-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
Avascular necrosis of the maxilla after orthognathic surgery, a devastating complication? A systematic review of reported cases and clinical considerations
Authors: E. N. Vitkos 1; N. E. Kounatidou 2; K. Agoropoulos 3; A. Kyrgidis 4
Authors place of work: Department of Ophthalmology, University Medical Center Hamburg-Eppendorf, Hamburg, Germany 2; Department of Internal Medicine, General Hospital of Larissa, Larissa, Greece 3; Department of Oral and Maxillofacial Surgery, George Papanikolaou General Hospital, Thessaloniki, Greece 4
Published in the journal: ACTA CHIRURGIAE PLASTICAE, 65, 3-4, 2023, pp. 117-127
doi: https://doi.org/10.48095/ccachp2023117Introduction
The need for treating discrepancies of the facial skeleton led to the development of orthognathic surgery, which has evolved to be a core, integral part of oral and maxillofacial surgery specialty [1–4]. Since the very first osteotomy performed to correct dentofacial discrepancies in 1927 by Herman Wassmund, the Le Fort I osteotomy has been used to treat any kind of midfacial deformities while at the same time provide a high level of patient satisfaction [2,5–7]. The first report of maxillary segmentation comes few years later, when Axhausen segmentized the maxilla to correct open bite discrepancy secondary to maxillary fractures in 1934 [8,9]. Since the mid-twentieth century, when orthognathic surgery became popular in Europe, Le Fort I osteotomy has been regularly used. It constitutes a very versatile procedure, which can separate the maxilla into one or more segments and manipulate its position in three axes [8,9].
Despite the progress in understanding maxillary mechanics and biology, Le Fort I osteotomy is still a procedure associated with complications in around 6% of the patients [2,5–7]. Among them, avascular maxillary necrosis (AMN) is a very rare entity, that ranges from minor soft tissue damage, to complete loss of the maxilla, which is devastating [5,10]. Clinical and laboratory studies have highlighted many local and systematic factors that predispose to maxillary hypoperfusion. These are related to cleft lip and palate, previous palatal surgery, segmentation of the maxilla and anatomical variations [11–14]. Cadaveric studies have also mapped maxillary vascular variations and suggest, they could have a possible impact in the perfusion of the Le Fort I segment [11]. Regarding the surgical technique, management of the descending palatine artery (DPA) is also a field of big controversy among authors [11,15–17]. Despite its contribution to the perfusion of the osteotomized segment, the ligation of the DPA is often performed, as it facilitates the procedure technically and reduces the risk of postoperative bleeding [10,15].
To our knowledge this is the first systematic review to summarize reported cases of AMN following maxillary osteotomy. Given the fact that there is not a typical pattern regarding post orthognathic AMN, our goal was to analyze the factors that led to this dreadful complication.
Materials and methods
Protocol and registration
This study was designed and conducted in accordance with the Preferred reporting items for systematic reviews and meta-analyses (PRISMA) guideline [18]. The research protocol was registered in the international PROSPERO under reference number CRD42023397723.
Eligibility criteria
We included case reports or case series that met the following criteria: (i) cases with secondary avascular maxillary necrosis; (ii) following maxillary osteotomy in the frame of orthognathic surgery; (iii) without any other possible explanation of this complication. As avascular maxillary necrosis we defined all reported ischemic complications that ranged from soft tissue ischemia to loss of bony segments of the maxilla following maxillary osteotomy for orthognathic surgery. We excluded (i) systematic reviews and meta-analyses and (ii) letters to the editor or editorials. The authors had agreed in advance that in the case of two studies reporting the same case or population only a single study with the better design or more detailed presentation would be included.
Search strategy
Two independent researchers (E. N. Vitkos, N. E. Kounatidou) performed a systematic search of MEDLINE (via PubMed), Scopus and Cochrane Library with last search date: November 08, 2022, using the following algorithm ((maxill*) OR (jaw)) AND ((osteotomy) OR (orthognathic) OR (lefort)) AND ((avascular) OR (ischemic) OR (necrosis) OR (aseptic)). All the results were then extracted to Microsoft Excel® spreadsheet and the duplicate articles were identified. The same independent researchers performed title and abstract screening of the resulting articles and then assessed selected articles for eligibility through full text evaluation. In case of any disagreement between the two researchers, the senior author (AK) would be summoned to resolve it. A manual search to the references of all included articles was also performed to identify any other potentially eligible study [19].
Data extraction and tabulation
The first and the second author (E. N. Vitkos, N. E. Kounatidou) independently extracted the data of the included studies in a pre-designed standardized formula for evidence collection. All the potential disagreements were resolved by reaching consensus with the senior author (AK). The following data were extracted: (i) study characteristics (author, year, study design, total number of patients, total number of patients with AMN; (ii) patients’ baseline characteristics (age, sex, possible predisposing factors, prior palatal surgery, type of intervention); (iii) type, extent, and management of the necrotized maxillary segment.
