-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
Correction of Myopia and Myopic Astigmatism by Implantation of a Phakic Posterior Chamber Implantable Collamer Lens
Authors: T. Řeháková; V. Veliká; P. Rozsíval; N. Jirásková
Authors place of work: Oční klinika Fakultní nemocnice Hradec Králové, Sokolská 581, Hradec Králové, 500 05 (přednostka: prof. MUDr. Naďa Jirásková Ph. D., FEBO)
Published in the journal: Čes. a slov. Oftal., 74, 2018, No. 4, p. 147-152
Category: Původní práce
doi: https://doi.org/10.31348/2018/1/4-4-2018Summary
Aim:
We analysed two years results in patients with correction of moderate-to-high myopia or myopic astigmatism by implantation of posterior chamber phakic intraocular Impantable Collamer Lens (ICL).
Methods:
To the retrospective study were included 63 eyes of 32 patients (3 men, 29 women), who underwent implantation of ICL between 2007 – 2016 in the outpatient Department of Ophthalmology clinic, University Hospital in Hradec Králové, to correction moderate-to-high myopia or myopic astigmatism. We assess uncorrected visual acuity (UCVA) and best spectacle-corrected visual acuity (BSCVA), subjective refraction, intraocular pressure (IOP), endothelial cell density (ECD) and the incidence of complications in the two years follow-up period.
Results:
At the time of implantation was the mean patient‘s age 28,14 ± 5,35 years (range 21 to 36 years). In 16 eyes was implanted ICM V4 model, in 23 eyes VICMO, in 2 eyes TICM V4, in 13 eyes VTICMO and in 9 eyes VTICM. The mean preoperative subjective refraction improved from -7,8 ± 2,7 D sf (range -14 to -3,25 D sf) and -0,65 ± 0,8 D cyl (range -3,25 to 0 D cyl) to -0,01 ± 0,1 D sf (range -0,75 to 0 D sf) and -0,05 ± 0,16 D cyl (range -0,55 až 0 D cyl). The mean ECD value was 3270,5 ± 454,7 cells/mm2 (range 2155 to 4201 cells/mm2) compared to 2803,4 ± 441,8 cells/mm2 (range 2079 to 4184 cells/mm2) at the end of our follow-up period. The percentage loss of ECD two years after the surgery was 13,5%. The mean preoperative value of intraocular pressure (IOP) was 15,0 ± 2,9 mmHg (range 10 to 20 mmHg) and 15,2 ± 2,5 mmHg (range 10 to 20 mmHg) at the end of follow-up period. We observed the elevation of IOP over 21 mmHg 1 month postoperatively in 11 eyes. In 9 eyes was IOP until 35 mmHg (27,7 ± 3,74, range 22 to 34 mmHg), in a one case (both eyes of the same patient) over 50 mmHg. Postoperatively we observed following complications: IOP elevation (11 eyes), decentration of ICL with development of subcapsular opacity in the crystallin lens periphery (1 eye), pigment dispersion without elevation of IOP (1 eye) and incidence of optical phenomenon (4 patients). The explantation of ICL was indicated in one patient bilaterally.
Conclusions:
According to our experience is the correction of moderate-to-high myopia or myopic astigmatism by implanation of ICL an effective, relative safe and predictable method. It has an important place in the modern refractive surgery, especially in patients, who are not able to undergo the photorefractive corneal surgery to correct their refractive errors.
Key words:
Implantable Collamer Lens, phakic intraocular lens, myopia, myopic astigmatism
INTRODUCTION
Refractive errors are among the most common ocular problems worldwide. Their presence influences several aspects of human life, not only the physical and mental health of individuals but also their social and economic status, and the costs of health insurance companies for the necessary care. Rose et al. examined the quality of life of patients with various degrees of myopia and keratoconus. The study demonstrates that a high degree of myopia and the associated dependence on correction represents a significant limiting factor for individuals in their everyday activities. Patients stated a negative influence on their psychological well-being upon wearing strong glasses correction, augmented by the cosmetic effect thereof, as well as a large economic burden upon procuring corrective aids and the connected high degree of social isolation (22).
Surgical options for the correction of myopia in young patients with preserved accommodation in first place incorporate photorefractive procedures on the cornea, either by the surface method of photorefractive keratectomy (PRK) and laser subepithelial keratomileusis (LASEK), or intrastromal procedures such as laser in situ keratomileusis (LASIK), LASIK with the assistance of femtosecond laser (FS-LASIK) or the method of small-incision lenticule extraction (ReLEx Smile). If laser treatment of the cornea is contraindicated, most often due to insufficient corneal thickness in combination with a higher degree of refractive error, myopia can be corrected while simultaneously preserving accommodation by the implantation of a phakic intraocular lens (P IOL) (18).
At present the most frequently implanted P IOLs include the Visian® Implantable Collamer® Lens (VICLTM, STAAR Surgical AG, Nidau, Switzerland). This is a single-piece P IOL produced from copolymer (Collamer®, a compound of collagen and hydroxyethylmethacrylate), which is highly biocompatible, flexible and highly permeable for gases and metabolites. It is supplemented with a filter for ultraviolet radiation (UV), which is capable of absorbing up to 90% of UV rays with a wavelength smaller than 387 nm. The phakic ICL is designated for implantation into the sulcus of the posterior chamber. In order to respect the individual variability of the anatomical dimensions of the eye, it is available in a number of lengths (for the latest model of VICLTM four lengths within the range of 12.1 to 13.7 mm). The design of the convex-concave optical part (for VICLTM diameter of 4.8-5.8 mm) and the plate haptic prevents contact with other intraocular structures, in particular with the anterior surface of the lens, above which it creates a space termed a vault, and the posterior surface of the iris (9, 23, 26).
