Tau Protein and Anti-Tau Antibodies in Patients with Multiple Sclerosis
Tau protein a anti-tau protilátky u pacientů s roztroušenou sklerózou
Cíl:
Poškození neuronů u roztroušené sklerózy (RS) může být doprovázeno uvolňováním cytoskeletálního tau proteinu, který interaguje s narušenými složkami imunitního systému za vzniku autoprotilátek. Koncentrace tau proteinu mohou být ovlivněny přítomností protilátek proti tau proteinu v séru a mozkomíšním moku (MMM).
Metody:
Tau protein i protilátky proti tau proteinu jsme stanovovali pomocí metod ELISA u 31 pacientů (14 pacientů s RS a 17 kontrolních neurologických pacientů). Vypočítali jsme tři poměry tau proteinu vztahující se ke koncentraci a) anti-tau protilátek v MMM, b) anti-tau protilátek v séru a c) intratékálně produkovaných anti-tau protilátek.
Výsledky:
Neprokázali jsme statisticky významné korelace mezi koncentracemi tau proteinu a protilátek proti tau proteinu v séru, v MMM ani anti-tau protilátek intratékálně produkovaných. Koncentrace tau proteinu v MMM ani žádný z vypočtených poměrů se nelišily mezi skupinou pacientů s RS a kontrolních pacientů.
Závěr:
Uvolňování tau proteinu do MMM a protilátková odpověď proti tau proteinu u RS jsou zřejmě na sobě nezávislé procesy, podobně jako u jiných neurologických pacientů.
Klíčová slova:
tau protein – autoprotilátky – mozkomíšní mok – roztroušená skleróz
Authors:
L. Fialová 1; A. Bartošihash2 2,3 1,4
Authors place of work:
Charles University in Prague, First Faculty of Medicine, Institute of Medical Biochemistry, Prague
1; Charles University in Prague, Third Faculty of Medicine, University Hospital Královské Vinohrady, Department of Neurology, Prague
2; Prague Psychiatric Center
3; Institute of Clinical Biochemistry and Laboratory Diagnostics, General University Hospital, Prague
4
Published in the journal:
Cesk Slov Neurol N 2012; 75/108(3): 310-313
Category:
Původní práce
Summary
Background:
Neuronal damage in multiple sclerosis (MS) may be associated with the release of cytoskeletal tau protein. This antigen interacts with dysregulated immune system and this results in anti-tau antibody synthesis. Tau-protein concentrations and anti-tau antibody levels in cerebrospinal fluid (CSF) and in serum may correlate.
Methods:
Total tau protein and anti-tau antibodies measured using ELISA methods were studied in 31 patients (14 MS patients and 17 neurological controls). We calculated three ratios: the CSF tau concentration to a) the CSF anti-tau antibody level, b) the serum anti-tau antibody concentration and c) the level of intrathecal anti-tau antibodies.
Results:
We did not find any significant correlation between the tau protein concentrations and anti-tau antibody levels (serum, CSF or intrathecal) in the MS group or in the controls. There was no difference in the CSF tau concentrations or any of the calculated ratios between MS patients and controls.
Conclusions:
The release of tau protein into CSF and anti-tau humoral response are independent processes in MS and do not differentiate the MS patients from other neurological patients.
Key words:
tau protein – autoantibodies – cerebrospinal fluid – multiple sclerosis
Introduction
The tau protein is a small low-molecular-weight protein abundantly present in the central nervous system. It is expressed predominantly in neurons but may also be found in glial cells [1,2].
The role of the tau protein has been intensively explored in various clinical conditions and it is considered to be a neurochemical marker of axonal degeneration or damage [3–9]. An increase in CSF total tau concentration has been reported in patients with the Alzheimer disease (AD), head trauma, acute stroke, encephalitis or neurosyphilis [3–6,10,11]. Multiple sclerosis (MS) is another disease were CSF tau protein elevations have repeatedly been observed [7,12–14]. However, other studies reported comparable CSF tau levels in MS patients and their controls [15–18]. The conflicting results were attributed to heterogeneity of MS and inter-individual variations.