Data analysis and visualization
Data extracted from the individual studies were summarized and presented in the relevant tables. Due to the descriptive nature of the included studies in this systematic review no meta-analysis was feasible. Continuous variables are reported as means and standard deviations. Data visualization was performed using R 4.2.1, ggplot2 package.
Results
Study selection and baseline characteristics
The systematic review of the literature retrieved a total of 701 articles. One additional study was identified through the manual search of the references of the retrieved articles, but it was rejected after full text evaluation. A total of 318 studies were identified as duplicates and therefore excluded from further investigation. Title and abstract screening were performed to the remaining 383 studies which deemed that 17 of them were eligible for full text evaluation. Through the manual search of the references of eligible studies one more study was retrieved but eventually excluded due to not reporting the number of patients with ischemic complication of the maxilla [20]. The study selection process is illustrated in the relevant PRISMA flow-chart (Scheme 1).
Scheme 1. PRISMA flow-chart of study selection process [18,45]. ![Scheme 1. PRISMA flow-chart of study selection process [18,45].](https://pl-master.mdcdn.cz/media/image/5af91acfb71b8fbc073f82935e50b3e1.png?version=1711550456)
Study characteristics and patient demographics
Ultimately 16 studies met our inclusion criteria which report a total of 65 patients from 10 different countries suffering from AMN following maxillary orthognathic surgery [2,5,10,12,21–26,27–32]. We present the baseline characteristics of the included studies and patients in Tab. 1. Most of the studies included were case report 10/16 (62.5%) [5,21,24,25,27–32] one was a case series study [10] and five more were prospective or retrospective cohort studies [2,12,22,23,26]. All but one study were available in the English language [24]. Four studies were originated from the USA [5,10,27,32], two studies from India [21,23], Australia [12,29], and UK [26,30], each and one study from Japan [25], Germany [2], Brazil [28], Singapore [31] and Spain [24], each.