The Implantable Collamer Lens (ICL) was first introduced onto the market in 1993 (model IC2020), and on the basis of experiences with its implantation the design of the ICL has undergone several modifications over time (for example in the thickness and size of the ICL itself, its configuration and height of the vault). The latest model of the VICLTM, implanted since 2011, has a KS AquaPORT® opening in the centre of the optical part with a size of 0.36 mm (Visian ICL CentraFLOW® technology). This design modification with CentraFLOW® technology improves the dynamics of flow of the chamber fluid). It is now not so essential to perform laser iridotomy or basal iridectomy in order to prevent postoperative increase of intraocular pressure (IOP), the metabolism of the lens is disturbed to a lesser degree, and perioperative removal of visco-elastic material has also been made easier (9, 23, 26).
With the aid of ICL it is possible to correct myopia (within the range of -0.5 to -20.0D), and simultaneously also myopic astigmatism up to +6.0 D (26).
The aim of this study is to evaluate the 2-year results in patients in whom correction of medium and high myopia or myopic astigmatism was resolved by means of implantation of an ICL phakic posterior chamber lens.
MATERIAL AND METHOD
We included in our retrospective study a total of 63 eyes of 32 patients (3 men, 29 women), in whom an ICL had been implanted at the Department of Ophthalmology at the University Hospital of Hradec Králové in the period of 2007-2016, with the aim of correcting medium and high myopia or myopic astigmatism.
The entry criteria for inclusion of the patients was fulfilment of the indication conditions set by the manufacturer (STAAR Surgical AG, Nidau, Switzerland), i.e. age of 21-45 years, anterior chamber depth (ACD) of at least 2.8 mm (measured from endothelium to anterior surface of lens), endothelial cell density (ECD) above 2000 cells/mm2, stable refractive error for at least 2 years.
Patients with other ocular pathologies (corneal pathology with low or abnormal ECD, intraocular hypertension syndrome, open or closed angle glaucoma, uveitis, cataract, retinal pathology) were excluded from the cohort, as well as patients with serious systemic pathologies (diabetes mellitus, autoimmune disorders), patients who had undergone any refractive laser procedure on the cornea in the past, and finally patients who did not attend regular postoperative follow-up examinations.
Before the procedure all patients underwent a complex eye examination, which incorporated anamnesis, determination of uncorrected (UCVA) and best corrected visual acuity (BCVA) on LCD optotype (NIDEK CP-90), manifest and cycloplegic refraction (autorefractometer, NIDEK AR 31OA), value of intraocular pressure (IOP), noncontact tonometry (NIDEK NT-530), corneal topography (Pentacam, Oculus Inc.), biometry (IOLMaster, Carl Zeiss Meditec AG), endothelometry (noncontact endothelial microscope CEM-530, Nidek, Japan), contrast sensitivity (CSV-1000, Vectorvision) and assessment of the ocular finding on the anterior and posterior segment of the eye on a slit lamp. Calculation of the dioptric strength of the ICL and selection of a toric variant was performed with the aid of an online calculator from the STAAR Surgical company. The length of the ICL was selected according to the measured values of two parameters, the horizontal dimension of white-to-white according to IOL-Master and ACD according to Pentacam.
The operation was performed by two surgeons (P.R. and J.U.) under local anaesthesia, using the standard procedure recommended by the manufacturer, making a 3 mm temporal incision and two service paracentheses. In the case of ICL models without CentraFLOWTM technology, i.e. in a total of 27 eyes (16 eyes model ICM V4, 2 eyes model TICM V4 and 9 eyes model VTICM), one basal iridectomy was performed perioperatively, usually at no. 12, as prevention of the development of a pupillary block or acute closure of the chamber angle. Up to the year 2015, rotation of the TICL to the correct axis was performed according to a diagram, and since that year we have used the Verion navigation system at our centre in order to ensure maximum precision of the position of the implanted IOL.
In 31 patients implantation of the ICL was performed bilaterally, the second eye was always operated on the following day. In the case of one patient only unilateral implantation was performed. In the second eye we chose a photorefractive procedure on the cornea, specifically the FS-LASIK method, with regard to sufficient corneal thickness with the given degree of refractive error. Postoperatively the patients applied a combined preparation of antibiotics and corticosteroid in the form of drops 5x per day for one week, and subsequently 3x per day for 14 days. Regular postoperative follow-up examinations were performed 1 day, 1 week, 1, 3 and 6 months after surgery, and subsequently at yearly intervals. The observed parameters were subjective refraction, UCVA, BCVA, values of IOP, ECD, incidence of complications during surgery and in the observation period of 2 years.
The measured values were statistically processed by the program SYSTAT 8.0. In order to determine the significance of the differences between the individual measurements over time, a non-parametric Wilcox pair test was used, in which the majority of parameters did not have normal distribution. The results were evaluated on a level of significance of 0.05.
RESULTS
The age of the patients at the time of implantation was on average 28.14 ± 5.35 years (within the range of 21 to 36 years).
