-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
Antiepleptic drug interactions: A clinical case demonstration
Antiepileptické léky a lékové interakce: klinická kazuistika
Epilepsie je závažné onemocnění postihující dětskou i dospělou část populace po celém světě. Vzhledem k btížnému zjišťování etiologie je prvotní nastavení léčebných postupů často řízené empirickými terapeutickými úvahami. Zvládnutí projevů vyžaduje použití antiepileptických léčiv, přičemž mnoho z ich vykazuje možné lékové interakce. Speciálně děti jsou vnímavé k ékovým interakcím a často vykazují atypické nežádoucí projevy vyžadující speciální péči.
Cíl.
Popis případu patnáctileté dívky trpící refrakterní epilepsií na podkladě fokální kortikální dysplazie, u níž bylo zhoršování klinického stavu s elkou pravděpodobností spojováno s ékovými interakcemi mezi běžně předepisovanými antiepileptiky, jmenovitě kyselinou valproovou, fenobarbitalem, nebo primidonem a karbamazepinem.Klíčová slova:
fokální kortikální dysplazie, léky na refrakterní epilepsii, léková interakce.
Authors: Hundie Tesfaye 1; E. Eva Klapková 1; Alena Tesfayeová 2; Vladimír Komárek 2
Authors place of work: Department of Clinical Biochemistry and Pathobiochemistry, Division of Clinical Pharmacology, University Hospital, Motol, nd Faculty of Medicine, Charles University, Prague, Czech Republic 1; Department of Paediatric Neurology, University Hospital, Motol, 2nd Faculty of Medicine, Charles University, Prague, Czech Republic 2
Published in the journal: Čas. Lék. čes. 2011; 150: 451-456
Category: Kazuistika
Summary
Epilepsy is a serious health disorder affecting both paediatric and adult population worldwide. Due to difficulties in identifying its aetiology, initial management is often guided by empiric therapy measures. Symptomatic control requires the use of antiepileptic drugs (AEDs), many of which have the potential for adverse drug interactions. Children are especially susceptible to drug interactions and frequently exhibit atypical adverse events, which may require special care.
Aim.
To demonstrate a case of a 15 year old girl suffering from refractory epilepsy with underlying focal cortical dysplasia (FCD), whose seizure deterioration was most probably associated with drug-drug interactions between prescribed common antiepileptic drugs, namely valproic acid, phenobarbital or the prodrug primidon and carbamazepine.Key words:
focal cortical dysplasia, refractory epilepsy drug, drug interaction.INTRODUCTION
Epilepsy is a common disorder approximately affecting 0.5–2% of the population worldwide. Approximately one-third of cases are resistant to drug treatment although the mechanisms underlying this drug resistance are not well understood (1). In a recent review, Reynolds and Rodin (2) brought attention to the history of clinical concepts of epilepsy and its classification, especially in the last 100 years. Throughout its recorded history, epilepsy has always been defined by its most dramatic symptoms, like falling, motor activity or loss of consciousness, but separation from other causes of the same paroxysmal symptoms has always proved challenging. A recent paper by Meencke (3) also describes the history of the clinical neuropathology of epilepsy in the last hundred years, from microscopy to molecular neuropathology, mainly focusing on the concepts of hippocampal sclerosis and causes or consequence of seizures, as well as the concept of developmental disturbances in respect to general epileptogenicity. Clinical neuropathology remains an important discipline in the future of epileptology and brain research especially in the area of molecular genetics, whereas, neuropathology may help to understand the stages of epileptogenesis and factors responsible for the progressive nature of the disease. Despite remarkable progress, the aetiology of the syndrome is often difficult to confirm. Focal cortical dysplasia (FCD) is a congenital abnormality where the neurons in an area of the brain fail to migrate in the proper formation in utero. It is one of the most common causes of intractable epilepsy in children and is a frequent cause of epilepsy in adults. FCD is associated with enlarged cells known as balloon cells for their large elliptical shape, displaced nucleus, and lack of dendrites or axons. It is hypothesized that balloon cells and dysplastic neurons contribute to seizures in patients with cortical dysplasia. Refractory epilepsy with underlying focal cortical dysplasia is usually resistant to drug treatment in approximately one-third of cases, but the mechanisms underlying this drug resistance are not understood. Previous studies in human epilepsy have shown that multidrug resistance (MDR-1) or multidrug resistance associated protein (MRP-1) may also be overexpressed in brain tissue (glia and neurones), which do not normally express these proteins.Thus in pathologies causing refractory epilepsy, the presence of overexpressed resistance proteins in lesional dysplastic neurones may lower the interstitial concentration of AEDs in the vicinity of the epileptogenic pathology leading to drug resistant epilepsy (1). As drug monotherapy rarely controls the disease, multi-drug approaches are common practice, especially in paediatric age groups. Symptomatic control requires the prophylactic use of antiepileptic drugs (AEDs), many of which have the potential for adverse drug interactions with other coprescribed medication. Drug-drug interactions are reported to give rise to adverse drug reactions (ADRs) in at least 6% of subjects with epilepsy (4). Children are especially susceptible to drug interactions and frequently exhibit atypical adverse events, which may require hospitalization or result in death (5). In primary care, 3.0% of children on chronic antiepileptic therapy are co-prescribed therapeutic agents, which could give rise to clinically serious drug-drug interactions (6). Within a 36 year period 331 children aged < 17 years died following suspected ADRs in the United Kingdom. AED therapy was associated with 65 of these deaths, and sodium valproate alone with 31 deaths. Even the ‘safer’ second-generation AEDs were involved in 20 fatal cases and have been identified as the most important cause of drug-related childhood death (7). In the present case we describe possible drug-drug interactions which may be responsible for clinical deterioration in a patient with refractory epilepsy.
CASE PRESENTATION
A female paediatic patient was on long term follow-up and general care for refractory epilepsy. No familial history association was reported with the diagnosis. Her epilepsy was classified as multi-drug resistant, where all available AEDs including combinations of new generation drugs failed to fully control the entity. Further investigation led to the diagnosis of FCD IIb Lsin. indicating surgery (stereotactic resection) as the most appropriate treatment. Post sugery, no significant long term improvement was observed, instead, hemianopsia and praparesis l.dx. has been observed as complication. Maintenance dose of AEDs was continued, but with poor control yet, where vagus nerve stimulation (VNS) has been provided in addtion to AEDs therapy. AEDs levels just four months before deterioration were within conventional therapeutic ranges (Table 1. first row), whereas phenobarbital levels during the clinical deterioration were within toxic ranges despite the rest of the AEDs being within therapeutic margins. Once upon admission her state had deteriorated and VPA was added to thre AEDs (PRM, PB i.v., CBZ) and this led to further deterioration. The main clinical manifestations potentially linked to ADRs were alteration of consciousness, halucination and delerium. AED levels revealed significantly high PB levels (Fig. 1.) despite significant reduction of the dose from intially 300 mg/day to only 100 mg/day during the hospital stay.
Tab. 1. Combined antiepileptic plasma concentrations (μmol/L) determined by flourescent polarization immunoassay (FPIA) method five months before (top column) and during the event requiring intensive care (bottom columns) 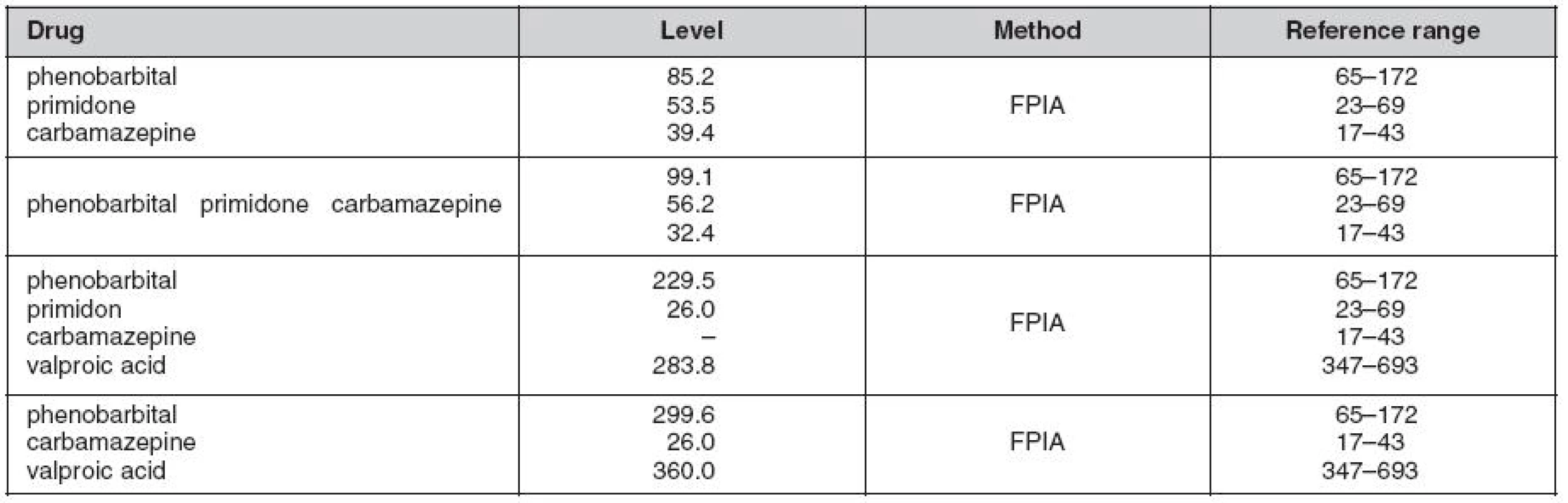
Fig. 1. Profile of series serum levels of concomitantly adminstered AEDs, namely, valproic acid (VPA), phenobarbital (PB) and carbamazepine (CBZ) in a 14 year old female patient with suspected drug-drug interaction for the three antiepileptic drugs. The concentrations of PB were above the therapeutic limit until dose reduction to a third of the intial dose. However, the other two drugs (VPA and CBZ) demonstrated levels within conventional therapeutic ranges (see Table 1). 
DISCUSSION
Drug-drug interactions at any level (both pharmacokinetic and pharmacodynamic) represent a major challenge in multi-drug treatment of epilepsy. Valproic acid is a minor substrate of CYP2A6, 2B6, 2C8/9, 2C19, 2E1. It weakly inhibits CYP2C8/9, 2C19, 2D6 and 3A4; whereas it induces CYP2A6. In the present case of concomitantly used drugs, phenobarbial and carbamazepine are inducers, but the inhibitory effect of valproate may be significant as demonstrated by evident cumulation of phenobarbital blood levels (Fig. 2). Valproic acid may increase, decrease, or have no effect on carbamazepine levels; but may increase serum concentrations of carbamazepine – epoxide (active metabolite) by inducing the metabolism of parent drug (carbamazepine). VPA can displace the protein binding of CBZ. VPA also inhibits microsomal epoxide hydrolase (mEH), the enzyme responsible for the breakdown of carbamazepine-10,11 epoxide into inactive metabolites. Valproic acid appears to inhibit the metabolism of phenobarbital; thus increasing its overall effect. (Cave: PB intoxication after adding PB/PRM to VPA, as a late effect). Phenobarbital/primidone, and carbamazepine may produce enzyme inducing effects that can lower the half-life of VPA thus leading to therapeutic failure. Although the mechanism is unknown, it should be considered that phenobarbital levels increase when valproic acid is given concomitantly, enhancing its sedative effects. It is well documented that carbamazepine serum concentrations may be elevated when valproic acid is added to the treatment regimen. This is largely due to an increase in carbamazepine epoxide levels. Phenobarbital, primidone, and carbamazepine may produce enzyme inducing effects that can temporarily lower the half-life of valproic acid. The mechanism of action of carbamazepine and its derivatives is relatively well understood i.e, voltage-gated sodium channels are the molecular pores that allow brain cells (neurons) to generate action potentials, the electrical events that allow neurons to communicate over long distances. After the sodium channels open, to start the action potential, they inactivate, essentially closing the channel. Carbamazepine stabilizes the inactivated state of sodium channels, meaning that fewer of these channels are available to open, making brain cells less excitable. Epileptic children exposed to oxidative stress and conventional antiepileptic drugs change the oxidative/antioxidative balance. Recently, Aycicek et al. confirmed the effects of carbamazepine, valproic acid and phenobarbital on the oxidative and antioxidative balance in epileptic children (8).
Tab. 2. Mechanisms of action of antiepileptic drugs (AEDs) used concomitantly in the present case report and consideration of synergism in terms of pharmacodynamics. As illustrated all the three drugs act by inflencing the gamma aminobutyric acid (GABA) system 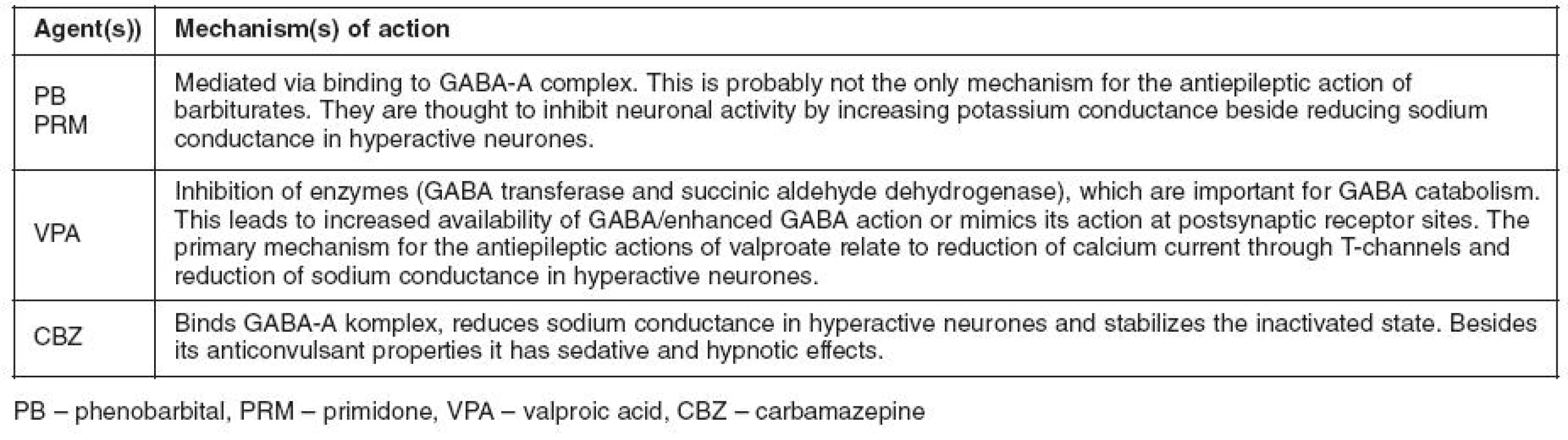
Fig. 2. Serum phenobarbital concentrations during concomitant therapy with valproate and restoration of levels close to conventional therapeutic range after dose adjustment in refractory epilepsy 
Common symptoms of AED overdose include coma, deep sleep, motor restlessness, and visual hallucinations, where supportive treatment is necessary. Valproic acid is usually well tolerated but has been associated with some side effects. Several cases of VPA induced non-hepatic hyperammonemic encephalopathy in subjects treated by VPA alone and other concomitant AED have been reported in the medical literature (9). Valproate-induced hyperammonemic encephalopathy is an unusual complication which may occur just after the beginning or during treatment. It is characterized by vomiting, drowsiness, lethargy and progressive impairment of consciousness, focal neurologic signs, cognitive slowing and increased seizure frequency. This is a poorly studied side effect independent of the drugs hepatotoxic action. The increase in serum ammonium level is due to several mechanisms, although the most important one appears to be the inhibition of carbamoylphosphate synthetase-I, the enzyme that begins the urea cycle. Hyperammonemia leads to an increase in the glutamine level in the brain, which produces astrocyte swelling and cerebral edema. Polytherapy with several drugs, such as phenobarbital, seems to contribute to the problem (10). Valproic acid-induced hyperammonemic encephalopathy may occur in people with normal liver function, despite normal doses and serum levels of VPA (11). Our patient also had significant hyperamonemia cca 109 μmol/L (normal range 14-55 μmol/L) which was gradually reversible. Carbamazepine may aggravate juvenile myoclonic epilepsy and has also been linked to serious adverse cognitive effects, including EEG slowing and cell apoptosis. These adverse effects have been said to be associated with the formation of CBZ metabolites. The problem of biounequivalence and/or therapeutic unequivalence may be demonstrated by toxicity or therapeutic failure in association with replacement of different generic products. Such a problem was first observed in Australia, when an outbreak of phenytoin intoxication occurred among epileptic patients (12, 13) as a result of single excipient change from calcium sulphate to lactose by the drug manufacturer. The consequence was a substantial increase in bioavailability and an increase of phenytoin serum concentrations by 80 to 100%. Patients presented with a typical clinical picture of phenytoin intoxication with ataxia, double vision and periods of vomiting, where a complete remission in all patients was achieved when the original excipient was restored (14). Similar cases have been reported, of patients, who had seizure frequency increase after they were switched to generic drug associated with significant drug level changes (15). In a recently published cases of breakthrough seizures, brand to generic substitution was blamed as the main cause (16). In Denmark, several patients who complained of adverse effects when switched to generic lamotrigine underwent hospitalization with blood levels assessed every 3 hours. One patient had a fall, a skull fracture, and an epidural hematoma after the switch and had elevated levels during testing that were consistent with toxicity while taking the generic drug. Another patient had status epilepticus and lower levels on the generic and a third patient had ataxia within the first hour of taking the generic associated with a higher Cmax (17). Two Canadian studies (18, 19) demonstrated that switchback rates from generic to brand are 5 to 10 times higher for AEDs than other classes of medications and significantly higher numbers of outpatient visits and mean length of hospital stays occurred in people taking generic AEDs compared with those taking brand name drug. In 2007, US case – controlled database analysis of healthcare for 12 - to 64-year-olds with epilepsy and AED formulation changes, indicated 81% greater odds of an AED formulation switch relative to the controls (20). Carbamazepine, phenobarbital, and valproic acid are among commonly used antiepileptic drugs that show complicated pharmacokinetic behavior. Immunoassays are used routinely to monitor these drugs, and assay specificity is important to obtain accurate results. Frank et al. (21) reported molar cross-reactivity of carbamazepine-10,11-epoxide of 12% in carbamazepine determination by immunoassay, indicating that high performance liquid chromatography (HPLC) may be useful for monitoring patients. This may be particularly relevant for those patients exhibiting symptoms of carbamazepine toxicity, whose serum carbamazepine concentration is within the therapeutic range but who may be producing significant levels of the active epoxide metabolite. Unfortunately we did not have access to this assay at the time of case manifestation. Matos et al. (22) reported cases in which false-positive antidepressive drug levels led to the diagnosis of carbamazepine intoxication. Dasgupta et al. (23) observed significant interference of carbamazepine with the FPIA method. Besides problems relating to interindividual and intra-individual variation and ideosyncratic undesired effects, shortcomings due to bioequivalence and/or therapeutic equivalence problems may also be a challenge associated with the introduction of different generic products (13–14). For the determination of bioequivalence, the so-called rule of inclusion is used, meaning that the 90% confidence intervals (CIs) for the new preparation should be within the limits: for AUC 80–125%. Proof of bioequivalence between reference and generic preparations of antiepileptic drugs does not mean that they are freely interchangeable. Generic formulations with proven bioequivalence to branded preparations can be used, for example, at the beginning of treatment or in poorly controlled patients with serum concentrations in the mid range. However, it has been demonstrated that the usual rules for bioequivalence and the range of acceptability for preparations of carbamazepine are problematic (23). Beside the pharmacokinetic variability, interaction at a pharmacodynamic level is also possible due to some similarities in mechanism of actions. The available data indicate that the anticonvulsant efficacy of these AEDs is mainly due to the inhibition of sodium channel activity (24). Similar targets or mechanism of action and other conditions affecting pharmacokinetic and toxicodynamic processes may be significant in terms of drug-drug interactions. The mechanism of action of valproic acid is not clearly defined; however, effects of the drug may be related at least in part to increased brain concentrations of the inhibitory neurotransmitter GABA, probably through inhibition of catabolic enzymes of GABA. Thus, valproate may cause increased availability of GABA, an inhibitory neurotransmitter, to brain neurones or may enhance or mimic its action at postsynaptic receptor sites. Animal studies have shown that valproic acid inhibits GABA transferase and succinic aldehyde dehydrogenase, enzymes which are important for GABA catabolism, whereas results of a study indicate the drug inhibits neuronal activity by increasing potassium conductance (25). In a study of 88 paediatric patients receiving sodium valproate monotherapy, side effects were noted in 71 patients. Although average doses in these patients were significantly higher than in the 17 patients with no side effects, no difference in plasma concentration were noted (26). In clinical practice, serum level monitoring of anticonvulsant drugs is usually adequate. When there is an alteration in the binding of the anticonvulsant drug to the plasma proteins, however, the relationship between the serum concentration and therapeutic efficacy or toxicity becomes difficult to interpret. Behavioural alterations, digestive disorders, and neurological changes are the common side effects observed and can occur with combinations such as non-steroidal anti-inflammatory drugs (NSAIDs), phenytoin, carbamazepine and valproic acid, or when albumin levels are low. A failure to rely on serum free levels of the anti-convulsant under these circumstances can easily result in poor clinical decisions. The technique of serum free level measurement and illustrative examples of specific cases are provided to document the usefulness of this invaluable laboratory test (27). The relationship between dose and total valproate concentration is nonlinear, i.e. concentration does not increase proportionally with dose, but increases to a lesser extent at higher doses due to saturable plasma protein binding. The kinetics of unbound drug are linear. Symptoms of overdose include coma, deep sleep, motor restlessness, and visual hallucinations. Valproic acid appears to inhibit the metabolism of phenobarbital; thus increasing its effect. Phenobarbital is a short-acting barbiturate with sedative, hypnotic, and anticonvulsant properties. Time to peak, serum on oral is 1–6 hours, whereas half-life elimination in children is 37–73 hours, with 20% to 50% excreted as unchanged drug in the urine. As is the case with barbiturates in general, phenobarbital depresses the sensory cortex, decrease motor activity, alter cerebellar function, and produces drowsiness, sedation, and hypnosis. In high doses, it exhibits anticonvulsant activity and may produce also dose-dependent respiratory depression. Valproic acid appears to inhibit the metabolism of phenobarbital, thus increasing its effect. One must beware of phenobarbital intoxication after adding PB/PRM to VPA, also as a late effect. Valproic acid and valnoctamide both interact with carbamazepine, as they inhibit microsomal epoxide hydrolase (mEH), the enzyme responsible for the breakdown of carbamazepine-10,11 epoxide into inactive metabolites. By inhibiting mEH, valproic acid and valnoctamide cause a buildup of the active metabolite, prolonging the effects of carbamazepine and delaying its excretion. In combinations with CBZ, VPA inhibits the reduction of the metabolite CBZ-epoxide, thus leading to an overdose of CBZ-epoxide, an interaction which happens more frequently when CBZ is added to VPA than the other way round. Carbamazepine itself is associated with a number of idiosyncratic adverse effects, including skin rash, blood disorders and hepatitis, in 30–50% of patients (28) These adverse effects have been associated with the formation of CBZ metabolites (29–30). Therefore, therapeutic drug monitoring (TDM) of CBZ metabolites also has important clinical implications. In addition CBZ is also a well known enzyme inducer up-regulating cytochrome P450 enzymes (31).
CONCLUSIONS
To enhance our understanding of epilepsy as a disease, antiepileptic drugs (AEDs) PK/PD principles, including drug interaction mechanisms, may allow more effective use of these drugs. If a high potential for metabolic drug-drug interactions exists between co-administered antiepileptic drugs, undesired effects may occur unexpectedly at any time during therapy. Carbamazepine, phenobarbital, and valproic acid are commonly used antiepileptic drugs that show complicated pharmacokinetic behavior. Therefore, dose adjustment based on clinical judgement assisted by therapeutic drug monitoring may be of vital importance.
Abbreviations
- ADRs – adverse drug reactions
- AEDs – antiepileptic drugs
- CBZ – carbamazepine
- CIs – confidence intervals
- FCD – focal cortical dysplasia
- GABA – gamma aminobutyric acid
- HPLC – high performance liquid chromatography
- MDR – multidrug resistance
- mEH – microsomal epoxide hydrolase
- NSAIDs – non-steroidal anti-inflammatory drugs
- PB – phenobarbital
- PRM – primidone
- TDM – therapeutic drug monitoring
- VNS – vagus nerve stimulation
- VPA – valproic acid
The corresponding autor is thankful to Dr. Gareth J. Veal from Newcastle University, UK, for reviewing the manuscript not only language wise also for stylistic advice.
ADRESA PRO KORESPONDENCI:
MUDr. Hundie Tesfaye, Ph.D.
Ústav klinické biochemie a patobiochemie 2. LF UK a FN Motol
V Úvalu 84, 150 06 Praha 5
e-mail: hundie.tesfaye@fnmotol.cz
Zdroje
1. Sisodiya SM, Lin WR, Harding BN, Squier MV, Thom M. Drug resistance in epilepsy: expression of drug resistance proteins in common causes of refractory epilepsy. Brain 2002; 125 : 22–31.
2. Reynolds EH, Rodin E. The clinical concept of epilepsy. Epilepsia 2009; 50(Suppl 3): 2–7.
3. Meencke HJ. Clinical neuropathology of the epilepsies in the 100 years of the ILAE (1909–2009). Epilepsia 2009; 50 (Suppl 3): 8–16.
4. Manon-Espaillat R, Burnstine TH, Remler B, et al. Antiepileptic drug intoxication: factors and their significance. Epilepsia 1991; 32 : 96–100.
5. Bourgeois BF. New antiepileptic drugs in children: which ones for which seizures. Clin Neuropharmacol 2000; 23 : 119–132.
6. Novak PH, Ekins-Daukes S, Simpson CR, Milne RM, Helms P, McLay JS. Acute drug prescribing to children on chronic antiepilepsy therapy and the potential for adverse drug interactions in primary care. Br J Clin Pharmacol 2005; 59 : 712–717.
7. Clarkson I, Choonara I. Surveillance for fatal suspected adverse drug reactions in the UK. Arch Dis Child 2002; 87 : 462–467.
8. Aycicek A, Iscan A. The effects of carbamazepine, valproic acid and phenobarbital on the oxidative and antioxidative balance in epileptic children Eur Neurol 2007; 57 : 65–69.
9. Mehndiratta MM, Mehndiratta P, Phul P, Garg S.Valproate induced non hepatic hyperammonaemic encephalopathy (VNHE) – a study from tertiary care referral university hospital, north India. J Pak Med Assoc 2008; 58 : 627–631.
10. Segura-Bruna N, Rodriguez-Campello A, Puente V, Roquer J. Valproate-induced hyperammonemic encephalopathy. Acta Neurol Scand 2006; 114 : 1–7.
11. Wadzinski J, Franks R, Roane D, Bayard M. Valproate-associated hyperammonemic encephalopathy. J Am Board Fam Med 2007; 20 : 499–502.
12. Balla J. “Dilantin” overdose Med J Aust 1968; 2 : 480–481.
13. Eadie MJ, Sutherland JM, Tyrer JH. “Dilantin” overdosage. Med J Aust 1968; 2 : 515.
14. Tyrer JH, Eadie MJ, Sutherland JM, Hooper WD. Outbreak of anticonvulsant intoxication in an Australian city. BMJ 1970; 4 : 271–273.
15. Burkhardt RT, Leppik IE, Blesi K, Scott S, Gapany SR, Cloyd JC. Lower phenytoin serum levels in persons switched from brand to generic phenytoin. Neurology 2004; 63 : 1494–1496.
16. Berg MJ, Gross RA, Tomaszewski KJ, Zingaro WM, Haskins LS. Generic substitution in the treatment of epilepsy: case evidence of breakthrough seizures. Neurology 2008; 71 : 525–530.
17. Nielsen KA, Dahl M, TŅmmerup E, Wolf P. Comparative daily profiles with different preparations of lamotrigine: a pilot investigation. Epilepsy Behav 2008; 13 : 127–130.
18. Andermann F, Duh MS, Gosselin A, Paradis PE. Compulsory generic switching of antiepileptic drugs: high switchback rates to branded compounds compared with other drug classes. Epilepsia 2007; 48 : 464–469.
19. LeLorier J, Duh MS, Paradis PE, Lefebvre P, Weiner J, Manjunath R, Sheehy O. Clinical consequences of generic substitution of lamotrigine for patients with epilepsy. Neurology 2008; 70(22 Pt 2): 2179–2186
20. Zachry WM 3rd, Doan QD, Clewell JD, Smith BJ. Case-control analysis of ambulance, emergency room, or inpatient hospital events for epilepsy and antiepileptic drug formulation changes. Epilepsia 2009; 50 : 493–500.
21. Frank EL, Schwarz EL, Juenke J, Annesley TM, Roberts WL. Performance characteristics of four immunoassays for antiepileptic drugs on the IMMULITE 2000 automated analyzer. Am J Clin Pathol 2002; 118 : 124–131.
22. Matos ME, Burns MM, Shannon MW. False-positive tricyclic antidepressant drug screen results leading to the diagnosis of carbamazepine intoxication. Pediatrics 2000; 105(5): E66.
23. Dasgupta A, McNeese C, Wells A. Interference of carbamazepine and carbamazepine 10,11-epoxide in the fluorescence polarization immunoassay for tricyclic antidepressants: estimation of the true tricyclic antidepressant concentration in the presence of carbamazepine using a mathematical model. Am J Clin Pathol 2004; 121 : 418–425.
24. Mayer T, May TW, Alternmüller DM, Sandmann M, Wolf P. Clinical problems with generic antiepileptic drugs. Clin Drug Invest 1999; 18 : 17–26.
25. McEvoy GK. (ed.) American hospital formulary service, drug information. Bethesda, MD: American Society of Hospitál Pharmacists 1991; 1147.
26. Reynolds JEF (ed.) Martindale, the extra pharmacopoeia, 29th ed. London: The Pharmaceutical Press 1989; 413.
27. Ambrósio AF, Soares-Da-Silva P, Carvalho CM, Carvalho AP. Mechanisms of action of carbamazepine and its derivatives, oxcarbazepine, BIA 2-093, and BIA 2-024. Neurochem Res 2002; 27 : 121–130.
28. Reynolds NC Jr, Murthy VS. Serum free levels and evaluation anticonvulsant drug interactions. Wis Med J 1989; 88 : 25–27.
29. Ju C, Uetrecht JP. Detection of 2-hydroxyiminostilbene in the urine of patients taking carbamazepine and its oxidation to a reactive iminoquinone intermediate. J Pharmacol Exp Ther 1999; 288 : 51–56.
30. Shear NH, Spielberg SP. Anticonvulsant hypersensitivity syndrome. In vitro assessment of risk. J Clin Invest 1988; 82 : 1826–1832.
31. Riley RJ, Kitteringham NR, Park BK. Structural requirements for bioactivation of anticonvulsants to cytotoxic metabolites in vitro. Br J Clin Pharmacol 1989; 28 : 482–487.
32. Luo G, Cunningham M, Kim S, et al. CYP3A4 induction by drugs: correlation between a pregnane X receptor reporter gene assay and CYP3A4 expression in human hepatocytes. Drug Metab Dispos 2002; 30 : 795–804.
Štítky
Adiktológia Alergológia a imunológia Angiológia Audiológia a foniatria Biochémia Dermatológia Detská gastroenterológia Detská chirurgia Detská kardiológia Detská neurológia Detská otorinolaryngológia Detská psychiatria Detská reumatológia Diabetológia Farmácia Chirurgia cievna Algeziológia Dentální hygienistka
Článek Laureáti Nobelovy ceny
Článok vyšiel v časopiseČasopis lékařů českých
Najčítanejšie tento týždeň
- Metamizol jako analgetikum první volby: kdy, pro koho, jak a proč?
- Kombinace metamizol/paracetamol v léčbě pooperační bolesti u zákroků v rámci jednodenní chirurgie
- Antidepresivní efekt kombinovaného analgetika tramadolu s paracetamolem
- Parazitičtí červi v terapii Crohnovy choroby a dalších zánětlivých autoimunitních onemocnění
- fSCIG v reálnej klinickej praxi u pacientov s hematologickými malignitami
-
Všetky články tohto čísla
- Reactive oxygen and nitrogen species in the clinical medicine
- Pelvic ring injuries: current concepts of management
- Clinical importance of the IgG4 related disease
- The most frequent methods used for DNA methylation analysis
- Udělení Ceny Jana Evangelisty Purkyně
- Our experience with maraviroc treatment in HIV positive patients
- Antiepleptic drug interactions: A clinical case demonstration
- Plánované akce odborných složek ČLS JEP
- Gambling in children and adolescents
-
Hygiena ženy II
Doba rudolfínská -
XI. jihočeské Timrovy dny
Hluboká nad Vltavou, 21.–22. dubna 2011 -
VI. sympozium o léčbě bolesti*
Brno, 29.–30. dubna 2011 -
26. pracovní dny – Dědičné metabolické poruchy
Mikulov, 11–13. května 2011 -
Evropské perspektivy v personalizované medicíně
Brusel, 12.–13. května 2011 - Jiří Mareš, Eva Vachková a kol.: PACIENTOVO POJETÍ NEMOCI II
- Laureáti Nobelovy ceny
- Časopis lékařů českých
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- Pelvic ring injuries: current concepts of management
- Clinical importance of the IgG4 related disease
- Reactive oxygen and nitrogen species in the clinical medicine
- The most frequent methods used for DNA methylation analysis
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



