-bearing and range of the motion is demanded (Figure 1). In addition, physical examination of the free flap in context of a patient’s overall condition is critical in order to determine whether the findings are related to the flap itself or to a systematic issue5.
Fig. 1. Nursing monitoring and management scheme 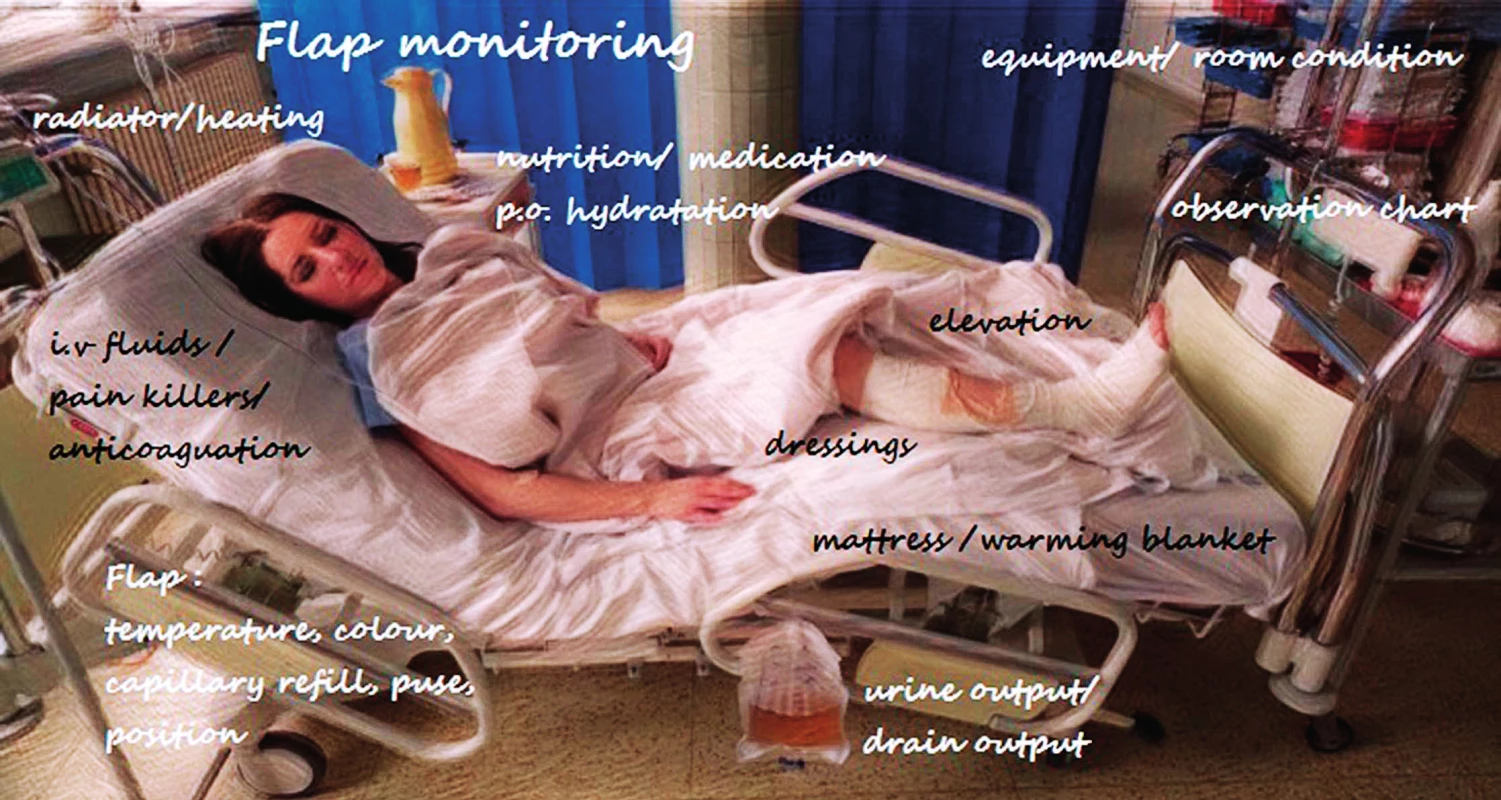
The ideal approach appears to be creating a teaching session to junior nursing staff and resident doctors with explanation of the basic principles of flaps and their postoperative care and generate a universal postoperative flap monitoring protocol to improve and standardize care provision (Table 2)12. A re-audit of procedure on the unit following the intervention by nurses improved the early detection (30% improvement in flap survival) of sings of flap ischemia as well as in the confidence of nursing staff and early availability for surgeons to offer clarification and undertake review of flap causing concern12. Another research result stated that reduced resident monitoring frequency did not alter flap salvage nor flap outcome11. Following the elemental principles of flap monitoring with patient’s general status can increase positive outcome, reduce the financial cost and affect the total lost rate.