Upon correction of myopia, an ICM V4 model was implanted in 16 eyes, a VICMO model in 23 eyes, and in the case of myopic astigmatism a TICM V4 model in 2 eyes, a VTICMO model in 13 eyes and a VTICM model in 9 eyes (table 1).
Tab. 1. Representation of implanted ICL models 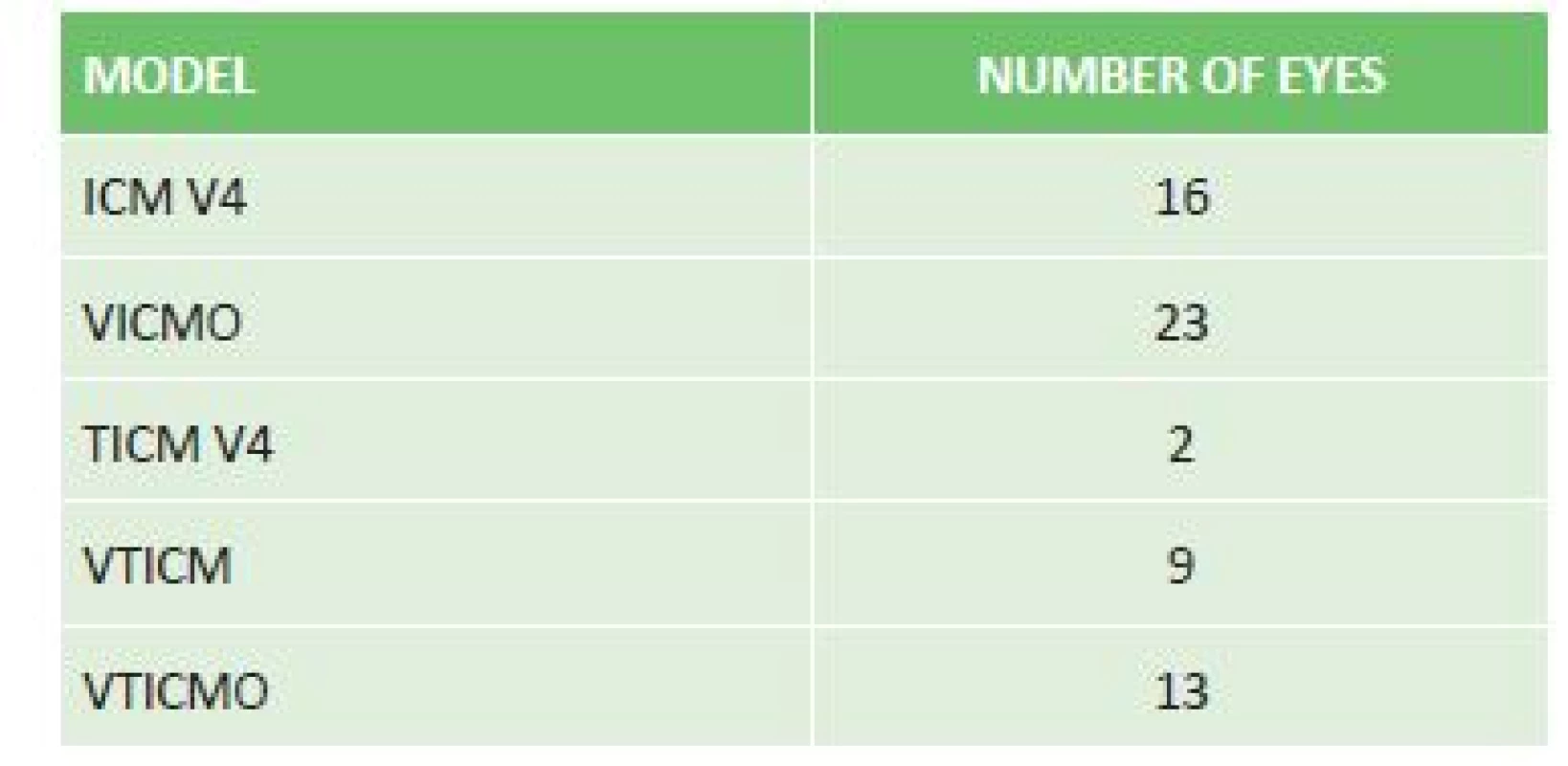
The average preoperative value of subjective refraction was -7.8 ± 2.7 D sf (within the range of -14 to -3.25 D sf) and -0.65 ± 0.8 D cyl (within the range of -3.25 to 0 D cyl), at the end of the observation period after 2 years it had been reduced to -0.01 ± 0.1 D sf (within the range of -0.75 to 0 D sf) and -0.05 ± 0.16 D cyl (within the range of -0.55 to 0 D cyl). The values of subjective refraction from all the postoperative follow-up examinations are illustrated in graph 1. Average UCVA (decimal values) improved from preoperative values of 0.04 ± 0.07 (within the range of 0.004 to 0.6) to 1.05 ± 0.2 (within the range of 0.7 to 1.5) 2 years after surgery. The difference between the preoperative and first postoperative values in the 1st month was statistically significant in both observed parameters, and improvement of the parameter remained stable in the further course of observation. In the case of BCVA there was no statistically significant difference between the preoperative and final values. A comparison of UCVA and BCVA before surgery and after 2 years of observation is illustrated in graph 2.