Of the total 65 reported patients, 35 (53%) underwent multisegmented LF I osteotomies, 24 (37%) underwent one segment LF I, 3 (3%) underwent anterior maxillary osteotomy, 1 (1.3%) patient underwent hemi Le Fort I, 1 (1.3%) underwent hemi LFI / hemi LF 3 (3%), while for 1 (1.3%) of the patients the type of osteotomy was not reported. Of the presented patients, 19 were males and 32 were females while the sex was not reported for 14 patients. A detailed presentation of the type of maxillary surgery, extent of necrosis, management of the necrosis and type of reconstructive method used is presented in Tab. 2. Bar plots visualizing the distribution of sex amongst patients, type of necrosis and type of osteotomies used are presented in Fig. 1, 2 and 3, respectively.
Tab. 1. Studies and patient characteristics. 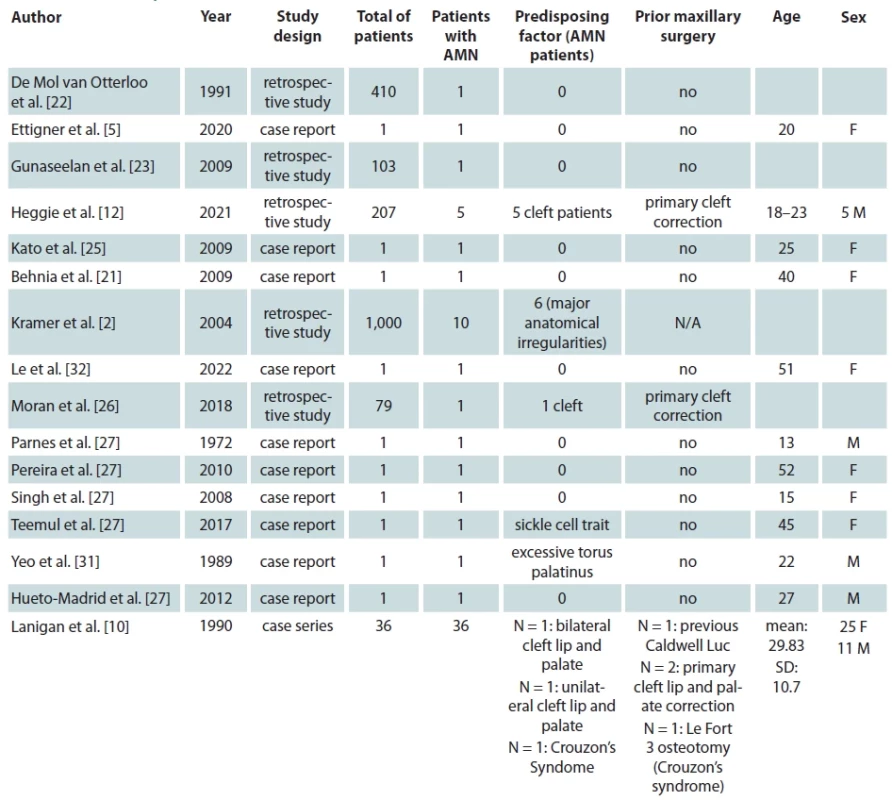
AMN – avascular maxillary necrosis, F – female, M – male, NA – not available, SD – standard deviation Tab. 2. Type of maxillary surgery, extent of necrosis, management of the necrosis and type of reconstructive method per included study. 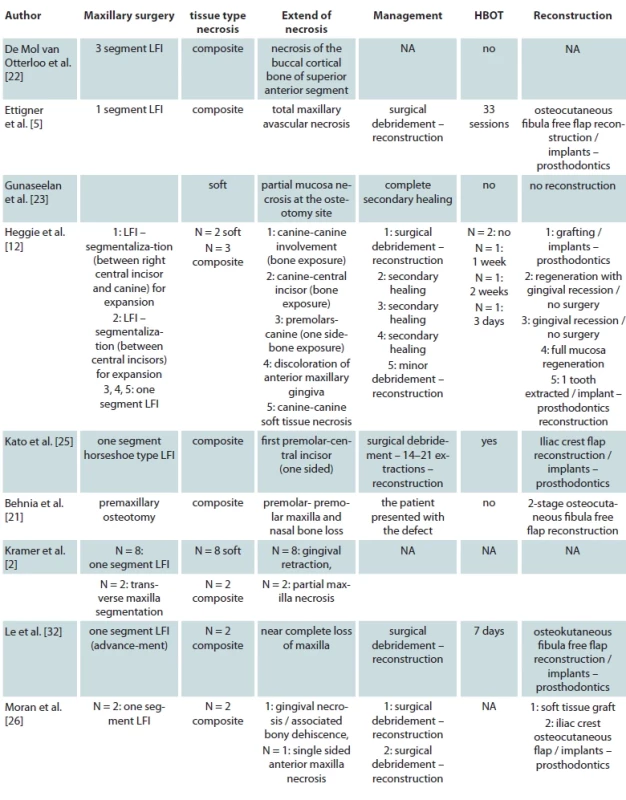
Tab. 2 – continuing. Type of maxillary surgery, extent of necrosis, management of the necrosis and type of reconstructive method per included study. 
HBOT – hyperbaric oxygen therapy, LF – Le Fort, LFI – Le Fort I, NA – not available, PRP – platelet-rich plasma Fig. 1. Bar plot describing the sex distribution amongst patients suff ering from avascular maxillary necrosis. 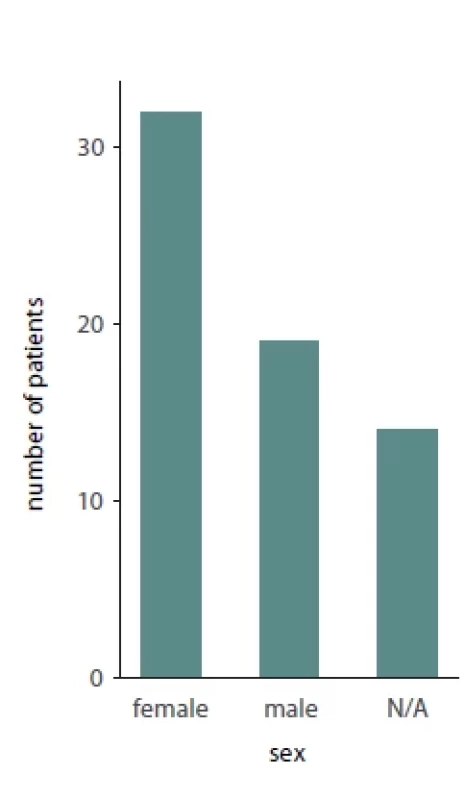
NA – not analyzed Fig. 2. Bar plot describing the osteotomy type used in patients suff ering from avascular maxillary necrosis. 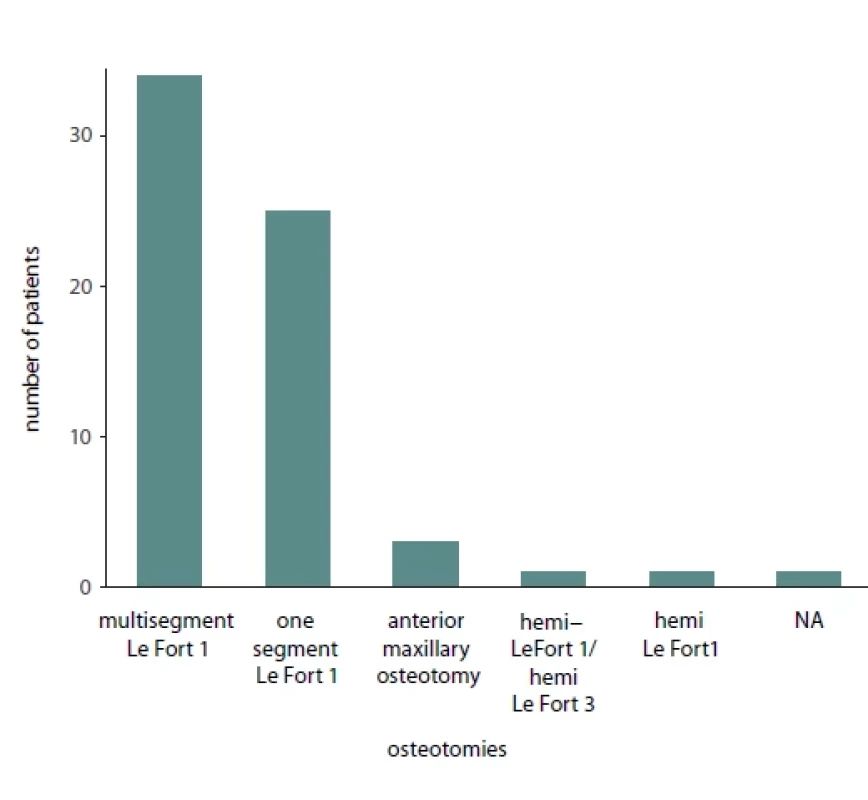
Fig. 3. Bar plot describing the extent of necrosis amongst patients suff ering from avascular maxillary necrosis. 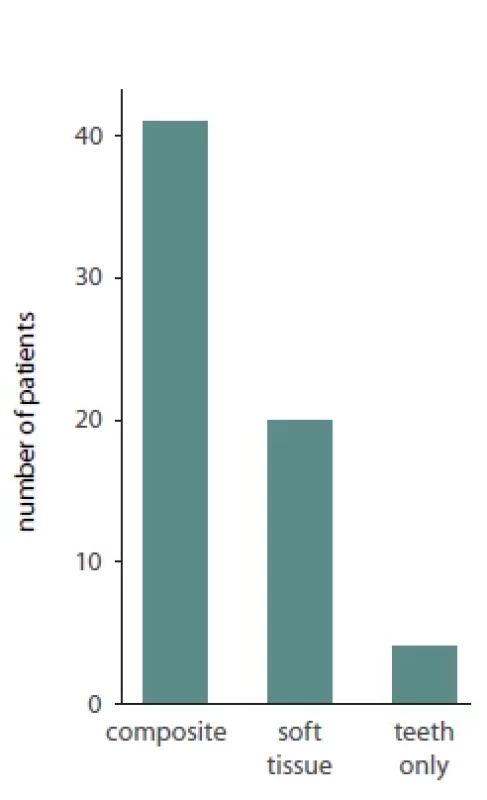
Quality assessment
Although case reports and case series are biased due to their design, standardized tools to assess their methodological quality have been created for the systematic review of reported cases. In our study we used the modified Newcastle-Ottawa scale (NOS) introduced by Murad et al. [33]. This tool assesses selection, ascertainment, casualties and reporting by a series of 8 questions. We have intentionally removed questions number 4, 5 and 6, as they are not applicable to the type of outcome we assess. Based on a cumulative score of 5, the risk of bias of the studies were classified as “low risk”, “medium risk” and “high risk” with scores 4–5, 2-3, 0–1 respectively. Nine studies were deemed as “low risk” for bias, six studies as “medium risk” and one study as “high risk”. The results are presented in Suppl. Tab. 1.
Discussion
