-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
Seborrheic keratoses and severe hypoinsulinemic hypoglycemia associated with insulin grow factor 2 secretion by a malignant solitary fibrous tumor
A rare sign of some malignant tumors is a sudden eruption of multiple seborrheic keratoses called Leser-Trélat sign. Overproduction of insulin-like growth factor-2 (IGF2) or its precursor is the main mechanism related to non-islet cell tumor hypoglycemia. Doege-Potter syndrome is the name given to paraneoplastic hypoinsulinemic hypoglycemia in presence of a solitary fibrous tumor. This report describes a case of a patient with hypoinsulinemic hypoglycemia and Leser-Trélat sign associated with a malignant solitary fibrous tumor with IGF2 secretion. Both conditions have improved after tumor excision.
Keywords:
Insulin-like growth factor 2, Hypoglycemia, Tumor, Seborrheic keratosis
Authors: Andreia Latanza Gomes Mathez; Debora Moroto; Sergio Atala Dib; Joao Roberto De Sa *
Published in the journal: Diabetol Metab Syndr (2016), 8:33
Category: Short report
doi: https://doi.org/10.1186/s13098-016-0148-2© Mathez et al. 2016
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
The electronic version of this article is the complete one and can be found online at: http://dmsjournal.biomedcentral.com/articles/10.1186/s13098-016-0148-2Summary
A rare sign of some malignant tumors is a sudden eruption of multiple seborrheic keratoses called Leser-Trélat sign. Overproduction of insulin-like growth factor-2 (IGF2) or its precursor is the main mechanism related to non-islet cell tumor hypoglycemia. Doege-Potter syndrome is the name given to paraneoplastic hypoinsulinemic hypoglycemia in presence of a solitary fibrous tumor. This report describes a case of a patient with hypoinsulinemic hypoglycemia and Leser-Trélat sign associated with a malignant solitary fibrous tumor with IGF2 secretion. Both conditions have improved after tumor excision.
Keywords:
Insulin-like growth factor 2, Hypoglycemia, Tumor, Seborrheic keratosisBackground
Cutaneous morphology can be modified by endocrine and metabolic diseases and skin lesions might serve as a window to the early diagnosis and treatment of various hormone-secreting tumors [1]. An example is a sudden eruption of multiple seborrheic keratosis called as Leser-Trélat sign and it is more associated with adenocarcinomas although it may be related to other tumors indicating worse prognosis [2].
Different tumors can cause hypoglycemia. Among tumoral etiologies the insulinoma is the most common cause [3]. Nevertheless, other tumors unrelated to β cell may cause hypoinsulinemic hypoglycemia by producing insulin growth factor-2 (IGF2) or its precursor named as big-IGF2 [3, 4, 5, 6, 7]. Doege-Potter syndrome is a paraneoplastic hypoglycemia caused by solitary fibrous tumor [8, 9]. This rare neoplasm can secrete an IGF2 precursor that is a pro-IGF2 or a big-IGF2 [9, 10].
This case report describes a non-diabetic 81-year-old man who presented with Leser-Trélat sign and hypoglycemia secondary to a retroperitoneal malignant solitary fibrous tumor.
Clinical case
An 81-year-old man with primary hypothyroidism complained about symptoms suggestive of hypoglycemia for 6 months, with progressive worsening, and abdominal pain. He reported rapidly progressive appearance of brownish warty lesions throughout his body, one of them already biopsied (seborrheic keratoses).
He showed, as important, in his physical examination, acromegaloid face, brown warty lesions on the face, trunk and upper limbs (Fig. 1a) and a palpable mass in the right flank. Hematologic, renal and liver function, electrolytes evaluation of his blood and sera were within normal range. The work-up to his hypoglycemia etiology is shown in the Table 1.
