-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
Efficacy and safety of switching from intravenous to subcutaneous CTP-13 treatment in IBD patients – a one-year retrospective study from large Slovak IBD centre
Účinnosť a bezpečnosť prechodu z intravenózneho na subkutánnu liečbu CTP-13 u pacientov s IBD – ročná retrospektívna štúdia z veľkého slovenského IBD centra
Úvod a ciele: Nedávno bol schválený prvý subkutánny infliximab (IFX), CTP-13, v indikácii IBD. Pilotná štúdia preukázala noninferioritu subkutánneho infliximabu voči intravenóznej forme z hľadiska účinnosti, pričom neboli pozorované žiadne neočakávané nežiaduce účinky liečby. Cieľom štúdie bolo zhodnotiť účinnosť liečby, farmakokinetiku a bezpečnosť po prechode z intravenóznej na subkutánnu liečbu infliximabom. Metódy: Táto retrospektívna kohortová štúdia zahàňala všetkých pacientov s Crohnovou chorobou (CD) a ulceróznou kolitídou (UC) sledovaných v IBD centre, ktorí prešli z intravenózneho na subkutánny IFX medzi septembrom 2022 a septembrom 2023. Klinická aktivita ochorenia podľa HBI a pMayo, sIBDQ, fekálny kalprotektín, perzistencia liečby, bezpečnosť a farmakokinetika boli hodnotené v 24. a 56. týždni po prechode na subkutánny infliximab. Výsledky: Kohorta pozostávala z 107 pacientov (63 CD a 44 UC). Indexy kvality života a klinickej aktivity ochorenia zostali nezmenené pri CD aj UC. Fekálny kalprotektín sa významne znížil pri CD, ale nie pri UC. Pacienti, ktorým bol IV IFX podávaný v štandardnom režime, ako aj tí na intenzifikovaných režimoch, vykazovali podobné výsledky pri štandardnom SC dávkovaní. Nevyskytli sa žiadne závažné nežiaduce udalosti. Prechodné injekčné reakcie sa vyskytli u 4,7 % pacientov. Medián najnižších hladín infliximabu sa významne zvýšil po prechode na subkutánnu formu. Záver: Prechod z intravenózneho infliximabu na subkutánny je bezpečnou a účinnou možnosťou liečby pacientov so zápalovým ochorením čriev vrátane tých s aktivitou ochorenia a/ alebo na intenzifikovanom intravenóznom režime infliximabu.
Klíčová slova:
ulcerózna kolitída – Crohnova choroba – biologiká – subkutánny – intravenózny – infliximab
Authors: A. Gojdičová 1
; I. Sturdik 1; J. Tóth 1; M. Gojdič 2; Z. Vrablicová 1; I. Čierna 1; D. Székyová 1; A. Krónerová 1
; T. Hlavatý 1
Authors place of work: Gastroenterology centre Bezručova, Bratislava , Slovak Republic 1; Urology Department, First Faculty of Medicine Charles University, Bulovka University Hospital, Prague, Czech Republic 2
Published in the journal: Gastroent Hepatol 2024; 78(1): 31-36
Category: IBD
doi: https://doi.org/10.48096/ccgh202431Summary
Background and aims: Recently, the first subcutaneous infliximab (IFX), CTP-13, was approved in the indication of IBD. Pivotal study established noninferiority of subcutaneous infliximab to intravenous formulation in terms of efficacy, with no unexpected safety signals observed. The aims of the study were to evaluate persistency of treatment, pharmacokinetics and safety following a switch from intravenous to subcutaneous infliximab treatment. Methods: This retrospective, single-centre cohort study, recruited all Crohn’s disease (CD) and ulcerative colitis (UC) patients who transitioned from intravenous to subcutaneous IFX between September 2022 and September 2023. Clinical disease activity according to HBI and pMayo, sIBDQ, faecal calprotectin, drug persistence, safety and pharmacokinetics were evaluated at week 24 and 56 after the switch to subcutaneous infliximab. Results: A number of 107 patients was included (63 CD and 44 UC). Quality of life and clinical disease activity scores remained unchanged both in CD and UC. Faecal calprotectin decreased significantly in CD but not in UC. Patients that had been on standard compared to intensified IV infliximab dosing displayed similar outcomes on standard SC dosing. No serious adverse events occurred. Transient local injection reactions were experienced by 4.7% of patients. Median infliximab trough levels significantly increased after the switch to subcutaneous formulation. Conclusion: The switch from intravenous to subcutaneous infliximab is a safe and effective option for the treatment of inflammatory bowel diseases including those with active disease and/ or on intensified intravenous infliximab regime.
Keywords:
ulcerative colitis – Crohn’s disease – biologics – subcutaneous – intravenous – infliximab
Introduction
Inflammatory bowel diseases (IBD) are characterized by chronic relapsing intestinal inflammation. It has been a worldwide healthcare problem with a continually increasing incidence [1]. Infliximab (IFX) is a chimeric IgG1 monoclonal antibody inhibiting tumour necrosis factor a (TNFa), which was first approved for the treatment of IBD 25 years ago. The COVID-19 pandemic has highlighted the benefits of ambulatory and home care. Route of delivery is recognized as an important factor associated with healthcare resource use – health care costs (no infusion unit necessary) and societal costs (no need to take time off work) [2]. The important thing is also the option of SC formulation that offers patients a choice regarding the route of administration. Recently, the first subcutaneous (SC) IFX, CTP-13, was approved in the indication of IBD. Pivotal study established non inferiority of IFX SC to CT-P13 IV in terms of efficacy, with no unexpected safety signals observed [3]. Smith et al. conducted a retrospective multicentre cohort study, which included stable patients who received treatment with IV infliximab and who were switched to SC CTP-13. In this study, the authors conclude that this change presents high rates of treatment persistence and low rates of immunogenicity. In addition, no changes were observed in disease clinical activity indices or in biomarkers. As in the pivotal study, infliximab levels increased after switching to SC CTP-13 [4].