Preoperatively the average ECD value was 3270.5 ± 454.7 cells/mm2 (within the range of 2155 to 4201 cells/mm2), at the end of the observation period it had decreased to 2803.4 ± 441.8 cells/mm2 (within the range of 2079 to 4184 cells/mm2). The percentage loss of ECD 2 years after surgery was 13.5%. This parameter was not statistically evaluated with regard to the use of two instruments for calculation of ECD during the observation (graph 3).
Graph 1. Average values of subjective refraction during the course of postoperative follow-ups. Statistically significant improvement from the first postoperative follow-up in the first month. 
Tab. 2. Average values of uncorrected and best corrected visual acuity during the course of the postoperative follow-ups. Statistically significant improvement of uncorrected visual acuity from the first postoperative follow-up in the first month. BCVA without statistically significant difference during the course of observation. 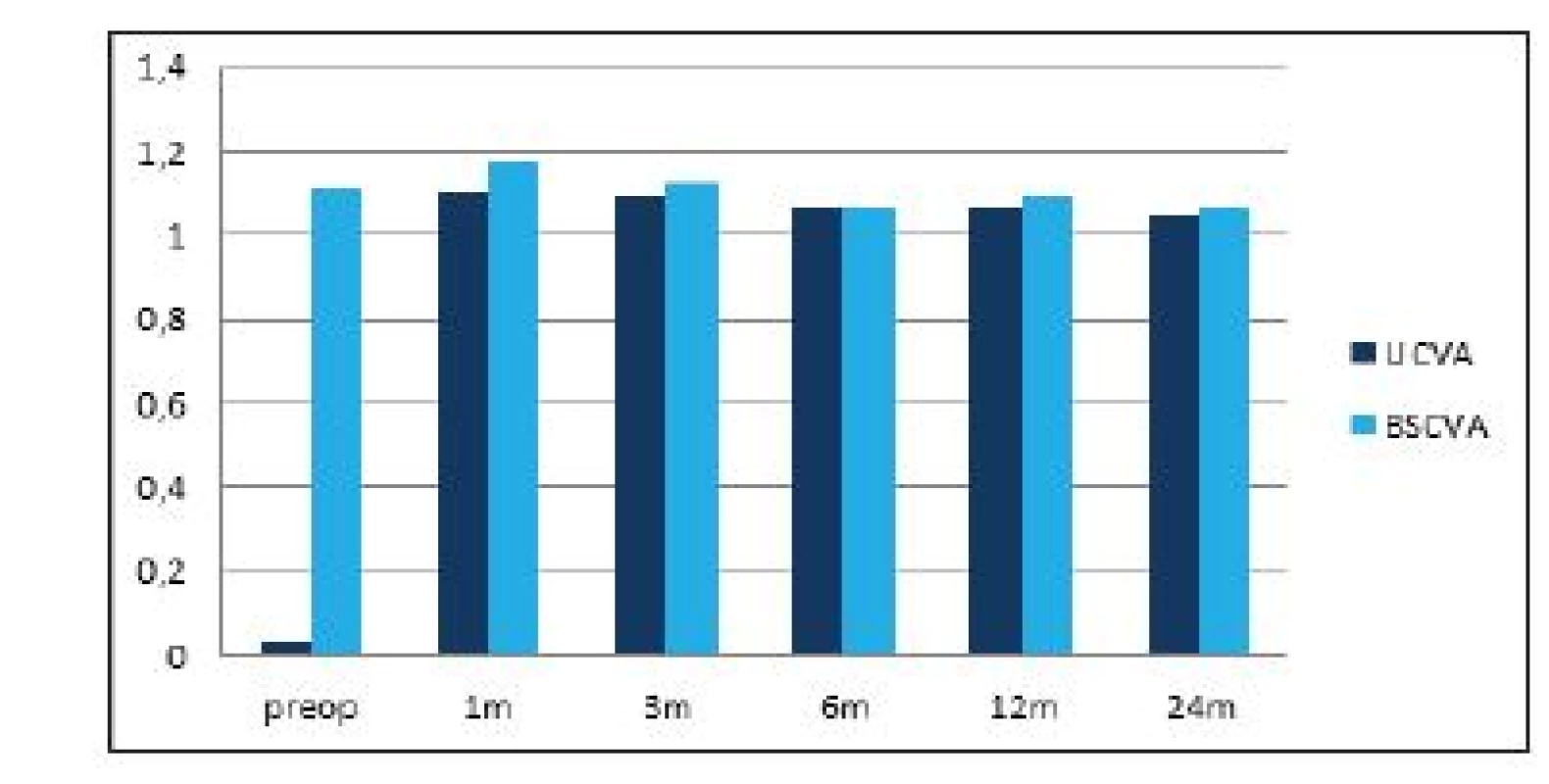
Graph 2. Percentage loss of endothelial cell density was 13.5% two years after surgery. The most pronounced difference was upon comparison of the preoperative and first postoperative values. 
Intraocular pressure (IOP) before surgery was on average 15.0 ± 2.9 mmHg (within the range of 10 to 20 mmHg), 2 years after surgery on average 15.2 ± 2.5 mmHg (within the range of 10 to 20 mmHg). We observed an increase of IOP above 21 mmHg in a total of 11 eyes in the first month after surgery. In 9 eyes the values did not exceed 35 mmHg (27.7 ± 3.74, within the range of 22 to 34 mmHg). In one patient there was an excessive bilateral increase of IOP above 50 mmHg (graph 4).