Fig. 1. Sign of Leser-Trélat. Multiple lesions on the face (<b>a</b>) and back of the patient (<b>b</b>) compatible with seborrheic keratoses 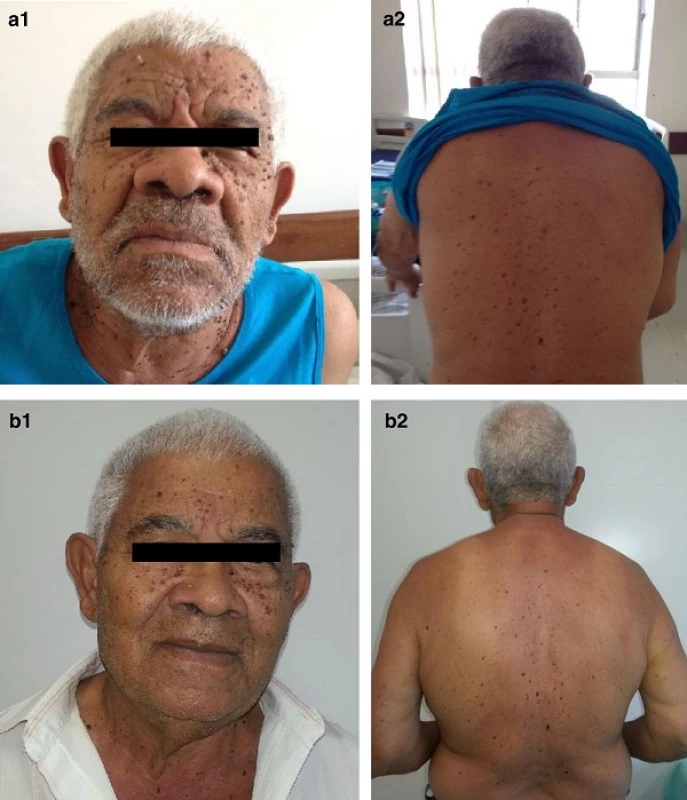
Tab. 1. Laboratory tests confirming non-ketotic hypoinsulinemic hypoglycemia by IGF2 production 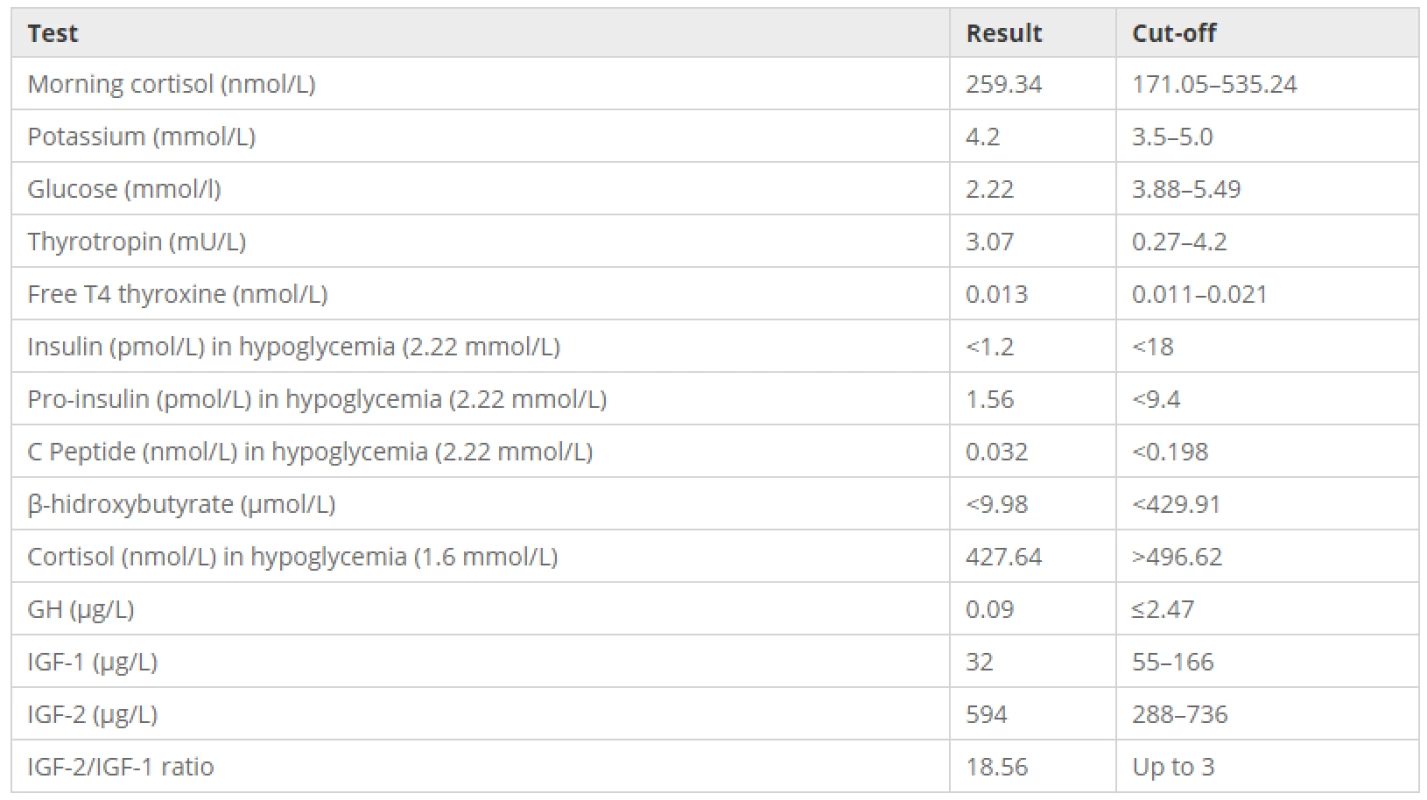
His abdominal magnetic nuclear resonance imaging showed a retroperitoneal expansive mass with 18.6 cm in diameter extremely closed to great abdominal vessels (Fig. 2). Distant metastatic lesions were excluded by tomography screening.
Fig. 2. Solitary fibrous tumor from this patient. <b>a</b> MRI appearance of the mass in the coronal plane