Tab. 2. Modified optimal flap monitoring protocol 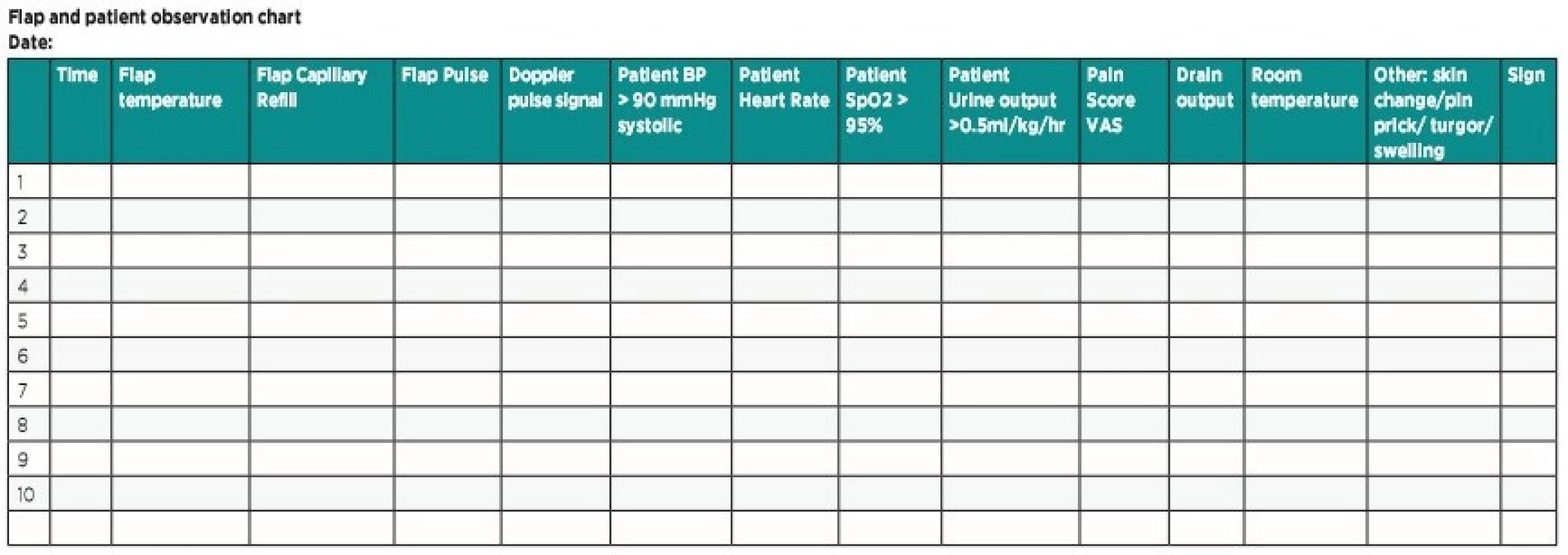
Ideal flap values:
Temperature: The flap should be as warm as the contralateral side. Difference greater than 1–3 C° between the flap and nearest non flap skin may indicate a vascular problem. If cold, move the patient to a warm environment (room heating/blanket/foil) – alert doctor.
Color: Pink is optimal. Pale/white indicates arterial insufficiency. Blue/purple indicates venous congestion – alert doctor.
Capillary refill: 2–3 seconds is ideal. Brisk refill time and livid or dark discoloration is a sign of venous congestion. If delayed >3 seconds/ absent and pale flap indicates arterial insufficiency – alert doctor.
Texture: An ideal flap is soft. If spongy/dehydrated/swollen/tense – alert doctor.
Skin changes: The skin should resemble the skin of the donor site. If blisters or other skin changes appear – alert doctor.
Edema/ swelling: This may be due to a collection in or under the flap, elevate the extremity above heart level and alert doctor.
Pulse: Not all flaps may have a palpable pulse. Use Doppler if required. If there is no pulse or acoustic signal – alert doctor.
Pressure on pedicle: Relieve all pressure on the flap pedicle by the combined use of pillow support, elevation and patient positioning.
Patient parameters: Pulse 60–100 beats per minute. Blood pressure > 90/60 and < 140/90 mmHg. Respiratory rate 16–20 per minute. SpO2 95%. Urine output > 0.5ml/kg/h.
Pain: The patient should be pain free. If the patient is in pain despite analgesia it could be due to flap ischemia – alert doctor.
Drain: If there is no output, check if the drain is in situ. If the output is more than 50 ml per hour alert the doctor because of possible active bleeding and pressure on the anastomosis.
Pin prick: Normal slow bright blood after pin prick/ dark fast bleeding in venous congestion/ no bleeding sign for arterial compromise.