With orthognathic surgery considered to be the standard of care for dentofacial discrepancies, the understanding of this potentially devastating complication is important. Maxillary avascular necrosis is amongst the rarest complications when maxillary osteotomy is performed [4,24]. Although a rare event, it remains a morbid complication, as it can lead to complete loss of the maxilla to an otherwise healthy patient [5]. Therefore, numerous anatomical, laboratory and clinical studies have been carried out to investigate and understand the possible mechanisms and suggest ways to prevent and manage this complication. With this systematic review we aim to collect and present all the available evidence regarding AMN following maxillary osteotomy for orthognathic. Understanding the complexity of maxillary perfusion in association with the individualized patient characteristics and the type of maxillary osteotomy will provide practitioners with useful clinical insights and lead to better surgical outcomes.
The understanding of maxillary blood supply is essential for the safe conduction of a maxillary osteotomy in orthognathic surgery although the optimal management of the vessels supplying the maxilla is not yet clear [32]. Siebert et al. in a cadaveric study have shown that the maxillary segment after Le Fort I osteotomy is mainly vascularized by the ascending palatine and ascending pharyngeal arteries [34]. They have also demonstrated a rich anastomotic network between those branches as well as the alveolar branches of the internal maxillary artery [34]. An additional cadaveric study performed by Brunener et al. tried to associate the avascular necrosis complication with anatomical variances of the Le Fort I segment blood supply [11]. In their study two types of maxillary arterial blood supply are described [11].The first one matches the one of the previous cadaveric studies of Siebert et al. However, Bruneder et al. identified and described the unilateral absence of the ascending palatine artery in some cadaveric specimens, with the presence of a welldeveloped anterior branch of the ascending pharyngeal artery instead. This led them to suggest, that ischemic complications may be associated with the anatomic variations of blood vessels supplying the Le Fort I segment [11]. No research has managed to identify a threshold for sufficient residual blood supply to the osteotomized maxillary segment, that could prevent those complications [10].

