-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
Electrogastrography in systemic sclerosis – a pilot study
Authors: Medkova H. 1; Jansova A. 1; Soukup T. 1; D. Kohoutová 1,2
; Janebova M. 1; Knoblochova V. 1; Peterová E. 1; Kupkova B. 1; Ilja Tachecí 1
; Bures J. 1
Authors place of work: 2nd Department of Internal Medicine – Gastroenterology, Charles University, Faculty of Medicine in Hradec Králové and University Hospital Hradec Králové, Czech Republic 1; The Royal Marsden Hospital NHS Foundation Trust, London, United Kingdom 2
Published in the journal: Gastroent Hepatol 2020; 74(4): 285-294
Category: Klinická a experimentální gastroenterologie: původní práce
doi: https://doi.org/10.14735/amgh2020285Summary
Introduction: Systemic sclerosis (SSc) is an autoimmune connective tissue disease with a chronic progressive course. Inflammation, vasculopathy and fibrosis play an important role in the pathogenesis of SSc. Gastrointestinal tract belongs to the most commonly affected organ systems and its involvement can be observed in up to 90% of individuals. Clinical manifestations are associated with dysmotility of all segments of the gastrointestinal tract, including the stomach. Electrogastrography (EGG) is a non-invasive method used for the assessment of gastric myoelectrical activity.
Aim: The aim of this prospective study was to investigate EGG in SSc patients in detail, including the analysis of one-minute intervals and assessment of EGG power.
Method: The study included 33 patients that fulfilled SSc ACR/EULAR classification criteria (5 men, 28 women; mean age 60 years). Patients suffered from a diffuse cutaneous form of SSc (dSSc) (N = 17), limited cutaneous form (lSSc) (N = 13) or they belonged to other subgroups (N = 3). The gastric myoelectric activity was investigated by means of an EGG Stand (MMS, Enschede, The Netherlands).
Results: Altogether 855 one-minute EGG intervals were evaluated, each one of them in dominant frequency, power and power ratio. Only one patient had a completely normal EGG. The EGG of most patients showed bradygastria (17 subjects) or gastric arrhythmia with a predominance of bradygastria (6 patients). The postprandial power decreased significantly. The power ratio was low in all intervals.
Conclusion: Bradygastria and the postprandial decrease of power were the most characteristic features. These findings were confirmed especially in dSSc subgroup.
Keywords:
systemic sclerosis
Introduction
Systemic sclerosis (SSc) is a connective tissue disease with a chronic and progressive course. Inflammation, vasculopathy and fibrosis play an important role in the pathogenesis of SSc [1]. The gastrointestinal tract is one of the most commonly involved organ systems in SSc and is present in up to 90% of patients with SSc. Any part of the gastrointestinal tract can be affected [2]. Most commonly, the involvement of oesophagus and stomach is observed; yet, an impairment of the small bowel function with the syndrome of intestinal bacterial overgrowth can complicate the condition [3,4].
The pathological processes contributing to the gastrointestinal involvement in SSc are complex and are leading todifferent gastrointestinal motor disorders. Neuropathy, followed by myopathy and later on by fibrosis production, is a crucial moment in the pathophysiology of the disease. The presence of specific antibodies support the above: autoantibodies against acetylcholine M3 receptor (anti-M3R) causing significant smooth muscle dysfunction in early phase of gastrointestinal involvement in SSc have been detected [2].
The stomach in SSc can be affected by various motility dysfunctions, including impaired interdigestive and postprandial gastric motor pattern, delayed gastric emptying and/or can be associated with chronic atrophic gastritis [5].
The surface electrogastrography (EGG) is a non-invasive method for assessment of gastric myoelectrical activity. A gastric pacemaker generates an electrical phenomenon termed “gastric slow waves”. They originate in a “pacemaker region” located on the greater curvature of the stomach near the junction of the fundus and proximal gastric body [6]. A method of examining a signal by frequency instead of time has been implemented mathematically and is called the Fourier transform. The dominant frequency is determined as a frequency with the greatest power in the Fourier transform during a specific time period. The power is defined as an area of waves (amplitudes) [6]. The normal gastric slow-wave frequency corresponds to 3 cycles per minute (a 3-cmp rhythm) [6–9]. The values of 0.5–2.0 cpm are referred to bradygastria, the values of 4–9 cpm are called tachygastria, and the last gastric dysrhythmia is an arrhythmia in which non-rhythmic gastric slow waves occur [9]. EGG is feasible both for experimental studies [10–16] and clinical assessment [6,7,17–24]. Only a few papers investigated EGG in SSc so far [25–33]. Bradygastria was the most important finding in those studies. To the best of our knowledge, no in-depth EGG analysis in SSc has been published so far. The aim of our prospective study was to investigate EGG of SSc in detail, including the analysis of one-minute intervals and evaluation of EGG power.
Materials and methods
Patients
The study group included 33 patients (5 men, 28 women, mean age 60 years, min. 22, max. 83 years). All patients met the criteria for SSc determination according to the American College of Rheumatology (ACR) and the European League Against Rheumatism (EULAR) from 2013 [34]. The limited SSc (lSSc) and diffuse SSc (dSSc) subsets were based on differences in the extent of skin involvement [35]. Totally 17 patients (including 2 men) suffered from dSSc and 13 patients (including 2 men) had lSSc. Sclerosis sine scleroderma was seen in two subjects, one male patient suffered from an overlap syndrome. The mean age of patients at the time of the diagnosis was 51 years (10–80 years). The average time from the onset of the first symptoms to the diagnosis was 5 years (the shortest period to the diagnosis was 1 month, the longest 34 years). The most common initial non-Raynaud’s symptom was the joint pain (11 patients; including 1 male) and skin stiffness (11 patients; including 1 male). Other symptoms were: dyspnoea (5 patients; including 1 male), heartburn (4 patients), digital ulcers (4 patients), joint swelling (3 patients; including 1 male), cough (3 patients), muscle weakness (3 patients), dysphagia (2 patients), oedemas of lower extremities (1 male patient), palpitations (1 patient), diarrhoea (1 patient), chest pain (1 patient), Sjögren’s syndrome (1 patient) and arterial hypertension (1 male patient). Totally 18 patients had more than one symptom. Five patients kept smoking, twelve stopped smoking and 16 individuals have never smoked.