shows 18.6 cm in maximal diameter. <b>b</b> MRI T2-weighted image in the sagittal plane demonstrates the retroperitoneal location of the tumor. <b>c</b> contrast-enhanced MRI transverse plane indicates a lobulated tumor with solid and cystic componentes. <b>d</b> Photograph of the dissected specimen weighing 1.5 kg presents two nodular masses measuring 14.3 and 13 cm with irregular shape and connected by a fibroadipose tissue. There were extensive areas of necrosis 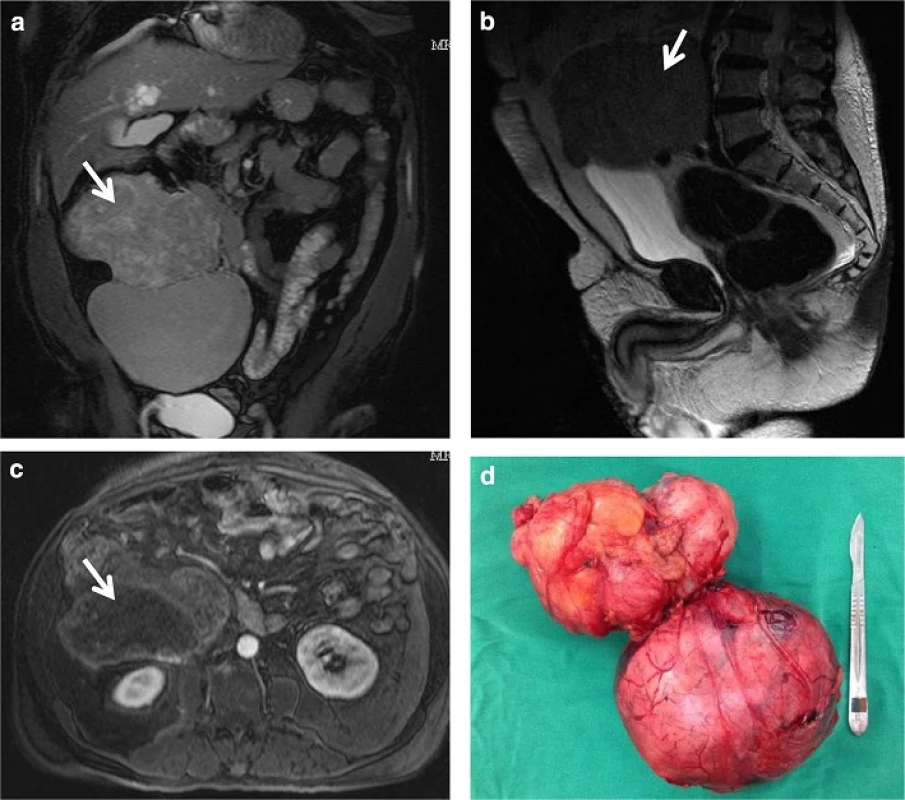
To test the tumor production of IGF2 were collected: growth hormone (GH), IGF1 and IGF2 (radioimmunoassay), confirming the hypothesis (Table 1).