Suppl. Tab. 1. Quality assessment of included non-randomized studies using the modified Newcastle-Ottawa scale (NOS) by Murad et al. [33]. Furthermore, the importance of the DPA in the blood supply of the Le Fort I segment is also highlighted by many studies [10,11,15]. The preservation of DPA is currently a point of conflict in the literature [10,15,28,35]. Some researchers advocate the preservation of those vessels whenever possible, as their contribution to the blood supply is considered to be significant [10,17,36]. It has been suggested, that the intraoperative ligation of those arteries could be the main cause of avascular maxillary necrosis [10,16,17]. DPA preservation has the theoretical benefit of maintaining a portion of the afferent blood supply [15]. In a randomized controlled trial, Dosdon et al. compared the outcomes after Le Fort I osteotomy in two groups of patients, one with ligated DPA and one with preserved [15]. They concluded there were no statistically significant differences in the incidence of avascular necrosis [15].
From a technical aspect the ligation of the DPA is considered to facilitate the operation, as the mobilization of the maxilla is performed easier [10, 15]. Furthermore, an unidentified laceration of the DPA can cause severe postoperative bleeding [15,16,37]. The ligation of DPA reduces the risk of postoperative bleeding that not only can be a life-threatening complication but bleeding can also lead to hypotension and thus to hypoperfusion of the maxilla, which increases the risk of avascular necrosis [11,12,15]. However, the management of DPA is complicated by both anatomical and biological concerns. The damage to the DPA can also be indirect during maxillary mobilization and especially during high maxillary protrusion [10,11,28,29]. The application of bending forces can damage the endothelium of the DPA and therefore lead to thrombosis of the vessel. This could also undermine maxillary perfusion even though rupture of the vessel is not observed [11, 28, 38]. Furthermore intraoperative manipulation can affect the palatal pedicle that significantly contributes to Le Fort I segment survival [37]. Specifically Epker et al suggest that the expansion of the maxilla for more than 3–5 mm, can risk avulsing part of the attached palatal pedicle and further undermine maxillary blood supply [37].
Another factor related to maxillary perfusion that has significant impact in the clinical application of orthognathic osteotomy are pre-existing maxillary anatomical variations. These include surgically corrected cleft lip and palate as well as other maxillary operations that induce the production of scar tissue in the healing process [2,10,12,26,28].
Regarding cleft patients, the congenital deformity, the alterations in blood supply of the maxilla due to surgical interventions and the scarring of the palate compromise the perfusion of the Le Fort I segment making it potentially less viable in comparison to that of noncleft patients [12,26,37]. In an angiographic study performed by Drommer et al. on cleft patients, smaller greater palatine arteries were also identified [39]. This is another important consideration, when osteotomy is applied to this subset of patients, in order to prevent ischemic complications [39]. It was also suggested that the preservation of DPA in those patients might be of big importance in the viability of the Le Fort segment as the collateral arterial network with superior alveolar and supraorbital arteries, developed to compensate for inferior palatal perfusion can be interrupted with the osteotomy [10,12]. A minimal periosteal elevation, specifically in the patients with premaxilla segment is also recommended, given its importance in Le Fort I segment perfusion [40–42]. Heggie et al. have reported outcomes of 207 cleft patients undergoing maxillary osteotomy, 5 of those were complicated with maxillary avascular necrosis [12]. For this reason they suggested the “delayed maxillary flap” technique, namely a preliminary procedure to assist maxillary perfusion [12].
Patients requiring segmental maxillary osteotomy for the management of their deformity are generally more prone to AMN [10,22,35,37]. Bruneder et al. suggested that patients usually tolerate a sagittal maxillary osteotomy in the Le Fort I segment, while additional segmentation may lead to interruption of the collateral blood supply of the segments and, thus, to an increased risk of ischemic complications [11]. As the major blood supply of the Le Fort I segment comes from the palatine vessels the anterior segment is more prone to ischemia, as its palatal perfusion is inadequate after segmentation [21,37]. In these cases, the contribution of the attached mucosa to the maxillary segments is vital [10,30, 37]. Perforations of the palatal mucosa and specifically horizontal tearing of the anterior part of the palatal mucosa undermine maxillary segments perfusion [10,22,37]. Thus, it is proposed that surgeons minimize the maxillary segmentation and care should be focused on preserving the soft tissue and periosteal attachment of the segments, when needed [10,22,37]. Specifically, Teemul and al. suggest segmental osteotomy to be in the paramedial site where bone and mucosa are thicker and, greater perfusion is provided to the additional segment [30].
While avascular maxillary necrosis can occur in healthy patients, some predisposing factors have been described [5]. Yeo et al. reported a case of a patient with extensive torus palatinus, suffering palatal perforation secondary to ischemia after Le Fort I osteotomy [31]. In the case of Teemul et al. a patient with sickle cell anemia trait suffered AMN after Le Fort I osteotomy and right posterior segmental osteotomy [30]. Previous maxillary operations may also be accounted for, as Lanigan et al. report a case of AMN in a patient with a Caldwell-Luc operation previously performed [10]. Finally, vertical posterior impactions of the maxilla, excessive soft tissue degloving, large maxillary advancement and other medical comorbidities have also been suggested to predispose to AMN [2,5,10,25,32].