In the study, we assessed the maintenance of remission, drug discontinuation, effectiveness, safety, and pharmacokinetics in a real‐world IBD cohort, who switched from IV IFX maintenance treatment to SC IFX.
Patients and methods
Study design
This retrospective, single-centre cohort study recruited all consecutive adult (18 years and over) patients with Crohn’s disease (CD) and ulcerative colitis (UC) transitioned from IV to SC IFX between September 2022 and September 2023 at Gastroenterology centre Bezručova, Bratislava, Slovakia.
All data in the research database were anonymised by replacing each subject’s personal identification data with a unique research ID number. Patients were transitioned at the discretion of the clinician managing care, with agreement from the patient. All patients have been established on maintenance IV IFX. Patients were included if they had completed at least 12 subcutaneous administrations of CTP-13 prior to the end of the study or discontinued treatment early due to an adverse event. The dose of IV IFX prior to the switch was variable, either from the standard IV IFX regimen 5 mg/ kg every 8 weeks or intensified regimens of 5 mg/ kg every 6 or 4 weeks. One patient switched from the regimen of 5 mg/ kg every 12 weeks.
Study cohort
The baseline cohort data collected included demographic and clinical characteristics such as age, sex, type of disease including Montreal classification (CD or UC). Details on treatments were also collected, including IV IFX initiation date, dose and frequency, use of concomitant immunomodulators, prior medication history. Baseline C-reactive protein (CRP), faecal calprotectin (FCP), IFX level, and IFX antibodies were extracted from the patient’s electronic health records in the centre information system at the time of switch and follow up visit in 24th and 56th week. We also recorded HBI and partial Mayo scores at the time of switch and at follow--up visits. All adverse drug events were collected and outcomes were recorded.
Outcome parameters
We defined clinical remission of CD patients as Harvey-Bradshaw index (HBI) less than 5 and no active fistula. Clinical remission of UC patients was defined as a partial Mayo score (pMayo) of less than 3. Biochemical remission was defined as a CRP level less than 10 mg/ L, and FCP less than 100 μg/ g, based on the recommendations on the management of IBD in adults [5]. Adverse events were collected at each follow-up visit. These included severe or opportunistic infections, acute or delayed hypersensitivity reactions, treatment discontinuation due to side effects, and IBD-related hospital admission or surgery.
Statistical analysis