We hypothesized that the anti-tau protein antibodies may contribute to these inconsistent results. We have previously investigated anti-tau antibodies in MS patients and observed the presence of anti-tau antibodies both in the CSF and serum. Compared to control neurological patients, MS patients had significantly elevated intrathecally synthesized antibodies against tau protein. Moreover, the CSF antibodies in MS patients were characterized by higher avidity [19]. It is known that an immune reaction is a complex process and the production of autoantibodies is influenced by a range of different factors. We were interested to ascertain whether the intensity of autoantibody response might be dependent on the tau antigen load. A relationship between antibody synthesis and antigen load has been established. Benner et al [20] found that both antigen and T-cell deprivation decreased the number of plasma blasts and plasma cells. Similarly, another study showed that low but persistent HIV antigen expression correlated with broad HIV-1 neutralizing antibody activity [21]. B cells maturation involving Ig class switching and somatic hypermutation represent complex processes [22]. The affinity of an antibody changes during this process. Humoral response is driven towards the most efficient combination of a concentration and affinity by a negative feedback interaction between these two variables under constant antigen challenge [23]. Therefore, it is difficult to expect a simple correlation between the amount of extracellularly released tau molecules and synthesis of specific antibodies against them. Therefore, we aimed to examine free anti-tau antibodies (not bound in immune complexes) concentration only in the CSF and serum in a group of MS patients and a group of patients with other non-immunological neurological diseases. Simultaneously, total tau protein levels were determined in the CSF of these patients. We were interested to establish whether there is an association between CSF tau protein levels and free antibodies against the tau antigen.
Patients and methods
Subjects
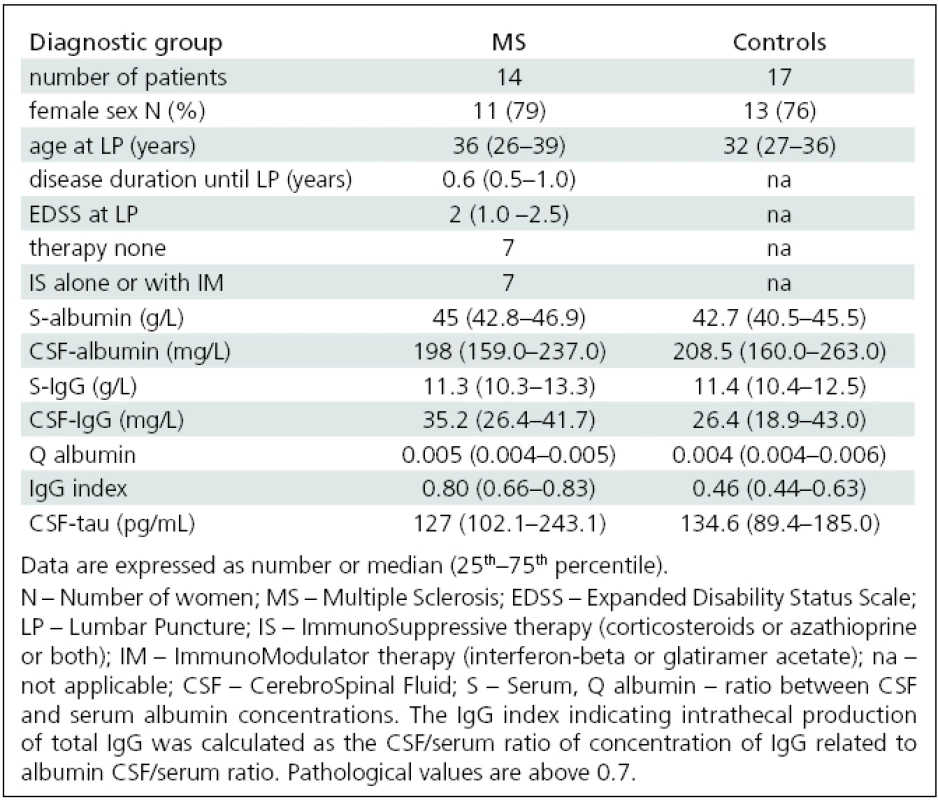
The study included paired CSF and serum samples obtained from 31 patients including 14 MS patients [clinically isolated syndrome (n = 4), relapsing-remitting form of MS (n = 9; relapse phase n = 5), secondary progressive (n = 1)] and 17 controls recruited from patients with neurological diseases (9× cervicocranial syndrome, 2× facial palsy, 3× aseptic neuroinfection, 1× epilepsy, 1× operation of inguinal hernia, 1× cervicobrachial syndrome). The diagnosis and the course of MS were determined at the time of lumbar puncture using established criteria [24–26]. All MS patients had disability score rated according to the Expanded Disability Status Scale (EDSS) [27]. The subject clinical data are presented in detail in Tab. 1.