Electrogastrography
Our original method of EGG has already been published elsewhere [14]. Briefly, six active self-adhesive electrodes were placed on the upper part of the abdomen; the 7th electrode (basal one) was placed in the left supraclavicular area. A special abdominal belt (respiratory sensor) was used to identify possible artefacts due to breathing and body movements (Fig. 1). The gastric myoelectric activity was investigated using an EGG stand (MMS, Enschede, the Netherlands). All EGGs were accomplished at a supine position in the morning after overnight fasting. The first 15-minute interval was recorded in patients who have been fasting and have been awake during the whole period of recording. Fifteen minutes after a standardized breakfast had been completed, the following 15 one-minute postprandial intervals were recorded. All files were archived for further assessment. EGG was evaluated by MMS software (version 8.19). Running spectral analysis was used for a standard evaluation of EGG. The results were expressed as a dominant frequency of the gastric slow waves and the power analysis and power ratio assessment as a fraction of the areas of the amplitudes after (numerator) and before (denumenator) the standardized breakfast.
Fig. 1. Six self-adhesive electrodes placed on the upper part of the abdomen, the ?th electrode (basal) placed in the left supraclavicular area (arrow). A special abdominal belt (respiratory sensor) is used to identify artefacts caused by breathing and body movements. Drawing: Hana Kotlandová.
Šest samolepících elektrod umístěných v horní části břicha, ?. elektroda (bazální) umístěná v levé supraklavikulární oblasti (šipka). Speciální břišní pás (respirační senzor) se používá k identifi kaci artefaktů způsobených dýcháním a pohyby těla. Kresba: Hana Kotlandová.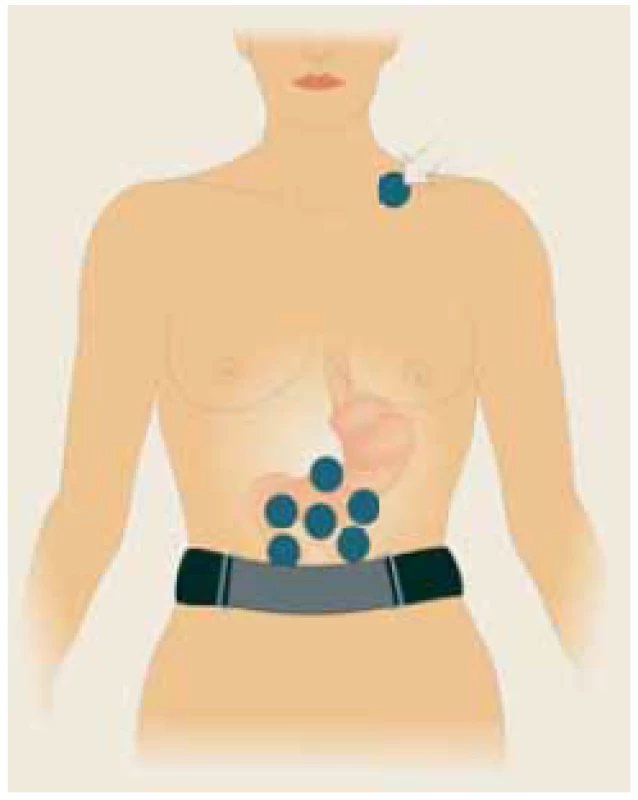
Statistical analysis
The data were statistically treated by means of descriptive statistics, non-paired t-test, Mann-Whitney rank sum test and Pearson product-moment correlation using the SigmaStat software (Version 3.1, Jandel Corp, Erkrath, Germany).
Ethics
The study was approved by the Joint University Ethics Committee (Protocol number DSP_GIT_SSc, ref. no. 201805 S07P). All procedures were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its latter amendments.
All patients signed a written consent. For all data obtained, all personal identification information was deleted in compliance with the laws for the protection of confidentiality of the Czech Republic.
Results
High-quality recordings were obtained and these enabled unequivocal final conclusions in all subjects. Altogether 855 one-minute EGG intervals were evaluated, each in dominant frequency, power and power ratio (Tab. 1, Fig. 2 and 3, Graphs 1–3). Only one patient (3%) had a completely normal EGG (Fig. 4). Most patients had bradygastria (17 patients, including 4 men) (Fig. 5) of which 59% suffered from dSSc and 24% lSSc; 12% had sclerosis sine scleroderma and 6% an overlap syndrome. Another 6 patients (including 1 man) had gastric arrhythmia with a predominance of bradygastria (Fig. 6), 6 patients had gastric arrhythmia and 2 patients had a non-specific abnormal EGG, with no differences between dSSc and lSSc. One patient with lSSc had a combination of gastric arrhythmia, bradygastria and tachygastria. There was a significant increase in postprandial dominant frequency compared to basal recording, with a peak at 8 minutes of postprandial EGG (P = 0.020) (Graph 1). The postprandial power decreased significantly, with a minimum observed at the 3rd minute (P = 0.009) (Graph 2). The power ratio was low in all intervals.
Tab. 1. Electrogastrography in 33 patients with systemic sclerosis.
Elektrogastrografie u 33 pacientů se systémovou sklerodermií.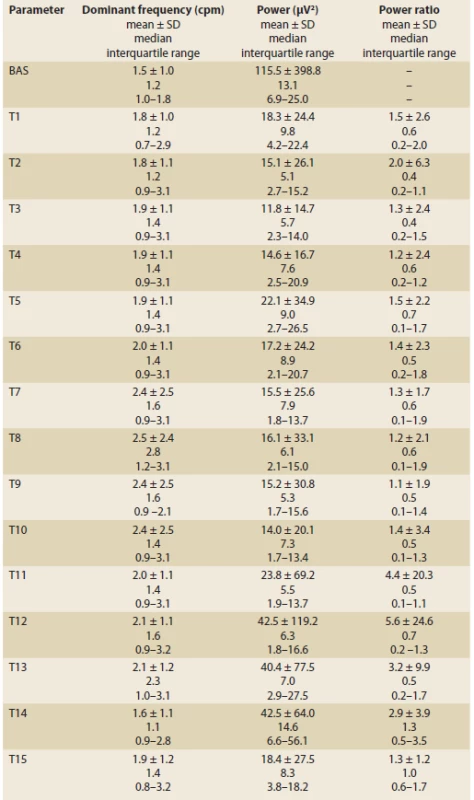
BAS – basic 15-minute recording in fasting waking patients, T1–T15 – one-minute intervals of recording, SD – standard deviation Dominant frequency (DF) is determined as a frequency with the greatest power in the Fourier transform during a specifi c time period (unit: cycles per minute, cpm). The power is defi ned as an area of waves (amplitudes; unit – μV2). The power ratio is ratio of the postprandial to fasting power of the DF, normally is greater than two and postulated to refl ect a postprandial increase in the electromechanical activity of the stomach with an additional component of gastric distention occurring after meal ingestion. Fig. 2. Electrogastrography (EGG). Bradygastria (0.5 to 2 cycles per minute). AUC: areas of amplitudes (EGG power) – upper left part of the picture; respiration channel at the right bottom (red line) is used to identify artefacts caused by breathing and body movements.