Statistical analysis was carried out using the SPSS statistics software package (IBM SPSS Inc., Chicago, Illinois, USA). Graphs were plotted using Analyse-it in Microsoft Excel (Analyse-it Software Ltd, Leeds, UK). Nominal and ordinal variables, such as clinical characteristics, clinical remission rate, and adverse event rates, are presented as frequency and percentage. Continuous variables, including age, duration of treatment, HBI score, pMayo score, faecal calprotectin, and CRP levels, were tested for normal distribution using the Kolmogorov-Smirnov test. All variables had non-normal distributions and are presented as median, quartiles, and range. The statistical significance of the change in HBI score, pMayo score, faecal calprotectin, CRP, IFX drug and IFX antibodies levels between baseline and follow-up visits was tested using the Wilcoxon rank sum test. Significance was defined as a P-value <0.05.
Tab. 1. Baseline clinical and demographic characteristics of the cohort. 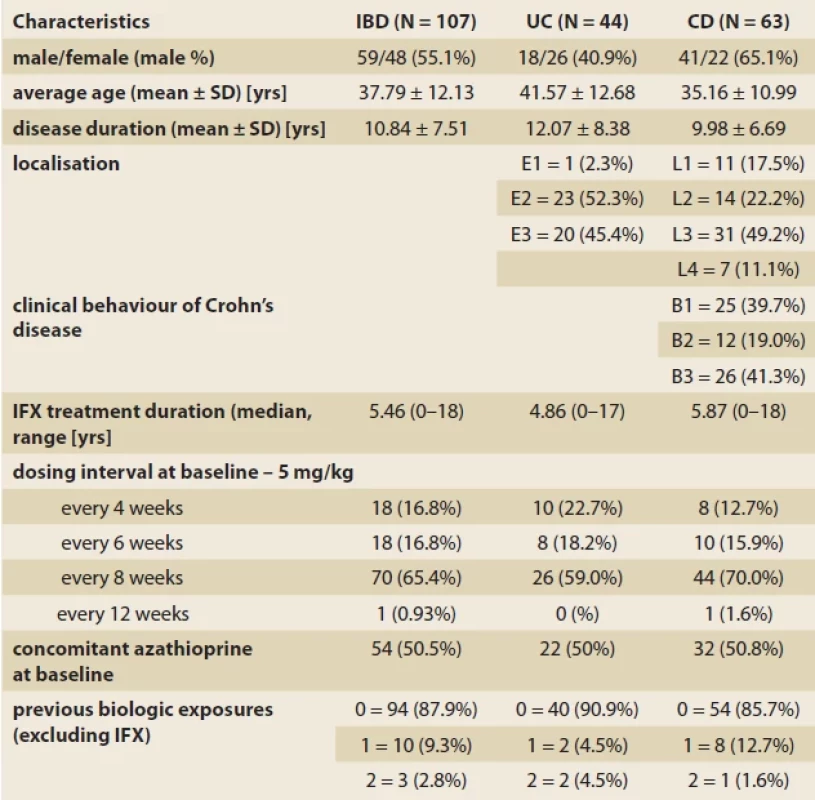
Tab. 1. Východiskové klinické a demografické charakteristiky kohorty. Fig. 1. Evolution of sIBDQ in IBD patients compared to baseline. 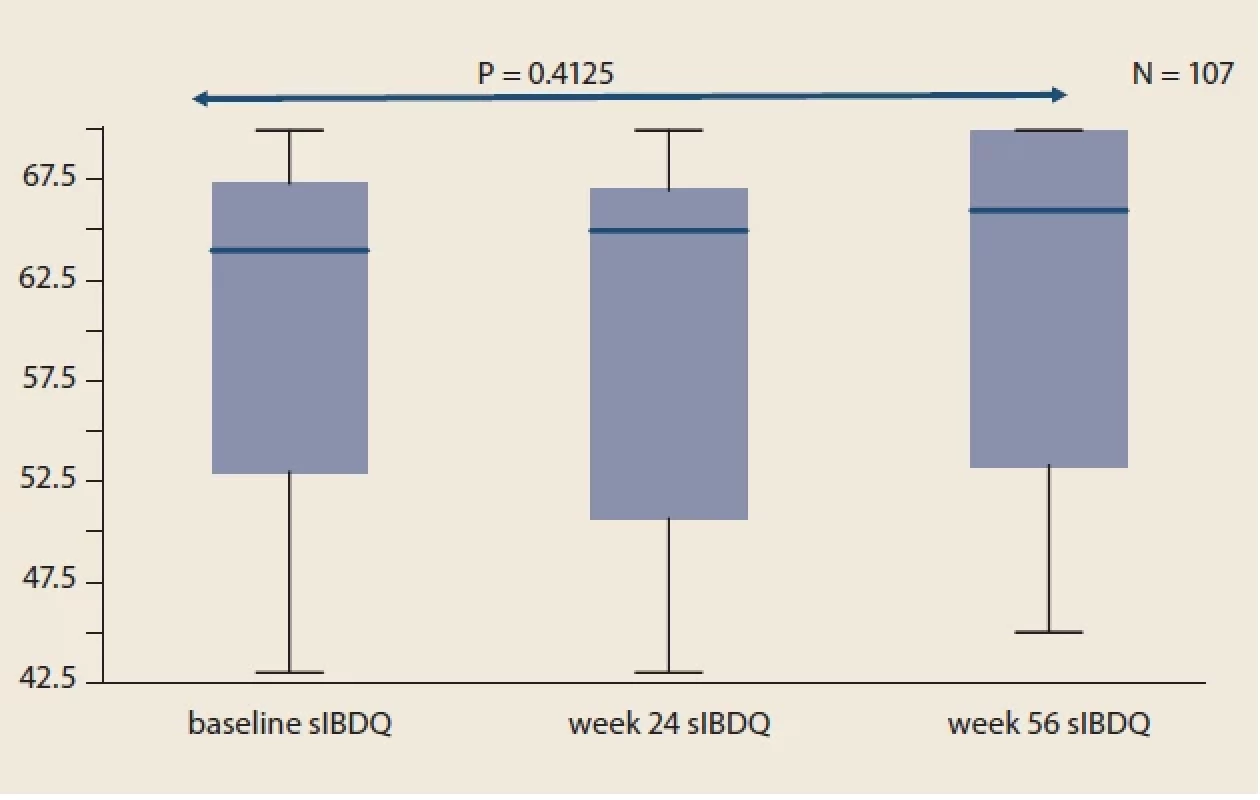
Obr. 1. Vývoj sIBDQ u pacientov s IBD v porovnaní s východiskovou hodnotou. Fig. 2. Evolution of HBI in CD patients compared to baseline. 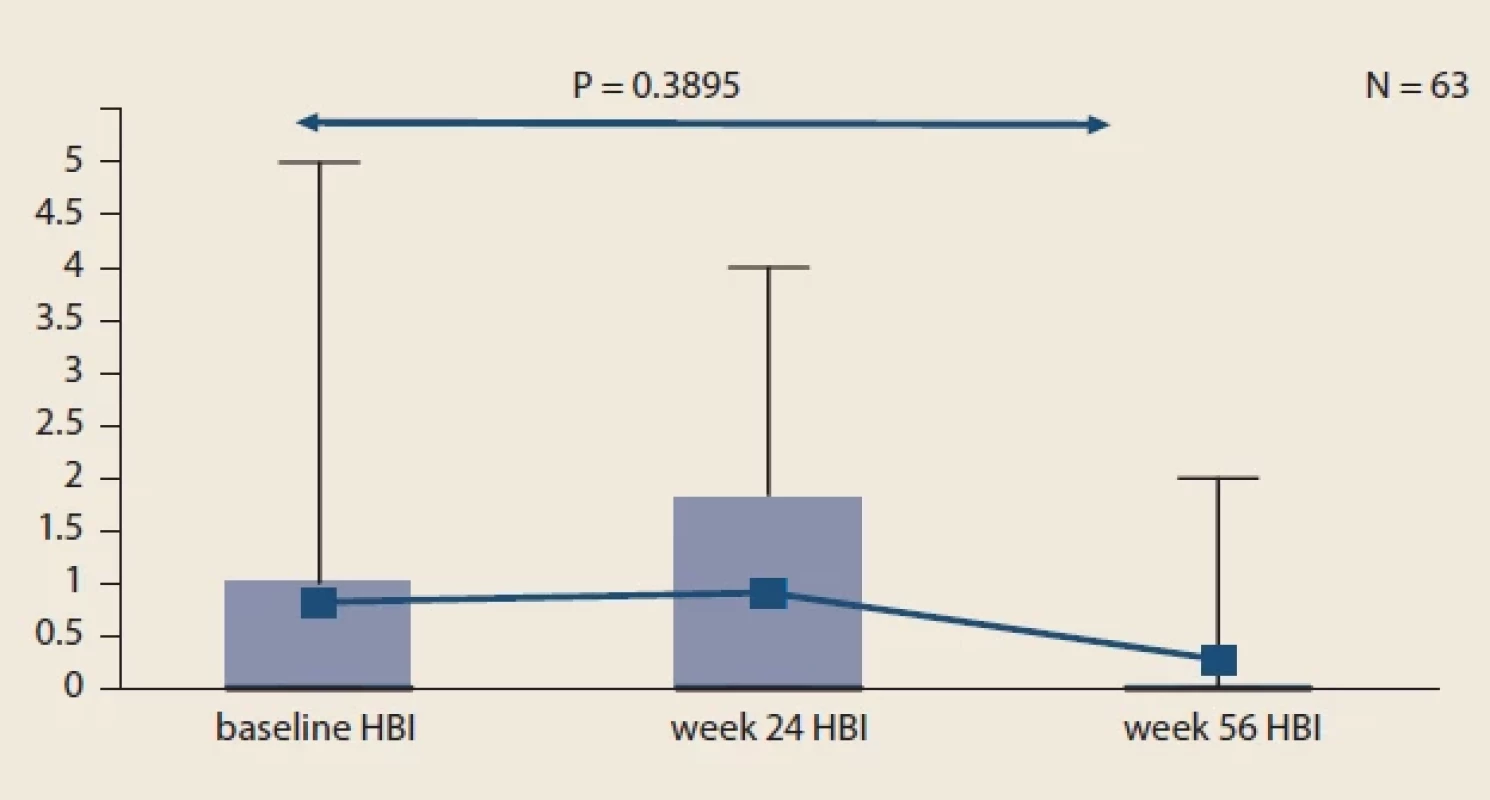
Obr. 2. Vývoj HBI u pacientov s CD v porovnaní s východiskovou hodnotou. Fig. 3. Evolution of pMayo in UC patients compared to baseline. 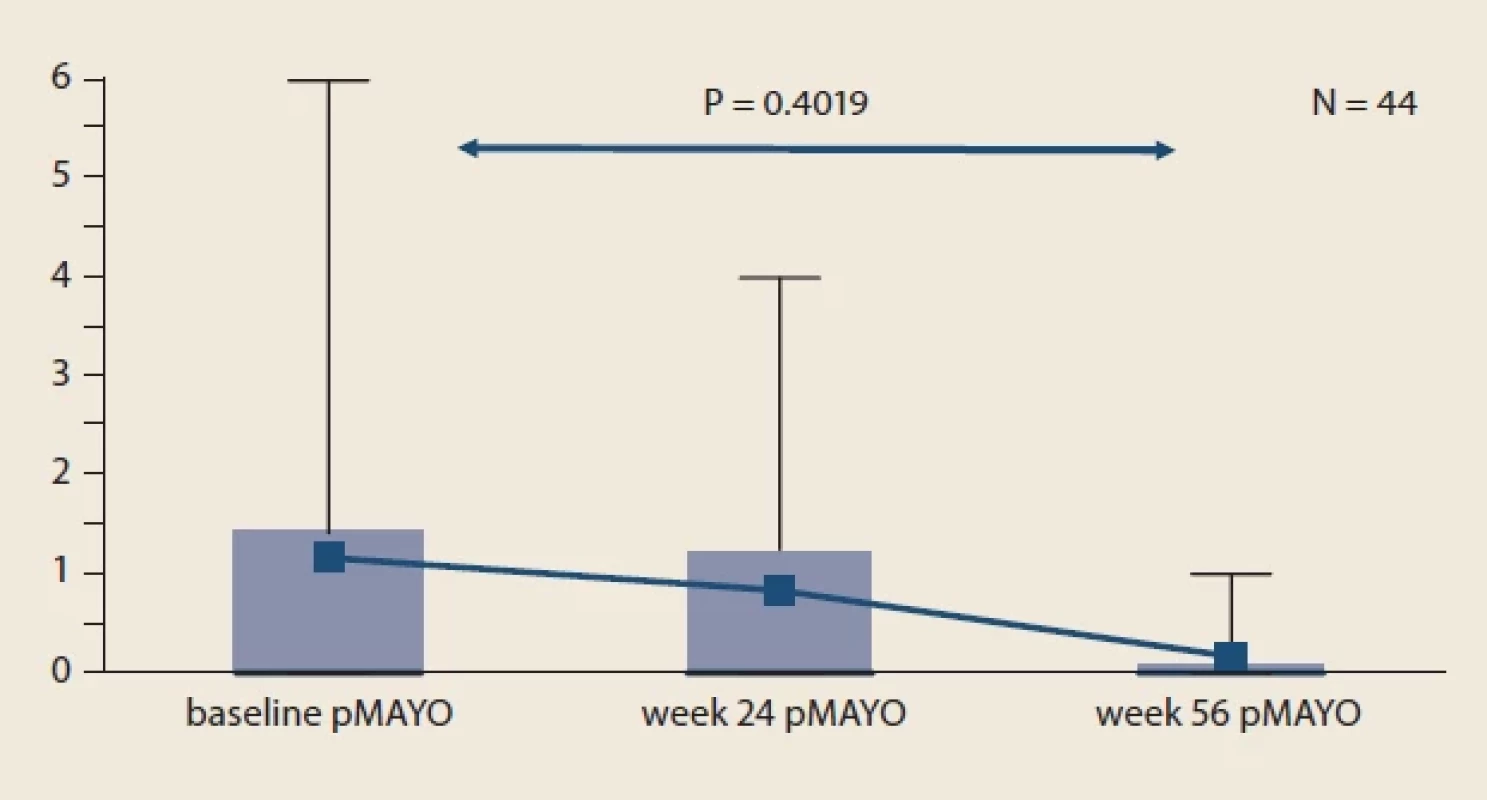