All patients provided a written informed consent regarding study participation. The Ethics Committee of the Third Faculty of Medicine, Charles University, Prague approved the study.
Specimens were stored at –20 °C until analysed but no longer than 12 month, and they were thawed only once.
Methods
CSF and serum IgG anti-tau antibodies were analysed using ELISA techniques newly developed from our prior experience [28,29]. Bovine tau protein (Cytoskeleton, USA) was used as the antigen for coating microplate wells. Before the analysis, the sera were diluted 1 : 400, CSFs were analysed undiluted. The same pool of human sera was used as a standard in all the analytical series for comparative purposes. The absorbances were transformed into arbitrary concentration units (AU) using a standard curve constructed from the pool of diluted human sera by geometrical series.
The serum and cerebrospinal fluid concentrations of albumin and total IgG were assayed using immunonephelometry.
Intrathecal (IT) synthesis of total IgG was calculated as IgG index according to the following formula: [(CSF IgG/serum IgG)/(CSF albumin/serum albumin)]. Intrathecal synthesis of specific anti-tau IgG antibodies was estimated in a similar way using the following formula: [(CSF anti-tau/serum anti-tau) / (CSF albumin//serum albumin)]. Compared to CSF antibodies, intrathecal synthesis is calculated as the proportion of the total CSF antibodies produced locally within the CNS compartment.
Tau protein concentrations in CSF were determined by commercial sandwich ELISA Innotest hTAU Antigen kit (Innogenetics GmbH, Ghent, Belgium). The assay was carried out according to the manufacturer’s instructions.
Since the data were not normally distributed, non-parametric statistical tests were applied. Differences among groups were analysed with the Mann-Whitney U test. The Spearman’s coefficient was used for correlation analyses. The significance level for all tests was p <0.05. Statistical analysis was performed using Statistica 9 software (StatSoft, Inc. Tulsa, OK, USA).
Results
The basic clinical and laboratory variables of MS patients and controls are shown in Tab. 1. There was no difference either in age and sex or biochemical variables between MS patients and controls with the exception of significantly higher IgG indices in the MS group (p <0.0001).
We could not demonstrate any correlation between the CSF tau protein and CSF, intrathecally synthesized or serum anti-tau antibodies in the MS group and the control. No relationship was found even when all subjects (MS group and neurological controls) were analysed together.
Three tau antigen to autoantibodies ratios were calculated – CSF tau/CSF anti--tau antibodies, CSF tau/serum anti-tau antibodies and CSF tau/IT anti-tau antibodies. We did not observe differences in any of these ratios between the MS group and controls.
No relationship was found between tau protein or CSF tau/anti-tau antibody ratios and patient age in either group. CSF tau protein levels or ratios did not correlate with disease duration or patient disability in the MS group.
A significant correlation between CSF-tau and CSF IgG was observed in the control group (r = 0.56; p <0.05) but not in the MS group. The relationship between CSF-tau and CSF IgG held true when we analyzed all subjects together (r = 0.53; p <0.05).
The levels of CSF tau protein did not differ between the MS group and control subjects (Fig. 1). We did not find any differences between the MS group and controls in serum anti-tau antibodies [median (25th–75th percentile); MS group 3,054 (2,545–5,407) AU vs controls 2,624 (1,204–4,189) AU n.s.)] and CSF [MS group 3.2 (2.0–7.4) AU vs controls 2.1 (0.9–3.3) AU n.s.)].