During the 2 months wait period before surgery it was prescribed fractionated diet, oral prednisone (30 mg/day) plus overnight intravenous glucose solution infusion with sporadic episodes of mild hypoglycemia. By the surgery time an encapsulated tumor was resected (Fig. 2). Histopathological and immunohistochemical analysis confirmed malignant solitary fibrous tumor.
The patient showed good health recovery and he was sent to home and to out-patient clinic follow-up without hypoglycemic crises and on oral prednisone treatment that was stopped 6 months later.
By out-patient clinic appointment he referred no hypoglycemic episodes and his acromegaloid features and cutaneous lesions shown an involution (Fig. 1b). He started on local radiotherapy but new tomography screening showed pulmonary nodules suggestive of metastatic disease though he did not present any further hypoglycemia. The patient decided for palliative care and died after 18 months from the diagnosis.
Discussion
Non-islet cell tumor hypoglycemia should be suspected in any patient with Whipple’s triad and hypoinsulinemic hypoglycemia [4], since several diseases, hormone deficiencies and alcohol intake have been excluded [1, 11]. This patient had a palpable tumor and progressive skin lesions favoring tumoral etiology [3, 4, 7, 12].
The presence of seborrheic keratosis contributed to the suspected of neoplastic syndrome in this patient. This rare situation, [13] due to a tumor, is known as the Leser-Trélat sign. The first description comes from 1956 by Hollander [2, 14]. Seborrheic keratosis usually appears as black or brown warty papules, sharply delineated, with 3 mm mainly on the chest and back. It is a sign of senility but the eruptive and abruptly form can result from inflammatory skin diseases, adverse drug reaction or paraneoplastic syndrome [14,15]. Lesions appear mainly on the chest and back, bigger than the usual and the pre-existing lesions can increase in size [12].
It is more frequent (68 %) that the skin condition precedes the diagnosis of the tumor, [12, 13, 16] and it is resulted of the release of tumor growth factors [12]. The most frequent tumors involved are adenocarcinomas, but other tumors have been described [2, 16]. The best treatment is the tumor resection, but the skin response is variable [13]. Although the exactly pathogenesis is uncertain it involves the cytokines produced by the neoplastic cells as IGF1 and epidermal growth factor that act in the surface of epidermal cells [16].
In half of the patients with non-islet cell tumor hypoglycemia, hypoglycemia comes after the tumor has been found [5] and fasting hypoglycemia and neuroglycopenic symptoms are more common. This tumor is most commonly diagnosed in the fifth or sixth decades of life and symptoms can be present for weeks or months before it [1, 3, 4, 6, 7, 12]. Weight loss, palpable mass and pain are the main clinical manifestations. In rare patients there is acromegaloid features [3, 7]. It was first described in 1929 but only in 1988 it was associated with IGF2 production [3, 7, 17, 18].