!!! Contact plastic surgeon immediately if the conditions are not met, or in doubt !!!ALTERNATIVE MONITORING TECHNIQUE
Acoustic Doppler
Acoustic Doppler ultrasonography (ED – External Doppler) is the most common method of monitoring, traditionally combined with a physical examination. The basic principle is the Doppler effect, which is generally the change in frequency of waves in relation to the movement of one object (probe) to one another (red blood cells) with a hand-held acoustic Doppler sonography probe that emits ultrasound waves, which change as a result of blood flow within the flap5. The device then generates sound that is appropriate to the volume of blood flow. To make flap monitoring using the Doppler probe easier, it is advantageous after the completed surgery to mark the place with the highest signal that corresponds to the nearest location of an arterial anastomosis to the skin paddle. The limitations are, of course, in buried flaps. The arterial blood flow then creates a characteristic pulsatile sound, whereas a venous signal produces a constant and quiet acoustic output. The disadvantages of this method are sometimes low specificity (false negative), because vessels can be overlooked or missed due to background noise from larger vessels in the vicinity and too high sensitivity, as often diminutive vessels inadequate to sustain a cutaneous flap can be found15. This portable device is cheap, simple to use, and usually easy to interpret. It can be also simply used for intra-operative verification of perforators, as well as assessment of the location of recipient vessels15. This technique is widely used in the Czech Republic.
Colour Duplex Ultrasonography
Colour Doppler ultrasonography directly shows a real-time visualization of the microvascular anastomosis using the same phenomenon as the previous method. The colour observed on the monitor depends on the direction of blood flow – red is usually chosen for the arteries and blue for the veins to simplify data interpretation15. Higher frequency transducers permit scanning of superficial depths just below the skin level, with sensitivity to detect vessels with a diameter as small as 0.2 mm15. Sensitivity in identifying perforators with colour duplex imaging is high, but specificity is low, as only a small region can be examined at any time15. However, the equipment needs be operated by a radiologist and often a microsurgeon is also needed to assist in anatomical orientation, or presence of skilled plastic surgeon trained in Colour Duplex Ultrasonography. Therefore, colour duplex ultrasound is not used for routine continuous bedside or intra-operative monitoring.
Laser Doppler Flowmetry
Surface Laser Doppler Flowmetry (LDF) utilizes the Doppler effect applied to a coherent laser light which illuminates flap tissue. A probe emits laser light that experiences a shift in frequency based on the velocity of blood flow within the flap and reports the real-time measurements as an average over a predetermined time frame in LDF unit5,15. Although there are no standard criteria, usually a value higher than 2.0 LDF is indicative of a normally perfused flap5. But the device can be calibrated via a computer regarding to the particular flap and patient. Usually detection of the blood flow velocity to a depth of up to 8 mm is possible. Probably the most critical time for assessing flap perfusion after completion of microanastomosis is during insetting15. LDF surveillance during this period helps to determine any pedicle compromise from kinking, stretching, or excessive pressure15. This non-invasive system provides an accurate, objective display that is almost an ideal monitoring system for intra-operative and postoperative monitoring of free flaps32.
Flow Coupler
A plastic device called a coupler (Synovis Flow Coupler) is designed for use in end-to-end venous anastomosis. This particular polyethylene apparatus is additionally fitted with a Doppler probe, whose signal is transduced through a wire connected to the coupler, which continues via the surgical wound and exits the incision where it is ultimately connected to a sound and power source5. The Doppler acoustic signal is then heard continuously and any loss of the sound indicates vascular compromise. The final disconnection is made before the hospital discharge by gentle traction on the wire, which is then detached from the coupler remaining in the patient. Monitoring the vein via flow coupler has high sensitivity in identifying vascular compromise compared to the arterial probe, especially for venous thrombosis and compared to the implantable Doppler, the flow coupler eliminates time to place a separate monitoring probe16.
Implantable Doppler
The implantable Doppler (ID – Implantable Doppler/Cook-Swartz Doppler) utilizes the Doppler principle applied directly to the microvascular anastomosis, but in contrast to the flow coupler, it can be used for arterial, venous or both anastomoses5. The ultrasound probe is similarly placed in a soft silicone sleeve and fixed by suture or tissue glue directly to the vessel distal to the vascular anastomosis. The wire is connected to the external sound and power source and produce the same auditory output as in the acoustic Doppler. The probe is usually placed there for approximately 7 postoperative days and then easily removed as in the flow coupler. The probe implanted around an artery can immediately detect compromised arterial blood flow, however, an arterial Doppler signal will persist for several hours after venous thrombosis. A probe implanted around the vein can detect compromised venous blood flow almost immediately; compromised arterial blood flow induces almost instantaneous loss of the venous Doppler signal. Placement of the probe around the vein therefore provides a marked advantage compared to a probe placed around the artery in terms of detection of venous thromboses and can also be used to monitor arterial blood flow9. Venous probes have been found to be able to detect venous occlusion immediately and arterial occlusion within a mean time of 6 +/ - 2.4 min. Arterial probes can detect arterial occlusion immediately but detection of venous occlusion is delayed by 220 +/ - 40 min3. Disadvantages associated with the use of the venous coupler include vessel twisting and endothelial trauma when applying the device. Despite these problems, several series have shown that the failure rates for sutured and coupled venous anastomoses are similar3. False positives were not observed with clinical assessment while 8–17% false positives were seen