Discussion
We were first to investigate the relationship between CSF total tau protein levels and the CSF or serum anti-tau antibodies in MS patients. We observed that the CSF tau load is independent of the free anti-tau antibodies both in MS patients and in neurological controls.
Our result is in a good agreement with a study on patients with Alzheimer disease. Rosenmann et al [30] also failed to find any difference in the anti-tau antibody levels between groups with low and high tau levels. It may be hypothesized that the autoimmune reaction against tau protein may have similar pattern in MS and AD patients as well as in other neurological patients used as controls. These findings suggest that there is no direct relationship between tau and free anti-tau antibodies in MS and AD patients. The anti-tau antibodies may form immune complexes with the tau protein molecules in the CSF or serum. It can be presumed that the tau protein bound in the circulating immune complex has an altered immunoreactivity because their epitopes are occupied by autoantibody binding sites. Moreover, anti-intracellular antigen antibodies may enter into the cells by receptor-mediated endocytosis [31] or by other ways. Additionally, clearance of tau-anti-tau immune complexes by macrophages might influence the CSF tau protein levels.
Brettschneider et al [14] reported that tau tended to increase in MS patients with higher intrathecal total IgG synthesis. A positive relationship between CSF tau protein and IgG index in MS patients has already been described [7]. We did not find a positive correlation between tau protein and intrathecally produced total IgG. However, a relationship with total CSF IgG was demonstrated in the control group as well as in the group of MS patients and neurological controls together. There also was a tendency to positive correlation in the MS group. These findings suggest that the association between the tau protein and IgG in the CSF is not only characteristic for multiple sclerosis but may exist in various neurological diseases. In addition, in our previous study we reported a positive correlation between total IgG in CSF with anti-tau antibodies in CSF [32]. In the context of these observations, it is surprising that no correlation between specific anti-tau antibodies and the tau protein was found. This discrepancy may partly be due to the formation of immune complexes.
Unlike some other studies, we did not observe significant elevation of the CSF tau protein in our MS patients [15,16]. The inconsistent results regarding CSF tau protein concentrations in MS studies [12–14] cannot be explained with the influence of anti-tau antibodies. Our patients had mild disability and short disease duration. Other reasons for different findings include heterogeneity of MS and various underlying processes.
In conclusion, we did not prove the relationship between tau and free anti-tau antibodies in MS patients or in neurological controls. CSF concentration of the tau protein was independent of anti-tau antibody CSF level and was not different between MS patients and patients with other neurological diseases.
The study was supported by the grant No. 10369-3 from the Czech Ministry of Health.
Acknowledgements
We wish to thank Dr. Benakova for albumin and total IgG level measurements in all our specimens and Dr. Soukupova for her help with tau protein analysis.
Assoc. Prof. Aleš Bartoš, MD, PhD
Charles University in Prague
Third Faculty of Medicine
University Hospital Královské Vinohrady
Department of Neurology
Šrobárova 50
100 34 Prague 10
e-mail: bartos@pcp.lf3.cuni.cz
Accepted for review: 1. 8. 2011
Accepted for print: 12. 1. 2012
Zdroje
1. LoPresti P, Szuchet S, Papasozomenos SC, Zinkowski RP, Binder LI. Functional implications for the microtubule-associated protein tau: localization in oligodendrocytes. Proc Natl Acad Sci U S A 1995; 92(22): 10369–10373.
2. Binder LI, Frankfurter A, Rebhun LI. The distribution of tau in the mammalian central nervous system. J Cell Biol 1985; 101(4): 1371–1378.
3. Paraskevas GP, Kapaki E, Liappas I, Theotoka I, Mamali I, Zournas C et al. The diagnostic value of cerebrospinal fluid tau protein in dementing and nondementing neuropsychiatric disorders. J Geriatr Psychiatry Neurol 2005; 18(3): 163–173.