The intraoperative assessment of the tissues in the Le Fort I segment has been suggested to be indicative of tissue ischemia [43]. Freihofer et al. recommended the monitoring of the mucosal color during operation and in the case of suspected ischemia they proposed the interruption of the operation and its completion a few weeks later for the improvement of the vascularization in the meantime. Alternatively, they suggested the application of elastic traction three days after the initial operation instead [43].
Regarding the management of the ischemic complications the application of optimal oral hygiene in the area with frequent irrigation with saline is essential [10,28,37]. Optimally, treatment with hyperbaric oxygen therapy (HBOT) should start as early as possible as studies have shown that it can confine the extent of the necrosis [37,44]. It should be noted though that HBOT cannot reverse the existing damage to maxillary tissues [12,44]. The administration of antibiotics should be considered to prevent secondary infection of the necrotized maxilla [10,12,28,37]. Surgical debridement with removal of the necrotic tissue, helps to speed up the healing process [10]. Regarding the secondary reconstruction of the defect the different authors used different kinds of techniques depending on the extent of the necrosis and the technical capabilities of their department at the time. Notably, Ettinger et al. in their case with total maxillary necrosis, used osteocutaneous fibula free flap reconstruction followed by dental implants and prosthodontics [5]. The same free flap was also used by Le et al. and Behnia et al. in their reported cases [21,32]. Bone grafting with iliac crest graft was also used in several patients [24–26,29]. Finally, in most of the cases, reconstruction with dental implants and prosthodontics was used after tissue healing as shown in Tab. 2.
Conclusion
Despite the advances in understanding maxillary perfusion after orthognathic surgery, avascular maxillary necrosis is still a complication seen in everyday practice. Great care should be taken by clinical practitioners when treating patients with previously performed maxillary surgery and specifically patients with surgically corrected cleft lip and palate. Furthermore, segmentation of the maxilla should be used, only when absolutely necessary, with care in order to preserve the vital tissues for the vascularization of the segments. Other systemic comorbidities should also be considered. Finally, in the case of AMN the management should be fast and secondary reconstruction depending on the extent of necrosis should be applied.
Limitations: A retrospective review of the reported cases has limitations. Not all the information needed was available in each case. The management of the DPA is not reported in most cases. Most authors do not report the protocol they used for antibiotics and fluid administration in AMN treatment. Finally, this complication may also be underreported as small marginal soft tissue necrosis might not be recorded by many surgeons.
Competing interests: The participating authors declare no competing interests.
Roles of authors
Conception and design – E. N. Vitkos; data collection – E. N. Vitkos, N. E. Kounatidou; data visualization – E. N. Vitkos; writing the article – E. N. Vitkos, N. E. Kounatidou, A. Kyrgidis; critical revision of the article – E. N. Vitkos, N. E. Kounatidou, K. Agoropoulos, A. Kyrgidis, final approval of the article – E. N. Vitkos, N. E. Kounatidou, K. Agoropoulos, overall responsibility – E. N. Vitkos.
Sources of financial report: The participating authors declare no sources of financial support that require acknowledgement.
Zdroje
1. Haas OL., Guijarro-Martínez R., de Sousa Gil AP., et al. Stability and surgical complications in segmental Le Fort I osteotomy: a systematic review. Int J Oral Maxillofac Surg. 2017, 46 (9): 1071–1087.
2. Kramer F., Baethge C., Swennen G., et al. Evaluation of 1000 patients. J Craniofac Surg. 2004, 15 (6): 971–977.
3. Van de Perre JPA., Stoelinga PJW., Blijdorp PA., et al. Perioperative morbidity in maxillofacial orthopaedic surgery: a retrospective study. J Craniomaxillofac Surg. 1996, 24 (5): 263–270.
4. Steel BJ., Cope MR. Unusual and rare complications of orthognathic surgery: a literature review. J Oral Maxillofac Surg. 2012, 70 (7): 1678–1691.
5. Ettinger KS., Nathan J., Guerrero LM., et al. Microvascular reconstruction of total maxillary avascular necrosis as a complication of routine orthognathic surgery. J Oral Maxillofac Surg. 2020; 78 (10): 1846–1858.
6. Maurer P., Otto C., Bock JJ., et al. Patient satisfaction with the outcome of surgical orthodontic intervention and effect of esthetic and functional criteria. Mund Kiefer Gesichtschir. 2002, 6 (1): 15–18.
7. Garg S., Kaur S. Evaluation of post-operative complication rate of Le Fort I osteotomy: a retrospective and prospective study. J Maxillofac Oral Surg. 2014, 13 (2): 120–127.