Obr. 3. Vývoj pMayo u pacientov s UC v porovnaní s východiskovou hodnotou. Fig. 4. Evolution of faecal calprotectin of the whole IBD cohort compared to baseline. 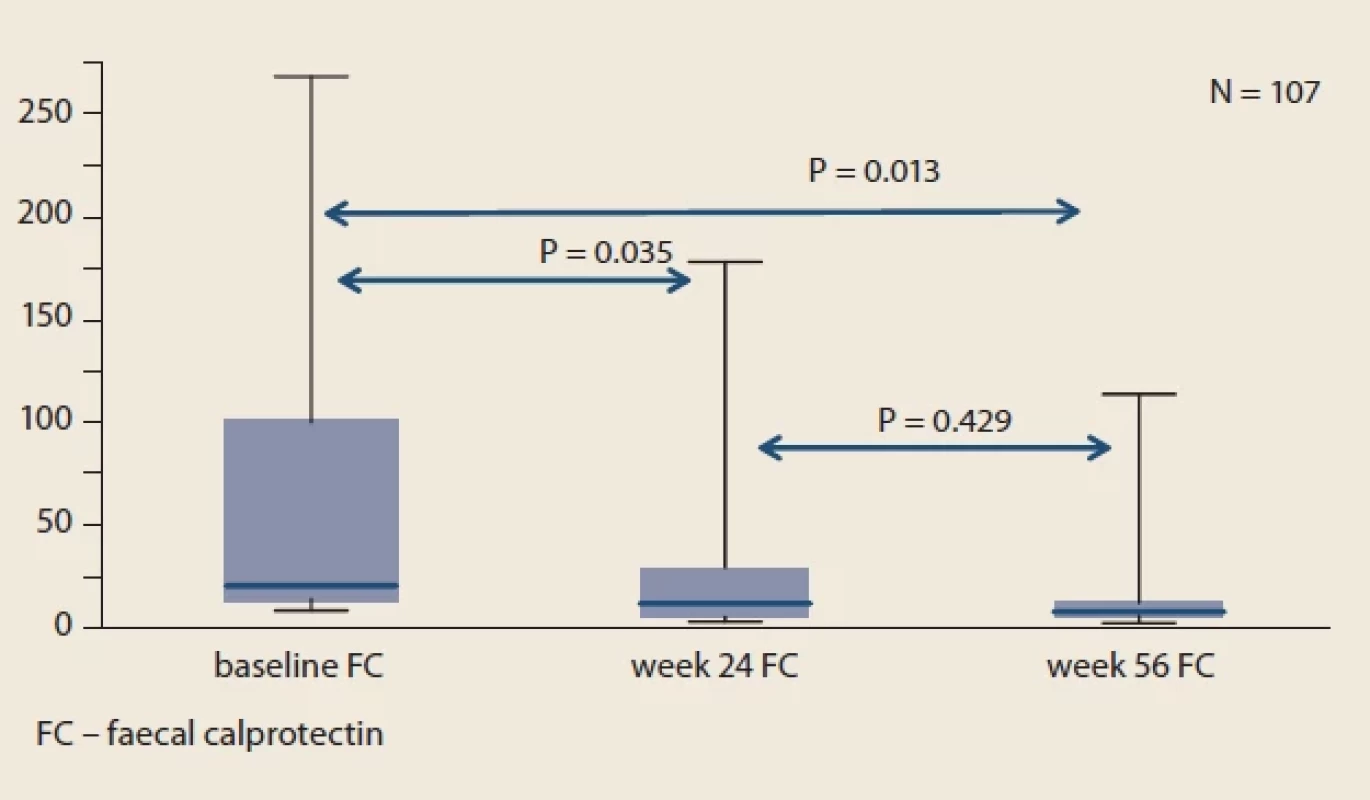
Obr. 4. Vývoj fekálneho kalprotektínu celej kohorty IBD v porovnaní s východiskovou
hodnotou.Fig. 5. The median serum levels of infliximab at each visit compared to baseline. 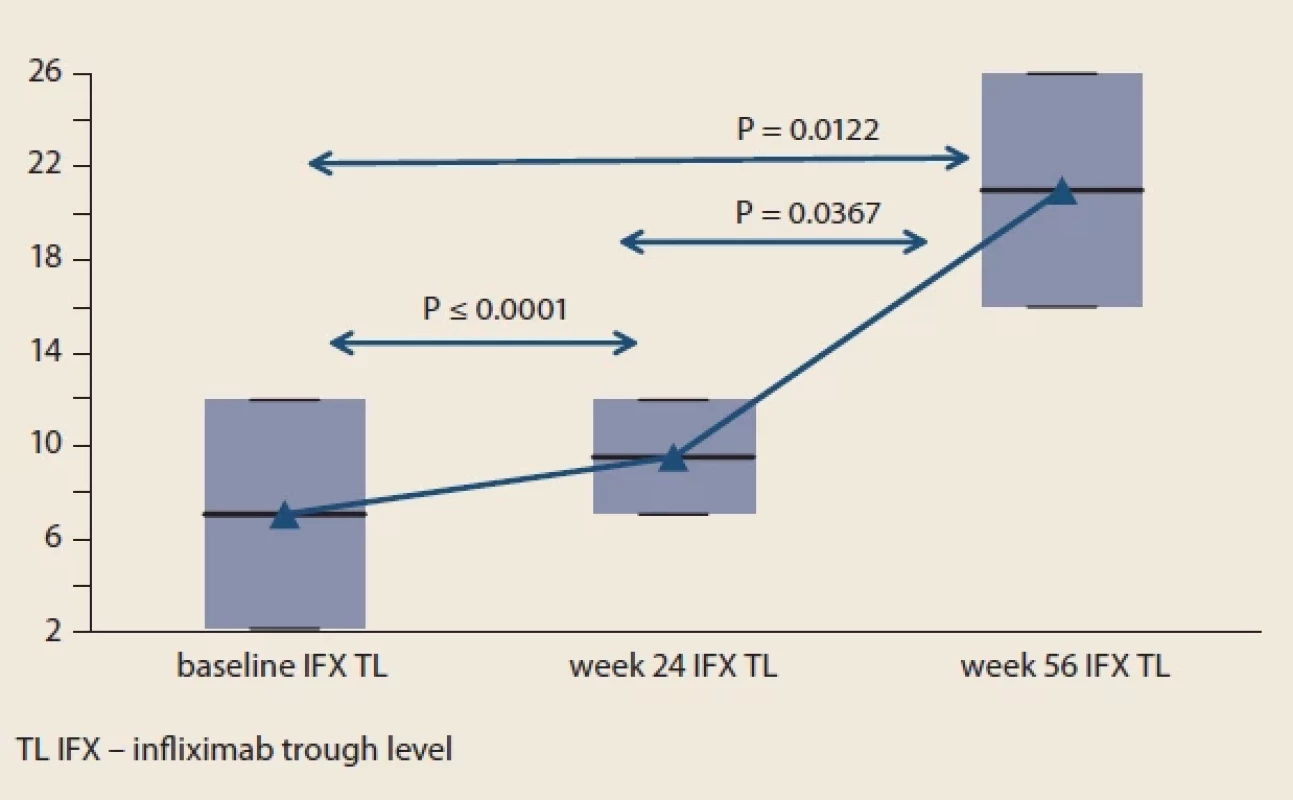
Obr. 5. Medián sérových hladín infliximabu pri každej návšteve v porovnaní s východiskovou hodnotou. Results
Among 167 patients who switched from IV to SC IFX, 107 met the inclusion criteria. Out of these, 63 had CD and 44 had UC. Fifty-nine (55.1%) patients were male, and the mean age was 37,79 ± 12.13 years. The demographic and clinical characteristics of the cohort are presented in Tab. 1.
Treatment efficacy
Most of the patients were on 5 mg/ kg IV IFX every 8 weeks (65.4%) at the time of switch. There were equally 18 patients (16.8%) on 5 mg/ kg every 6 and 4 weeks at the time of switch. One patient switched from the 12-week dosing interval. All patients were switched to 120 mg of SC IFX fortnightly, regardless of the IV regime they had previously. Fifty-four (50.5%) patients were using combination treatment with an immunomodulator – azathioprine at the time of switch. Thirteen patients had received a biologic therapy apart from IV infliximab at the time of switch. No patients were using steroids at the time of switch to SC.
