-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
Where Will the Next Generation of Stroke Treatments Come From?
article has not abstract
Published in the journal: Where Will the Next Generation of Stroke Treatments Come From?. PLoS Med 7(3): e32767. doi:10.1371/journal.pmed.1000224
Category: Research in Translation
doi: https://doi.org/10.1371/journal.pmed.1000224Summary
article has not abstract
The Extent of the Problem
Stroke, about 80% of which is ischaemic caused by occlusion of an intracerebral artery and 20% caused by intracerebral bleeding, is the second most common cause of death and disability globally. WHO statistics indicate that stroke and other cerebrovascular diseases kill approximately 5.7 million people each year. In the United States alone it is estimated that the 780,000 symptomatic strokes detected each year may be accompanied by a further 11 million asymptomatic strokes [1]. The need to reduce this burden by better use of existing therapies and identification of new ones is pressing.
Stroke Mechanisms and Pathophysiology: Heterogeneity Is the Key
A unique feature of stroke that creates opportunities for new therapies is the heterogeneity of its mechanisms. These range from large artery to artery embolism, cardiac embolism to in situ small vessel disease and even arterial dissection. Intracerebral haemorrhage may be caused by hypertensive small vessel disease, amyloid angiopathy, or rupture of saccular aneurysms. Risk factors such as atrial fibrillation, hypertension, smoking, diabetes, and disordered lipid metabolism contribute to underlying atherosclerosis or embolus formation [2]. The sequence of events, termed the ischaemic cascade, that follows an ischaemic stroke has also been established [3]. Here, neurons exposed to extreme reductions in blood flow (the “ischaemic core”) lose their membrane potential, undergo irreversible structural damage, and die. In surrounding regions (the “ischaemic penumbra”) the reduction in blood flow is sufficient to compromise neuronal function but not immediately cause neuronal death. A balance between energy supply and consumption exits and tissue survival is determined by the depth and duration of ischaemia [3],[4]. An understanding of this process has led to the concept of reperfusion and neuroprotective therapies.
Twenty Years of Rapid but “Inherited” Advances
Interestingly, many therapeutic advances in stroke have come from research in other disciplines. For example, blood pressure lowering agents such as the ACE inhibitors, developed originally to reduce the risk of vascular injury and myocardial infarction were found to reduce stroke incidence [5],[6]. Similarly for the statins, designed to reduce LDL-cholesterol were found to protect against stroke [7]. Thrombolysis and anti-platelet therapies developed from ischaemic heart disease management [8], and hemicraniectomy to relieve pressure in some cases of ischaemic stroke was used in head trauma [9]. Even some stroke care unit management practices have come from approaches developed in cardiology, oncology, burns, and transplant medicine [10]. We have “inherited” the majority of four categories of acute and five of secondary prevention interventions with level 1 evidence of benefit in stroke since 1978 in this way (see Table 1). As this approach has been successful in the past, abandoning it now would be unwise: we suggest a continued monitoring of other disciplines, while also pursuing novel stroke-specific research. We will address the likely wins from existing classes of intervention and then speculate from where the next therapeutic classes may emerge.
Tab. 1. Acute interventions and secondary prevention strategies of proven benefit based on level I evidence. 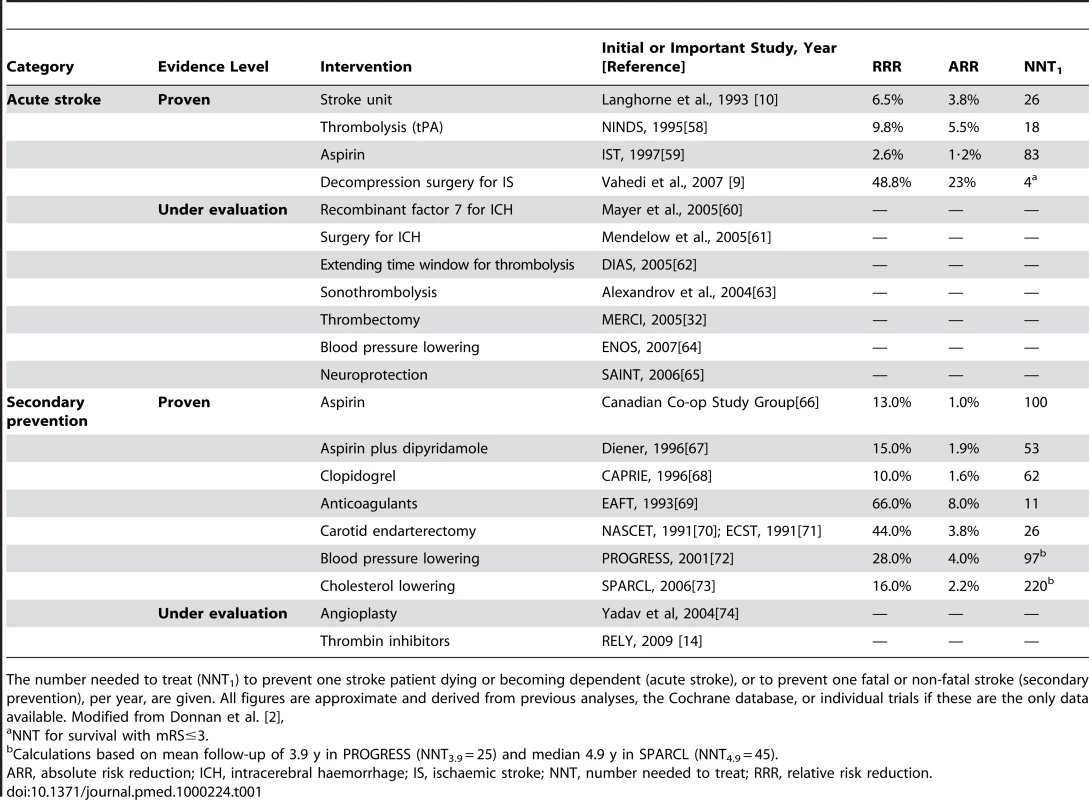
The number needed to treat (NNT1) to prevent one stroke patient dying or becoming dependent (acute stroke), or to prevent one fatal or non-fatal stroke (secondary prevention), per year, are given. All figures are approximate and derived from previous analyses, the Cochrane database, or individual trials if these are the only data available. Modified from Donnan et al. [2], The Next Generation of Treatments: New Twists on Existing Therapies
Primary and Secondary Prevention
The greatest early gains are likely to come from enhancing existing strategies. Declines in stroke incidence and mortality in developed countries are most likely due to better risk factor control [11]. However, not all of the reduction in stroke mortality may have come from better blood pressure control. Benefits may have accrued from the recently recognised anti-inflammatory effects of ACE inhibitors and statins [12], and an inflammatory genesis of atherosclerosis may create opportunities for new therapeutic targets. Better recognition of atrial fibrillation (AF), the risk factor that is often overlooked in spite of its high age-specific attributable risk [13], is necessary and may lead to new opportunities. Prevention of stroke in AF with new classes of drugs such as the thrombin inhibitors is a reality [14]. With the growing impact of metabolic syndrome [15], incretin-based therapies, which help control hyperglycaemia and hyperlipidemia [16], combined with better lifestyle management may also be useful in the future. Anti-platelet agents have been a mainstay of secondary stroke prevention (Table 1). However, a ceiling may have been reached of about 20% relative risk reduction, and further anti-platelet effects may cause unacceptable bleeding [17]. Compounds with actions “beyond the platelet” need to be identified, such as thromboxane receptor antagonists.
Can imaging and other biomarkers assist in the search for new therapies? Although currently little evidence indicates that this would be cost effective, screening to detect asymptomatic aneurysms, variations in Circle of Willis anatomy [18] and arterial collateralization [19] may ultimately prove useful. For example, patients with reduced capacity to redistribute cerebral blood flow and thus maintain perfusion above ischemic thresholds are likely to be susceptible to larger strokes. Reports that variation in expression of molecules such as thrombin activatable fibrinolysis inhibitor (TAFI) [20] and plasminogen activator inhibitor-1 (PAI-1) [21] might define risk in specific stroke subsets needs evaluation in the general population. Bioinformatics may help assess panels of protein or mRNA biomarkers to assess stroke risk. Systems controlling clotting and fibrinolysis and regulating inflammation or oxidative stress may be particularly informative.
Acute Stroke
Novel approaches to thrombolysis
Recanalisation and restoration of blood flow by thrombolysis with tissue plasminogen activator (tPA) benefits only a small proportion of stroke patients [22]. The narrow 3-hour time window and the risk of bleeding limit its use [23]. The time window has now been extended to 4.5 hours, and penumbral imaging with MR or CT may extend this further [24],[25]. The development of biomarker assays for stroke duration or individual risk of bleeding may increase the proportion of eligible patients. Biomarker assays may also be used to improve the toxicity profile of thrombolytic agents and help develop ways to make thrombolytics safer, for example by using the platelet-derived growth factor receptor, alpha (PDGFR-α) antagonist imatinib to reduce tPA-induced bleeding [26]. Another possibility is TAFI, with a genotype associated with stroke risk [27], circulating activity that modifies outcome after thrombolytic therapy [28] and the capacity to make clots resistant to heparins [29]. With the potential to reduce time to artery opening by up to 90 minutes [30], TAFI inhibitors might be effective and safe profibrinolytic agents for use with existing thrombolytic therapies [31].
Mechanical clot removal/disruption
The use of mechanical devices to remove clots after the acute stroke event is a logical approach but it is highly labour - and capital-intensive. Early recanalisation success was demonstrated with the MERCI Retriever embolectomy device [32] and has been followed by a number of others, most recently the Penumbra device [33]. While it remains to be proved that clinical benefit accrues, based on the shift from thrombolysis to more direct catheter-based intervention in acute myocardial ischemia management, a similar pattern for acute stroke is likely. Opportunities exist to develop improved clot-retrieval devices and, more importantly, health care system changes to allow deployment effectively in a timely manner.
Neuroprotection
Despite disappointments in the area of neuroprotection, the rhetoric “neuroprotection is dead” seems premature. Systematic reviews and meta-analyses have revealed deficiencies in experimental designs. Failure to consider bias [34] and comorbidities common in human stroke [35] all lead to over-optimistic interpretations of preclinical animal testing. A more rational approach is needed in the sequence of animal to human studies. The heterogeneity of stroke supports the breadth of preclinical evaluation recommended by the Stroke Therapy Academic Industry Roundtable (STAIR) [36], while the fundamentals of good science demand careful bias avoidance [34]. In addition it would seem desirable to have evidence that new drugs reach their hypothesised targets and elicit at least a surrogate response once there. Hypothermia does tick all the appropriate preclinical boxes [37] and has been found to be effective in protecting against the neurological sequelae of cardiac arrest [38]; a large-scale Phase III trial in stroke is needed in spite of logistical difficulties. Also, compounds that directly depress body temperature should perhaps be considered. For example, improgan, a member of a new class of nonopioid analgesics, can reduce core temperature in rodents by 1°C within 10 minutes [39], and hydrogen sulphide can rapidly induce a suspended animation-like state [40]. Drugs that alter the thermoregulatory set-point and make hypothermia more tolerable by reducing shivering are already being considered [41]. We should also entertain alternative mechanisms of action of hypothermia such as control of oedema and local compression.
Timing of treatment following stroke is critical. It may be that neuroprotection is of value only if reperfusion ultimately occurs. In other disciplines, graft ischaemia times for renal, cardiac, and lung transplantation due to developments in effective cold storage and preservative fluids are impressive. Hence “freezing” the penumbra with neuroprotectants may be a realistic goal while waiting for reperfusion. An example is with normobaric oxygen, which increases penumbral oxygen partial pressure and reduces infarct volume in animals [42]. Prolongation of penumbral survival has been inferred in Phase II MR-based studies and pilots of therapy performed in humans [43].
Where May the Next New Class of Therapies Come From?
Although a number of avenues of research may bring rewards in terms of completely new classes of intervention for the prevention and treatment of stroke, we believe two areas are most likely to generate completely new classes of therapeutic targets.
Stimulation of Plasticity
One of the most important advances in neuroscience has been the recognition of activity-dependent plasticity. Synapses, the base units of connectivity, form and disappear depending upon activity and experience, and axons and dendrites can reach out to and withdraw from new targets [44]. Although it had become established that astrocytes and microglia respond rapidly to injury, the realisation that new neurons and supporting oligodendroglia could be generated from pools of neural stem cells and progenitors was a paradigm shift in neurology [45],[46]. These processes offer new and exciting therapeutic opportunities. At the simplest level, we can use observations of benefit after enriching the environment or increasing motor activity to improve traditional rehabilitation strategies [47]. Effective delivery of growth-promoting factors, including the nerve growth factor and glial cell line-derived neurotrophic factor families of proteins, to enhance plasticity and regeneration may also prove effective. Alternatively, mobilization and activation of endogenous neurogenesis/plasticity with drugs such as granulocyte colony-stimulating factor (G-CSF) may be attractive [48]. Although stem cell implants into the brain may currently deliver only a supportive or plastic environment that aids recovery, these cells can mature and integrate into the host neural circuitry [49]. Given that repopulation of connective tissue scaffolds by stem cells can reconstitute a beating animal heart [50], the same may ultimately be possible for regions of damaged brain.
Importantly, evidence is emerging that neural recovery and immune function are intimately linked [51]. Neural outputs seem to regulate bone marrow and spleen activity, while cytokines and related molecules act both locally and systemically to facilitate neuroimmune communication. While this interaction provides considerable scope for clinical intervention, for example by using G-CSF to mobilise neural stem cells [48] or manipulating microglial or macrophage mediated axonal plasticity [52], it is a double-edged sword. Many of the candidate molecular targets directly influence both acute injury development and later neurovascular remodelling [53]. Our approaches need to be sophisticated enough to deal with these critical temporal issues.
Unravelling the Genetic Code to the Heterogeneity of Stroke
There is about a 40% gap in the population attributable risk for stroke when all known risk factors are considered [54]. Much of the gap may consist of genetic contributions to risk in some form. Fortunately, there have been enormous advances in the genetics of stroke. Initial linkage analysis studies identified, rare conditions such as CADASIL (cerebral autosomal dominant arteriopathy with subcortical infarcts and leucoencephalopathy) due to mutations in the NOTCH3 gene [55], which is involved in cell signalling and fate during embryonic development. Subsequently, a candidate gene approach using case-control designs produced a large number of potential gene polymorphisms, many of which could not be replicated and were probably the product of underpowered studies. The emergence of the concept of polygenic contributions to the stroke syndrome, gene chip technology and genome-wide association studies (GWASs) has revolutionized the area. Large international cooperative studies with sample sizes in the thousands have enabled investigators to produce reliable data. For example, by genotyping more than 310,000 single-nucleotide polymorphisms (SNPs) in more than 1,700 intracranial aneurysms and 7,400 controls, SNPs on Chromosomes 2q, 8q, and 9p were associated with aneurysmal presence. The biological implications come from an understanding of the function of these genes as our research effort explores their biology. Chromosomes 8q and 9p both have genes that are associated with progenitor cells and expressed in blood vessels. The main candidate gene on 8q is SOX17, which is required for endothelial formation and maintenance [56]. The implications for the development of gene-based or other therapies are obvious. Similarly, investigators of the International Stroke Genetics Consortium found an association between SNPs in the Chromosome 9p21.3 region and large-artery stroke [57]. GWASs are still in their infancy and are dependent on careful phenotyping and large sample sizes. However, the likelihood that completely novel therapeutic classes emerge from these studies is extremely high.
Summary
Remarkable progress has occurred over the last two decades in stroke interventions. Many have been developed on the basis of their efficacy in other disorders. This “inheritance” approach should continue, but two areas where completely novel therapeutic targets might emerge are the stimulation of neuroplasticity and unraveling the genetic code of stroke heterogeneity (Table 2). For the former, the next steps are to identify small-molecule, nontoxic compounds that most effectively enhance plasticity in animal models, and then subject them to clinical trial in humans. For the latter, more and larger-scale cooperative GWASs in carefully phenotyped stroke populations are required to better understand the polygenic nature of cerebrovascular disease. Then, the physiological relevance of genetic abnormalities can be determined in in vitro and in vivo systems before candidate compounds are developed.
Tab. 2. Five key papers in the field of stroke. 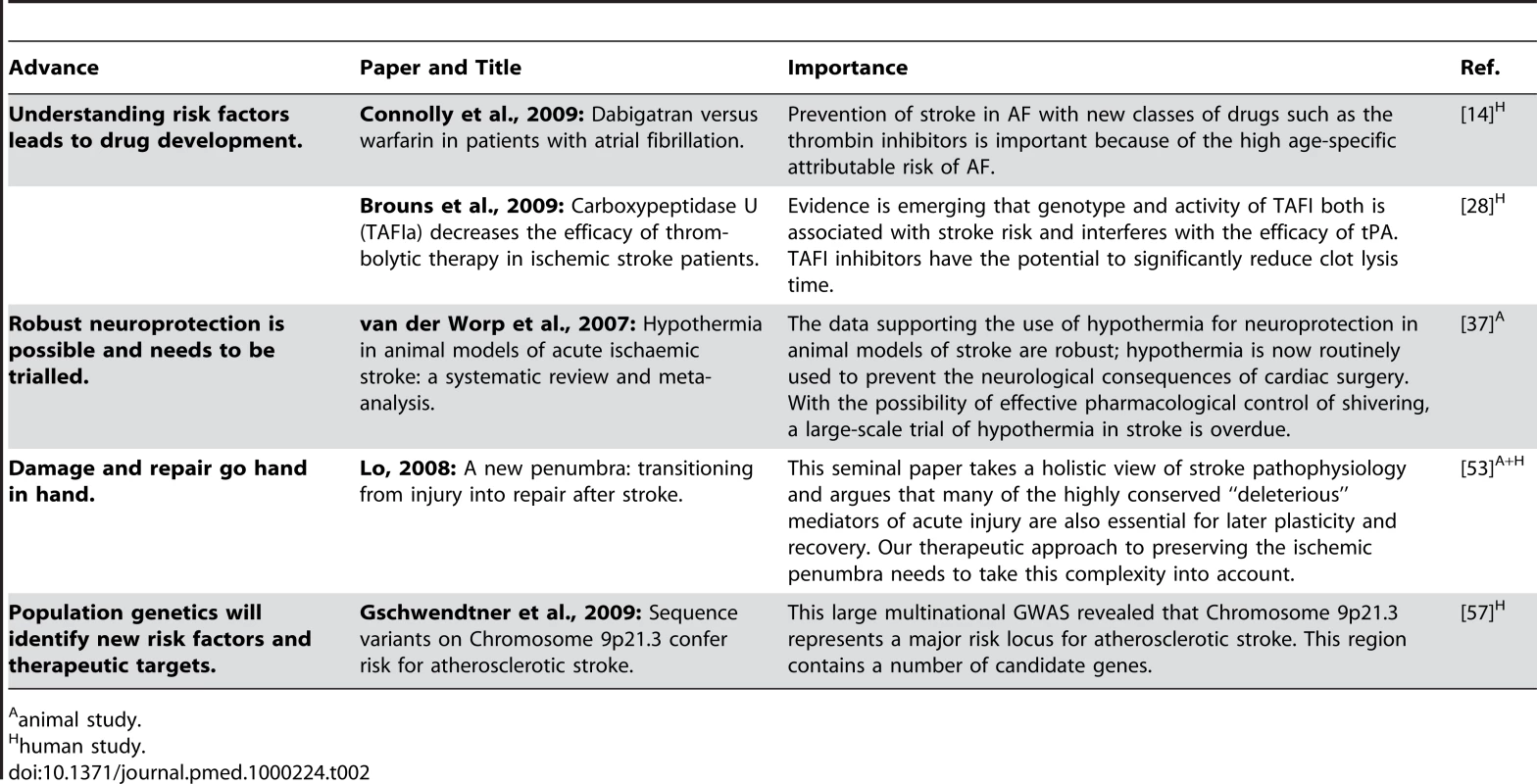
animal study.
Zdroje
1. LearyMC
SaverJL
2003 Annual incidence of first silent stroke in the United States: a preliminary estimate. Cerebrovasc Dis 16 280 285
2. DonnanGA
FisherM
MacleodM
DavisSM
2008 Stroke. Lancet 371 1612 1623
3. MergenthalerP
DirnaglU
MeiselA
2004 Pathophysiology of stroke: lessons from animal models. Metab Brain Dis 19 151 167
4. DonnanG
BaronJ
DavisS
SharpF
2006 The Ischaemic Penumbra: history, current status and implications for therapy. New York Informa Health Care Inc
5. PROGRESS Collaborative Group 2001 Randomised trial of a perindopril-based blood-pressure-lowering regimen among 6,105 individuals with previous stroke or transient ischaemic attack. Lancet 358 1033 1041
6. KizerJR
DahlofB
KjeldsenSE
JuliusS
BeeversG
2005 Stroke reduction in hypertensive adults with cardiac hypertrophy randomized to losartan versus atenolol: the Losartan Intervention For Endpoint reduction in hypertension study. Hypertension 45 46 52
7. AmarencoP
BogousslavskyJ
CallahanA3rd
GoldsteinLB
HennericiM
2006 High-dose atorvastatin after stroke or transient ischemic attack. N Engl J Med 355 549 559
8. GrangerCB
WhiteHD
BatesER
OhmanEM
CaliffRM
1994 A pooled analysis of coronary arterial patency and left ventricular function after intravenous thrombolysis for acute myocardial infarction. Am J Cardiol 74 1220 1228
9. VahediK
HofmeijerJ
JuettlerE
VicautE
GeorgeB
2007 Early decompressive surgery in malignant infarction of the middle cerebral artery: a pooled analysis of three randomised controlled trials. Lancet Neurol 6 215 222
10. LanghorneP
WilliamsBO
GilchristW
HowieK
1993 Do stroke units save lives? Lancet 342 395 398
11. FeiginVL
LawesCM
BennettDA
AndersonCS
2003 Stroke epidemiology: a review of population-based studies of incidence, prevalence, and case-fatality in the late 20th century. Lancet Neurol 2 43 53
12. BonitaR
BeagleholeR
1989 Increased treatment of hypertension does not explain the decline in stroke mortality in the United States, 1970–1980. Hypertension 13 I69 73
13. BejotY
Ben SalemD
OssebyGV
CouvreurG
DurierJ
2009 Epidemiology of ischemic stroke from atrial fibrillation in Dijon, France, from 1985 to 2006. Neurology 72 346 353
14. ConnollySJ
EzekowitzMD
YusufS
EikelboomJ
OldgrenJ
2009 Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 361 1139 1151
15. KwonHM
KimBJ
ParkJH
RyuWS
KimCK
2009 Significant association of metabolic syndrome with silent brain infarction in elderly people. J Neurol
16. LovshinJA
DruckerDJ
2009 Incretin-based therapies for type 2 diabetes mellitus. Nat Rev Endocrinol 5 262 269
17. DienerHC
2006 Secondary stroke prevention with antiplatelet drugs: have we reached the ceiling? Int J Stroke 1 4 8
18. HoksbergenAW
MajoieCB
HulsmansFJ
LegemateDA
2003 Assessment of the collateral function of the circle of Willis: three-dimensional time-of-flight MR angiography compared with transcranial color-coded duplex sonography. AJNR Am J Neuroradiol 24 456 462
19. MiteffF
LeviCR
BatemanGA
SprattN
McElduffP
2009 The independent predictive utility of computed tomography angiographic collateral status in acute ischaemic stroke. Brain 132 2231 2238
20. BiswasA
TiwariAK
RanjanR
MeenaA
AkhterMS
2009 Prothrombotic polymorphisms, mutations, and their association with pediatric non-cardioembolic stroke in Asian-Indian patients. Ann Hematol 88 473 478
21. AdamskiMG
TurajW
SlowikA
Wloch-KopecD
WolkowP
2009 A-G-4G haplotype of PAI-1 gene polymorphisms −844 G/A, HindIII G/C, and −675 4G/5G is associated with increased risk of ischemic stroke caused by small vessel disease. Acta Neurol Scand 120 94 100
22. GilliganAK
ThriftAG
SturmJW
DeweyHM
MacdonellRA
2005 Stroke Units, Tissue Plasminogen Activator, Aspirin and Neuroprotection: Which Stroke Intervention Could Provide the Greatest Community Benefit? Cerebrovasc Dis 20 239 244
23. DawsonTM
DawsonVL
2006 Taming the clot-buster tPA. Nat Med 12 993 994
24. BluhmkiE
ChamorroA
DavalosA
MachnigT
SauceC
2009 Stroke treatment with alteplase given 3.0–4.5 h after onset of acute ischaemic stroke (ECASS III): additional outcomes and subgroup analysis of a randomised controlled trial. Lancet Neurol 8 1095 1102
25. HackeW
KasteM
BluhmkiE
BrozmanM
DavalosA
2008 Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med 359 1317 1329
26. SuEJ
FredrikssonL
GeyerM
FolestadE
CaleJ
2008 Activation of PDGF-CC by tissue plasminogen activator impairs blood-brain barrier integrity during ischemic stroke. Nat Med 14 731 737
27. LadenvallC
GilsA
JoodK
BlomstrandC
DeclerckPJ
2007 Thrombin activatable fibrinolysis inhibitor activation peptide shows association with all major subtypes of ischemic stroke and with TAFI gene variation. Arterioscler Thromb Vasc Biol 27 955 962
28. BrounsR
HeylenE
SheorajpandayR
WillemseJL
KunnenJ
2009 Carboxypeptidase U (TAFIa) decreases the efficacy of thrombolytic therapy in ischemic stroke patients. Clin Neurol Neurosurg 111 165 170
29. SemeraroF
AmmolloCT
SemeraroN
ColucciM
2009 Tissue factor-expressing monocytes inhibit fibrinolysis through a TAFI-mediated mechanism, and make clots resistant to heparins. Haematologica 94 819 826
30. BajzarL
NesheimME
TracyPB
1996 The profibrinolytic effect of activated protein C in clots formed from plasma is TAFI-dependent. Blood 88 2093 2100
31. BirdE
TamuraJ
BostwickJS
SteinbacherTE
StewartA
2007 Is exogenous tissue plasminogen activator necessary for antithrombotic efficacy of an inhibitor of thrombin activatable fibrinolysis inhibitor (TAFI) in rats? Thromb Res 120 549 558
32. SmithWS
SungG
StarkmanS
SaverJL
KidwellCS
2005 Safety and efficacy of mechanical embolectomy in acute ischemic stroke: results of the MERCI trial. Stroke 36 1432 1438
33. BoseA
HenkesH
AlfkeK
ReithW
MayerTE
2008 The Penumbra System: a mechanical device for the treatment of acute stroke due to thromboembolism. AJNR Am J Neuroradiol 29 1409 1413
34. MacleodMR
FisherM
O'CollinsV
SenaES
DirnaglU
2009 Good laboratory practice: preventing introduction of bias at the bench. Stroke 40 e50 52
35. SenaE
van der WorpHB
HowellsD
MacleodM
2007 How can we improve the pre-clinical development of drugs for stroke? Trends Neurosci 30 433 439
36. STAIR 1999 Recommendations for standards regarding preclinical neuroprotective and restorative drug development. Stroke 30 2752 2758
37. van der WorpHB
SenaES
DonnanGA
HowellsDW
MacleodMR
2007 Hypothermia in animal models of acute ischaemic stroke: a systematic review and meta-analysis. Brain 130 3063 3074
38. BernardS
2006 Therapeutic hypothermia after cardiac arrest: now a standard of care. Crit Care Med 34 923 924
39. SalussoliaCL
NalwalkJW
HoughLB
2007 Improgan-induced hypothermia: a role for cannabinoid receptors in improgan-induced changes in nociceptive threshold and body temperature. Brain Res 1152 42 48
40. AslamiH
SchultzMJ
JuffermansNP
2009 Potential applications of hydrogen sulfide-induced suspended animation. Curr Med Chem 16 1295 1303
41. SesslerDI
2009 Defeating normal thermoregulatory defenses: induction of therapeutic hypothermia. Stroke 40 e614 621
42. HenningerN
BouleyJ
NelliganJM
SicardKM
FisherM
2007 Normobaric hyperoxia delays perfusion/diffusion mismatch evolution, reduces infarct volume, and differentially affects neuronal cell death pathways after suture middle cerebral artery occlusion in rats. J Cereb Blood Flow Metab 27 1632 1642
43. SinghalAB
BennerT
RoccatagliataL
KoroshetzWJ
SchaeferPW
2005 A pilot study of normobaric oxygen therapy in acute ischemic stroke. Stroke 36 797 802
44. HoltmaatA
SvobodaK
2009 Experience-dependent structural synaptic plasticity in the mammalian brain. Nat Rev Neurosci 10 647 658
45. CurtisMA
ErikssonPS
FaullRL
2007 Progenitor cells and adult neurogenesis in neurodegenerative diseases and injuries of the basal ganglia. Clin Exp Pharmacol Physiol 34 528 532
46. KuhlmannT
MironV
CuoQ
WegnerC
AntelJ
2008 Differentiation block of oligodendroglial progenitor cells as a cause for remyelination failure in chronic multiple sclerosis. Brain 131 1749 1758
47. JohanssonBB
2000 Brain plasticity and stroke rehabilitation. The Willis lecture. Stroke 31 223 230
48. MinnerupJ
HeidrichJ
WellmannJ
RogalewskiA
SchneiderA
2008 Meta-analysis of the efficacy of granulocyte-colony stimulating factor in animal models of focal cerebral ischemia. Stroke 39 1855 1861
49. DaadiMM
MaagAL
SteinbergGK
2008 Adherent self-renewable human embryonic stem cell-derived neural stem cell line: functional engraftment in experimental stroke model. PLoS One 3 e1644
50. OttHC
MatthiesenTS
GohSK
BlackLD
KrenSM
2008 Perfusion-decellularized matrix: using nature's platform to engineer a bioartificial heart. Nat Med 14 213 221
51. DirnaglU
SchwabJM
2009 Brain-immune interactions in acute and chronic brain disorders. Neuroscience 158 969 971
52. BatchelorPE
LiberatoreGT
WongJY
PorrittMJ
FrerichsF
1999 Activated macrophages and microglia induce dopaminergic sprouting in the injured striatum and express brain-derived neurotrophic factor and glial cell line-derived neurotrophic factor. J Neurosci 19 1708 1716
53. LoEH
2008 A new penumbra: transitioning from injury into repair after stroke. Nat Med 14 497 500
54. WhisnantJ
1997 Modeling of risk factors for ischemic stroke. The Willis Lecture. Stroke 28 1840 1844
55. LouviA
Arboleda-VelasquezJF
Artavanis-TsakonasS
2006 CADASIL: a critical look at a Notch disease. Dev Neurosci 28 5 12
56. BilguvarK
YasunoK
NiemelaM
RuigrokYM
von Und Zu FraunbergM
2008 Susceptibility loci for intracranial aneurysm in European and Japanese populations. Nat Genet 40 1472 1477
57. GschwendtnerA
BevanS
ColeJW
PlourdeA
MatarinM
2009 Sequence variants on chromosome 9p21.3 confer risk for atherosclerotic stroke. Ann Neurol 65 531 539
58. 1995 Tissue plasminogen activator for acute ischemic stroke. The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. N Engl J Med 333 1581 1587
59. 1997 The International Stroke Trial (IST): a randomised trial of aspirin, subcutaneous heparin, both, or neither among 19435 patients with acute ischaemic stroke. International Stroke Trial Collaborative Group. Lancet 349 1569 1581
60. MayerSA
BrunNC
BegtrupK
BroderickJ
DavisS
2005 Recombinant activated factor VII for acute intracerebral hemorrhage. N Engl J Med 352 777 785
61. MendelowAD
GregsonBA
FernandesHM
MurrayGD
TeasdaleGM
2005 Early surgery versus initial conservative treatment in patients with spontaneous supratentorial intracerebral haematomas in the International Surgical Trial in Intracerebral Haemorrhage (STICH): a randomised trial. Lancet 365 387 397
62. HackeW
AlbersG
Al-RawiY
BogousslavskyJ
DavalosA
2005 The Desmoteplase in Acute Ischemic Stroke Trial (DIAS): a phase II MRI-based 9-hour window acute stroke thrombolysis trial with intravenous desmoteplase. Stroke 36 66 73
63. AlexandrovAV
MolinaCA
GrottaJC
GaramiZ
FordSR
2004 Ultrasound-enhanced systemic thrombolysis for acute ischemic stroke. N Engl J Med 351 2170 2178
64. 2006 Glyceryl trinitrate vs. control, and continuing vs. stopping temporarily prior antihypertensive therapy, in acute stroke: rationale and design of the Efficacy of Nitric Oxide in Stroke (ENOS) trial (ISRCTN99414122). Int J Stroke 1 245 249
65. LeesKR
ZivinJA
AshwoodT
DavalosA
DavisSM
2006 NXY-059 for acute ischemic stroke. N Engl J Med 354 588 600
66. 1978 A randomized trial of aspirin and sulfinpyrazone in threatened stroke. The Canadian Cooperative Study Group. N Engl J Med 299 53 59
67. DienerHC
CunhaL
ForbesC
SiveniusJ
SmetsP
1996 European Stroke Prevention Study. 2. Dipyridamole and acetylsalicylic acid in the secondary prevention of stroke. J Neurol Sci 143 1 13
68. CAPRIE Steering Committee 1996 A randomised, blinded, trial of clopidogrel versus aspirin in patients at risk of ischaemic events (CAPRIE). Lancet 348 1329 1339
69. 1993 Secondary prevention in non-rheumatic atrial fibrillation after transient ischaemic attack or minor stroke. EAFT (European Atrial Fibrillation Trial) Study Group. Lancet 342 1255 1262
70. 1991 Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. North American Symptomatic Carotid Endarterectomy Trial Collaborators. N Engl J Med 325 445 453
71. 1991 MRC European Carotid Surgery Trial: interim results for symptomatic patients with severe (70–99%) or with mild (0–29%) carotid stenosis. European Carotid Surgery Trialists' Collaborative Group. Lancet 337 1235 1243
72. 2001 Randomised trial of a perindopril-based blood-pressure-lowering regimen among 6,105 individuals with previous stroke or transient ischaemic attack. Lancet 358 1033 1041
73. AmarencoP
BogousslavskyJ
CallahanA
GoldsteinLB
HennericiM
2006 High-dose atorvastatin after stroke or transient ischemic attack. New England Journal of Medicine 355 549 559
74. YadavJS
WholeyMH
KuntzRE
FayadP
KatzenBT
2004 Protected carotid-artery stenting versus endarterectomy in high-risk patients. N Engl J Med 351 1493 1501
Štítky
Interné lekárstvo
Článok vyšiel v časopisePLOS Medicine
Najčítanejšie tento týždeň
2010 Číslo 3- Parazitičtí červi v terapii Crohnovy choroby a dalších zánětlivých autoimunitních onemocnění
- Intermitentní hladovění v prevenci a léčbě chorob
- Statinová intolerance
- Co dělat při intoleranci statinů?
- Monoklonální protilátky v léčbě hyperlipidemií
-
Všetky články tohto čísla
- CONSORT 2010 Statement: Updated Guidelines for Reporting Parallel Group Randomised Trials
- BMI and Risk of Serious Upper Body Injury Following Motor Vehicle Crashes: Concordance of Real-World and Computer-Simulated Observations
- Preventing Road Deaths—Time for Data
- Essential Surgery at the District Hospital: A Retrospective Descriptive Analysis in Three African Countries
- Drivers of Inequality in Millennium Development Goal Progress: A Statistical Analysis
- Where Will the Next Generation of Stroke Treatments Come From?
- Unravelling the Genetics of Ischaemic Stroke
- Chronic Obstructive Pulmonary Disease: Effects beyond the Lungs
- The Promise of Prevention: The Effects of Four Preventable Risk Factors on National Life Expectancy and Life Expectancy Disparities by Race and County in the United States
- Can Animal Models of Disease Reliably Inform Human Studies?
- New Approaches to Preventing, Diagnosing, and Treating Neonatal Sepsis
- Protecting Vulnerable Road Users from Injury
- Providing Alcohol-Related Screening and Brief Interventions to Adolescents through Health Care Systems: Obstacles and Solutions
- Effects on Coronary Heart Disease of Increasing Polyunsaturated Fat in Place of Saturated Fat: A Systematic Review and Meta-Analysis of Randomized Controlled Trials
- Accelerating Policy Decisions to Adopt Type b Vaccine: A Global, Multivariable Analysis
- Human Resource and Funding Constraints for Essential Surgery in District Hospitals in Africa: A Retrospective Cross-Sectional Survey
- PLOS Medicine
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- BMI and Risk of Serious Upper Body Injury Following Motor Vehicle Crashes: Concordance of Real-World and Computer-Simulated Observations
- Unravelling the Genetics of Ischaemic Stroke
- Providing Alcohol-Related Screening and Brief Interventions to Adolescents through Health Care Systems: Obstacles and Solutions
- Human Resource and Funding Constraints for Essential Surgery in District Hospitals in Africa: A Retrospective Cross-Sectional Survey
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