with Cook Schwarz implantable Doppler among the included studies. In contrast, the rate of false negatives is higher in the conventional method compared with the Cook Schwarz Doppler. Most of the false-positive cases reported in older studies (before 2006) were essentially due to the learning curve associated with the use of the device, such as incorrect placement or fixation of the probe, incorrect interpretation of the Doppler signal or a battery problem, or accidental displacement of the probe by pressure exported on the lead9. Arterial implantable Doppler has, however, an additional benefit of less false-positives than venous Doppler6. In the recent literature, there is focus on the comparison between two most common techniques – ED versus ID. Implantable Doppler would avoid the need for new free flap surgery in two out of every 100 patients and by buried flaps four to five cases per 100 9. ID has an advantage in monitoring buried flaps where clinical or ED monitoring would be virtually impossible without exteriorizing a vascular component or creating a surgical window for monitoring on the surface of the skin above the flap3. Although there is an additional cost associated with its use, many authors have justified the expense in comparison to the huge cost associated with flap failure3. Nowadays, only about 16% of surgeons utilize ID in routine practice compared with 75% who use the ED device14. From a financial point of view, it could be advantageous to use ID when the failure rate before re-operation is greater than or equal to 6–10%14. Next to the cost analysis, the psychological stress related to the flap perfusion monitoring procedure for residents and healthcare personnel should be taken into account, continual monitoring since the surgery, improved comfort for the patient avoiding frequent examination including pin-prick test, quality of sleep during the patient’s stay in the hospital, and, last but not least, the physical and emotional devastation of the patient in case of complete flap loss and additional expenses of replacing a new flap3,9. ID probe provides a continuous signal since the anastomosis is made and can detect a subclinical vascular compromise, also re-explorative time is shorter compared with an ED device, resulting in higher flap salvage3. The sensitivity and specificity of the ID was found to be superior to that of the ED (100%, 98.7% versus 89%, 97%) and the flap survival rate was higher in the ID group (98%) then in the ED group (89.3%)3. The difference in salvage rates is even more marked in surgical specialities in which free flaps are very often buried, such as head and neck surgery. In their study the difference in salvage rates for this subcategory was therefore 94.12% with implantable Doppler versus 40% with conventional monitoring9. Examined records of 1317 flaps monitored by clinical methods and 147 flaps monitored with Cook-Schwarz Implantable Doppler found successful salvage rates of 100% in the implantable Doppler group and 71.3% in the group that relied solely on clinical monitoring as well as four false positive cases but no false negative in the implantable Doppler group9,13. In conclusion, ID is especially recommended for buried flaps for direct continuous monitoring. ID system can be used to monitor the pedicle artery or vein. With regards to the observation outcomes the preference is predominantly for the vein only, because venous thrombosis is more common than arterial and the artery will be patent for a certain period after venous thrombosis9. Intra-operative Dopplers increase the detection of immediate or incipient vascular problems. Unfortunately, for this unique device, the excessive cost compared to conventional methods still remains the significant drawback for most plastic surgery institutions. This technique is the first-choice option in San Antonio Military Medical Centre.
Fluorescence angiography
Fluorescence laser angiography (SPY Elite) using indocyanine green is a feasible and safe technique which provides real-time perioperation assessment of tissue perfusion in multiple settings. The device includes a laser light source and a camera, which records ICG fluorescence induced by the near-infrared light after the dye solution has flowed through the vessels of the observed area22. The ICG molecule strongly binds to plasma proteins, causing them to remain in the intravascular space. It also has a short plasma half-life of 3 to 5 minutes in humans which allows repeated injections for flap monitoring without ever reaching toxic levels and allowing for repetitive evaluations during the same operative procedure22,23. The technique allows visualization of the arterial inflow, venous return, and tissue perfusion during the intra-operative and postoperative period as well as flap planning or identifying perforators and their perfusion zones, assessing tissue perfusion, and identifying tissue at risk for necrosis. Early identification of insufficiently perfused tissue with the potential to develop ischemia or postoperative necrosis will help guide intra-operative surgical decision making, such as the need for flap revision, tissue resection, or a delayed procedure. The surgeon is able to significantly reduce necrosis rates and provide ideal flap making22.
Near-Infrared and Visible Light Spectroscopy
In spectroscopy, a source probe emits light of a specific wavelength (650–900 nm in IR ViOptix and 475–600nm in Visible Light T-Stat Spectroscopy) towards an object (chromophore – haemoglobin), and the detector measures changes in the reflected light, such as reduction in intensity5. In free flaps is the measurement of tissue oxygen dependent on relative changes of oxygenated, deoxygenated and total haemoglobin concentration, which vary depending on the blood flow rate within the flap. The light probe is fixed to the skin paddle with a dressing in a specific location chosen during the surgery. With thrombosis, deoxygenated and total haemoglobin increased whereas oxygenated haemoglobin and oxygen saturation decreased and drop to zero from baseline in a mean of 43 minutes with venous thrombosis and 37 minutes with arterial thrombosis14. The positive signs for vascular compromise are tissue oxygen saturation <30% or a decrease rate of >20% per hour and sustained for more than 30 minutes and the device can be set to the alarm when these criteria are met5,14. Even though this method is sensitive, specific, non-invasive and is able to identify the vascular problem before clinical sings are obvious, the main drawback still remains the cost34,35.