Perioperatively we recorded a single complication, when an ICM V4 lens began to develop in a reversed position in the anterior chamber, and it was necessary to extend the temporal incision, remove the lens and re-implant it into the correct position, with subsequent suture of the wound. The further course was uncomplicated.
Of postoperative complications we observed elevation of IOP in 11 eyes, decentration of the ICL with occurrence of subcapsular opacity in the periphery of the lens in 1 eye, dispersion of pigment in 1 eye without elevation of IOP, and in 4 patients occurrence of secondary optic phenomena (glare, halo) which did not reduce quality of vision. Explantation was performed in 2 eyes of one patient due to decentration of the ICL and development of acute glaucoma attack with insufficient effect of conservative and surgical solution of the condition (table 2).
Graph 3. Average preoperative and postoperative values of intraocular pressure with statistically significant elevation in first month after surgery. 
Tab. 3. Incidence and type of postoperative complications 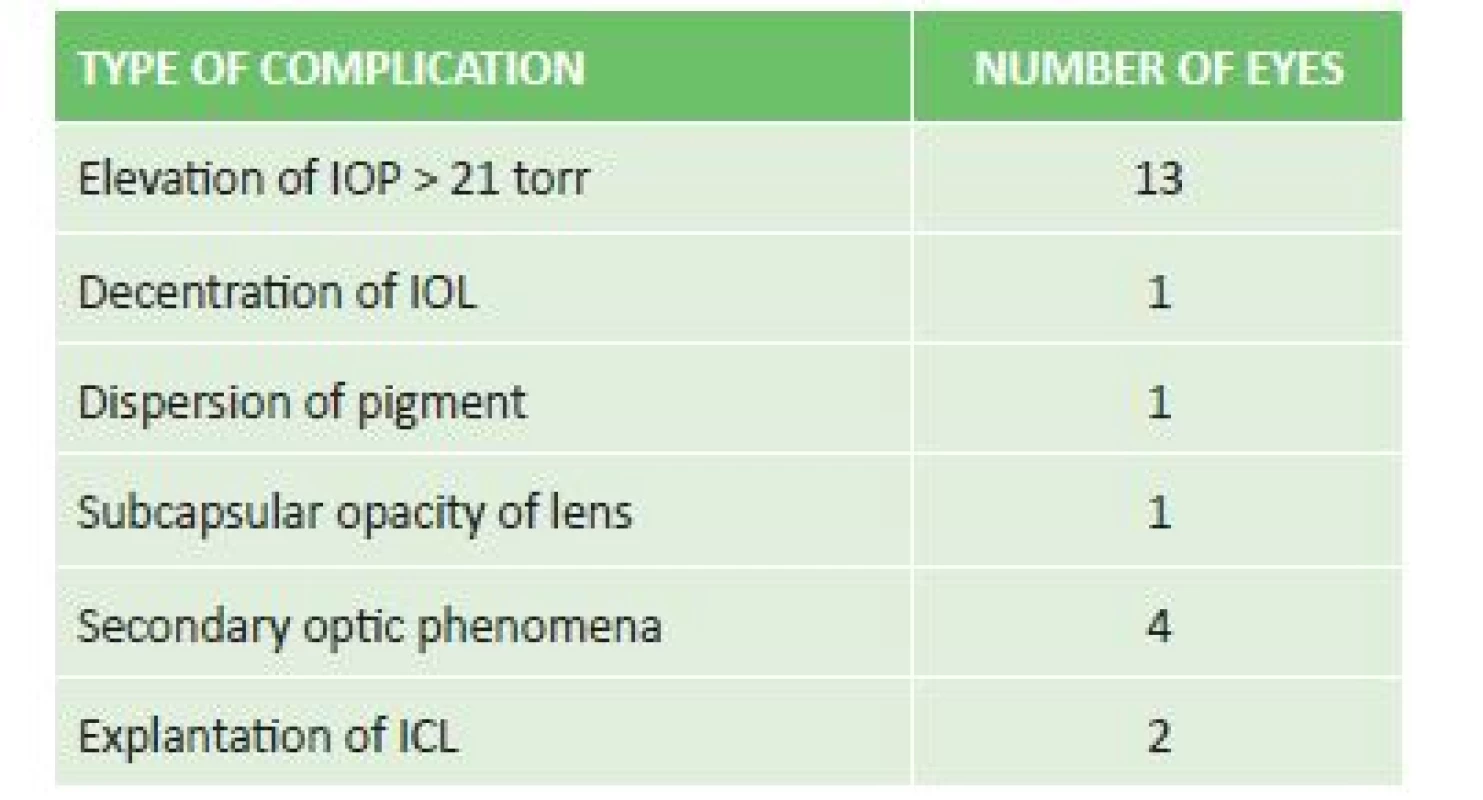
DISCUSSION
The main aim of modern refractive surgery is an improvement in the quality of life of patients with a refractive error connected to a high degree of dependence on glasses correction or contact lenses. With the arrival of the excimer laser more than 25 years ago, photorefractive procedures on the cornea became far more precise with regard to the predictability of the postoperative refractive result, as well as safer in comparison with the previously used technique of radial keratectomy and ketatomileusis. Despite the fact that it is possible to resolve a large number of refractive errors by means of modification of the shape of the cornea, for a section of patients this procedure is not suitable. Thanks to new and better-quality materials of P IOL, visco-elastic material, surgical micro-techniques and possibilities of instrument display, at the end of the 1980s and beginning of the 1990s the idea of correcting higher refractive errors by means of implantation of a P IOL reappeared (5, 12).