Throughout the study, we did not observe significant changes in mean sIBDQ (Fig. 1), HBI score in CD patients (Fig. 2) and pMayo score in UC patients (Fig. 3). For the whole IBD cohort (baseline 58.4 ± 73.6 μg/ g vs. week 24 26.7 ± 45.4 μg/ g vs. week 56 20.7 ± 32.7 μg/ g) (Fig. 4), the subgroup on standard IV IFX regime (baseline 76.9 ± 80.5 μg/ g vs. week 24 29.5 ± 53.8 μg/ g vs. week 56 16.7 ± 34.1 μg/ g) and the subgroup of CD patients (baseline 77.8 ± 89.2 μg/ g vs. week 24 34.0 ± 60.0 μg/ g vs. week 56 19.7 ± 38.0 μg/ g), significant decrease in mean faecal calprotectin were observed following the switch, whereas no changes were seen in UC patients or patients on intensified IV regime.
Pharmacokinetics
Considering the whole cohort, the median IFX trough level at baseline was 7.1 (IQR, 2.2 to 12.0) mg/ mL. Serum levels of IFX significantly increased after the switch from IV to SC infliximab. Median IFX trough level was 9.55 (IQR 7.1 to 12.0) mg/ mL and 21.0 (IQR 16.0–36.0) mg/ mL at week 24 and 56, respectively (Fig. 5). At the baseline, 8 IBD patients (4 UC and 4 CD) receiving IV 5 mg/ kg every 8 weeks had detectable level of anti-infliximab antibodies. Their neutralization occurred in 87.5% (7/ 8) and in the 56th week anti-infliximab antibodies were detectable in only one of these patients. although in a lower level (>200 ng/ mL vs. 17.9 ng/ mL). No additional patient developed anti-infliximab antibodies throughout the study.