Photoplethysmography
Photoplethysmography (PPG) uses an optical technique to detect changes in arterial blood volume as the vessels change in diameter due to the pulsation22,23. This variation of blood volume in arteries is detected by illuminating the tissue under observation using LEDs, usually in the range of 600 nm to 940 nm23. The transmitted or reflected light is detected using a photodetector which is sensitive to the emitted wavelengths. Photoplethysmography has widespread clinical applications such as pulse oximetry, where the technology is utilized to determine the oxygen saturation of arterial blood (SpO2) by measuring the ratio of the red and infrared light which is absorbed by oxygenated and deoxygenated haemoglobin23. PPG has been modified to allow real-time estimation of SpO2 levels of the free flap. During the monitoring, an additional PPG probe is placed on the finger to compare both signals and validate the estimated arterial oxygen saturation values. This system is non-invasive, low at cost, simple, and easy to use. It promises reliable monitoring of SpO2 and change in volume of arterial blood in free flaps in the post-operative period.
Microdialysis
Microdialysis technique (ISCUS Flex) monitor the metabolism of a flap by continuous sampling of interstitial fluid. Ischemia can be detected 1–2 hours before it becomes clinically obvious by the changes in glucose, lactate, and pyruvate levels. The system consists of a small double-lumen catheter with a semipermeable membrane that is placed into subcutaneous tissue and the dialysis is then analysed in the bedside analyser26. The sutured catheter is easily removed before discharge by pulling it out after releasing the stitches. Analysis of each sample takes about 15 minutes and dialysis samples were taken on from 30 minutes to an hourly basis for 72 hours postoperatively26,27. During flap ischemia, the lactate/pyruvate ratio increased above 25 (expression of the redox reactions in the tissue), glucose concentrations reduced below 0.4 mmol/L (marker of the supply of substrate), whereas glycerol level increased (marker of more pronounced ischemia leading to breakdown of cell walls) as well as lactate level increased to about 7 mmol/L (marker of ischemia with anaerobic metabolism), when compared with normal values26,27. The machine must be carried out by experienced nursing staff, however, the data are displayed on the bedside analyser as numerical values and the interpretation is clear. Microdialysis is a new, useful, and safe tool for monitoring especially buried flaps or intraorally located flaps when clinical monitoring is difficult and may also reduce the patient discomfort caused by repeated clinical examination of the flap.
Surface temperature and IR spectroscopy
Flap temperature monitoring can be used more as an additional alternative monitoring technique, which is mostly a part of the standard clinical examination and it is also a sensitive tool in monitoring venous congestion of digital replantations. In this case, we can apply specific colorimetric tapes on the side of the flap and on the control skin side. It has been reported that a change of more than 1.8°C over a particular period is significant for flap circulation compromise14,29. This method is simple to use and one of the oldest and cheapest options. The principle of IR spectroscopy is detecting infrared radiation from an object, producing an image based on the local temperature, and can be used as a non-invasive and non-contact technique for indirect monitoring of skin blood perfusion in the preoperative planning, intra-operative evaluation of perfusion and postoperative monitoring of perfusion dynamics14,30,33. It uses a long-wave (8 to 14 μm) infrared sensor that has a working temperature range of 0° to 100°C. Thermal imaging has been applied to many purposes including the detection of cutaneous perforators or assessment of burn depth30. New generation thermal imaging cameras are becoming more sensitive with high-resolution cameras enabling taking pictures or video recording. The practical thermographic camera weights approximately 300g, and thus is quite easy to carry around for monitoring flap. It should be used in conjunction with existing technologies to provide additional clinical information for tissue transfer monitoring with minimal training requirements.
Video-based application
Eulerian Video Magnification (EVM) is quite clinically unknown video-based application that allows users “to see” perfusion throughout the flap by analysing physiological signals from video data. This computational technique is able to visualize subtle colour and motion variations in ordinary videos by making small changes that are hard or impossible to see by ourselves to be larger and visible. These small changes can be then quantitatively analysed. Tiny motions and small colour changes are isolated and amplify signals of interest, so we are able to see for example pulsation on the skin or colour changes from white to pink during the blood pulsatile flows within the flap31. This very new technology for free flap monitoring is free, sensitive, simple to use and it can evaluate flap viability in a safe, effective, and inexpensive fashion.
Telemedicine
Telemedicine is the term for using information technology (high data internet) and telecommunication (smartphones) to provide clinical health care from a distance, in this case for flap monitoring. Communication, sharing information, and correct interpretation also rank among the most important duties of medicinal professionals. One study compared flap assessing via photographs sent by smartphone with personal monitoring and the accuracy rate was 98.7% and 94.2%, respectively14. Another study confirmed higher rate of flap salvage due to significantly shorter interval between the first notification of flap problem and the start of re-exploration (4 hours vs. 1.4 hours) using mobile messenger applications14. This method is easy, accessible and it can reduce the response time of surgeon’s answer and solution of the vascular compromise.