At our centre, in the case of correction of myopia the first choice is laser treatment on the cornea, upon fulfilment of the indication criteria and sufficient corneal thickness, with regard to the degree of the rectified refractive error with the goal of emmetropia. If, on the basis of a preoperative examination, it is not possible to perform laser correction, then in the case of suitable ocular conditions our choice is implantation of a P IOL or posterior chamber ICL. The effectiveness, relative safety and good predictability of the refractive results has already been published several times both for the V4 model and for the newer V4c with KS AquaPORT® and toric variants of the ICL (4, 14, 15, 20).
The advantage of the use of an ICL is above all the solution of high myopia. However, like every surgical procedure, implantation of an ICL also has its potential risks. The incidence of the most common complications in connection with the implantation of an ICL is described in a meta-analysis (cohort of 2592 eyes) by Fernandes et al.: development of anterior subcapsular opacities to cataract in 136 eyes (5.2%), there is also a relatively frequent early postoperative increase in IOL, and the occurrence of a pupillary block is one of the most feared complications (3, 19, 25), which has been described also in the case of the V4c with KS AquaPORT®(9). Further complications are pigment dispersion syndrome with the risk of transition to secondary pigment glaucoma, loss of ECD (9.9% after 2 years) and the general risks of surgical intraocular procedures such as endophthalmitis, haemorrhage and vitreoretinal complications. The necessity to perform explantation of the ICL is stated in 1.62% of cases, most often due to development of cataract and elevation of IOP (11). Al Sabaani et al., in a cohort of 787 eyes, state explantation in a total of 30 eyes (3.8%). The most frequently stated cause is selection of incorrect size of ICL (22 eyes), followed by cataract (4 eyes), high residual astigmatism (2 eyes), retinal detachment (1 eyes) and occurrence of intolerable glare (1 eye) (1).
In our cohort we observed an elevation of IOP in a total of 11 eyes at a postoperative follow-up examination 1 month after surgery, in which the difference was statistically significant (p=0.002). In 9 eyes of 6 patients with elevation of IOP up to 35 mmHg (model V4c in 8 cases, model V4b in one case), we explain the increase with reference to the effect of local corticosteroids in combination with preoperative values around the upper limit of the norm (up to 21 mmHg), the average values in this group of patients were initially 17.22 ± 3.74 mmHg (within the range of 10-20 mmHg). In these cases we compensated IOP by administering temporary general (acetazolamide 250 mg tbl) and local antiglaucomatous therapy (dorzolamide 20.0. mg and timolol 5.0 mg). Surgical intervention was not required for compensation of IOP, nevertheless in two patients local antiglaucomatous therapy is being applied long-term (in one patient there is a positive family history of glaucoma). Increase of IOP in the 1st month up to 30 mmHg (model V4c) with a good response to conservative therapy has also been published by other authors (6, 12, 21).
We observed an excessive increase in IOP above 50 mmHg soon after surgery in one patient bilaterally (V4b). In the right eye (RE) the elevation of IOP was conditioned by the decentration of the ICL upward, with shallowing of the interior chamber in its lower half. The condition was initially addressed by the application of antiglaucomatous therapy, supplemented with laser iridotomy and repositioning of the lens. Subsequently IOP in RE was compensated, but spontaneous decentration of the ICL in RE reoccurred, with with onset of subcapsular opacity of the lens in the periphery, while simultaneously an acute glaucoma attack developed in the left eye (LE), with inadequate response to treatment. Bilateral explantation of the ICL was subsequently performed on the patient. After pacification of the finding in both eyes, partial correction of the refractive error using the LASIK method was performed upon the patient's wishes, 6 months after explantation. At the last follow-up examination (7 years after surgery), UCVA in RE was 0.4, with correction -1.5 sf = -1.0 cyl 1.0 and LE 0.4, with correction -1.5 sf = -0.75 cyl 1.2, and the IOP values were within the norm (RE 13 mmHg, LE 11 mmHg). The aforementioned postoperative course was conditioned by the implantation of the incorrect size of ICL. On the basis of the preoperative examination we selected the correct size, nevertheless upon the insistence of the consultant from the manufacturer of the intraocular lens who was present at our centre during the first operations, an ICL of larger dimensions was implanted. Soon after the procedure, excessive ventral buckling occurred due to the influence of the inappropriate length of the ICL, with the development of acute pupillary block not responding to treatment, with the necessity of bilateral explantation of the lenses. A similar postoperative course upon implantation of a larger ICL is also stated by McCaughey et alc. (19).
We recorded subcapsular opacity in the periphery of the lens in 1 eye, which was conditioned by decentration of the ICL upward. The occurrence of cataract is described generally in the literature as a later complication following the procedure, which is caused by a lowering vault. Sanders et al. (24) state a frequency of 6-7% after 7 years, and Kocová et al. (17) published a report stating incidence of cataract with a decrease in visual acuity at 16.1% over an average observation period of 10.5 years. Dispersion of pigment appeared in one eye, and was not complicated by an increase in IOP.