Usually, 80 % of the circulating mature IGF2 stays inactive bounded to insulin like growth factor binding protein 3 (IGFBP3) and to acid labile subunit [7, 19, 20, 21]. In patients with non-islet cell tumor the pool of big-IGF2 increases and 80 % of this molecule is bounded to IGFBP3 only, forming a lower molecular weight that crosses the endothelial barrier and acts on insulin receptors [4, 7, 20, 22].
Increased glucose uptake, lower insulin and glucagon secretion, decreased lipolysis and liver gluconeogenesis are some effects of the massive secretion of IGF2 that lead to hypoglycemia [3, 5]. Autocrine and paracrine action also promotes tumor growth. The big-IGF2 suppresses GH secretion and it causes synthesis reduction of IGF1, IGFBP2 [1, 3, 18, 20] increasing the bioavailability of IGF2 [1, 3, 4, 6, 20,22].
Laboratorial tests can show normal level of IGF2 but elevated ratio of IGF2/IGF1. A ratio up to three is considered normal; if it is higher than ten, in the presence of non-ketotic hypoglycemia and suppressed GH, it is reported to be virtually pathognomonic of non-islet cell tumor hypoglycemia [7, 9, 11]. Measurement of free IGF2 or its precursors are not widely available but it is known that free IGF2 and the ratio pro-IGF2/IGF1 are increased [1, 3, 4, 9]. The presence of lower levels of GH and IGF1 and a positive response to glucagon-stimulation test can support the diagnosis when the IGF2 assays are unavailable [4].
The tumors are usually large and well differentiated, with epithelial or mesenchymal origin, slow growing, arising in thorax, pelvis, or retroperitoneum [1, 3, 6]. The most common epithelial tumor is hepatocellular carcinoma and the mesenchymal are fibrosarcomas and solitary fibrous tumor [3, 5, 7, 10, 23]. The latter is a rare neoplasm, with a widespread distribution, involving pleura, lungs, pelvic and abdominal organs or retroperitoneum [9, 10, 23]. It affects men and women equally, 50 % of then were asymptomatic and the majority were found as incidentalomas on diagnosis. The most symptomatic tumors have extrathoracic location and mass effects (urinary retention, venous thrombosis and abdominal pain) [23].
Solitary fibrous tumor usually follow a benign clinical course although progress with distant metastasis or local recurrence may arise in some cases of large tumors. Extra thoracic location, paraneoplastic hypoglycemia, positive margins, high cellularity and mitotic activity, necrosis and nuclear pleomorphism are predictors of worse clinical outcome [10, 23].
Hypoglycemia due to a solitary fibrous tumor was first reported by Doege and Potter in 1929. Since then, this finding is called Doege-Potter Syndrome but is very rare (less than 5 % of all solitary fibrous tumors) [8,9, 10]. Among 79 cases of solitary fibrous tumors reported by Jason et al. only two presented hypoglycemia [23].
Regardless of histology, surgical resection is the therapy of choice. Complete surgical resection solves hypoglycemia [3, 7, 9, 24] and reverses those cases of acromegaloid fenotype [4]. When total resection is impossible the debulking technic can be used to ameliorate hypoglycemia or totally solve it in some cases [7]. Adjuvant therapy like tumor embolization, radiotherapy or chemotherapy may bring success in other cases [3, 7].
Short - term measures for avoiding hypoglycemia include corticoid, glucagon, diazoxide and glucose intake. When hypoglycemia persists after surgical intervention, multiple medical modalities have been employed. Although many of these tumors have somatostatin receptors, the use of analogs was not effective in controlling hypoglycemia and somatostatin analogue scintigraphy could not predict treatment response [3].
There are many reports of successful treatment with recombinant growth hormone, although it does not normalize IGF2 levels. Nevertheless, this therapy is expensive, requires high doses, and has many adverse effects [4, 12]. It’s use should be avoided when there are acromegaloid features [4].