4. Paraskevas GP, Kapaki E, Kararizou E, Mitsonis C, Sfagos C, Vassilopoulos D. Cerebrospinal fluid tau protein is increased in neurosyphilis: a discrimination from syphilis without nervous system involvement? Sex Transm Dis 2007; 34(4): 220–223.
5. Hesse C, Rosengren L, Andreasen N, Davidsson P, Vanderstichele H, Vanmechelen E et al. Transient increase in total tau but not phospho-tau in human cerebrospinal fluid after acute stroke. Neurosci Lett 2001; 297(3): 187–190.
6. Franz G, Beer R, Kampfl A, Engelhardt K, Schmutzhard E, Ulmer H et al. Amyloid beta 1–42 and tau in cerebrospinal fluid after severe traumatic brain injury. Neurology 2003; 60(9): 1457–1461.
7. Bartosik-Psujek H, Archelos JJ. Tau protein and 14-3-3 are elevated in the cerebrospinal fluid of patients with multiple sclerosis and correlate with intrathecal synthesis of IgG. J Neurol 2004, 251(4): 414–420.
8. Bitsch A, Horn C, Kemmling Y, Seipelt M, Hellenbrand U, Stiefel M et al. Serum tau protein level as a marker of axonal damage in acute ischemic stroke. Eur Neurol 2002; 47(1): 45–51.
9. Blennow K, Hampel H, Weiner M, Zetterberg H. Cerebrospinal fluid and plasma biomarkers in Alzheimer disease. Nat Rev Neurol 2010; 6(3): 131–144.
10. Sussmuth SD, Reiber H, Tumani H. Tau protein in cerebrospinal fluid (CSF): a blood-CSF barrier related evaluation in patients with various neurological diseases. Neurosci Lett 2001; 300(2): 95–98.
11. Hampel H, Blennow K, Shaw LM, Hoessler YC, Zetterberg H, Trojanowski JQ. Total and phosphorylated tau protein as biological markers of Alzheimer‘s disease. Exp Gerontol; 45(1): 30–40.
12. Terzi M, Birinci A, Cetinkaya E, Onar MK. Cerebrospinal fluid total tau protein levels in patients with multiple sclerosis. Acta neurologica Scandinavica 2007; 115(5): 325–330.
13. Kapaki E, Paraskevas GP, Michalopoulou M, Kilidireas K. Increased cerebrospinal fluid tau protein in multiple sclerosis. Eur Neurol 2000; 43(4): 228–232.
14. Brettschneider J, Maier M, Arda S, Claus A, Sussmuth SD, Kassubek J et al. Tau protein level in cerebrospinal fluid is increased in patients with early multiple sclerosis. Mult Scler 2005; 11(3): 261–265.
15. Hein Nee Maier K, Kohler A, Diem R, Sattler MB, Demmer I, Lange P et al. Biological markers for axonal degeneration in CSF and blood of patients with the first event indicative for multiple sclerosis. Neurosci Lett 2008; 436(1): 72–76.
16. Guimaraes I, Cardoso MI, Sá MJ. Tau protein seems not to be a useful routine clinical marker of axonal damage in multiple sclerosis. Mult Scler 2006; 12(3): 354–356.
17. Jimenez-Jimenez FJ, Zurdo JM, Hernanz A, Medina-Acebron S, de Bustos F, Barcenilla B et al. Tau protein concentrations in cerebrospinal fluid of patients with multiple sclerosis. Acta Neurol Scand 2002; 106(6): 351–354.
18. Valis M, Talab R, Stourac P, Andrys C, Masopust J. Tau protein, phosphorylated tau protein and beta-amyloid42 in the cerebrospinal fluid of multiple sclerosis patients. Neuro Endocrinol Lett 2008; 29(6): 971–976.
19. Fialová L, Bartos A, Svarcová J, Malbohan I. Increased intrathecal high-avidity anti-tau antibodies in patients with multiple sclerosis. PLoS One 2011; 6(11): e27476.