CONCLUSION
Postoperative free flap monitoring remains a crucial part of successful result of demanding microsurgical transfer. The salvage rate of impaired flaps is closely related to the time between onset of vascular compromise and surgical intervention and this depends on the multidisciplinary well-educated team while maintaining the patient. Despite the advances in development of microsurgical equipment, improved materials, pharmacotherapy and more experienced surgical teams, there is still lack of one ideal device that meets all criteria for applicability to all flap types, rapid response to blood flow changes and providing real time information, accuracy, affordability and ease of interpretation and use. No single monitoring technique fulfilled all these requirements. Clinical monitoring alone has proven to be so effective that it is difficult to demonstrate better outcomes with alternative methods6. The simplest postoperative monitoring protocol was associated with the highest rate of flap success (99%) so the board range of flap monitoring techniques is not associated with statistically significant or clinically meaningful differences rates of flap success11. It is also important to take into consideration the difference between techniques with regards to overall flap survival, cost and ease to use (Table 3)6. None of all the previously mentioned newest techniques provided better results than clinical examination combined with external acoustic Doppler ultrasound, however, there is a potential benefit for the ID probe in the postoperative monitoring of free flap tissue transfer as a minimal invasive, reliable method3,6. The new technologies are not superior to traditional clinical assessment. To sum up, physical examination simultaneously with acoustic Doppler remains the cornerstone as well as the simplest and cheapest option5.
Tab. 3. Cost analysis of different free flap monitoring techniques 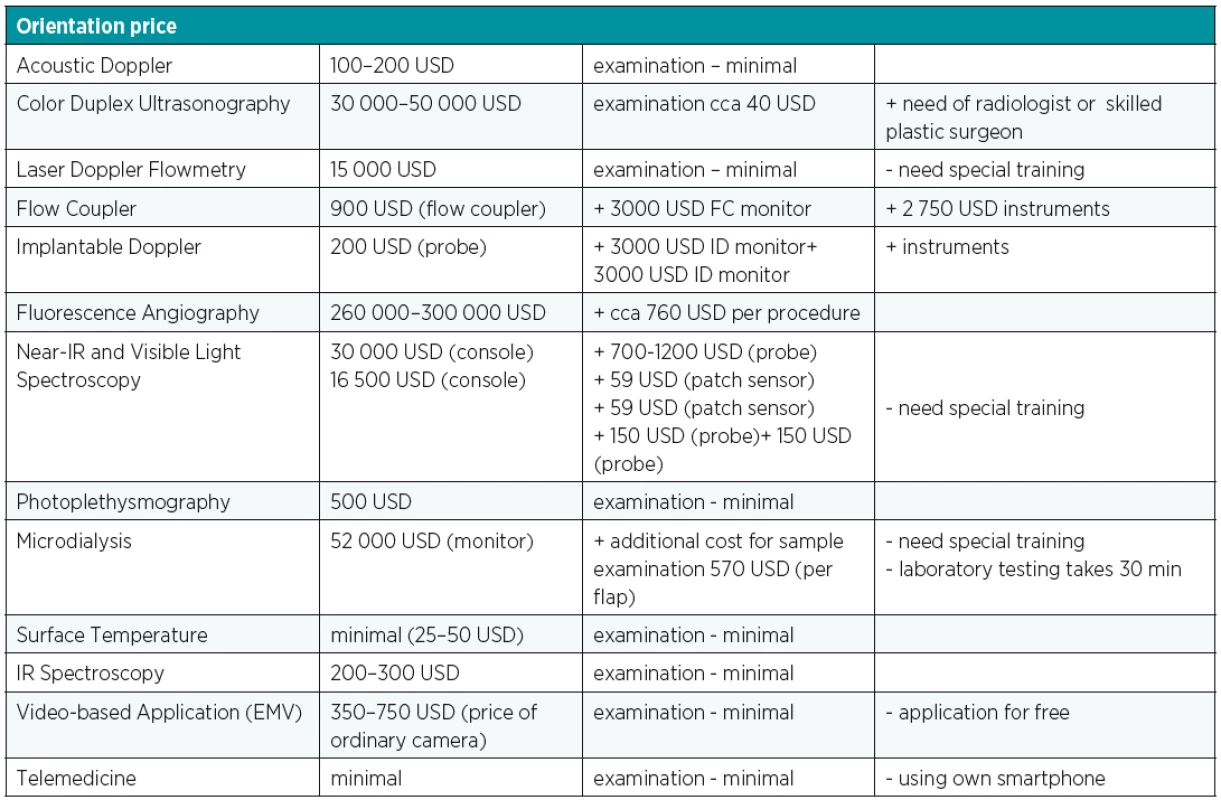
Acknowledgement
The author is grateful to Burn Centre at Brook Army Medical Centre for their hospitality and gained experience with the state-of the art technologies and latest surgical approaches.