The best clinical treatment option is the use of glucocorticoids with immediate effect on hypoglycemia in doses equivalent to 30–60 mg/day of prednisone [3, 4]. Moderate to high doses also could promote tumor reduction [7]. It is the only medical treatment that can suppress tumor production of IGF2, leading to normal levels of C-peptide, insulin and glucose [7, 14].
According to the English literature review, Leser-Trélat sign and hypoinsulinemic hypoglycemia by a solitary fibrous tumor is extremely rare. Whipple’s triad and hypoinsulinemic hypoglycemia, with low levels of GH and IGF1 suggest the diagnosis and the increase of IGF2/IGF1 rate confirms. Some weeks after the surgery the patient showed remission of hypoglycemia without glucocorticoids use and improvement of seborrheic keratosis and acromegaloid features.
Authors’ contributions
All authors were involved in patient care during hospitalization. They have assessed the diagnosis, discussed the clinical management and discussed the exams needed for a conclusive diagnosis. ALGM was the first endocrinologist contacted during patient hospitalization. Besides she conducted the literature review and drafted this manuscript. DM gave the medical support to the patient and also contributed to get the necessary exams. She also collected the medical records for this clinical case report. SAD has been involved in revising this manuscript. JRS coordinated the patient management and directed the steps to confirm the diagnosis. He has been involved in revising this manuscript. All authors followed the patient evolution and approved the final version of this manuscript.
Received: 4 February 2016
Accepted: 6 April 2016
Published: 29 April 2016
*Correspondence:
Joao Roberto de Sa
Endocrinology Division, Escola Paulista de Medicina, Federal University of São Paulo, São Paulo, SP, Brazil
jrsa@uol.com.br
Zdroje
1. Ngonga GF, Ferrari D, Lorusso L, Gasparetto C, Neznama E, D’Abramo M, Ricevuti G. Paraneoplastic syndromes: pathogenetics theories, clinical aspects and therapeutic approach. Ann Ital Med Int. 2005;20(1):28–38.
2. Bártholo RM, Bártholo TP, Florião RA. Leser-Trélat—Um sinal clínico revisitado. Pulmão RJ. 2009;18(1):53–6.
3. Dynkevich Y, Rother KI, Whitford I, et al. Tumors, IGF-2, and hypoglycemia: insights from the clinic, the laboratory, and the historical archive. Endocr Rev. 2013;34(6):798–826.
4. Bodnar TW, Acevedo MJ, Pietropaolo M. management of non-isletcell tumor hypoglycemia: a clinical review. J Clin Endocrinol Metab. 2014;99(3):713–22.
5. Iglesias P, Diez JJ. A clinical update on tumor-induced hypoglycemia. Eur J Endocrinol. 2014;170(4):R147–57.
6. Hizuka N, Fukuda I, Takano K, Asakawa-Yasumoto K, Okubo Y, Demura H. Serum high molecular weight form of insulin-like growth factor ii from patients with non-islet cell tumor hypoglycemia is o-glycosylated*. J Clin Endocrinol Metab. 1998;83(8):2875–7.
7. Dutta P, Aggarwal A, Gogate Y, et al. Non-islet cell tumor-induced hypoglycemia: a report of five cases and brief review of the literature. Endocrinol Diabetes Metab Case Rep. 2013;2013 : 130046.
8. Roy TM, Burns MV, Overly DJ, Curd BT. Solitary fibrous tumor of the pleura with hypoglycemia: the Doege-Potter syndrome [abstract]. J Ky Med Assoc. 1992;90(11):557–60.
9. Schutt RC, Gordon TA, Bhabhra R, et al. Doege-Potter syndrome presenting with hypoinsulinemic hypoglycemia in a patient with a malignant extrapleural solitary fibrous tumor: a case report. J Med Case Rep. 2013;7(1):1.
10. Hadju M, Singer S, Maki RG, Schwartz GK, Keohan ML, Anonescu CR. IGF2 overexpression in solitary fibrous tumors is independent of anatomic location and is related to loss of imprinting. J Pathol. 2010;221(3):300–7.