8. Wolfe SA., Berkowitz S. LeFort I osteotomy. Cleft Lip Palate Diagnosis Manag. 2013, 537–554.
9. Drommer RB. The history of the “Le Fort I osteotomy.” J Maxillofac Surg. 1986, 14 (3): 119–122.
10. Lanigan DT., Hey JH., West RA. Aseptic necrosis following maxillary osteotomies: report of 36 cases. J Oral Maxillofac Surg. 1990, 48 (2): 142–156.
11. Bruneder S., Wallner J., Weiglein A., et al. Anatomy of the Le Fort I segment: are arterial variations a potential risk factor for avascular bone necrosis in Le Fort I osteotomies? J Craniomaxillofac Surg. 2018, 46 (8): 1285–1295.
12. Heggie A., Robertson K., Shand J. Avascular necrosis in cleft maxillary repositioning: a review of cases and introduction of the “delayed maxillary flap”. Int J Oral Maxillofac Surg. 2021, 50 (2): 185–190.
13. Gauthier A., Lézy JP., Vacher C. Vascularization of the palate in maxillary osteotomies: anatomical study. Surg Radiol Anat. 2002, 24 (1): 13–17.
14. Ferri J., Druelle C., Schlund M., et al. Complications in orthognathic surgery: a retrospective study of 5025 cases. Int Orthod. 2019, 17 (4): 789–798.
15. Dodson TB., Bays RA., Neuenschwander MC. Maxillary perfusion during Le Fort I osteotomy after ligation of the descending palatine artery. J Oral Maxillofac Surg. 1997, 55 (1): 51–55.
16. Bell WH., You ZH., Finn RA., et al. Wound healing after multisegmental le fort i osteotomy and transection of the descending palatine vessels. J Oral Maxillofac Surg. 1995, 53 (12): 1425–1433.
17. Regan BO., Bharadwaj G. The identification and protection of the descending palatine artery in Le Fort I osteotomy: a forgotten technique? Br J Oral Maxillofac Surg. 2007, 45 (5): 412–414.
18. Page MJ., McKenzie JE., Bossuyt PM., et al. The PRISMA. 2020 statement: an updated guideline for reporting systematic reviews. PLoS Med. 2021, 18 (3): e1003583.
19. Wohlin C. Guidelines for snowballing in systematic literature studies and a replication in software engineering. [online]. Available from: https: //www.wohlin.eu/ease14.pdf.
20. Robl MT., Farrell BB., Tucker MR. Complications in orthognathic surgery a report of 1000 cases. Oral Maxillofac Surg Clin North Am. 2014, 26 (4): 599–609.
21. Behnia H., Nazerani S., Kalantar Motamedi MH., et al. Comprehensive reconstruction of the maxilla after a failed premaxillary osteotomy: a case report with long-term follow-up. Ann Plast Surg. 2009, 62 (1): 59–62.
22. de Mol van Otterloo JJ., Tuinzing DB., Greebe RB., et al. Intraand early postoperative complications of the le fort I osteotomy. A retrospective study on 410 cases. J Craniomaxillofac Surg. 1991, 19 (5): 217–222.
23. Gunaseelan R., Anantanarayanan P., Veerabahu M., et al. Intraoperative and perioperative complications in anterior maxillary osteotomy: a retrospective evaluation of 103 patients. J Oral Maxillofac Surg. 2009, 67 (6): 1269–1273.
24. Hueto-Madrid JA., Gutierrez-Santamaria J. Complicaciones quirúrgicas de la cirugía ortognática: presentación de tres casos y revisión de la literatura. Rev Esp Cir Oral Maxilofac. 2012, 34 (2): 56–74.
25. Kato H., Watanabe A., Takano M., et al. A case of maxillary partial aseptic necrosis after Le Fort I osteotomy. J Oral Maxillofac Surg Med Pathol. 2020, 32 (1): 53–56.
26. Moran I., Virdee S., Sharp I., et al. Postoperative complications following LeFort 1 maxillary advancement surgery in cleft palate patients: a 5-year retrospective study. Cleft Palate Craniofacial J. 2018, 55 (2): 231–237.
27. Parnes EI., Becker ML. Necrosis of the anterior maxilla following osteotomy. Report of a case. Oral Surg Oral Med Oral Pathol. 1972, 33 (3): 326–330.
28. Pereira FL., Yaedú RYF., Sant’Ana AP., et al. Maxillary aseptic necrosis after Le Fort I osteotomy: a case report and literature review. J Oral Maxillofac Surg. 2010, 68 (6): 1402–1407.
29. Singh J., Doddridge M., Broughton A., et al. Reconstruction of post-orthognathic aseptic necrosis of the maxilla. Br J Oral Maxillofac Surg. 2008, 46 (5): 408–410.
30. Teemul TA., Perfettini J., Morris DO., et al. Post-operative avascular necrosis of the maxilla: a rare complication following orthognathic surgery. J Surg Case Reports. 2017, 2017 (1): rjw240.
31. Yeo JF., Loh FC., Egyedi P., et al. Serious circulatory disturbance after Le Fort I osteotomy. A case report. J Craniomaxillofacial Surg. 1989, 17 (5): 222–225.