20. Benner R, van Oudenaren A, Haaijman JJ, Slingerland-Teunissen J, Wostmann BS, Hijmans W. Regulation of the „spontaneous‘ (background) immunoglobulin synthesis. Int Arch Allergy Appl Immunol 1981; 66(4): 404–415.
21. Sajadi MM, Guan Y, DeVico AL, Seaman MS, Hossain M, Lewis GK. Correlation between circulating HIV-1 RNA and broad HIV-1 neutralizing antibody activity. J Acquir Immune Defic Syndr 2011; 57(1): 9–15.
22. MacLennan IC. Germinal centers. Annu Rev Immunol 1994; 12: 117–139.
23. Jackola DR, Liebeler CL, Lin CY, Chiu YK, Blumenthal MN, Rosenberg A. Evidence that negative feedback between antibody concentration and affinity regulates humoral response consolidation to a non-infectious antigen in infants. Mol Immunol 2005; 42(1): 19–30.
24. Lublin FD, Reingold SC. Defining the clinical course of multiple sclerosis: results of an international survey. National Multiple Sclerosis Society (USA) Advisory Committee on Clinical Trials of New Agents in Multiple Sclerosis. Neurology 1996; 46(4): 907–911.
25. Poser CM, Paty DW, Scheinberg L, McDonald WI, Davis FA, Ebers GC et al. New diagnostic criteria for multiple sclerosis: guidelines for research protocols. Ann Neurol 1983; 13(3): 227–231.
26. McDonald WI, Compston A, Edan G, Goodkin D, Hartung HP, Lublin FD et al. Recommended diagnostic criteria for multiple sclerosis: guidelines from the International Panel on the diagnosis of multiple sclerosis. Ann Neurol 2001; 50(1): 121–127.
27. Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology 1983; 33(11): 1444–1452.
28. Bartos A, Fialová L, Soukupová J, Kukal J, Malbohan I, Pitha J. Antibodies against light neurofilaments in multiple sclerosis patients. Acta Neurol Scand 2007; 116(2): 100–107.
29. Bartos A, Fialová L, Soukupová J, Kukal J, Malbohan I, Piťha J. Elevated intrathecal antibodies against the medium neurofilament subunit in multiple sclerosis. J Neurol 2007; 254(1): 20–25.
30. Rosenmann H, Meiner Z, Geylis V, Abramsky O, Steinitz M. Detection of circulating antibodies against tau protein in its unphosphorylated and in its neurofibrillary tangles-related phosphorylated state in Alzheimer‘s disease and healthy subjects. Neurosci Lett 2006; 410(2): 90–93.
31. Mohamed HA, Mosier DR, Zou LL, Siklos L, Alexianu ME, Engelhardt JI et al. Immunoglobulin Fc gamma receptor promotes immunoglobulin uptake, immunoglobulin-mediated calcium increase, and neurotransmitter release in motor neurons. J Neurosci Res 2002; 69(1): 110–116.
32. Bartos A, Fialova L, Svarcova J, Cechova L, Dolezil D, Malbohan IM. Serum and cerebrospinal fluid antibodies against tau protein in multiple sclerosis. Eur J Neurol 2009; 16 (Suppl 3): 251.
Štítky
Detská neurológia Neurochirurgia NeurológiaČlánok vyšiel v časopise
Česká a slovenská neurologie a neurochirurgie

2012 Číslo 3
- Metamizol jako analgetikum první volby: kdy, pro koho, jak a proč?
- Kombinace metamizol/paracetamol v léčbě pooperační bolesti u zákroků v rámci jednodenní chirurgie
- Kombinace kodein/paracetamol prokázala stejný analgetický účinek jako hydrokodon/paracetamol
- 100 let s metamizolem: jaké je jeho současné postavení v léčbě bolesti
Najčítanejšie v tomto čísle
- Neurosyphilis
- Surgical Treatment of a Tarsal Tunnel Syndrome
- Bilateral Phrenic Nerve Lesion Manifesting as an Orthopnea – Three Case Reports
- Diagnosis and Treatment Options for Niemann-Pick Disease Type C