At the last follow-up examination, the reduction of ECD was 13.5% in our cohort. However, the data were not statistically evaluated due to the fact that part of the measurements was performed on a more modern noncontact endothelial microscope, with different software for calculation of ECD. The obtained data nevertheless indicate the most pronounced reduction in comparison with the averages of preoperative and first postoperative values in the 3rd month, specifically by 10.25%. In the further observation period the decrease was slight, upon an observation of the averages of ECD in the 3rd and 24th month 3.6%, and the lowest measured value at the end of the observation period was 2079 cells/mm2. Other studies with a two-year observation period state a lower degree of reduction, i.e. 9.9% (7) and 6.57% (11).
We evaluated subjective satisfaction with an inquiry into the incidence of complaints and the quality of vision. The patients were informed within the framework of the preoperative examination regarding the possible postoperative incidence of secondary optic phenomena (glare, halo). In the postoperative period we did not actively determine these phenomena, with the aim of minimising the perception of these subjective complaints, which cannot be therapeutically influenced. In our cohort their presence was spontaneously stated by 4 patients (in all cases V4c), who nevertheless negated a reduction of quality of vision and stated overall satisfaction with the procedure. In the literature the authors mention the incidence of optic phenomena especially in the form of a comparison of the frequency in the case of the V4c with KS AquaPORT®, and V4b. With the arrival of the V4c model, there were initial concerns that the central opening would cause more pronounced perception of optic phenomena in comparison with the V4b. Bhandaria et al. described optic phenomena in 23% of patients with V4b and 25% with V4c (2), and further studies comparing the quality of vision in these two models do not state a statistically significant difference (10, 13, 16).
CONCLUSION
On the basis of our experience, correction of medium and high myopia and myopic astigmatism by the method of implantation of an ICL is effective, relatively safe, and with a predictable refractive result. The fundamental prerequisite for attaining good postoperative results is a thorough and comprehensive preoperative examination, respect for the indication criteria, designation of the correct size of ICL and regular, long-term postoperative observation. In modern refractive surgery this method is therefore appropriate in particular for patients who are unable to undergo correction of a refractive error by means of laser procedure on the cornea for whatever reason. In this group of patients, correction by means of an ICL is practically the only option for solution (on the precondition of preserving accommodation), and provides a substantial improvement in quality of life.
The authors of the study declare that no conflict of interest exists in the compilation, theme and subsequent publication of this professional communication, and that it is not supported by any pharmaceuticals company.
Received: 20. 2. 2018
Accepted: 9. 8. 2018
Available on-line: 18. 3 2019
MUDr. Tereza Řeháková
Oční klinika FN Hradec Králové
Sokolská 581
500 05 Hradec Králové
Zdroje
1. AlSabaani, NA., Behrens, A., Jastanieah, S. et al.: Causes of Phakic Implantable Collamer Lens Explantation/Exchange at King Khaled Eye Specialist Hospital. Middle east Afr J Ophthalmol, 23 (4); 2016 : 293-295.
2. Bhandari, V., Karandikar, S., Reddy, JK. et al.: Implantable collamer lens V4b and V4c for correction of high myopia. J Curr Ophthalmol, 27 (3-4); 2015 : 76-81.
3. Bylsma, SS., Zalta, AH., Foley, E. et al.: Phakic posterior chamber intraocular lens pupillary block. J Cataract Refract Surg, 28 (12); 2002 : 2222-2228.
4. Cao, X., Wu, W., Wang, Y. et al.: Posterior chamber collagen copolymer phakic intraocular lens with central hole for moderate-to-high myopie: First experience in China. Medicine (Baltimore), 95 (36); 2016: e4641.
5. Chaudhry, IA., El Danasoury, MA.: Phakic intraocular lenses. Saudi J Ophathalmol, 24 (4); 2013 : 231-233.
6. Eissa, SA., Sadek, AH., El-Deeb, MW.: Anterior chamber angle evaluation following phakic posterior chamber collamer lens with CentraFLOW and its correlation with ICL vault and intraocular pressure. J Ophthalmol, 2016 : 1383289.
7. Fernandes, P., González-Méijome, JM., Madrid-Costa, D. et al.: Implantable collamer posterior chamber intraocular lenses: a review of potentional complications. J Refract Surg, 27 (10); 2011 : 765-776.
8. Grover, IG., Senthil, S., Murthy, S. et al.: A rare case of pupillary block glaucoma following CentraFLOW implantable collamer lens surgery. J Glaucoma, 26 (8); 2017 : 694-696.
9. Güell, JL., Morral, M., Kook, D. et al.: Phakic intraocular lenses part 1: historical overview, current models, selection kriteria and surgical techniques. J Cataract Refract Surg, 36 (11); 2010 : 1976-93.
10. Huseynova, T., Ozaki, S., Ishizuka, T. et al.: Comparative study of 2 types of implantable collamer lenses, 1 with and 1 without a central arteficial hole. Am J Ophthalmol, 157 (6); 2014 : 1136-43.
11. Jiménez-Alfaro, I., Gomez-Telleria, G., Bueno, JL. et al: Contrast sensitivity after posterior chamber phakic intraocular lens implantation for high myopia. J Refract Surg, 17 (6); 2001 : 641-5.
12. Ju, Y., Gao, XW., Ren, B. et al.: Posterior chamber phakic intraocular lens implantation for high myopia. Int J of Ophathalmol, 6 (6); 2013: 831-835.