Safety
At the time of switch, 81/ 107 (75.7%) IBD patients were in a clinical and laboratory remission and 26/ 107 (24.3%) had a mild clinical and laboratory disease activity. At week 24 after switch to SC IFX, sixteen of these patients (61.5%) re-entered clinical remission. Three IBD patients (1 CD and 2 UC) experienced loss of response and discontinued the IFX treatment. SC IFX dose was at week 24 escalated in six patients (2 CD and 4 UC) to 120 mg weekly due to worsening of disease activity. All patients on intensified regime re-entered and maintained remission by week 56. Seven patients (7/ 107, 6.54%) discontinued SC IFX maintenance due to adverse event. One CD patient experienced itching of the whole body and serum IFX antibodies were high. One UC patient discontinued SC IFX due to an acute hypersensitivity reaction after the fourth injection. Five IBD patients (3CD and 2 UC) experienced local pain, redness and swelling at the injection site. All patients with local reaction were switched to IV IFX and continued with treatment to 56th week of follow-up in clinical and laboratory remission.
Discussion
This real‐world cohort study assessed the drug survival, effectiveness, safety, IFX drug and drug-antibodies serum concentrations relating to the transition of patients from IV to SC infliximab. Ten IBD patients (9.34%) had to discontinue SC IFX treatment. Patients who discontinued SC treatment for local adverse events successfully switched back to IV IFX therapy. In patients who continued SC IFX treatment, clinical and biochemical disease activity remained stable compared to baseline. The rate of immunogenicity and secondary loss of response to IV infliximab is variable, with an estimated risk of 10% to 20% per patient-year, that is comparable to our discontinuation rate [6]. In the Liverpool multicentre study, 92.3% patients were still on IFX SC treatment 12 months after switching from IV regime. There were no analysed variables found to be significantly associated with persistence [4]. In the multicentre prospective study with different IV regimes switching to SC formulation, the rates of maintaining clinical remission after 6 months were high for patients switching from IFX IV 10 mg/ kg Q8W (38/ 41 [93%]) or 10 mg/ kg Q6W (15/ 18 [83%]), although only 5/ 15 (33%) patients switching from 10 mg/ kg Q4W had not relapsed at this timepoint [7]. Of the 22 patients who relapsed, 15 received an off-label IFX SC escalation to 240 mg Q2W, leading to recapture of clinical remission in 14 (93%) patients [7]. In the prospective cohort study, comparing SC and IV IFX formulation, the SC IFX group showed higher 1-year durable remission that the IV IFX group (N = 31 of 38, 82% vs. N = 11 of 23, 48%; P = 0.013) [8].
We observed significant increase of IFX serum levels at the follow up visits after switching to SC treatment. We have also demonstrated the neutralization of anti-drug antibodies in 87.5% of IBD patients after switching to SC formulation. Notably, no additional patient developed anti-infliximab antibodies throughout the study. This is consistent with a prospective multicentre Spanish study, Korean prospective study, and multicentre Liverpool study, that have also demonstrated improved pharmacokinetic profiles after switching to IFX SC in different IBD patient populations and IV IFX regimes [4,8,9]. In the CTP-13 SC 1.6 pilot study, there was a significantly lower incidence of drug-neutralizing antibodies in patients with SC administered IFX [3]. The Liverpool study found that 8% patients developed de novo anti-drug antibodies within 12 months after switching to IFX SC [4]. In the REMSWITCH study, none of the patients developed de novo anti-infliximab antibodies throughout the study, while they observed the disappearance of high-level anti-drug antibodies in 1 patient shortly after switching to SC formulation [7].
During the follow-up, we did not observe significant changes in mean HBI score in CD patients and pMayo score in UC patients, but we found significant drop of mean faecal calprotectin, from 58.4 ± 73.6 at the baseline to 20.68 ± 32.7 at week 56, P = 0.0103. In a single centre Spanish study, clinical Mayo scores significantly reduced from baseline to 24 weeks, while Harvey-Bradshaw Index (HBI) and clinical remission rates were maintained [10]. Faecal calprotectin levels reduced from baseline to 24 weeks, while CRP levels and erythrocyte sedimentation rate were maintained [10]. A post hoc analysis of a pilot study reported a statistically significant improvement in CDAI in CD patients (P = 0.04) and pMayo score in UC patients (P = 0.025) after switching from IV to SC form of IFX [11]. They also observed a significant decrease in faecal calprotectin after switching to SC IFX [11].
Considering safety, we have demonstrated a good safety profile for SC infliximab, with no major adverse side effects over 56 weeks of follow-up. Main adverse event reported by was administration-related reaction, localized injection-site reaction. Real-world studies have reported that switching to IFX SC was well tolerated and rates of IFX SC discontinuation due to adverse events were low [4,7,9,10]. In the Liverpool multicentre study, 2 patients switched back to IFX IV following localized skin rashes [4]. In REMSWITCH, 2 patients requested a switch back to IFX IV due to intolerance [7].
The main study limitations were the relatively short follow-up (56 weeks), the relatively small sample size for analysing subgroups, the low event number to perform the multivariable analysis and the lack of endoscopic assessment.
Conclusion
In summary, the evidence suggests that switching to IFX SC results in low risk of relapse in IBD patients, including those on an intensified IV regime. Evidence from real-world clinical practice as well as our study has demonstrated the clinical benefits of SC IFX after switching to IV IFX in terms of pharmacokinetics, clinical and biological response. SC IFX formulation is well tolerated, with no new safety signals reported beyond the well-characterized safety profile for IFX IV identified over decades of use. The data suggest that SC IFX if a safe and effective formulation that meets the definition of bio-better.
Zdroje
1. Zhang YZ, Li YY. Inflammatory bowel disease: pathogenesis. World J Gastroenterol 2014; 20(1): 91–99. doi: 10.3748/ wjg.v20.i1.91.
2. Stoner KL, Harder H, Fallowfield LJ et al. Intravenous versus Subcutaneous Drug Administration. Which Do Patients Prefer? A Systematic Review. Patient 201. doi: 10.1007/ s40271-014-0075-y.