Conflict of interest: Authors do not have any potential conflicting interests to declare.
Funding: The authors declare that this study has received no financial support.
Ethical requirements: All procedures in this study were in accordance with ethical standards of the institutional and/or national research committee and with the Helsinki declaration and its later amendments or comparable ethical standards.
Vanesa Lovětínská, MD
Department of Orthopaedic Trauma, First Faculty of Medicine and Central Military University Hospital
U Vojenské nemocnice 1200
169 02 Prague
Czech Republic
E-mail: vanesa.lovetinska@uvn.cz
Zdroje
1. Taylor GI, Daniel RK. The free flap: composite tissue by vascular anastomosis. Aust N Z J Surg. 1973, 43 : 1–3.
2. Chiu YH, Chang DH, Perng CK. Vascular complications and free flap salvage in head and neck reconstructive surgery: analysis of 150 cases of reexploration. Ann Plast Surg. 2017, 78 : 83–8.
3. Hosein RC, Cornejo A, Wang HT. Postoperative monitoring of free flap reconstruction: Comparison of external Doppler ultrasonography and the implantable Doppler probe. Plast Surg. 2016, 24 : 11–9.
4. Schiltz D, Geis S, Kehrer A, Dolderer J, Prantl L, Taeger CD. Video Tutorial for Clinical Flap-Monitoring in Plastic Surgery. Plast Reconstr Surg Glob Open. 2017, 5 : 1478.
5. Chao AH, Lamp S. Current approaches to free flap monitoring. Plast Surg Nurs. 2014, 34 : 57–8.
6. Cervenka B, Bewley AF. Free flap monitoring: a review of the recent literature. Curr Opin Otolaryngol Head Neck Surg. 2015, 23 : 393–8.
7. Cusano A, Fernandes R. Technology in microvascular surgery. Oral Maxilofac Surg Clin North Am. 2010, 22 : 73–90.
8. Kruse AL, Luebbers HT, Grätz KW, Obwegeser JA. Free flap monitoring protocol. J Craniofac Surg. 2010, 21 : 1262–3.
9. Poder TG, Fortier PH. Implantable Doppler in monitoring free flaps: a cost-effectiveness analysis based on a systematic review of the literature. Eur Ann Otorhinolaryngol Head Neck Dis. 2013, 130 : 79–85.
10. Abdel-Gail K, Mitchell D. Postoperative monitoring of microsurgical free tissue transfer for head and neck reconstruction: a systematic review of current techniques – Part I. Non-invasive techniques. Br J Oral Maxilofac Sur. 2009, 47 : 351–5.
11. Patel UA, Hernandez D, Shnayder Y, Wax MK, Hanasono MM, Hornig J, Ghanem TA, Old M, Jackson RS, Ledgerwood LG, Pipkorn P, Lin L, Ong A, Greene JB, Bekeny J, Yiu Y, Noureldine S, Li DX, Fontanarosa J, Greenbaum E, Richmon JD. Free Flap Reconstruction Monitoring Techniques and Frequency in the Era of Restricted Resident Work Hours. JAMA Otolaryngol Head Neck Surg. 2017, 143 : 803–9.
12. Khan MA, Mohan A. Ahmed W, Rayatt S. Nursing monitoring and management of free and pedicled flaps-outcomes of teaching sessions on flap care. Plast Surg Nurs. 2010, 30 : 213–6.
13. Kind GM, Buntic RF, Buncle GM, Cooper TM. Siko PP, Buncke Jr HJ. The effect of an implantable Doppler probe on the salvage of microvascular tissue transplants. Plast. Reconstr. Surg. 1998, 101 : 1268–1273.
14. Peering CHK. Recent advances in postoperative free microvascular flap monitoring. Formosan Journal of Surgery. 2013, 46 : 145–8.
15. Hallock GG. Acoustic Doppler sonography, color duplex ultrasound, and laser Doppler flowmetry as tools for successful autologous breast reconstruction. Clin Plast Surg. 2011, 38 : 203–11.
16. Fujiwara RJT, Dibble JM, Larson SV, Pierce ML, Mehra S4. Outcomes and reliability of the flow coupler in postoperative monitoring pf head and neck free flaps. Laryngoscope. 2018, 128 : 812–17.
17. Wax MK. The role of the implantable Doppler probe in free flap surgery. Laryngoscope. 2014, 124 : 1–12.
18. Lohman RF, Langevin CJ, Boykurt M, Kundu N, Djohan R. A postoperative analysis of free flap monitoring techniques: physical examination, external Doppler, implantable doppler, and tissue oximetry. J Reconstr Microsurg. 2013, 29 : 51–6.
19. Han ZF, Guo LL, Liu LB, Li Q, Zhou J, Wei AZ, Guo PF. A postoperative analysis of free flap monitoring techniques: physical examination, external Doppler, implantable doppler, and tissue oximetry. Int J Surg. 2016, 32 : 109–15.
20. Salgado CJ, Moran SL, Madrini S. Flap monitoring and patient management. Plast Reconstr Surg. 2009, 124 : 295–302.
21. Zenn MR. Fluorescent angiography. Clin Plast Surg. 2011, 38 : 293–300.
22. Hitier M, Cracowski JL, Hamou C, Righini C, Bettega G. Indocyanine green fluorescence angiography for free flap monitoring: A pilot study. J Craniomaxillofac Surg. 2016, 44 : 1833–41.
23. Zama T, Kyriacou PA, Pal SK. Free flap pulse oxymetry utilizing reflectance photoplethysmography. Cons Proc IEEE Eng Med Biol Soc. 2013, 4046–9.
24. Futran ND, Stack BR Jr, Hollenbeak C, Scharf JE. Green light photoplethysmography monitoring of free flaps. Arch Otolaryngol Head Neck Surg. 2000, 126 : 659–62.
25. Gurtner GC, Jones G, Neligan PC, Newman MI, Phillips BT, Sacks JM, Zenn MR. Intraoperative laser angiography using the SPY system: review of the literature and recommendation for use. Ann Surg Innov Res. 2013, 7(1):1. doi: 10.1186/1750-1164-7-1.
26. Jyänki J, Suominen S, Vuola J, Bäck L. Microdialysis in clinical practice: monitoring intraoral free flaps. Ann Plast Surg. 2006, 56 : 387–93.
27. Nielsen HT, Gutberg N, Birke-Sorensen H. Monitoring of intraoral free flaps with microdialysis. Br J Oral Maxillifac Surg. 2011, 49 : 521–6.
28. Green JM, Thomas S, Sabino J, Howard R, Basile P, Dryden S, Crecelius C, Valerio I. Use of intraoperative fluorescent angiography to assess and optimize free tissue transfer in head and neck reconstruction. J Oral Maxillofac Surg. 2013, 71 : 1439–49.
29. Khouri RK, Shaw WW. Monitoring of free flaps with surface-temperature recordings: is it reliable? Plast Reconstr Surg. 1992, 89 : 495–9.
30. Hardwicke JT, Osmani O, Skillman JM. Detection of Perforators Using Smartphone Thermal Imaging. Plast Reconstr Surg. 2016, 137 : 39–41.
31. Bennett S, Harake TN, Goubran R, Knoefel F. Adaptive Eulerian Video Processing of Thermal Video: An Experimental Analysis. IEEE Transaction on Instrumentation and Measurement. 2017, 66 : 2516–24.
32. Micheels J, Alsbjorn B, Sorensen B. Laser doppler flowmetry. A new non-invasive measurement of microcirculation in intensive care? Resuscitation. 1984, 12 : 31–9.
33. John HE, Niumsawatt V, Rozen WM, Whitaker IS. Clinical applications of dynamic infrared thermography in plastic surgery: a systematic review. Gland Surg. 2016, 5 : 122–32.
34. Kagaya Y, Ohura N, Kurita M, Takushima A, Harii K. Examination of tissue oxygen saturation (StO2) changes associated with vascular pedicle occlusion in a rat Island flap model using near-Infrared spectroscopy. Microsurgery. 2015, 35 : 393–8.
35. Takasu H, Hashikawa K, Nomura T, Sakakibara S, Osaki T, Terashi H. A Novel Method of Noninvasive Monitoring of Free Flaps with Near-Infrared Spectroscopy. Eplasty. 2017, 17–37.
Štítky
Chirurgia plastická Ortopédia Popáleninová medicína Traumatológia
Článek České souhrnyČlánek Acknowledgemets to reviewers
Článok vyšiel v časopiseActa chirurgiae plasticae
Najčítanejšie tento týždeň
2019 Číslo 1-4- Metamizol jako analgetikum první volby: kdy, pro koho, jak a proč?
- Kombinace metamizol/paracetamol v léčbě pooperační bolesti u zákroků v rámci jednodenní chirurgie
- Antidepresivní efekt kombinovaného analgetika tramadolu s paracetamolem
- Fixní kombinace paracetamol/kodein nabízí synergické analgetické účinky
- Metamizol v terapii akutních bolestí hlavy
-
Všetky články tohto čísla
- The impact of aesthetic plastic surgery on body image, body satifaction and self-esteem
- Bilobed flap in facial reconstruction
- Free-flap monitoring: review and clinical approach
- Giant basal carcinoma on the forehead and why should prevent them - case report
- Use of medial femoral condyle flap and anterolateral thigh free flap in proxymal tibial posttraumatic non-union with multiple anastomosis - case report
- Reconstruction of deep head burn with a free flap - a case study
- České souhrny
- Acknowledgemets to reviewers
- Acta chirurgiae plasticae
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- The impact of aesthetic plastic surgery on body image, body satifaction and self-esteem
- Free-flap monitoring: review and clinical approach
- Bilobed flap in facial reconstruction
- Use of medial femoral condyle flap and anterolateral thigh free flap in proxymal tibial posttraumatic non-union with multiple anastomosis - case report
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