13. Kamiya, K., Shimizu, K., Ando, W. et al.: Comparison of vault after implantation of posterior chamber phakic intraocular lens with and without a central hole. J Cataract Refract Surg, 41 (1); 2015 : 67-72.
14. Kamiya, K., Shimizu, K., Igarashi, A. et al.: Posterior chamber phakic intraocular lens implantation: comparative, multicentre study in 351 eyes with low-to-moderate or high myopia. Br J of Ophthalmol, 102 (2); 2018 : 177-181.
15. Kamiya, K., Shimizu, K., Kobashi, H. et al.: Three-Year Follow-Up of Posterior Chamber Toric Phakic Intraocular Lens Implantation for Moderate to High Myopic Astigmatism. PloS One, 8 (2); 2013: e56453.
16. Karandikar, S., Bhandari, V., Reddy, J.: Outcomes of implantable collamer lens V4 and V4c for correction of high myopia - a case series. Nepal J Ophthalmol, 7 (14); 2015 : 164-72.
17. Kocová, H., Vlková E., Michalcová, L. et al.: Incidence of cataract following implantation of a posterior - chamber phakic lens ICL (Implantable Collamer Lens) – long -term results. Cesk a Slov Oftalmol, 73 (3); 2017 : 87-93.
18. Krohnen, T., Strengen, A., Klaproth, OK. et al.: Basic Knowledge of Refractive Surgery. Dtsch Arzteblt Int, 105 (9); 2008 : 163-172.
19. McCaughey, MV., Mifflin, T., Fenzl, CR. et al.: Pseudophacomorphic glaucoma along with pupillary block after Visian Implantable Collamer Lens Implantation for High Myopia. Open J Ophthalmol, 4 (4); 2014 : 107-111.
20. Pjano, MA., Pidro, A., Biscevic, A. et al.: Refractive Outcomes of Chamber Phakic Intraocular Lens Implantation for Correction of Myopia and Myopic Astigmatism. Medical Archives, 71 (2); 2017 : 93-93.
21. Repplinger, B., Kohnen, T.: Intraocular pressure after implantation of an ICL with aquaport: Development of intraocular pressure after implantation of an ICL (model V4c) with aquaport without iridotomy. Ophthalmologe, 115 (1 ; 2018 : 29-33.
22. Rose, K., Harper, R., Tromans, C. et al.: Quality of life in myopia. Br J Ophathalmol, 84 (9); 2000 : 1031-4.
23. Rozsíval P. a kol.: Trendy soudobé oftalmologie, svazek 5, Galén 2008, str. 105
24. Sanders, DR.: Anterior subcapsular opacities and cataracts 5 years after surgery in the visian implantable collamer lens FDA trial. J Refract Surg, 24 (6); 2008 : 566-70.
25. Smallman, DS., Probst, L., Rafuse, PE. et al.: Pupillary block glaucoma secondary to posterior chamber phakic intraocular lens implantation for high myopia. J Cataract Refract Surg, 30 (4); 2004 : 905-907.
26. STAAR Surgical Company: Visian ICL Patient Information Booklet 2017 [Internet]. Dostupné z: https://staar.com/file/Patient-Information-Booklet-MKT-0125-Rev-2-9.7.17.pdf
Štítky
Oftalmológia
Článok vyšiel v časopiseČeská a slovenská oftalmologie
Najčítanejšie tento týždeň
2018 Číslo 4- Cyklosporin A v léčbě suchého oka − systematický přehled a metaanalýza
- Dlouhodobé výsledky lokální léčby cyklosporinem A u těžkého syndromu suchého oka s 10letou dobou sledování
- Účinnost a bezpečnost 0,1% kationtové emulze cyklosporinu A v léčbě těžkého syndromu suchého oka − multicentrická randomizovaná studie
- Pomocné látky v roztoku latanoprostu bez konzervačních látek vyvolávají zánětlivou odpověď a cytotoxicitu u imortalizovaných lidských HCE-2 epitelových buněk rohovky
- Konzervační látka polyquaternium-1 zvyšuje cytotoxicitu a zánět spojený s NF-kappaB u epitelových buněk lidské rohovky
-
Všetky články tohto čísla
- Diagnosis of Familial Hypercholesterolaemia on First Sight? The Role of the Ophthalmologist in Identifying Patients with Familial Hypercholesterolaemia
- Vertical Strabismus – Indication of Surgical Techniques on the Inferior Rectus Muscle
- Keratopigmentation (Corneal Tattoo) – Our First Experience
- Correction of Myopia and Myopic Astigmatism by Implantation of a Phakic Posterior Chamber Implantable Collamer Lens
- Digital Eye Strain in a Population of Young Subjects
- EFFECT OF MULTIPLE VARIABLES ON THE REFRACTIVE ERROR AFTER CATARACT SURGERY
- Česká a slovenská oftalmologie
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- Vertical Strabismus – Indication of Surgical Techniques on the Inferior Rectus Muscle
- Keratopigmentation (Corneal Tattoo) – Our First Experience
- Diagnosis of Familial Hypercholesterolaemia on First Sight? The Role of the Ophthalmologist in Identifying Patients with Familial Hypercholesterolaemia
- Correction of Myopia and Myopic Astigmatism by Implantation of a Phakic Posterior Chamber Implantable Collamer Lens
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