3. Schreiber S, Ben-Horin S, Leszczyszyn J et al. Randomized Controlled Trial: Subcutaneous vs Intravenous Infliximab CT-P13 Maintenance in Inflammatory Bowel Disease. Gastroenterology 2021; 160(7): 2340–2353. doi: 10.1053/ j.gastro.2021.02.068.
4. Smith PJ, Critchley L, Storey D et al. Efficacy and Safety of Elective Switching from Intravenous to Subcutaneous Infliximab [CT-P13]: A Multicentre Cohort Study. J Crohns Colitis 2022; 16(9): 1436–1446. doi: 10.1093/ ecco-jcc/ jjac053.
5. Lamb CA, Kennedy NA, Raine T et al. IBD guidelines eDelphi consensus group. British Society of Gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults. Gut 2019; 68(3): s1–s106. doi: 10.1136/ gutjnl-2019-318483.
6. Qiu Y, Chen BL, Mao R et al. Systematic review with meta-analysis: loss of response and requirement of anti-TNFa dose intensification in Crohn’s disease. J Gastroenterol 2017; 52(5): 535–554. doi: 10.1007/ s00535-017-1324-3.
7. Buisson A, Nachury M, Reymond M et al. Effectiveness of Switching From Intravenous to Subcutaneous Infliximab in Patients With Inflammatory Bowel Diseases: the REMSWITCH Study. Clin Gastroenterol Hepatol 2023; 21(9): 2338–2346. doi: 10.1016/ j.cgh.2022.08.011.
8. Hong SN, Song JH, Kim SJ et al. One-Year Clinical Outcomes of Subcutaneous Infliximab Maintenance Therapy Compared With Intravenous Infliximab Maintenance Therapy in Patients With Inflammatory Bowel Disease: A Prospective Cohort Study. Inflamm Bowel Dis 2023: izad094. doi: 10.1093/ ibd/ izad094.
9. Huguet JM, García-Lorenzo V, Martí L et al. Subcutaneous infliximab [CT-P13], a true biologic 2.0. Real clinical practice multicentre study. Biomedicines 2022; 10(9): 2130. doi: 10.3390/ biomedicines10092130.
10. Argüelles-Arias F, Fernández Álvarez P, Castro Laria L et al. Switch to subcutaneous infliximab during the SARS-CoV-2 pandemic: pre-liminary results. Rev Esp Enferm Dig 2022; 114(2): 118–119. doi: 10. 17235/ reed.2021.8320/ 20212.
11. Smith PJ, Fumery M, Leong RW et al. Real-world experience with subcutaneous infliximab: broadening treatment strategies for inflammatory bowel disease. Expert Rev Clin Immunol 2023; 19(9): 1143–1156. doi: 10.1080/ 174 4666X.2023.2231148.
ORCID authors
A. Gojdičová 0000-0003-1124-4628,
A. Krónerová 0009-0008-2786-2870,
T. Hlavatý 0000-0003-2555-9081.
Submitted/ Doručené: 10. 1. 2024
Accepted/ Prijaté: 15. 1. 2024Anna Gojdičová, MD, PhD
Gastroenterology centre Bezručova
Bezručova 2531/ 5
811 09 Bratislava
krajann@hotmail.comŠtítky
Detská gastroenterológia Gastroenterológia a hepatológia Chirurgia všeobecná
Článok vyšiel v časopiseGastroenterologie a hepatologie
Najčítanejšie tento týždeň
2024 Číslo 1- Metamizol jako analgetikum první volby: kdy, pro koho, jak a proč?
- Kombinace metamizol/paracetamol v léčbě pooperační bolesti u zákroků v rámci jednodenní chirurgie
- Antidepresivní efekt kombinovaného analgetika tramadolu s paracetamolem
- Parazitičtí červi v terapii Crohnovy choroby a dalších zánětlivých autoimunitních onemocnění
- Fixní kombinace paracetamol/kodein nabízí synergické analgetické účinky
-
Všetky články tohto čísla
- Editorial
- Heuréka!
- Jaké bude vaše doporučení pro terapii těžké ulcerózní kolitidy?
- Doporučení Pracovní skupiny pro idiopatické střevní záněty pro diagnostiku Crohnovy choroby
- Snížená hladina magnezia v moči po ileokolické resekci u pacientů s Crohnovou chorobou
- Mirikizumab – první protilátka anti-IL12 p19 v terapii ulcerózní kolitidy
- Efficacy and safety of switching from intravenous to subcutaneous CTP-13 treatment in IBD patients – a one-year retrospective study from large Slovak IBD centre
- Metotrexát – znovuobjevený lék u Crohnovy nemoci
- Hidradenitis suppurativa, intestinal microbiome and SIBO – a comprehensive overview of the issue
- Idiopatický hypereozinofilní syndrom s postižením zažívacího traktu
- Simultánní robotická resekce kolorektálního karcinomu a jaterní metastázy – naše první zkušenosti
- Výběry z mezinárodních časopisů
- Hoffmanová I. Abdominální sonografie žlučníku a žlučových cest
- Kreditovaný autodidaktický test: IBD
- Gastroenterologie a hepatologie
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- Metotrexát – znovuobjevený lék u Crohnovy nemoci
- Idiopatický hypereozinofilní syndrom s postižením zažívacího traktu
- Mirikizumab – první protilátka anti-IL12 p19 v terapii ulcerózní kolitidy
- Doporučení Pracovní skupiny pro idiopatické střevní záněty pro diagnostiku Crohnovy choroby
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy




