-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
A Nonsynonymous Polymorphism in as a Risk Factor for Human Unexplained Cardiac Arrest with Documented Ventricular Fibrillation
Unexplained cardiac arrest (UCA) with documented ventricular fibrillation (VF) is a major cause of sudden cardiac death. Abnormal sympathetic innervations have been shown to be a trigger of ventricular fibrillation. Further, adequate expression of SEMA3A was reported to be critical for normal patterning of cardiac sympathetic innervation. We investigated the relevance of the semaphorin 3A (SEMA3A) gene located at chromosome 5 in the etiology of UCA. Eighty-three Japanese patients diagnosed with UCA and 2,958 healthy controls from two different geographic regions in Japan were enrolled. A nonsynonymous polymorphism (I334V, rs138694505A>G) in exon 10 of the SEMA3A gene identified through resequencing was significantly associated with UCA (combined P = 0.0004, OR 3.08, 95%CI 1.67–5.7). Overall, 15.7% of UCA patients carried the risk genotype G, whereas only 5.6% did in controls. In patients with SEMA3AI334V, VF predominantly occurred at rest during the night. They showed sinus bradycardia, and their RR intervals on the 12-lead electrocardiography tended to be longer than those in patients without SEMA3AI334V (1031±111 ms versus 932±182 ms, P = 0.039). Immunofluorescence staining of cardiac biopsy specimens revealed that sympathetic nerves, which are absent in the subendocardial layer in normal hearts, extended to the subendocardial layer only in patients with SEMA3AI334V. Functional analyses revealed that the axon-repelling and axon-collapsing activities of mutant SEMA3AI334V genes were significantly weaker than those of wild-type SEMA3A genes. A high incidence of SEMA3AI334V in UCA patients and inappropriate innervation patterning in their hearts implicate involvement of the SEMA3A gene in the pathogenesis of UCA.
Published in the journal: A Nonsynonymous Polymorphism in as a Risk Factor for Human Unexplained Cardiac Arrest with Documented Ventricular Fibrillation. PLoS Genet 9(4): e32767. doi:10.1371/journal.pgen.1003364
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1003364Summary
Unexplained cardiac arrest (UCA) with documented ventricular fibrillation (VF) is a major cause of sudden cardiac death. Abnormal sympathetic innervations have been shown to be a trigger of ventricular fibrillation. Further, adequate expression of SEMA3A was reported to be critical for normal patterning of cardiac sympathetic innervation. We investigated the relevance of the semaphorin 3A (SEMA3A) gene located at chromosome 5 in the etiology of UCA. Eighty-three Japanese patients diagnosed with UCA and 2,958 healthy controls from two different geographic regions in Japan were enrolled. A nonsynonymous polymorphism (I334V, rs138694505A>G) in exon 10 of the SEMA3A gene identified through resequencing was significantly associated with UCA (combined P = 0.0004, OR 3.08, 95%CI 1.67–5.7). Overall, 15.7% of UCA patients carried the risk genotype G, whereas only 5.6% did in controls. In patients with SEMA3AI334V, VF predominantly occurred at rest during the night. They showed sinus bradycardia, and their RR intervals on the 12-lead electrocardiography tended to be longer than those in patients without SEMA3AI334V (1031±111 ms versus 932±182 ms, P = 0.039). Immunofluorescence staining of cardiac biopsy specimens revealed that sympathetic nerves, which are absent in the subendocardial layer in normal hearts, extended to the subendocardial layer only in patients with SEMA3AI334V. Functional analyses revealed that the axon-repelling and axon-collapsing activities of mutant SEMA3AI334V genes were significantly weaker than those of wild-type SEMA3A genes. A high incidence of SEMA3AI334V in UCA patients and inappropriate innervation patterning in their hearts implicate involvement of the SEMA3A gene in the pathogenesis of UCA.
Introduction
Unexpected sudden death in healthy individuals remains a daunting problem. Unexplained cardiac arrest with documented ventricular fibrillation (UCA) including idiopathic ventricular fibrillation (IVF) is defined as spontaneous VF that is not associated with a known structural or electrical heart disease. IVF is diagnosed in up to 10% of survivors of out-of-hospital cardiac arrest [1].
Many reports have documented the role of abnormal sympathetic innervations as a trigger of VF [2]–[6]. Sympathetic innervation of the heart is determined during development by chemoattractive and chemorepulsive factors. Semaphorins, members of a conserved family of both secreted and integral membrane proteins, are typical chemorepulsive factors acting on the growth cone as guidance cues to control the establishment of neural connections [7], [8]. Recently, SEMA3A was shown to form an epicardial-to-endocardial transmural sympathetic innervation pattern in the heart. In addition, disruption of innervation patterning in both SEMA3A -deficient and SEMA3A-overexpressing mice resulted in sudden death or lethal arrhythmias [9], [10].
Identification of the genes responsible for UCA may further increase our understanding of the pathophysiology of UCA and facilitate the diagnosis and prophylactic treatment, especially in asymptomatic, disease-carrying relatives of the proband. In the current study, we investigated the significance of the SEMA3A gene polymorphisms in the etiology of UCA.
Results
Genetic analysis of the SEMA3A gene in UCA patients
The subjects were divided into two geographic regions based on their birthplace information, as shown in Figure S1. The characteristics of the two regional groups of UCA patients enrolled in this study are listed in Table 1. There was no significant difference in the clinical characteristics among the two UCA groups. As for control groups, the gender distribution was similar in the two groups, but individuals were older in Eastern Japan as compared to Western Japan (70±9 years vs. 47±16 years).
Tab. 1. Characteristics of UCA with VF patients. 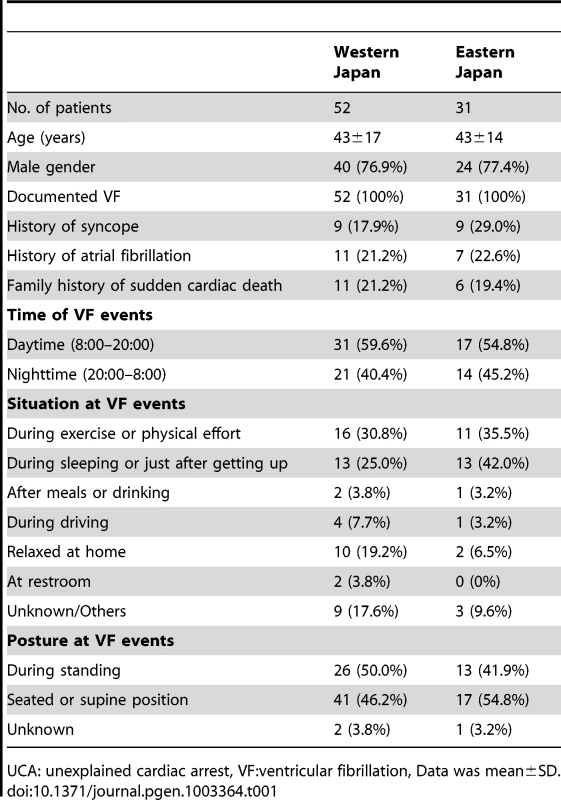
UCA: unexplained cardiac arrest, VF:ventricular fibrillation, Data was mean±SD. Only one nonsynonymous polymorphism was identified in exon 10 of the SEMA3A gene through resequencing of the coding region. This polymorphism causes an amino acid substitution from isoleucine to valine (I334V, SEMA3AI334V) and is identical with the SNP that was recently submitted to dbSNP (rs138694505). There was a significant difference in genotype frequencies between UCA cases and controls in the western Japan (dominant model P = 0.007). This association was replicated in the Eastern Japan (P = 0.008). The Breslow-Day test showed no heterogeneity among the groups, and the overall degree of association by the Mantel-Haenszel test was P = 0.0004 (OR 3.08, 95%CI 1.67–5.70) (Table 2). Collectively, 13 of the 83 UCA patients (15.7%) carried the risk genotype G, whereas only 5.6% did in the controls. The SEMA3AI334V carrier frequency appeared to be relatively stable throughout the age classes (Data not shown).
Tab. 2. SEMA3A polymorphism (SEMA3AI334V: rs138694505) in patients with UCA and controls. 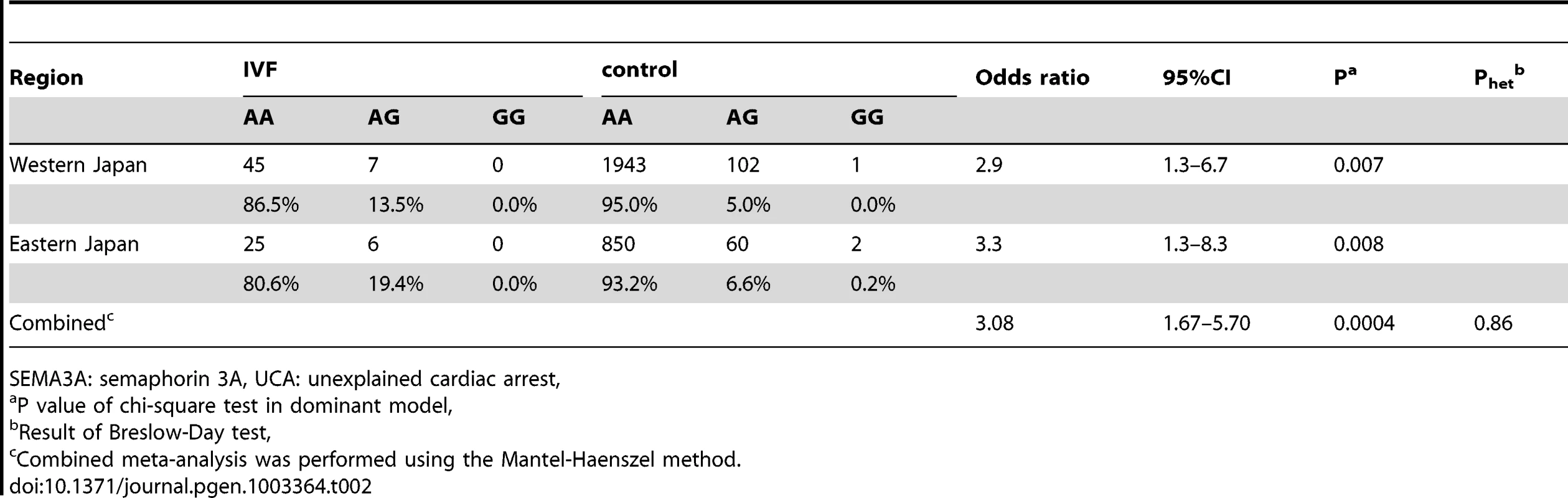
SEMA3A: semaphorin 3A, UCA: unexplained cardiac arrest, Genotype distribution of SEMA3A polymorphisms (rs138694505) among ethnicity
According to the 1000 Genomes Project, regional differences in the SEMA3AI334V (rs138694505) frequency are evident among populations. For example, the frequency of the G allele is 2.1% in East Asians (3.93% in Japanese), 1.35% in West Africans, 1.86% in Americans, and 0% in Europeans (Table 3).
Tab. 3. Regional differences in rs138694505 G allele frequency among populations. 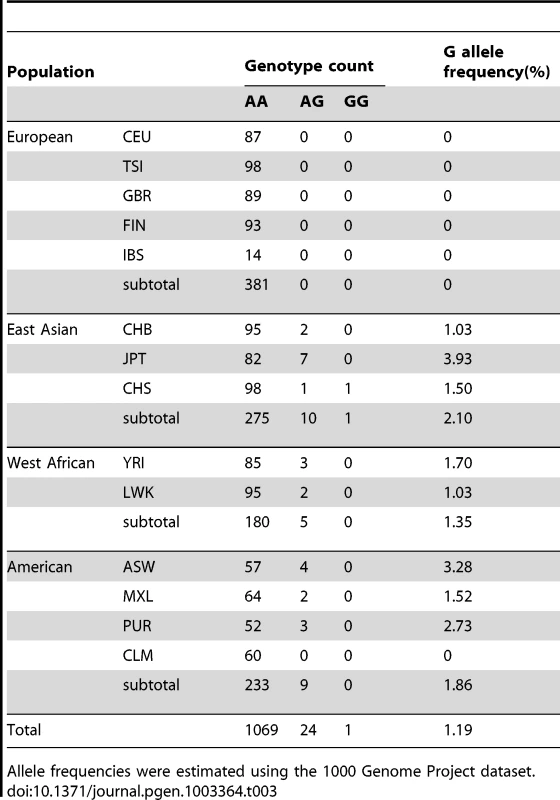
Allele frequencies were estimated using the 1000 Genome Project dataset. Phenotype characterization of UCA patients and clinical findings of UCA patients with and without SEMA3AI334V (rs138694505)
Two UCA cases were severe (Figure 1, patients 1 and 2). They suffered from VF at a young age and had a family history of sudden cardiac death. VF attacks recurred on several occasions in these patients. In one patient (Patient 1), VF recurred twice after discharge and was terminated by an implanted cardioverter defibrillator (ICD) shock (Figure 2 upper panel). According to the ICD records, a preceding transient bradycardia was followed by short coupled ectopic ventricular beats, finally leading to VF. Another patient (Patient 2) went into an electrical storm at midnight one day after hospitalization (Figure 2 lower). VF occurred suddenly during sinus bradycardia. She had been suffering repeated epileptic seizures with loss of consciousness from the age of 15. Most patients with SEMA3AI334V were found to have sinus bradycardia and sinus node dysfunction by an electrophysiological study. Figure 1 shows the ECGs before the ICD implantation in patients 1, 2 and 3. Because of the sinus bradycardia and in order to prevent a VF recurrence, the ICD was set to the AAI+ mode at 60–75 bpm. The number of tests that was performed in the UCA patients is shown in Table S2. The phenotype characterization in each UCA patient with SEMA3AI334V is shown in Table S3. Patient 3 had persistent AF and patient 13 had chronic AF. Patients 1,2,5,7 and 9 had 1st degree atrioventricular block.
Fig. 1. Twelve-lead ECG of patients with SEMA3AI334V. 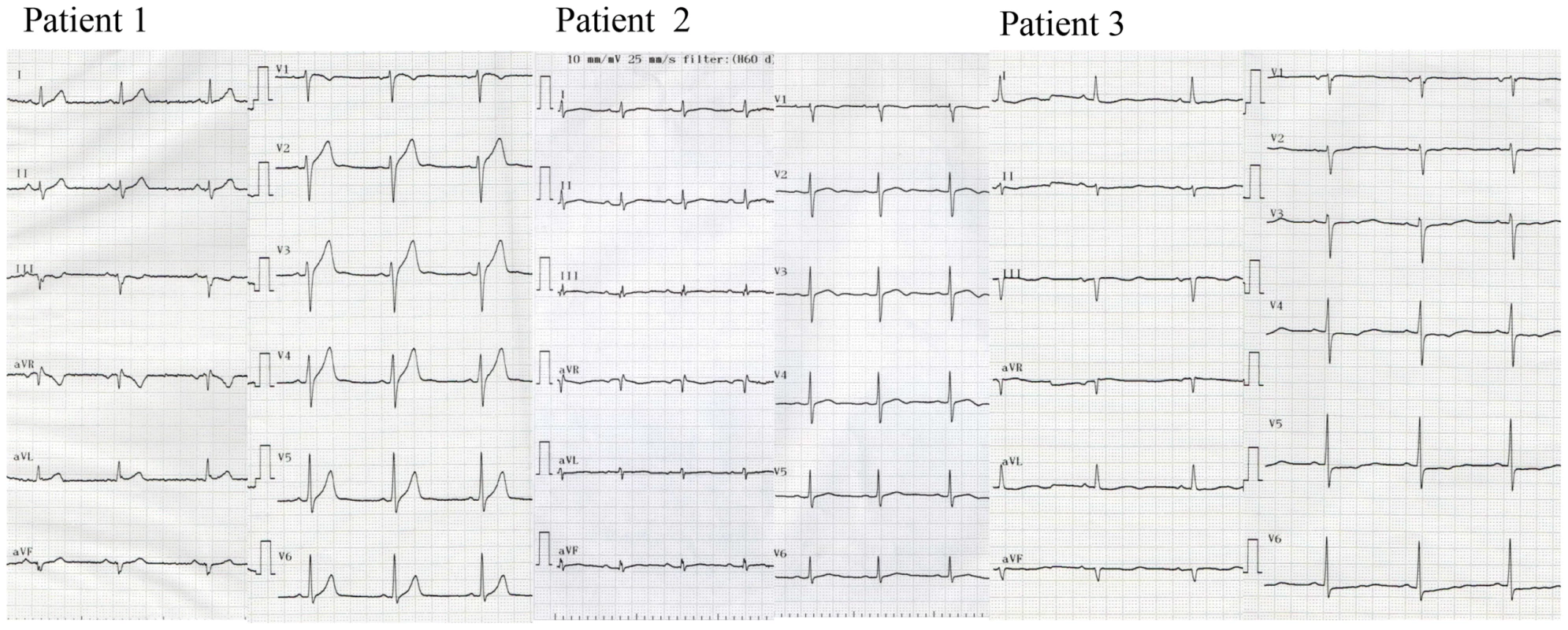
Twelve-lead ECG of typical patients wth sinus bradycardia (left to right, each patient 1–3) before the ICD implantation. Fig. 2. Ventricular fibrillation in patients with SEMA3AI334V. 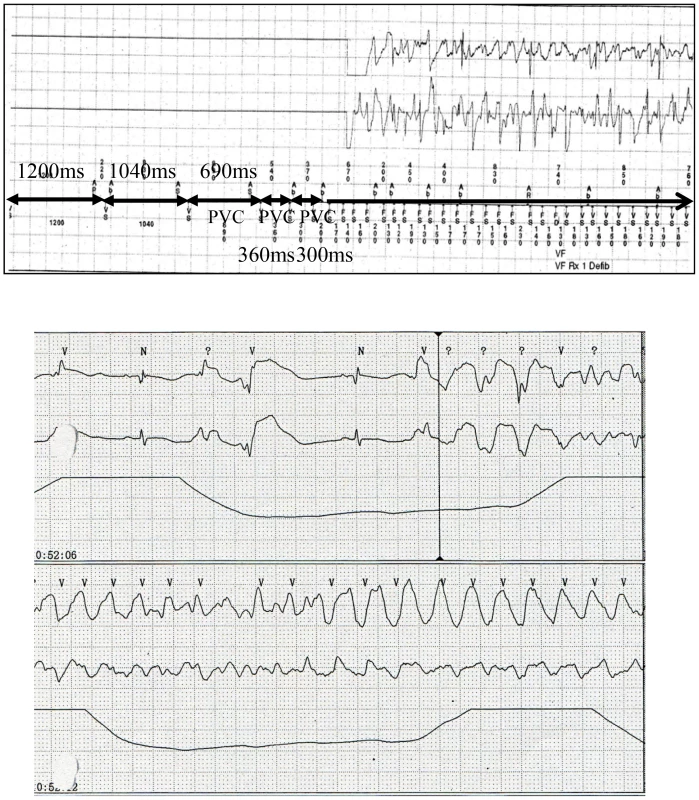
After discharge, VF recurred twice and was terminated by ICD shocks in one male patient (patient 1). According to the ICD records, a preceding transient bradycardia was followed by short coupling ectopic ventricular beats and finally VF occurred (upper). The day after admission to the emergency unit, another female patient (patient 2) went into an electrical storm. VF occurred suddenly during sinus bradycardia (lower). In Table 4 we present the clinical, electrocardiographic, and echocardiographic findings between the UCA patients with and without SEMA3AI334V. VF occurred predominantly at rest and during the night in the patients with SEMA3AI334V. In contrast, it occurred during exercise and during the day in most patients without SEMA3AI334V (VF occurred during the night 69.2% vs. 37.1%, P = 0.032, VF occurred at rest 69.2% vs. 34.3%, P = 0.015).
Tab. 4. Comparison of the clinical and electrocardiographic findings in UCA patients with and without SEMA3AI334V. 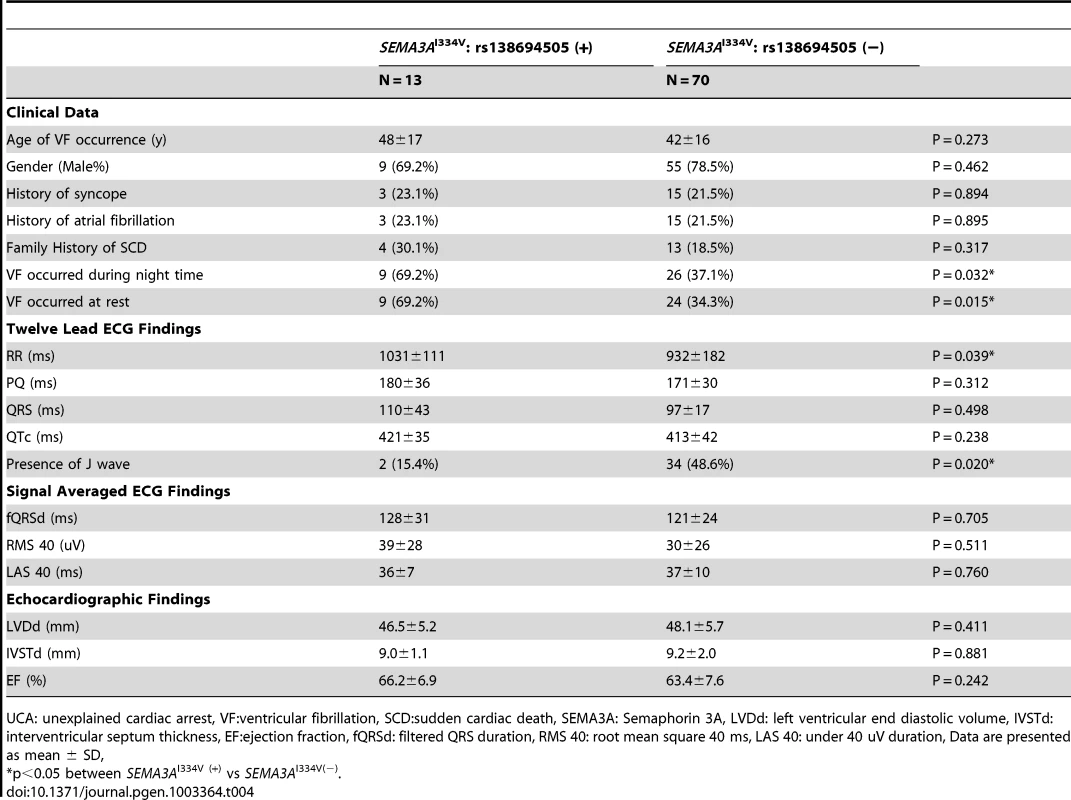
UCA: unexplained cardiac arrest, VF:ventricular fibrillation, SCD:sudden cardiac death, SEMA3A: Semaphorin 3A, LVDd: left ventricular end diastolic volume, IVSTd: interventricular septum thickness, EF:ejection fraction, fQRSd: filtered QRS duration, RMS 40: root mean square 40 ms, LAS 40: under 40 uV duration, Data are presented as mean ± SD, Some of the patients with SEMA3AI334V had sinus bradycardia, and their RR intervals on the 12-lead ECG tended to be longer than those without (1031±111 ms vs. 932±182 ms, P = 0.039). None of the UCA subjects regularly took β-blockers during their ECG recordings. One patient without Sema3aI334V took 100 mg/day of oral amiodarone when recording the ECG. The other cases did not have any anti-arrhythmic agents. Early repolarization (ER) was evident in only two SEMA3AI334V cases (15.4%), whereas 34 patients (48.6%) without SEMA3AI334V demonstrated ER (P = 0.02). The other 12-lead ECG parameters, signal-averaged ECG, and echocardiographic findings, were similar in the patients with and without SEMA3AI334V.
Screening of the SEMA3A region using tag SNPs
To screen the entire SEMA3A gene, 47 tag SNPs were additionally genotyped in the UCA patients from Hiroshima/Nagasaki University and the healthy controls from Hiroshima University (Table S1). All SNPs were successfully genotyped in >98% of the samples. Among them, one SNP, rs1533996, was not polymorphic. The other SNPs were within the Hardy-Weinberg equilibrium (P>0.01) in the controls except for rs13437857 (P = 0.0031) and rs10280701 (P = 0.000086). The p value of the I334V in the population (p = 4.53E-08) was still significant even if a Bonferroni correction for the tag-SNP approach was applied (p = 2.12E-06). None of the 47 tag SNPs were significantly associated with UCA after the Bonferroni correction. The I334V variant showed a moderate linkage disequilibrium only with rs740948 (r2 = 0.43). A haplotype analysis revealed that no haplotype had a stronger association with UCA than the single marker analysis (data not shown).
Sympathetic nerve localization and nerve growth factor (NGF) expression in UCA patients with and without SEMA3AI334V (rs138694505)
Representative immunofluorescence images for vinculin (a cell surface marker) and anti-tyrosine hydroxylase (TH) in the sympathetic nerves in the subendocardial layer of patients with and without SEMA3AI334V are shown in Figure 3. Under normal conditions, the TH nerves were reported to exist in the subepicardial layer of cardiomyocytes, not in the subendocardial layer (9). In patients without SEMA3AI334V, no TH nerves were observed in the subendocardial layer, consistent with earlier findings in normal subjects. In patients with SEMA3AI334V, in contrast, TH nerves were distributed in the subendocardial layer (right panel, the arrowheads indicate TH positive nerves). This finding was consistently observed in patients with SEMA3AI334V (N = 4) but not without SEMA3AI334V (N = 8), suggesting abnormal sympathetic innervation in the heart of UCA patients with SEMA3AI334V. On the other hand, NGF, a neural attractant factor, was similarly expressed in the subendocardial layer in patients with and without SEMA3AI334V (Figure 4).
Fig. 3. Immunofluorescence staining for Vinculin and anti-TH in the subendocardial layer of patients with and without SEMA3AI334V. 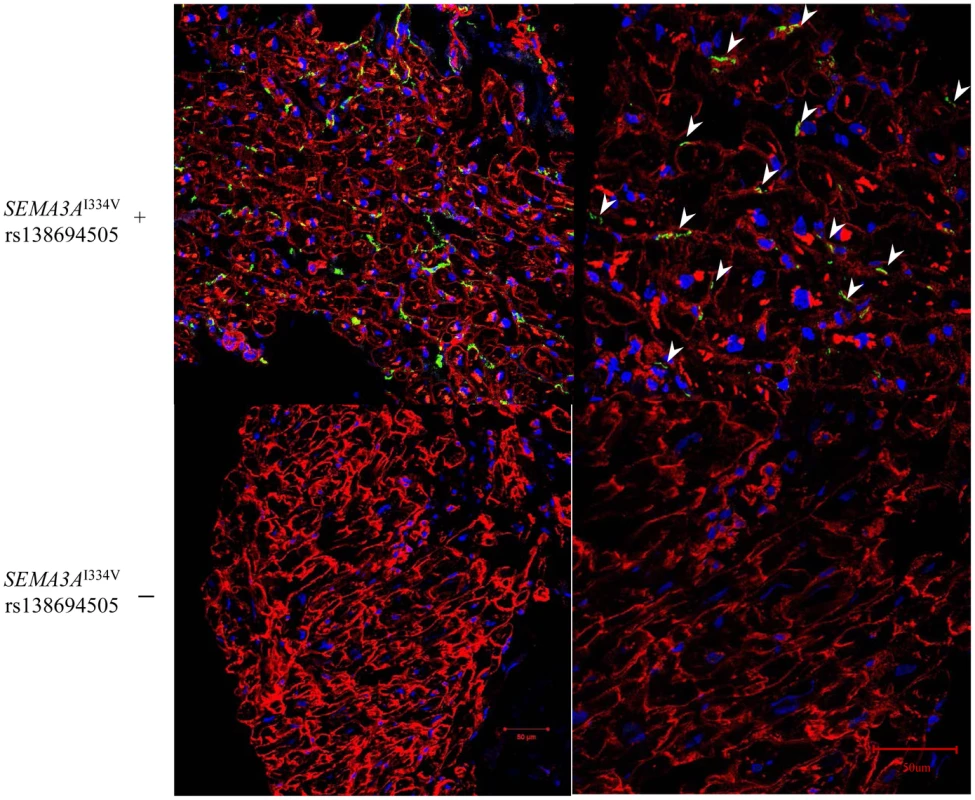
Immunofluorescence staining of cardiac biopsy specimens revealed that TH positive nerves, as sympathetic nerves, which are absent in the subendocardial layer in normal hearts, extended to the subendocardial layer only in patients with SEMA3AI334V (red; anti-Vinculin, green; anti-TH ). The samples were examined using a confocal microscope and captured with a 20×objective lens in the figures on the left and with a 40× objective lens in the figures on the right. The arrowheads show the TH positive nerves (Upper panels: SEMA3AI334V+, Lower panels: SEMA3AI334V−). Fig. 4. Immunofluorescence staining for Vinculin and NGF in the subendocardial layer of patients with and without SEMA3AI334V. 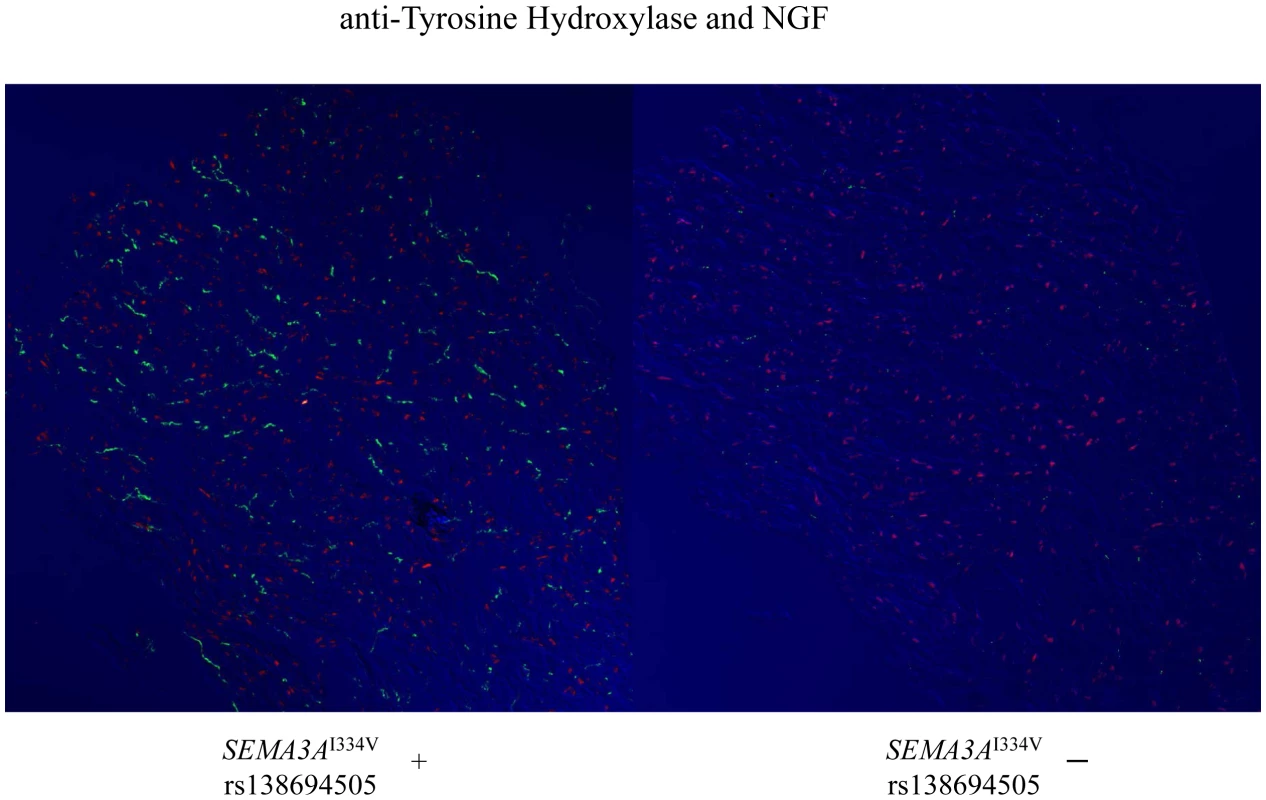
On the other hand, the levels of the NGF, a neural attractant factor, were expressed in the subendocardial layer and are comparable between patients with (left panel) and without (right panel) SEMA3AI334V (red; anti-NGF; green: anti-TH). Expression and function of SEMA3A and SEMA3AI334V
As a result of a DRG repulsion assay, SEMA3AWT-expressing cells repelled the DRG axons on the proximal side of the ganglia (Figure 5, left). In contrast, DRG explants were less responsive to SEMA3AI334V (Figure 5, middle).
Fig. 5. DRG repulsion assay of the SEMA3AWT, SEMA3AI334V, or control. 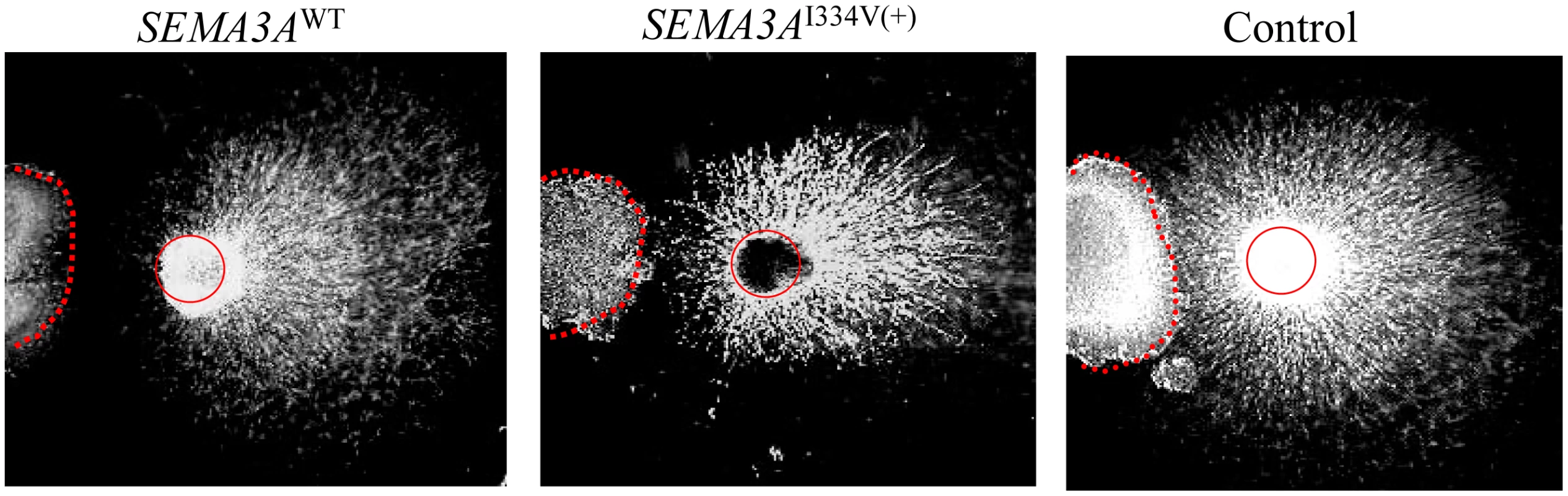
SEMA3AWT expressing cells repelled DRG axons on the proximal side of the ganglia (left). In contrast, DRG explants were less responsive to SEMA3AI334V (middle). Figure 6 shows the percentage of collapsed growth cones in the E8 chick embryos incubated with media containing SEMA3AWT, SEMA3AI334V and vector only (negative control) at 0.3, 0.1, and 0.03 dilutions of a concentrated media, respectively. At all dilutions, SEMA3AWT, and SEMA3AI334V were similarly expressed and secreted (Figure 6). The secreted proteins for both SEMA3AWT and SEMA3AI334V were similar in size (approximately 65 kDa). The growth cone collapse by SEMA3AI334V was less frequent than that of SEMA3AWT at all concentrations. (SEMA3AWT vs. SEMA3AI334V: 84.8±1.5% vs. 75.8±1.8% at a dilution of 0.3, P = 0.009, and 70.2±1.1% vs. 57.2±2.4% at a dilution of 0.1, P = 0.009; Figure 6, lower).
Fig. 6. Growth cone collapse assay of the SEMA3AWT, SEMA3AI334V, or control. 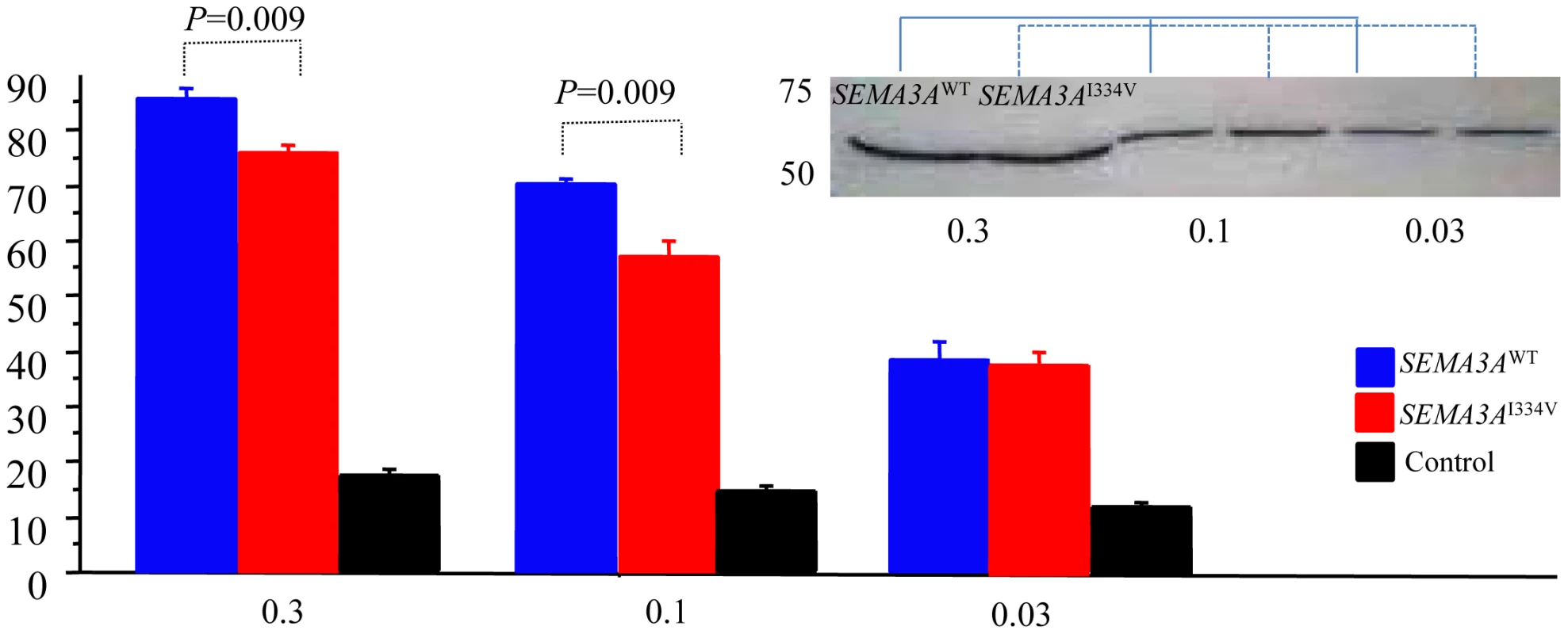
The percent of collapsed growth cones of the E8 chick embryos incubated with medium containing vector only, SEMA3AWT, or SEMA3AI334V at dilutions (0.03, 0.1, and 0.3) of a concentrated media. All dilutions of the concentrated media of the SEMA3A or SEMA3AI334V expressed in HEK293T cells were similarly secreted. SEMA3AWT and SEMA3AI334V led to a collapse of the DRG neuron growth cones in all concentrations, but growth cone collapses by SEMA3AI334V (red bar) were significantly less than those by SEMA3AWT (blue bar) at the dilutions (0.3, 0.1) of the concentrated media (P = 0.009). Discussion
To the best of our knowledge, this is the first report demonstrating that UCA patients have a high incidence of I334V SNP (rs138694505) in the SEMA3A located at chromosome 5. Furthermore, new experimental data presented here indicates that SEMA3AI334V disrupts the SEMA3A function of inhibiting neural growth and impaired appropriate innervation patterning in the heart. Finally, this study suggested that SEMA3AI334V is a risk factor for human UCA and contributes to the pathogenesis of UCA.
Many studies have reported the relationship between abnormal autonomic nerve activity and lethal ventricular arrhythmias, and in most of them I123-MIBG imaging was used to aid in the detection of sympathetic innervation abnormalities [3]–[5], [11]. However, the molecular mechanisms determining these innervation densities in patients with lethal arrhythmia have not been fully clarified. Elucidation of underlying genetic defects will provide further insight into the pathogenesis of UCA, but identification of the genes involved in UCA is very difficult because of its high mortality rate and subsequent diagnostic difficulties. Unlike other monogenic arrhythmia syndromes (e.g., long QT syndrome, catecholaminergic polymorphic ventricular tachycardia and Brugada syndrome), the diagnosis of UCA cannot be made on the basis of ECG abnormalities prior to the occurrence of VF. In addition, UCA is only diagnosed by excluding any identifiable structural or functional cardiac diseases among the few survivors of VF. One case report indicated that a missense variant of the KCNJ8 gene, a subunit of the KATP channel, conferred a predisposition to dramatic depolarization changes and ventricular vulnerability [12]. In another report, Alders et al. demonstrated that a haplotype on chromosome 7, which includes the DPP6 gene (associated with potassium channel Ito subunits), was the causal gene of IVF [13], [14].
Sympathetic innervation of the heart is sculpted during development by chemoattractive factors such as NGF and chemorepulsive factors such as SEMA3A. NGF acts through the Trk A and p75 neurotrophin receptors in sympathetic neurons. Lorenz et al. reported heterogeneous ventricular sympathetic innervation, altered β-adrenergic receptor expression, and rhythm instability in mice lacking the p75 neurotrophin receptor within the heart [15]. Ieda et al. [9], [10] reported that cardiac innervation patterning is disrupted in SEMA3A-deficient and SEMA3A-overexpressing mice, leading to lethal arrhythmias and sudden death. On the basis of this background information, we focused on SEMA3A, which plays a crucial role in cardiac innervation patterning [7]–[10], [16], as abnormal sympathetic innervations have been demonstrated in patients with UCA. We observed that a polymorphism in exon 10 of the SEMA3A gene (i.e., SEMA3AI334V), located in the semaphorin domain, which plays an essential role in SEMA3A [17], was highly prevalent in patients with UCA and strongly associated with UCA pathophysiology. To our knowledge, this is the first report that investigates the relevance of functional mutations or polymorphisms in SEMA3A with respect to human diseases.
We divided the case and control subjects into two geographical groups based on their birthplace in Japan. Significant results observed in Western Japan were replicated in the Eastern Japan group, and the combined P value and odds ratio calculated by the Mantel-Haenszel test were 0.0004 and 3.08, respectively.
According to publicly available data from the 1000 Genomes Project, the frequency of this risk allele of SEMA3A is similar among populations other than Europeans, suggesting that this variant may be relevant to the etiology of UCA across these populations. In our study, the G allele frequency was 2.8% in the controls, which was consistent with that reported in Japanese (3.9%) and East Asian populations (2.1%) in the 1000 Genomes Project.
Haïssaguerre et al. reported an increased prevalence of ER characterized by J-point elevation among patients with a history of UCA [18]. Antzelevitch et al. classified ER patterns for risk stratification of VF [19]. The genetic basis for ER is slowly coming into better focus. Burashnikow et al. identified loss of function mutations in the α1, β2, and α2δ subunits of the cardiac L-type calcium channels (CACNA1C, CACNB2, and CACNA2D1) in patients with ER syndrome [20]. Abe et al. reported that ER may be closely associated with depolarization abnormalities and autonomic modulation [21]. In this study, only two UCA cases with SEMA3AI334V demonstrated ER. Instead, the characteristics of the cases with SEMA3AI334V suffered VF attacks in a relaxed state and presented with sinus bradycardia/sinus node dysfunction. These findings are consistent with the report by Ieda et al. [9], [10] that SEMA3A−/− mice lacked a cardiac sympathetic innervation gradient and exhibited satellite ganglia malformations, which led to marked sinus bradycardia due to sympathetic dysfunction. Some of the UCA cases in our study may have a mild degree of depolarization or repolarization abnormalities, although we could not detect any obvious organic diseases such as cardiomiopathy by diagnostic imaging or manifest conduction disturbances. The other patients did not have any depolarization or repolarization abnormalities. The patients with SEMA3AI334V do not have a homogeneous phenotype and we have to follow up the clinical course of the UCA patients with SEMA3AI334V for a long period.
The frequency of AF was 21.6% and rather high in the UCA subjects of our study for unknown reasons and the frequency was similar in the patients with and without SEMA3AI334V. One possible reason was that the episodes of AF after resuscitation were included in the past history of AF.
In our study, immunofluorescence staining of the RV revealed that sympathetic nerves were distributed in the subendocardial layer only in patients with SEMA3AI334V. If SEMA3A exists in adequate quantities in the endocardial layer and functions normally, sympathetic nerves extending to the endocardial layer are suppressed. We assumed that in UCA patients with SEMA3AI334V, the epicardial-to-endocardial transmural sympathetic innervation patterning had deteriorated.
An SEMA3AWT - and SEMA3AI334V-concentrated media did not grossly affect the expression, stability, or secretion of the ligand. As for the molecular weight of SEMA3A, when it was expressed in HEK293, the full semaphorin domain (65 kDa) was cleaved and detected in a conditioned media [22]. The sizes of the secreted proteins in both SEMA3AWT and SEMA3AI334V were equal and coincident with the semaphorin domain including a dimerization interface and Neurolipin-1 (Nrp-1)-binding residue, and the biological activity was sufficient for the acquisition of a high repulsive activity [22].
The function of repelling the DRG axons was weaker and growth cone collapse was less frequent in SEMA3AI334V than in SEMA3AWT. Therefore, one allele of SEMA3A leads to a disruption of the sympathetic innervation of the heart under relevant conditions. These findings were consistent with immunofluorescence observations strongly suggesting that SEMA3AI334V can disrupt the ability of SEMA3A to repel or collapse DRG axons and sensory neuron growth cones under equal conditions of the neural attractant NGF.
Merte et al. reported that a forward genetic screen in mice identified a novel loss of function SEMA3AK108N mutation, which bound to Nrp-1 but failed to repel or collapse DRG axons in vitro [23]. SEMA3AI334V exists in blade 5 of the 7-bladed propeller structure of the semaphorin domain and performs a crucial function in SEMA3A. Residues 333–335 in 5S of SEMA3A constitute the dimerization interface. The SEMA3A-65K dimerization interface overlaps with sites responsible for the initial high-affinity binding to the domain of Nrp-1. Binding of SEMA3A to Nrp-1 leads to a conformational change in Plexin-A1, which is transmitted to the cytosolic domain [17].
In the association analysis, SEMA3AI334V was highly prevalent in patients with UCA and associated with the UCA pathophysiology. On the other hand, none of the control subjects with SEMA3AI334V had any signs of disease at the time of the study, indicating incomplete penetrance or additional environmental or genetic factors.
Our study had several limitations. First, it was very difficult to congregate many UCA cases and therefore the size of our study population was too small to obtain any robust findings. Secondly, we were not able to study the segregation data in the UCA patients with SEMA3AI334 because their families refused screening. A future prospective study with a larger cohort will be required to obtain these data. A further functional study would also be desirable to determine whether any abnormal innervation can be observed in healthy carriers by using autopsy specimens
In conclusion, a polymorphism of SEMA3AI334V diminishes the cardiac sympathetic innervation gradient and partially contributes to the etiology of UCA. This finding is important in elucidating the pathogenesis of UCA.
Materials and Methods
Subjects
We recruited a total of 83 UCA patients (64 male and 19 female, mean age 43±16 years) from Hiroshima University Hospital, Nagasaki University Hospital, Shiga University of Medical Science, and the National Cerebral and Cardiovascular Center. We recruited 2958 controls (1540 male and 1452 female, mean age 54±18 years) from Hiroshima University Hospital, Osaka-Midosuji Rotary Club (Osaka, Japan), Shiga University of Medical Science, and Niigata University Graduate School of Medical and Dental Sciences. All patients and controls in this paper were unrelated Japanese individuals. Case and control subjects were collected from various regions of Japan. Although the Japanese population has rather low genetic diversity, it has been shown that population structures may lead to spurious associations [24]. Therefore, to eliminate the possibility of a population stratification, we divided case and control subjects into two groups geographically based on their birthplace information (i.e., Western Japan and Eastern Japan) (Figure S1).
The Institutional Ethics Committee of the Graduate School of Biomedical Science at Hiroshima University approved all procedures involving human tissue usage. Written informed consent was obtained from all subjects prior to participation.
Twelve subjects enrolled in the study were diagnosed and treated at the Hiroshima University Hospital; the other subjects were diagnosed and treated at other affiliated hospitals and their information was provided to us.
Diagnosis of UCA
We defined UCA as that without structural heart disease and in the absence of signs of an arrhythmia syndrome such as Brugada syndrome, catecholaminergic polymorphic ventricular tachycardia and long QT syndrome. All patients with cardiac arrest underwent a physical examination, 12 lead ECG [25], echocardiography and coronary angiography to rule out any underlying heart disease. Those who met the inclusion criteria were enrolled and underwent additional testing (signal averaged ECG, T wave alternance, cardiac magnetic resonance imaging, computer tomography, provocation tests, cardiac biopsy or an electrophysiological study), if possible. The numbers of further noninvasive or invasive tests against UCA patients varied from institute to institute. Patients with exonic mutations in SCN5A and a positive pilsicainide challenge test were excluded from the sample. Early repolarization (ER) was defined as a QRS slurring or notching of ≥0.1 mV in more than two consecutive leads of the 12-lead ECG.
Sequence analysis of SEMA3A genomic DNA and genotyping
Peripheral blood was obtained from all the subjects. Genomic DNA was extracted from leukocytes using a QIAamp DNA Blood Mini Kit (QIAGEN, Hilden, Germany) according to the standard protocol. Using Go Taq (Promega, Madison, WI, USA), all coding regions of the SEMA3A located at chromosome 5 were amplified by PCR from 2.5-ng genomic DNA using our original primers in 17 UCA patients and 15 healthy controls entered from Hiroshima University. These amplified coding regions were then resequenced using an ABI PRISM 310 Genetic Analyzer (Applied Biosystems, Foster City, CA, USA) to identify mutations and polymorphisms.
Subsequently, SNP genotypes were genotyped in All of the UCA subjects and healthy control subjects using the Invader assay or the TaqMan assay, as described previously [26], [27].
Tag SNP selection
The 47 tag SNPs were genotyped only in the UCA patients and the healthy controls entered from Hiroshima University and Nagasaki University. Using the HapMap database (public release #27, hapmap.ncbi.nlm.nih.gov) and the Haploview program (www.broad.mit.edu/mpg/haploview) and based on selection criteria of r2>0.8 and a minor allele frequency of >0.01 for the Japanese population, tagging-SNPs were selected from the SEMA3A region spanning approximately 247 kb, from approximately 5 kb upstream of the transcription start site to 5 kb downstream of the 3′ untranslated region.
Plasmid construction
The complete coding region of human SEMA3A was amplified from cDNA with forward (tgttagtgttgccatgaggtct) and reverse (gcattcacctgtgttctctgttag) primers. To generate Flag - SEMA3A, the coding sequence DYKDDDD was introduced between the codons for G25 and K26 (NM_006080.2). The I334V mutation was introduced by site-directed mutagenesis using the QuickChange (Stratagene, La Jolla, CA, USA). Full-length human wild-type (SEMA3AWT) or mutant SEMA3A (SEMA3AI334V) cDNA was cloned into pcDNA3.1(+) (Invitrogen, Carlsbad, CA, USA).
Immunofluorescence staining of anti-tyrosine hydroxylase (TH), nerve growth factor (NGF), and vinculin
Transverse sections of a septal site of the RV outflow tract were obtained by biopsy from 12 UCA subjects (4 patients with SEMA3AI334V and 8 patients without SEMA3AI334V). These sections were embedded in an OCT compound (Sakura, Torrance, CA, USA) and frozen with liquid nitrogen. Immunofluorescence staining was performed using the frozen sections with rabbit anti-TH (AB152, Millipore, Billerica, MA, USA) antibodies and mouse anti-vinculin (Sigma-Aldrich, St. Louis, MO, USA) antibodies diluted at concentrations of 1∶100 and 1∶200, respectively, in 1% BSA/PBS. Alexa 488-conjugated goat anti-rabbit and Alexa 568-conjugated goat anti-mouse antibodies (Invitrogen) were used as secondary antibodies. As for NGF, sheep polyclonal to NGF (ab49205, Abcam, Cambridge, MA, USA) and rabbit anti-TH (ab152, Millipore) were used as primary antibodies at concentrations of 1∶100 in 1% BSA/PBS. Alexa568 donkey anti-sheep (A21099) and Alexa488 donkey anti-rabbit (A21206) antibodies were used as secondary antibodies. Nuclei were stained with 10 µM of Hoechst 33342 (Molecular probes). Samples were examined using a confocal microscope and captured with a 20× and 40× objective lens on a Zeiss LSM 510 laser scanning microscopy system (Carl Zeiss, Thornwood, NY, USA).
DRG repulsion assay and growth cone collapse assay of SEMA3AWT and SEMA3AI334V
The DRG were dissected from E8 chick embryos. HEK293T cells were transfected with Flag - SEMA3AWT or SEMA3AI334V expression vector or equal amounts of empty vector (control) using Gene Juice Transfection Reagent (Novagen, Madison, WI, USA). The DRG and SEMA3A -expressing HEK293T cell aggregates were embedded as described previously [28]. Samples were incubated at 37°C in a 5% CO2 humidified incubator for 48 h and examined using an inverted microscope. For DRG repulsion assays, 10–15 DRG cells were examined, each with Sema3AWT, Sema3AI334V, or a control.
For the purpose of a growth cone collapse assay, the conditioned medium of the SEMA3A -expressing HEK293T cells was concentrated [22]. A Western blot analysis was performed using both dilutions of the SEMA3AWT and SEMA3AI334V concentrated media with anti-FlagM2 (Sigma). Growth cone collapse assays were performed as previously described using chick E8 DRG explants grown on laminin (Invitrogen) - and poly-L-lysine (Sigma)-coated 48-well plates (BD Falcon/353078). The dilution series of the SEMA3AWT, SEMA3AI334V and vector only concentrates were added to each well and incubated at 37°C in a 5% CO2 humidified incubator for 30 min. The explants were fixed with 4% paraformaldehyde in 10% sucrose PBS (pH 7.4), and the samples were examined using an inverted microscope [29]. In each dilution series, 5 or 6 growth cone collapse assays were investigated. Each in vitro assay was performed in triplicate.
For quantification, we counted at least 50 growth cones to score on each explant. We assigned each growth cone as either collapsed or not collapsed, and the results were expressed as the percentage of collapsed to all counted growth cones. We compared the percentage of those collapsed between the SEMA3AWT and SEMA3AI334V.
Statistical analysis
Normally distributed continuous variables are presented as the mean ± SD. Continuous data between the two groups were analyzed using the nonparametric Mann–Whitney U test. For testing the genetic associations in the case–control studies, the chi-square test and Cochran–Armitage trend test were used. Tests for the Hardy–Weinberg equilibrium among the cases and controls were conducted for observed and expected genotype frequencies using an ordinary chi-square test, where a P-value of <0.05 was considered statistically significant. For a meta-analysis of 3 individual cases and controls, we used the Mantel-Haenszel test.
Supporting Information
Zdroje
1. No author listed (1997) Consensus Statement of the Joint Steering Committees of the Unexplained Cardiac Arrest Registry of Europe and of the Idiopathic Ventricular Fibrillation Registry of the United States. Survivors of out-of-hospital cardiac arrest with apparently normal heart. Need for definition and standardized clinical evaluation. Circulation 95 : 265–272.
2. SmithML, HamdanMH, WasmundSL, KneipCF, JoglarJA, et al. (2010) High-frequency ventricular ectopy can increase sympathetic neural activity in humans. Heart Rhythm 7 : 497–503.
3. NishisatoK, HashimotoA, NakataT, DoiT, YamamotoH, et al. (2010) Impaired cardiac sympathetic innervation and myocardial perfusion are related to lethal arrhythmia quantification of cardiac tracers in patients with ICDs. J Nucl Med 51 : 1241–1249.
4. PaulM, SchäfersM, KiesP, AcilT, SchäfersK, et al. (2006) Impact of sympathetic innervation on recurrent life-threatening arrhythmias in the follow-up of patients with idiopathic ventricular fibrillation. Eur J Nucl Med Mol Imaging 33 : 866–870.
5. BiffiM, FallaniF, BorianiG, FantiS, KowollL, et al. (2003) Abnormal cardiac innervation in patients with idiopathic ventricular fibrillation. Pacing Clin Electrophysiol 26 : 357–360.
6. AndréNG, BrackKE, PatelVH, CooteJH (2007) Autonomic modulation of electrical restitution, alternans and ventricular fibrillation initiation in the isolated heart. Cardiovascular Research 73 : 750–760.
7. TanelianDL, BarryMA, JohnstonSA, LeT, SmithGM (1997) Semaphorin III can repulse and inhibit adult sensory afferents in vivo. Nat Med 3 : 1398–1401.
8. KawasaakiT, BarryMA, JohnstonSA, LeT, SmithGM (2002) Requirement of neuropilin 1-mediated Sema3A signals in patterning of the sympathetic nervous system.. Development 129 : 671–680.
9. IedaM, KanazawaH, KimuraK, HattoriF, IedaY, et al. (2007) Sema3A maintains normal heart rhythm through sympathetic innervation patterning. Nature Med 13 : 604–612.
10. KimuraK, IedaM, FukudaK (2012) Development, maturation, and transdifferentiation of cardiac sympathetic nerves. Circ Res 110 ((2)) 325–36.
11. ChirumamillaA, TravinMI (2011) Cardiac applications of 123I-MIBG imaging. Semin Nucl Med 41 : 374–387.
12. HaïssaguerreM, ChatelS, SacherF, WeerasooriyaR, ProbstV, et al. (2009) Ventricular fibrillation with prominent early repolarization associated with a rare variant of KCNJ8/KATP channel. Cardiovasc Electrophysiol 20 : 93–98.
13. AldersM, KoopmannTT, ChristiaansI, PostemaPG, BeekmanL, et al. (2009) Haplotype-sharing analysis implicates chromosome 7q36 harboring DPP6 in familial idiopathic ventricular fibrillation. Am J Hum Genet 84 : 468–476.
14. PostemaPG, ChristiaansI, HofmanN, AldersM, KoopmannTT, et al. (2011) Founder mutations in the Netherlands familial idiopathic ventricular fibrillation and DPP6. Neth Heart J 19 : 290–296.
15. LorentzCU, AlstonEN, BelcikT, LindneJR, GiraudGD, et al. (2010) Heterogeneous ventricular sympathetic innervation, altered beta-adrenergic receptor expression, and rhythm instability in mice lacking the p75 neurotrophin receptor. Am J Physiol Heart Circ Physiol 298: H1652–1660.
16. IedaM, FukudaK (2009) Cardiac innervation and sudden cardiac death. Curr Cardiol Rev 5 : 289–295.
17. AntipenkoA, HimanenJP, van LeyenK, Nardi-DeiV, LesniakJ, et al. (2003) Structure of the semaphorin-3A receptor binding module. Neuron 39 : 589–598.
18. HaïssaguerreM, DervalN, SacherF, JeselL, DeisenhoferI, et al. (2008) Sudden cardiac arrest associated with early repolarization. N Engl J Med 358 : 2016–2023.
19. AntzelevitchC, YanGX (2010) J wave syndromes. Heart Rhythm 7 : 549–558.
20. BurashnikovE, PfeifferR, Barajas-MartinezH, DelpónE, HuD, et al. (2010) Mutations in the cardiac L-type calcium channel associated J wave syndrome and sudden cardiac death. Heart Rhythm 7 : 1872–1882.
21. AbeA, IkedaT, TsukadaT, IshiguroH, MiwaY, et al. (2010) Circadian variation of late potentials in idiopathic ventricular fibrillation associated with J waves insights into alternative pathophysiology and risk stratification. Heart Rhythm 7 : 675–682.
22. AdamsRH, LohrumM, KlostermannA, BetzH, PüschelAW (1997) The chemorepulsive activity of secreted semaphorins is regulated by furin-dependent proteolytic processing. EMBO J 16 : 6077–6086.
23. MerteJ, WangQ, Vander KooiCW, SarsfieldS, LeahyDJ, et al. (2010) A forward genetic screen in mice identifies Sema3A (K108N), which binds to neuropilin-1 but cannot signal. J Neurosci 30 : 5767–5775.
24. Yamaguchi-KabataY, et al. Japanese Population Structure, Based on SNP Genotypes from 7003 Individuals Compared to Other Ethnic Groups:Effects on Population-Based Association Studies(2008). Am J Hum Genetics 83 : 445–456.
25. KrahnAD, HealeyJS, ChauhanV, BirnieDH, SimpsonCS, et al. (2009) Systematic assessment of patients with unexplained cardiac arrest: Cardiac Arrest Survivors With Preserved Ejection Fraction Registry (CASPER). Circulation 120 ((4)): 278–85.
26. OhnishiY, TanakaT, OzakiK, YamadaR, SuzukiH, et al. (2001) A high-throughput SNP typing system for genome-wide association studies. J Hum Genet 46 : 471–477.
27. SuzukiA, YamadaR, ChangX, TokuhiroS, SawadaT, et al. (2003) Functional haplotypes of PADI4, encoding citrullinating enzyme peptidylarginine deiminase 4, are associated with rheumatoid arthritis. Nat Genet 34 : 395–402.
28. MooreSW, KennedyTE (2008) Dissection and coculture of embryonic spinal commissural neurons. Curr Protoc Neurosci 4 : 3.20.1–3.20-17.
29. KapfhammerJP, XuH, RaperJA (2007) The detection and quantification of growth cone collapsing activities. Nature Protocols 2 : 2005–2011.
Štítky
Genetika Reprodukčná medicína
Článek The G4 GenomeČlánek Mondo/ChREBP-Mlx-Regulated Transcriptional Network Is Essential for Dietary Sugar Tolerance inČlánek RpoS Plays a Central Role in the SOS Induction by Sub-Lethal Aminoglycoside Concentrations inČlánek Tissue Homeostasis in the Wing Disc of : Immediate Response to Massive Damage during DevelopmentČlánek Disruption of TTDA Results in Complete Nucleotide Excision Repair Deficiency and Embryonic LethalityČlánek DJ-1 Decreases Neural Sensitivity to Stress by Negatively Regulating Daxx-Like Protein through dFOXO
Článok vyšiel v časopisePLOS Genetics
Najčítanejšie tento týždeň
2013 Číslo 4- Gynekologové a odborníci na reprodukční medicínu se sejdou na prvním virtuálním summitu
- Je „freeze-all“ pro všechny? Odborníci na fertilitu diskutovali na virtuálním summitu
-
Všetky články tohto čísla
- Epigenetic Upregulation of lncRNAs at 13q14.3 in Leukemia Is Linked to the Downregulation of a Gene Cluster That Targets NF-kB
- A Big Catch for Germ Cell Tumour Research
- The Quest for the Identification of Genetic Variants in Unexplained Cardiac Arrest and Idiopathic Ventricular Fibrillation
- A Nonsynonymous Polymorphism in as a Risk Factor for Human Unexplained Cardiac Arrest with Documented Ventricular Fibrillation
- The Hourglass and the Early Conservation Models—Co-Existing Patterns of Developmental Constraints in Vertebrates
- Smaug/SAMD4A Restores Translational Activity of CUGBP1 and Suppresses CUG-Induced Myopathy
- Balancing Selection on a Regulatory Region Exhibiting Ancient Variation That Predates Human–Neandertal Divergence
- The G4 Genome
- Extensive Natural Epigenetic Variation at a Originated Gene
- Mouse Oocyte Methylomes at Base Resolution Reveal Genome-Wide Accumulation of Non-CpG Methylation and Role of DNA Methyltransferases
- The Environment Affects Epistatic Interactions to Alter the Topology of an Empirical Fitness Landscape
- TIP48/Reptin and H2A.Z Requirement for Initiating Chromatin Remodeling in Estrogen-Activated Transcription
- Aconitase Causes Iron Toxicity in Mutants
- Tbx2 Terminates Shh/Fgf Signaling in the Developing Mouse Limb Bud by Direct Repression of
- Mondo/ChREBP-Mlx-Regulated Transcriptional Network Is Essential for Dietary Sugar Tolerance in
- Sex-Differential Selection and the Evolution of X Inactivation Strategies
- Identification of a Tissue-Selective Heat Shock Response Regulatory Network
- Phosphorylation-Coupled Proteolysis of the Transcription Factor MYC2 Is Important for Jasmonate-Signaled Plant Immunity
- RpoS Plays a Central Role in the SOS Induction by Sub-Lethal Aminoglycoside Concentrations in
- Six Homeoproteins Directly Activate Expression in the Gene Regulatory Networks That Control Early Myogenesis
- Rtt109 Prevents Hyper-Amplification of Ribosomal RNA Genes through Histone Modification in Budding Yeast
- ATP-Dependent Chromatin Remodeling by Cockayne Syndrome Protein B and NAP1-Like Histone Chaperones Is Required for Efficient Transcription-Coupled DNA Repair
- Iron-Responsive miR-485-3p Regulates Cellular Iron Homeostasis by Targeting Ferroportin
- Mutations in Predispose Zebrafish and Humans to Seminomas
- Cytotoxic Chromosomal Targeting by CRISPR/Cas Systems Can Reshape Bacterial Genomes and Expel or Remodel Pathogenicity Islands
- Tissue Homeostasis in the Wing Disc of : Immediate Response to Massive Damage during Development
- All SNPs Are Not Created Equal: Genome-Wide Association Studies Reveal a Consistent Pattern of Enrichment among Functionally Annotated SNPs
- Functional 358Ala Allele Impairs Classical IL-6 Receptor Signaling and Influences Risk of Diverse Inflammatory Diseases
- The Tissue-Specific RNA Binding Protein T-STAR Controls Regional Splicing Patterns of Pre-mRNAs in the Brain
- Neutral Genomic Microevolution of a Recently Emerged Pathogen, Serovar Agona
- Genetic Requirements for Signaling from an Autoactive Plant NB-LRR Intracellular Innate Immune Receptor
- SNF5 Is an Essential Executor of Epigenetic Regulation during Differentiation
- Dialects of the DNA Uptake Sequence in
- Reference-Free Population Genomics from Next-Generation Transcriptome Data and the Vertebrate–Invertebrate Gap
- Senataxin Plays an Essential Role with DNA Damage Response Proteins in Meiotic Recombination and Gene Silencing
- High-Resolution Mapping of Spontaneous Mitotic Recombination Hotspots on the 1.1 Mb Arm of Yeast Chromosome IV
- Rod Monochromacy and the Coevolution of Cetacean Retinal Opsins
- Evolution after Introduction of a Novel Metabolic Pathway Consistently Leads to Restoration of Wild-Type Physiology
- Disruption of TTDA Results in Complete Nucleotide Excision Repair Deficiency and Embryonic Lethality
- Insulators Target Active Genes to Transcription Factories and Polycomb-Repressed Genes to Polycomb Bodies
- Signatures of Diversifying Selection in European Pig Breeds
- The Chromosomal Passenger Protein Birc5b Organizes Microfilaments and Germ Plasm in the Zebrafish Embryo
- The Histone Demethylase Jarid1b Ensures Faithful Mouse Development by Protecting Developmental Genes from Aberrant H3K4me3
- Regulates Synaptic Development and Endocytosis by Suppressing Filamentous Actin Assembly
- Sensory Neuron-Derived Eph Regulates Glomerular Arbors and Modulatory Function of a Central Serotonergic Neuron
- Analysis of Rare, Exonic Variation amongst Subjects with Autism Spectrum Disorders and Population Controls
- Scavenger Receptors Mediate the Role of SUMO and Ftz-f1 in Steroidogenesis
- DNA Double-Strand Breaks Coupled with PARP1 and HNRNPA2B1 Binding Sites Flank Coordinately Expressed Domains in Human Chromosomes
- High-Resolution Mapping of H1 Linker Histone Variants in Embryonic Stem Cells
- Comparative Genomics of and the Bacterial Species Concept
- Genetic and Biochemical Assays Reveal a Key Role for Replication Restart Proteins in Group II Intron Retrohoming
- Genome-Wide Association Studies Identify Two Novel Mutations Responsible for an Atypical Hyperprolificacy Phenotype in Sheep
- The Genetic Correlation between Height and IQ: Shared Genes or Assortative Mating?
- Comprehensive Assignment of Roles for Typhimurium Genes in Intestinal Colonization of Food-Producing Animals
- An Essential Role for Zygotic Expression in the Pre-Cellular Drosophila Embryo
- The Genome Organization of Reflects Its Lifestyle
- Coordinated Cell Type–Specific Epigenetic Remodeling in Prefrontal Cortex Begins before Birth and Continues into Early Adulthood
- Improved Detection of Common Variants Associated with Schizophrenia and Bipolar Disorder Using Pleiotropy-Informed Conditional False Discovery Rate
- Site-Specific Phosphorylation of the DNA Damage Response Mediator Rad9 by Cyclin-Dependent Kinases Regulates Activation of Checkpoint Kinase 1
- Npc1 Acting in Neurons and Glia Is Essential for the Formation and Maintenance of CNS Myelin
- Identification of , a Retrotransposon-Derived Imprinted Gene, as a Novel Driver of Hepatocarcinogenesis
- Aag DNA Glycosylase Promotes Alkylation-Induced Tissue Damage Mediated by Parp1
- DJ-1 Decreases Neural Sensitivity to Stress by Negatively Regulating Daxx-Like Protein through dFOXO
- Asynchronous Replication, Mono-Allelic Expression, and Long Range -Effects of
- Differential Association of the Conserved SUMO Ligase Zip3 with Meiotic Double-Strand Break Sites Reveals Regional Variations in the Outcome of Meiotic Recombination
- Focusing In on the Complex Genetics of Myopia
- Continent-Wide Decoupling of Y-Chromosomal Genetic Variation from Language and Geography in Native South Americans
- Breakpoint Analysis of Transcriptional and Genomic Profiles Uncovers Novel Gene Fusions Spanning Multiple Human Cancer Types
- Intrinsic Epigenetic Regulation of the D4Z4 Macrosatellite Repeat in a Transgenic Mouse Model for FSHD
- Bisphenol A Exposure Disrupts Genomic Imprinting in the Mouse
- Genetic and Genomic Architecture of the Evolution of Resistance to Antifungal Drug Combinations
- Transposable Elements Are Major Contributors to the Origin, Diversification, and Regulation of Vertebrate Long Noncoding RNAs
- Functional Dissection of the Condensin Subunit Cap-G Reveals Its Exclusive Association with Condensin I
- PLOS Genetics
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- The G4 Genome
- Neutral Genomic Microevolution of a Recently Emerged Pathogen, Serovar Agona
- The Histone Demethylase Jarid1b Ensures Faithful Mouse Development by Protecting Developmental Genes from Aberrant H3K4me3
- The Tissue-Specific RNA Binding Protein T-STAR Controls Regional Splicing Patterns of Pre-mRNAs in the Brain
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