Elektrogastrografi e. Bradygastrie (0,5–2 cykly/min). AUC: plocha pod křivkou (elektrogastrografi cká síla) – levá horní část obrázku; záznam elektrody snímající dechové pohyby, tak aby byly při vyšetření odlišeny artefakty způsobené dýcháním – záznam je na obrázku vpravo dole (červená křivka).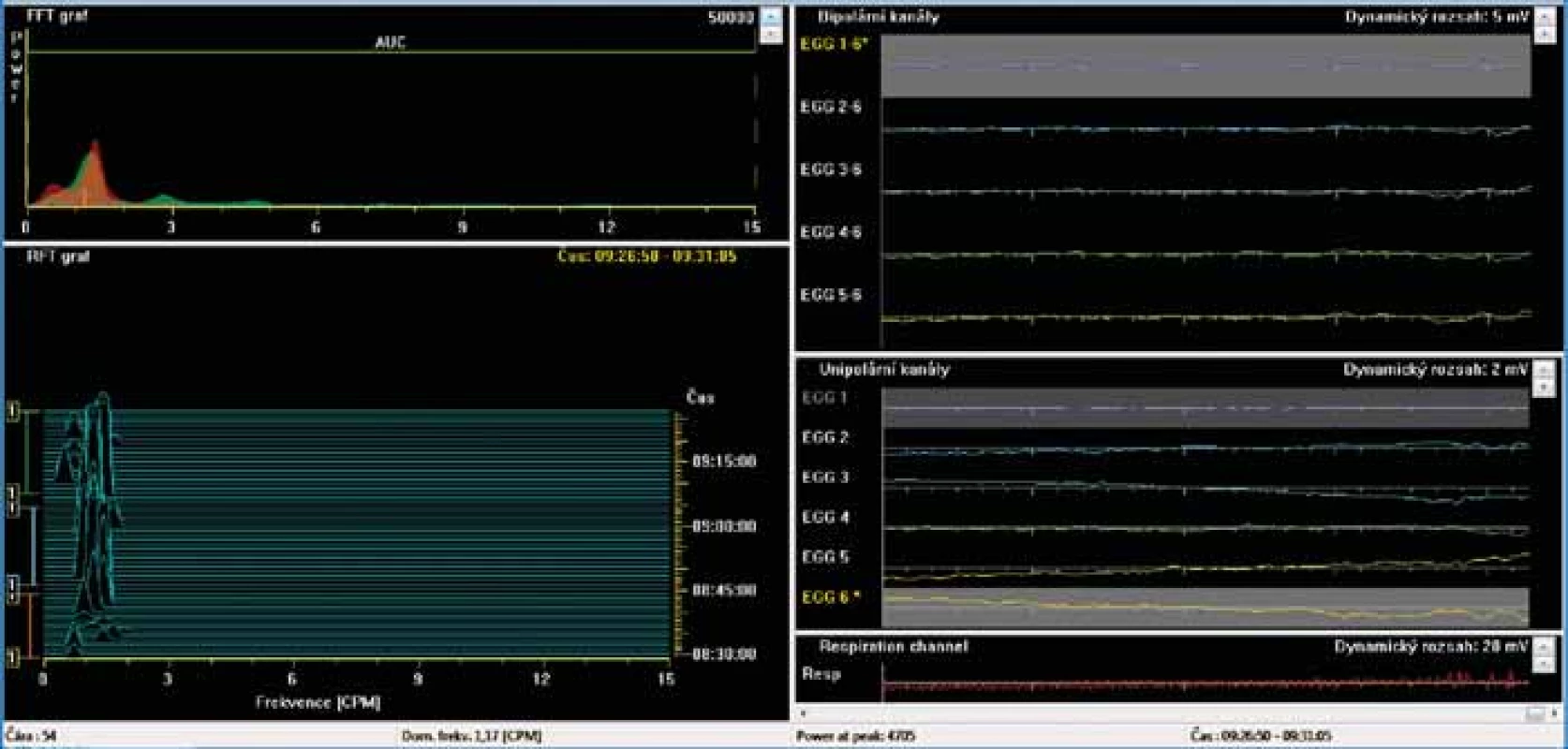
Fig. 3. Electrogastrography. Bradygastria. Low power (areas of amplitudes).
Elektrogastrografie. Bradygastrie. Nízká elektrogastrografická síla (plocha pod křivkou).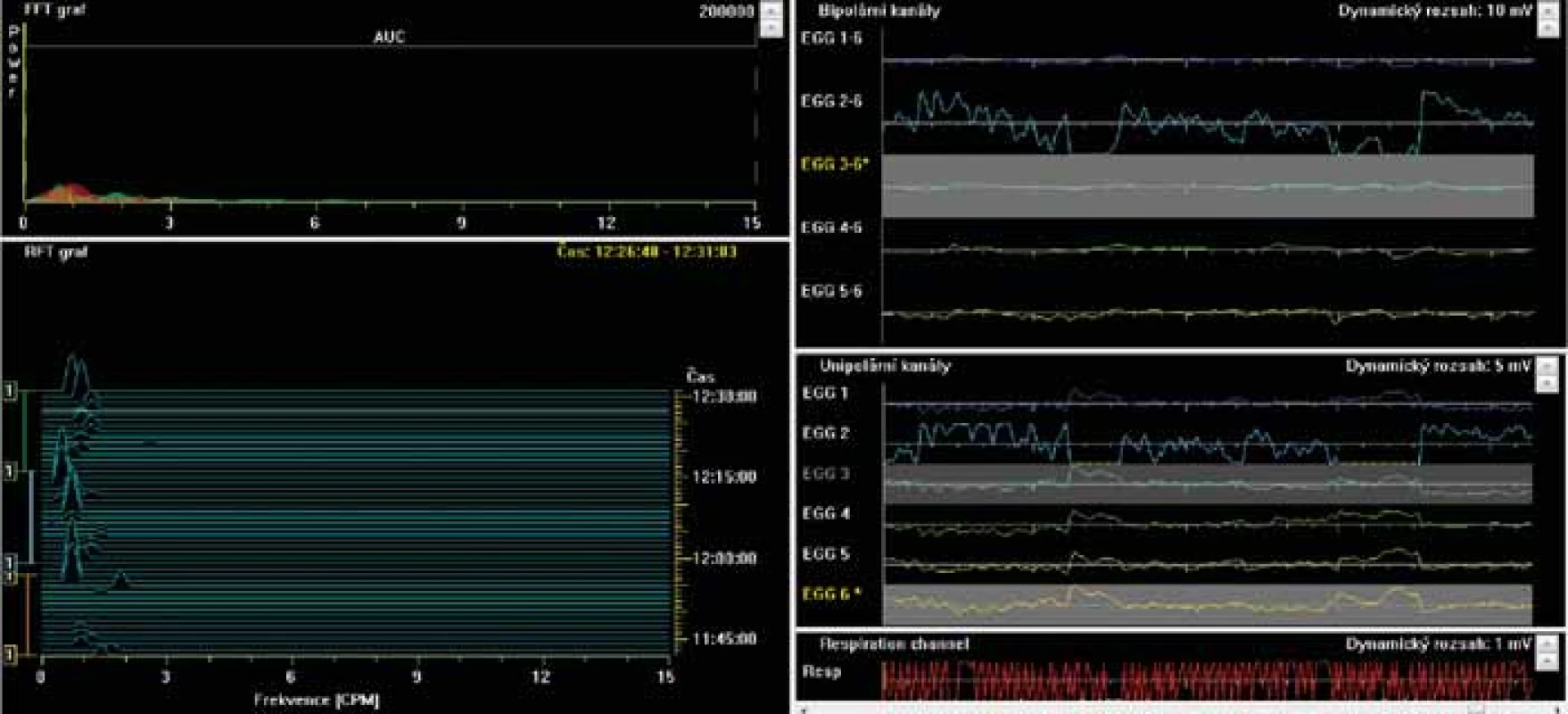
Fig. 4. Electrogastrography (EGG), postprandial recording. Fourier transform. Only one patient had completely normal EGG in all analysed settings, with three cycles per minute, during all the recording. It was a ??-year-old woman with systemic sclerosis (SSc) diagnosed three months before the EGG investigation. Initial clinical manifestations were sclerodactyly, fi ngertip lesions with pitting scars, abnormal nailfold capillaries found by nailfold capillaroscopy and SSc related antibodies (anticentromera antibodies presence). Patient’s clinical presentation did correspond to a cutaneous limited form of SSc.
Elektrogastrografi e (EGG), postprandiální záznam. Fourierova transformace. Pouze jeden pacient měl během celého záznamu se třemi cykly ve všech svodech zcela normální záznam. Jednalo se o 70letou ženu se systémovou sklerodermií (SSc) diagnostikovanou 3 měsíce před vyšetřením EGG. Počáteční klinické projevy byly sklerodaktylie, jizvy na konečcích prstů, abnormální kapiláry nehtových lůžek zjištěné kapilaroskopií a protilátky spojené s SSc (přítomnost protilátek proti centromerám). Klinická prezentace pacienta odpovídala limitované kožní formě SSc.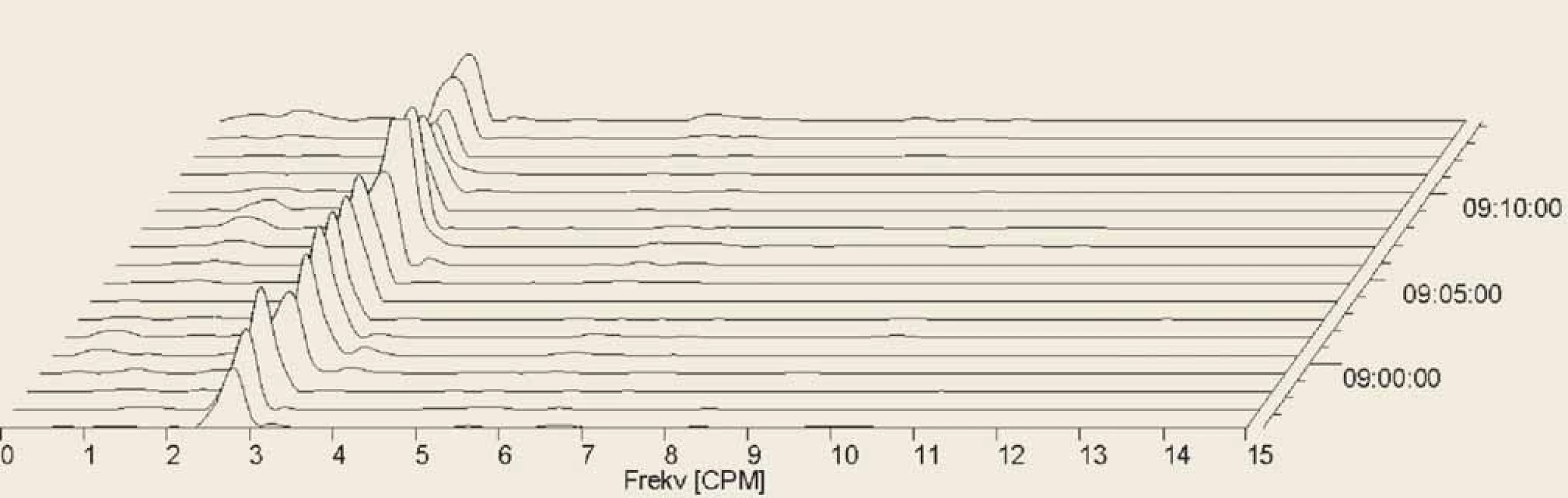
Fig. 5. Electrogastrography. Fourier transform. Postprandial bradygastria in a patient with systemic sclerosis.
Elektrogastrografi e. Fourierova transformace. Postprandiální bradygastrie u pacienta se systémovou sklerodermií.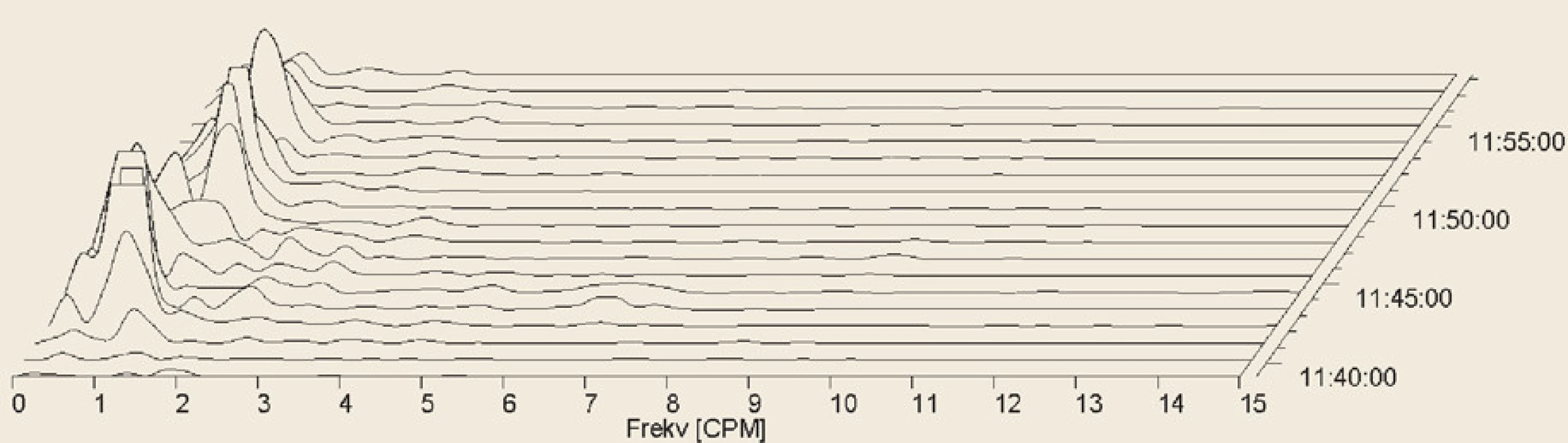
Fig. 6. Electrogastrography. Fourier transform. Severe postprandial gastric arrhythmia with predominant bradygastria.
Elektrogastrografie. Fourierova transformace. Těžká postprandiální žaludeční arytmie s převládající bradygastrií.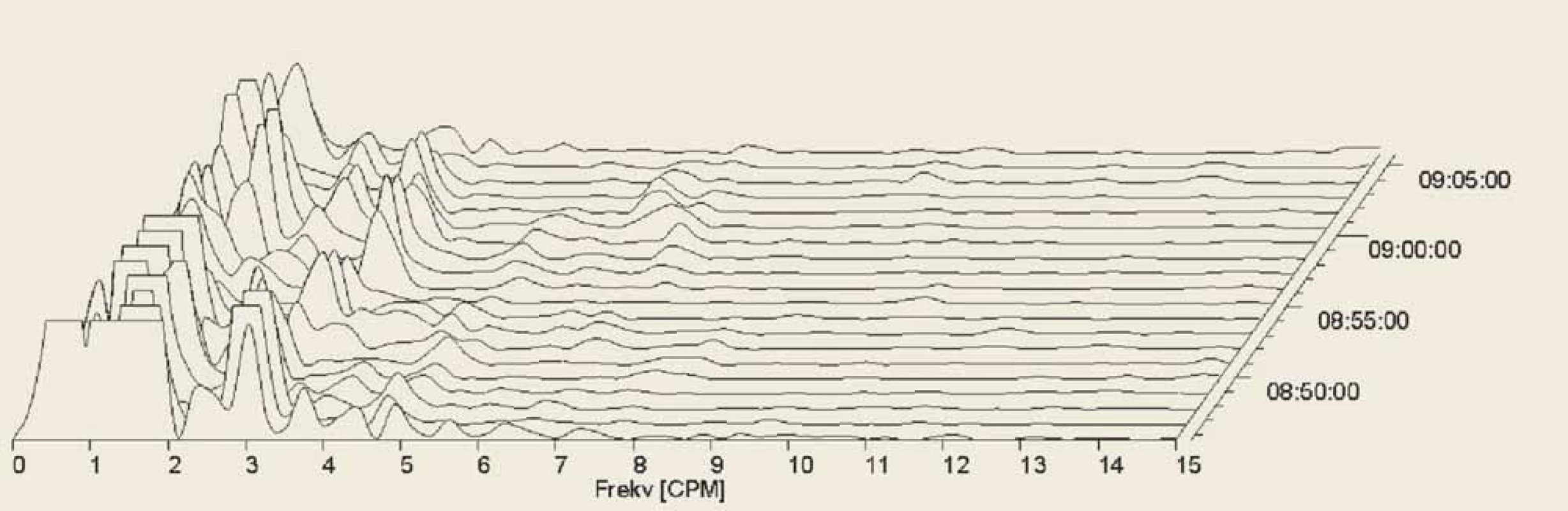
Graph 1. Dominant frequency (mean + standard deviation). Y-axis: linear scale. BAS: basic 15-minute recording during the fasting period. T1–T15: 1-minute intervals of recording.
Dominantní frekvence (průměr + směrodatná odchylka). Osa Y: lineární stupnice. BAS: klidový 15minutový záznam během lačnění. T1 – T15: 1minutové intervaly záznamu.
Discussion
Our current study has shown novel important data on gastric myoelectric activity in SSc. This is the first study providing a detailed analysis of EGG recordings in SSc. We evaluated particular one-minute intervals during the fasting period and after a test meal. Pathological or abnormal EGGs were found in all patients with the exception of one individual. Bradygastria was the most common finding, which is in a good agreement with other studies published so far [25–29,32]. Yet, our most important finding was the low postprandial EGG power. In a healthy adult subject, twofold increase in EGG power should be observed after a test meal [6–8]. In SSc, we found a significant decrease in the postprandial power compared to the recordings during the fasting period. The power ratio was low in all the evaluated time intervals. This is an important proof of both structural involvement and functional disorder of the stomach in SSc.
On the other hand, EGG was completely normal in one patient only. This was a 70-year old woman with a lSSc diagnosed 3 months before EGG was accomplished. There were no gastrointestinal symptoms at the initial clinical manifestation of SSc. According to the theory, which is taking into account the anti-M3R antibodies, neuropathy is observed in the early phase of the gastrointestinal involvement. This is followed by a myopathic (atrophy of smooth muscle) phase and a fibrotic phase. This would be in agreement with our study, where bradygastria was less frequent in lSSc forms of the disease. Moreover, patients usually do not develop a multiple organ involvement simultaneously in the very early stages of SSc (Graphs 2,3).
Graph 2. Power (areas of amplitudes; mean + standard deviation). Axis Y: natural log scale. BAS: basic 15-minute recording during the fasting period. T1–T15: 1-minute intervals of recording.
Elektrogastrografi cká síla (plocha pod křivkou; průměr + směrodatná odchylka). Osa Y: stupnice v přirozených logaritmech. BAS: klidový 15minutový záznam během lačnění. T1 – T15: 1minutové intervaly záznamu.
Graph 3. Power ratio: fraction of the areas of amplitudes after (numerator) and before (denumenator) the test meal. Axis Y: natural log scale. T1–T15: one-minute intervals.
Elektrogastrografická síla: zlomek plochy pod křivkou po testovacím jídle (čitatel) a před (jmenovatel). Osa Y: stupnice v přirozených logaritmech. T1 – T15: ?minutové intervaly.
Bradygastria is also typically observed in anorexia nervosa [36,37], in diseases associated with neuropathy, in connective tissue diseases group [38–40] and in the early phases after an abdominal surgery [18,19,22–24]. This is a broader basis for the study of the etiopathogenesis of gastric dysrhythmias including bradygastria.
In the literature, authors have focused on plasma levels of various substances that could affect dysrhythmias. For example, the gastrointestinal peptides motilin, ghrelin, vasoactive intestinal peptide or interleukin-6 (IL-6), antibodies against muscarinic acetylcholine receptors (AChRs) and myenteric neurons and specific antibodies which inhibit acetylcholine receptor anti-M3R have been studied in this context [2,22,23,32,33,36–45].
In SSc, plasma concentrations of motilin have shown positive correlation with dysrhythmia and negative correlation with normal slow wave rhythm [32]. Ghrelin was studied in anorexia nervosa, where bradygastria also occurs [36,37]. Insufficient release of gastrointestinal peptides, especially ghrelin, has been confirmed in anorexia nervosa [41,42].
Specific antibodies against muscarinic AChRs and myenteric neurons have also been found to have an association with gastrointestinal dysmotility; not only in SSc but also in rheumatoid arthritis and other systemic diseases [38–40]. Specific antibodies against M3R cause a significant smooth muscle dysfunction in SSc patients by inhibiting acetylcholine binding to anti-M3R [2,43–45]. The neutralization of anti-M3R antibodies by human intravenous immunoglobulins and its antigen-binding fragment F (ab) 2 might reverse the intestinal dysfunction and are being considered as a potential targeted therapy [2,46].
Surgical studies in which bradygastria correlated with IL-6 serum concentrations demonstrate that IL-6 also affects the gastric motility. It has been shown that IL-6 can promote early systemic inflammatory response to gastric myoelectric activity [22,23]. Studies have documented that bradygastria disappeared after a certain period (1 to 2 days) after the operation [18,19,23,24] and concentrations of IL-6 decreased [23]. Similar results were also demonstrated by others [32,33]. Interestingly, anti-IL-6 treatment improved autonomic dysfunction in patients with rheumatoid arthritis [47].
Current options for the diagnosis of motor dysfunction of the stomach are gastric scintigraphy, 13C-octanoic acid breath tests, wireless motility capsule, antroduodenal manometry, ultrasonography and magnetic resonance imaging [48–54]. EGG is a non-invasive, reliable, potentially useful method in the diagnosis of gastric motility disorders in SSc.
We are aware of possible limits of this pilot study. We have not enrolled a group of healthy individuals. We relied on our previous healthy controls [8] and on the data from literature [6,7]. Nevertheless, our findings were very consistent in the majority of our patients. We did not correlate EGGs with either subjective dyspeptic symptoms or other possible variables. We plan to repeat a detailed investigation of the gastric myoelectric activity in SSc during a follow-up of the patients in the near future.
Conclusion
A pathological EGG was a constant finding in our set of patients suffering from SSc. Bradygastria and postprandial decrease of the power were the most characteristic features. These findings were confirmed especially in the dSSc subgroup. Further studies are needed to characterize clinical significance of these features precisely.
Acknowledgment
The authors are much obliged to Bc. Dita Staníčková and Mgr. Veronika Balášková for their brilliant skill and excellent technical cooperation.
Conflict of Interest: The authors declare that the article/ manuscript complies with ethical standards, patient anonymity has been respected, and they state that they have no
fi nancial, advisory or other commercial interests in relation to the subject matter.
Publication Ethics: This article/ manuscript has not been published or is currently being submitted for another review. The authors agree to publish their name and e-mail in
the published article/ manuscript.
Dedication: This work was supported by the project SVV 260542/ 2020 from Charles University and by the research project Progres Q40-15 from Charles University.
The Editorial Board declares that the manuscript met the ICMJE „uniform requirements“ for bio medical papers.
Zdroje
1. Soukup T, Veleta T. Systemic sclerosis in 2017. Vnitř Lék 2018; 64 (2): 146–154.
2. Oreska S, Tomcik M. Gastrointestinal involvement in systemic sclerosis: overview, neglected aspects, malnutrition, body composition and management. In: Tomcik M (Ed). New Insights into Systemic Sclerosis. London: IntechOpen London 2019 : 63–86. doi: 10.5772/intechopen.78254.
3. Cyrany J, Soukup T, Branda P et al. Hydrogen and methane breath tests in patients with systemic sclerosis. Gut 2009; 58 (Suppl 2): A111.
4. Bures J, Cyrany J, Kohoutova D et al. Small interstinal bacterial overgrowth. World J Gastroenterol 2010; 16 (24): 2978–2990. doi: 10.3748/wjg.v16.i24.2978.
5. Stoichita S, Suteano S, Pirvu V et al. Digestive disorders in sclerosis. Čs. Gastroenterol 1973; 27 (6): 367–370.
6. Koch KL, Stern RM. Handbook of Electrogastrography. Oxford: University Press 2004.
7. Parkman HP, Hasler WL, Barnett JL et al. Electrogastrography: a document prepared by the gastric section of the American motility society clinical GI motility testing task force. Neurogastroenterol Motil 2003; 15 (2): 89–102. doi: 10.1046/j.1365-2982.2003.00396.x.
8. Bures J, Kopacova M, Vorisek V et al. Correlation of electrogastrography and gastric emptying rate estimated by 13C-octanoic acid breath test in healthy volunteers. Folia Gastroenterol Hepatol 2007; 5 (1): 5–11.
9. Yin J, Chen JD. Electrogastrography: methodology, validation and applications. J Neurogastroenterol Motil 2013; 19 (1): 5–17. doi: 10.5056/jnm.2013.19.1.5.
10. Bures J, Kvetina J, Pavlik M et al. Impact of paraoxon followed by acetylcholinesterase reactivator HI-6 on gastric myoelectric activity in experimental pigs. Neuro Endocrinol Lett 2013; 34 (Suppl 2): S79–S83.
11. Tacheci I, Kvetina J, Kunes M et al. The effect of general anaesthesia on gastric myoelectric activity in experimental pigs. BMC Gastroenterol 2013; 13 : 48. doi: 10.1186/1471-230X-13-48.
12. Bures J, Tacheci I, Kvetina J et al. Experimental electrogastrography. Gastroent Hepatol 2014; 68 (3): 237–242.
13. Bures J, Jun D, Hrabinova M et al. Impact of tacrine and 7-methoxytacrine on gastric myoelectrical activity assessed using electrogastrography in experimental pigs. Neuro Endocrinol Lett 2015; 36 (Suppl 1): S150–S155.
14. Bures J, Kvetina J, Tacheci I et al. The effect of different doses of atropine on gastric myoelectrical activity in fasting experimental pigs. J Appl Biomed 2015; 13 (4): 273–277. doi: 10.1016/j.jab.2015.04.004.
15. Kvetina J, Tacheci I, Pavlik M et al. Use of electrogastrography in preclinical studies of cholinergic and anticholinergic agents in experimental pigs. Physiol Res 2015; 64 (Suppl 5): S647–S652. doi: 10.33549/physiolres.933227.
16. Bures J, Kvetina J, Radochova V et al. The pharmacokinetic parameters and the effect of a single and repeated doses of memantine on gastric myoelectric activity in experimental pigs. PLoS One 2020; 15 (1): e0227781. doi: 10.1371/journal.pone.0227781.
17. Dolina J, Hep A, Prasek J et al. Is electrogastrography going to be a new diagnostic method in gastroenterology? Čes Slov Gastroenterol 1997; 51 (5): 177–180.
18. Frasko R, Maruna P, Gürlich R et al. Percutaneous electrogastrography in the perioperative period in laparoscopic gastric banding at the First Surgical Clinic of the General Faculty Hospital in Prague. Rozhl Chir 2001; 80 (11): 596–601.
19. Frasko R, Maruna P, Gürlich R et al. Percutaneous electrogastrography in the perioperative period in laparoscopic and classical cholecystectomy and in laparoscopic nonadjustable gastric banding. Sb Lek 2002; 103 (2): 247–255.
20. Dolina J, Hep A, Kroupa R. Contribution of oesophageal manometry and electrogastrography in diagnosis of functional disorders. Gastroent Hepatol 2005; 59 (Suppl 1): S33–S34.
21. Bures J, Kabelac K, Kopacova M et al. Electrogastrography in patients with Roux-en-Yreconstruction after previous Billroth gastrectomy. Hepatogastroenterology 2008; 55 (85): 1492–1496.
22. Frasko R, Maruna P, Gürlich R et al. Transcutaneous electrogastrography in patients with ileus. Relations to interleukin-1beta, interleukin-6, procalcitonin, and C-reactive protein. Eur Surg Res 2008; 41 (2): 197–202. doi: 10.1159/000134918.
23. Maruna P, Frasko R, Lindner J. Disturbances of gastric electrical control activity after laparotomic cholecystectomy are related to interleukin-6 concentrations. Eur Surg Res 2009; 43 (4): 317–324. doi: 10.1159/000235569.
24. Gürlich R, Maruna P, Frasko R. Transcutaneous electrogastrography in the perioperative period in patients undergoing laparoscopic cholecystectomy and laparoscopic non-adjustable gastric banding. Obes Surg 2003; 13 (5): 714–720. doi: 10.1381/096089203322509273.
25. Pfaffenbach B, Adamek RJ, Hagemann D et al. Effect of progressive systemic sclerosis on antral myoelectrical activity and gastric emptying. Z Gastroenterol 1996; 34 (9): 517–521.
26. Marycz T, Muehldorfer SM, Gruschwitz MS et al. Gastric involvement in progressive systemic sclerosis: electrogastrographic and sonographic findings. Eur J Gastroenterol Hepatol 1999; 11 (10): 1151–1156. doi: 10.1097/00042737-199910000-00013.
27. Hocke M, Seidel T, Sprott H et al. Ambulatory electrogastrography in patients with sclerodermia, delayed gastric emptying, dyspepsia, and irritable bowel syndrome. Is there any clinical relevance? Eur J Intern Med 2001; 12 (4): 366–371. doi: 10.1016/s0953-6205 (01) 00138-8.
28. Marie I, Levesque H, Ducrotté P et al. Gastric involvement in systemic sclerosis: a prospective study. Am J Gastroenterol 2001; 96 (1): 77–83. doi: 10.1111/j.1572-0241.2001.03353.x.
29. McNearney T, Lin X, Shrestha J et al. Characterization of gastric myoelectrical rhythms in patients with systemic sclerosis using multichannel surface electrogastrography. Dig Dis Sci 2002; 47 (4): 690–698. doi: 10.1023/a: 1014759109982.
30. Franck-Larsson K, Hedenström H, Dahl R et al. Delayed gastric emptying in patients with diffuse versus limited systemic sclerosis, unrelated to gastrointestinal symptoms and myoelectric gastric activity. Scand J Rheumatol 2003; 32 (6): 348–355. doi: 10.1080/03009740410005016.
31. Wollaston DE, Xu X, Tokumaru O et al. Patients with systemic sclerosis have unique and persistent alterations in gastric myoelectrical activity with acupressure to Neiguan point PC6. J Rheumatol 2005; 32 (3): 494–501.
32. McNearney TA, Sallam HS, Hunnicutt SE et al. Gastric slow waves, gastrointestinal symptoms and peptides in systemic sclerosis patients. Neurogastroenterol Motil 2009; 21 (12): 1269-e120. doi: 10.1111/j.1365-2982.2009.01350.x.
33. McNearney TA, Sallam HS, Hunnicutt SE et al. Prolonged treatment with transcutaneous electrical nerve stimulation (TENS) modulates neuro-gastric motility and plasma levels of vasoactive intestinal peptide (VIP), motilin and interleukin-6 (IL-6) in systemic sclerosis. Clin Exp Rheumatol 2013; 31 (2 Suppl 76): S140–S150.
34. van den Hoogen F, Khanna D, Fransen J et al. 2013 classification criteria for systemic sclerosis: an American College of Rheumatology/European league against Rheumatism collaborative initiative. Arthritis Rheum 2013; 65 (11): 2737–2747. doi: 10.1002/art.38098.
35. LeRoy EC, Medsger TA Jr. Criteria for the classification of early systemic sclerosis. J Rheumatol 2001; 28 (7): 1573–1576.
36. Diamanti A, Bracci F, Gambarara M et al. Gastric electric activity assessed by electrogastrography and gastric emptying scintigraphy in adolescents with eating disorders. J Pediatr Gastroenterol Nutr 2003; 37 (1): 35–41. doi: 10.1097/00005176-200307000-00006.
37. Ogawa A, Mizuta I, Fukunaga T et al. Electrogastrography abnormality in eating disorders. Psychiatry Clin Neurosci 2004; 58 (3): 300–310. doi: 10.1111/j.1440-1819.2004.01236.x.
38. Howe S, Eaker EY, Sallustio JE et al. Antimyenteric neuronal antibodies in scleroderma. J Clin Invest 1994; 94 (2): 761–770. doi: 10.1172/JCI117395.
39. Berger M, Steen VD. Role of anti-receptor autoantibodies in pathophysiology of scleroderma. Autoimmun Rev 2017; 16 (10): 1029–1035. doi: 10.1016/j.autrev.2017.07.019.
40. Kumar S, Singh J, Rattan S et al. Review article: pathogenesis and clinical manifestations of gastrointestinal involvement in systemic sclerosis. Aliment Pharmacol Ther 2017; 45 (7): 883–898. doi: 10.1111/apt.13963.
41. Méquinion M, Langlet F, Zgheib S et al. Ghrelin: central and peripheral implications in anorexia nervosa. Front Endocrinol (Lausanne) 2013; 4 : 15. doi: 10.3389/fendo.2013.00015.
42. Cuntz U, Enck P, Frühauf E et al. Cholecystokinin revisited: CCK and the hunger trap in anorexia nervosa. PLoS One 2013; 8 (1): e54457. doi: 10.1371/journal.pone.0054457.
43. Goldblatt F, Gordon TP, Waterman SA. Antibody-mediated gastrointestinal dysmotility in scleroderma. Gastroenterology 2002; 123 (4): 1144–1150. doi: 10.1053/gast.2002.36057.
44. Singh J, Mehendiratta V, Del Galdo F et al. Immunoglobulins from scleroderma patients inhibit the muscarinic receptor activation in internal anal sphincter smooth muscle cells. Am J Physiol Gastrointest Liver Physiol 2009; 297 (6): G1206–G1213. doi: 10.1152/ajpgi.00286.2009.
45. Singh J, Cohen S, Mehendiratta V et al. Effects of scleroderma antibodies and pooled human immunoglobulin on anal sphintcter and colonic smooth muscle function. Gastroenterology 2012; 143 (5): 1308–1318. doi: 10.1053/j.gastro.2012.07.109.
46. Kumar S, Singh J, Kedika R et al. Role of muscarinic-3 receptor antibody in systemis sclerosis: correlation with dissease duration and effect of IVIG. Am J Physiol Gastrointest Liver Physiol 2016; 310 (11): G1052–G1060. doi: 10.1152/ajpgi.00034.2016.
47. Syngle A, Verma I, Krishan P. Interleukin-6 blockade improves autonomic dysfunction in rheumatoid arthritis. Acta Reumatol Port 2015; 40 (1): 85–88.
48. Byrne KG, Quigley EM. Antroduodenal manometry: an evaluation of an emerging methodology 1997. Dig Dis 1997; 15 (Suppl 1): S53–S63. doi: 10.1159/000171621.
49. Bures J, Kopacova M, Vorisek V et al. Examination of gastric emptying rate by means of 13C-octanoic acid breath test. Methods of the test for adults and results of the investigation of healthy volunteers. Čas Lek Čes 2005; 144 (Suppl 3): S18–S22.
50. Kuo B, McCallum RW, Koch Kl et al. Comparison of gastric emptying of a nondigestible capsule to a radio-labelled meal in healthy and gastroparetic subjects. Aliment Pharmacol Ther 2008; 27 (2): 186–196. doi: 10.1111/j.1365-2036.2007.03564.x.
51. de Zwart IM, de Roos A. MRI for the evaluation of gastric physiology. Eur Radiol 2010; 20 (11): 2609–2616. doi: 10.1007/s00330-010-1850-3.
52. Dickman R, Zilpera T, Steinmetz A et al. Comparison of continuous breath test and gastric scintigraphy for the measurement of gastric emptying rate in healthy and dyspeptic individuals. Eur J Gastroenterol Hepatol 2013; 25 (3): 291–295. doi: 10.1097/MEG.0b013e32835c075d.
53. Bruno G, Lopetuso LR, Ianiro G et al. 13C-octanoic acid breath test to study gastric emptying time. Eur Rev Med Pharmacol Sci 2013; 17 (Suppl 2): S59–S64.
54. Mazzawi T, Bartsch E, Benammi S. Gastric emptying of low - and high-caloric liquid meals measured using ultrasonography in healthy volunteers. Ultrasound Int Open 2019; 5 (1): E27–E33. doi: 10.1055/a-0783-2170.
Štítky
Detská gastroenterológia Gastroenterológia a hepatológia Chirurgia všeobecná
Článok vyšiel v časopiseGastroenterologie a hepatologie
Najčítanejšie tento týždeň
2020 Číslo 4- Metamizol jako analgetikum první volby: kdy, pro koho, jak a proč?
- Kombinace metamizol/paracetamol v léčbě pooperační bolesti u zákroků v rámci jednodenní chirurgie
- Antidepresivní efekt kombinovaného analgetika tramadolu s paracetamolem
- I „pouhé“ doporučení znamená velkou pomoc. Nasměrujte své pacienty pod křídla Dobrých andělů
- Parazitičtí červi v terapii Crohnovy choroby a dalších zánětlivých autoimunitních onemocnění
-
Všetky články tohto čísla
- Klinická a experimentální gastroenterologie
- Electrogastrography in systemic sclerosis – a pilot study
- Portopulmonální hypertenze u pacientů indikovaných k transplantaci jater – zkušenosti transplantačního centra IKEM
- EUS navigované gastrointestinální anastomózy – nové možnosti terapeutické endoskopie
- Akutní pankreatitida u pacientů po transplantaci jater
- Applicability of the Glasgow-Blatchford score in predicting low-risk patients with upper gastrointestinal bleeding – first data from the Czech Republic
- Autonómna dysregulácia pri syndróme dráždivého čreva, funkčnej dyspepsii a globus pharyngeus – prehľad literatúry a pilotné výsledky
- Whippleova choroba – dvě kazuistiky
- Akutní poškození ledvin v gastroenterologii
- Výběr z mezinárodních časopisů
- Kreditovaný autodidaktický test: klinická a experimentální gastroenterologie
- Jorveza® – očekávaný preparát k léčbě eozinofilní ezofagitidy
- Hemové železo v substituci sideropenie a sideropenní anémie u pacientů s IBD
- Gastroenterologie a hepatologie
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- EUS navigované gastrointestinální anastomózy – nové možnosti terapeutické endoskopie
- Jorveza® – očekávaný preparát k léčbě eozinofilní ezofagitidy
- Whippleova choroba – dvě kazuistiky
- Portopulmonální hypertenze u pacientů indikovaných k transplantaci jater – zkušenosti transplantačního centra IKEM
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