32. Le JM., Gigliotti J., Ying Y., et al. Computer-assisted microvascular free flap reconstruction and implant rehabilitation of the maxilla-treatment of a rare post-orthognathic complication. J Maxillofac Oral Surg. 2022, 21 (1): 82–87.
33. Murad MH., Sultan S., Haffar S., et al. Methodological quality and synthesis of case series and case reports. Evid Based Med. 2018, 23 (2): 60–63.
34. Siebert JW., Angrigiani C., McCarthy JG., et al. Blood supply of the Le Fort I maxillary segment: an anatomic study. Plast Reconstr Surg. 1997, 100 (4): 843–850.
35. Omura S., Iwai T., Honda K., et al. Vital staining of palatal soft tissue in horseshoe Le Fort I osteotomy for superior repositioning of the maxilla. J Craniofac Surg. 2015, 26 (3): 911–913.
36. Nelson RL., Path MG., Ogle RG., et al. Quantitation of blood flow after Le Fort I osteotomy. J Oral Surg. 1977, 35 (1): 10–16.
37. Epker BN. Vascular considerations in orthognathic surgery. II. Maxillary osteotomies. Oral Surg Oral Med Oral Pathol. 1984, 57 (5): 473–478.
38. Blann AD. How a damaged blood vessel wall contibutes to thrombosis and hypertenasion. Pathophysiol Haemost Thromb. 2003, 33 (5–6): 445–448.
39. Drommer R. Selective angiographic studies prior to Le Fort I osteotomy in patients with cleft lip and palate. J Maxillofac Surg. 1979, 7 (4): 264–270.
40. Roy AA., Rtshiladze MA., Stevens K., et al. Orthognathic surgery for patients with cleft lip and palate. Clin Plast Surg. 2019, 46 (2): 157–171.
41. Kretschmer WB., Baciut G., Baciut M., et al. (2009) Changes in bone blood flow in segmental LeFort I osteotomies. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009, 108 (2): 178–183.
42. Kretschmer WB., Baciut G., Dinu C., et al. The influence of expansion on intraoperative bone blood flow in multisegmental maxillary osteotomies: an experimental study. Int J Oral Maxillofac Surg. 2010, 39 (3): 282–286.
43. Freihofer HP. Latitude and limitation of midface movements. Br J Oral Maxillofac Surg. 1984, 22 (6): 393–413.
44. Nilsson LP., Granström G., Röckert HOE. Effects of dextrans, heparin and hyperbaric oxygen on mandibular tissue damage after osteotomy in an experimental system. Int J Oral Maxillofac Surg. 1987, 16 (1): 77–89.
45. PRISMA. [online]. Available from: http: //www.prisma-statement.org/.
Evangelos N. Vitkos
Department of Oral and Maxillofacial Surgery
George Papanikolaou General Hospital
Thessaloniki, Greece
e-mail: envitkos@gmail.comSubmitted: 4. 4. 2023
Accepted: 20. 5. 2023Štítky
Chirurgia plastická Ortopédia Popáleninová medicína Traumatológia
Článok vyšiel v časopiseActa chirurgiae plasticae
Najčítanejšie tento týždeň
2023 Číslo 3-4- Metamizol jako analgetikum první volby: kdy, pro koho, jak a proč?
- Kombinace metamizol/paracetamol v léčbě pooperační bolesti u zákroků v rámci jednodenní chirurgie
- Antidepresivní efekt kombinovaného analgetika tramadolu s paracetamolem
- Srovnání analgetické účinnosti metamizolu s ibuprofenem po extrakci třetí stoličky
- Fixní kombinace paracetamol/kodein nabízí synergické analgetické účinky
-
Všetky články tohto čísla
- Editorial
- Diagnosis and treatment of Eagle’s syndrome and possible complications
- Scalp arteriovenous malformations – 20 years of experience in a tertiary healthcare centre
- The comparison of effectivity in breast cancer prevention between skin sparing and subcutaneous mastectomy – 20 years of experience
- Avascular necrosis of the maxilla after orthognathic surgery, a devastating complication? A systematic review of reported cases and clinical considerations
- 3D maxillofacial surgery planning – one decade development of technology
- A primary cutaneous carcinosarcoma of the retro auricular region, how to treat and literature review
- Combination of cable ties and barbed sutures for fasciotomy closure – two case reports
- Skin grafting on amputated lower limb, norepinephrine-induced ischemic limb necrosis – case report
- Abdominal wall reconstruction for extensive necrosis following abdominoplasty in a patient with subcostal scars – case report
- Acta chirurgiae plasticae
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- Diagnosis and treatment of Eagle’s syndrome and possible complications
- Avascular necrosis of the maxilla after orthognathic surgery, a devastating complication? A systematic review of reported cases and clinical considerations
- 3D maxillofacial surgery planning – one decade development of technology
- Skin grafting on amputated lower limb, norepinephrine-induced ischemic limb necrosis – case report
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