11. Teale JD, Marks V. Inappropriately elevated plasma insulin-like growth factor II in relation to suppressed insulin-like growth factor I in the diagnosis of non-islet cell tumour hypoglycaemia [abstract]. Clin Endocrinol. 1990;33(1):87–98.
12. Mohammedi K, Khalil CA, Olivier S, Benabad I, Roussel R, Marre M. Paraneoplastic hypoglycemia in a patient with a malignant solitary fibrous tumor. Endocrinol Diabetes Metab Case Rep. 2014;2014 : 140026.
13. Husain Z, Ho JK, Hantash BM. Sign and pseudo-sign of Leser-Trélat: case reports and a review of the literature [abstract]. J Drugs Dermatol. 2013;12(5):e79–87.
14. Baxter RC, Holman SR, Corbould A, Stranks S, Ho PJ, Braund W. Regulation of the insulin-like growth factors and their binding proteins by glucocorticoid and growth hormone in nonislet cell tumor hypoglycemia [abstract]. J Clin Endocrinol Metab. 1995;80(9):2700–8.
15. Eastman KL, Knezevich SR, Raugi GJ. Eruptive seborrheic keratoses associated with adalimumab use. J Dermatol Case Rep. 2013;7(2):60–3.
16. Silva JA, Mesquita Kde C, Igreja AC, et al. Paraneoplastic cutaneous manifestations: concepts and updates. An Bras Dermatol. 2013;88(1):9–22.
17. Nadler WH, Wolfer JA. Hepatogenic hypoglycemia associated with primary liver cell carcinoma [abstract]. Arch Intern Med. 1929;44(5):700–10.
18. Mohammedi K, Khalil CA, Olivier S, Benabad I, Roussel R, Marre M. Synthesis and secretion of insulin-like growth factor ii by a leiomyosarcoma with associated hypoglycemia [abstract]. N Engl J Med. 1988;319 : 1434–40.
19. Marks AG, Carroll JM, Purnell JQ, Roberts CT Jr. Plasma distribution and signaling activities of IGF-II precursors. Endocrinology. 2011;152(3):922–30.
20. Miraki-Moud F, Grossman AB, Besser M, Monson JP, Camacho-Hüber C. A rapid method for analyzing serum pro-insulin-like growth factor-II in patients with non-islet cell tumor hypoglycemia. J Clin Endocrinol Metab. 2005;90(7):3819–23.
21. Tsuro K, Kojima H, Okamoto S, et al. Glucocorticoid therapy ameliorated hypoglycemia in insulin-like growth factor-II-producing solitary fibrous tumor. Intern Med. 2006;45(8):525–9.
22. Steigen SE, Schaeffer DF, West RB, Nielsen TO. Expression of insulinlike growth factor 2 in mesenchymal neoplasms. Mod Pathol. 2009;22(7):914–21.
23. Gold JS, Antonescu CR, Hajdu C, et al. Clinicopathologic correlates of solitary fibrous tumors. Cancer. 2002;94(4):1057–68.
24. Alkemade GM, Bakker M, Rikhof B, et al. Hypoglycemia in a patient with a big “big”-IGF-II-producing tumor. J Clin Endocrinol Metab. 2013;98(8):3113–4.
Štítky
Diabetológia Kardiológia Obezitológia
Článok vyšiel v časopiseDiabetology & Metabolic Syndrome
Najčítanejšie tento týždeň
2016 Číslo 33- Statinová intolerance
- Co dělat při intoleranci statinů?
- Metabolit živočišné stravy produkovaný střevní mikroflórou zvyšuje riziko závažných kardiovaskulárních příhod
- Jak zlepšit záchyt a péči o osoby s prediabetem v primární péči?
- Jakým způsobem hydroresponzivní krytí napomáhá hojení rány?
Najčítanejšie v tomto čísle
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



