-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
What Will It Take to Eliminate Pediatric HIV? Reaching WHO Target Rates of Mother-to-Child HIV Transmission in Zimbabwe: A Model-Based Analysis
Background:
The World Health Organization (WHO) has called for the “virtual elimination” of pediatric HIV: a mother-to-child HIV transmission (MTCT) risk of less than 5%. We investigated uptake of prevention of MTCT (PMTCT) services, infant feeding recommendations, and specific drug regimens necessary to achieve this goal in Zimbabwe.Methods and Findings:
We used a computer model to simulate a cohort of HIV-infected, pregnant/breastfeeding women (mean age, 24 y; mean CD4, 451/µl; breastfeeding duration, 12 mo). Three PMTCT regimens were evaluated: (1) single-dose nevirapine (sdNVP), (2) WHO 2010 guidelines' “Option A” (zidovudine in pregnancy, infant nevirapine throughout breastfeeding for women without advanced disease, lifelong combination antiretroviral therapy for women with advanced disease), and (3) WHO “Option B” (pregnancy/breastfeeding-limited combination antiretroviral drug regimens without advanced disease; lifelong antiretroviral therapy with advanced disease). We examined four levels of PMTCT uptake (proportion of pregnant women accessing and adhering to PMTCT services): reported rates in 2008 and 2009 (36% and 56%, respectively) and target goals in 2008 and 2009 (80% and 95%, respectively). The primary model outcome was MTCT risk at weaning.
The 2008 sdNVP-based National PMTCT Program led to a projected 12-mo MTCT risk of 20.3%. Improved uptake in 2009 reduced projected risk to 18.0%. If sdNVP were replaced by more effective regimens, with 2009 (56%) uptake, estimated MTCT risk would be 14.4% (Option A) or 13.4% (Option B). Even with 95% uptake of Option A or B, projected transmission risks (6.1%–7.7%) would exceed the WHO goal of less than 5%. Only if the lowest published transmission risks were used for each drug regimen, or breastfeeding duration were shortened, would MTCT risks at 95% uptake fall below 5%.Conclusions:
Implementation of the WHO PMTCT guidelines must be accompanied by efforts to improve access to PMTCT services, retain women in care, and support medication adherence throughout pregnancy and breastfeeding, to approach the “virtual elimination” of pediatric HIV in Zimbabwe.
: Please see later in the article for the Editors' Summary
Published in the journal: What Will It Take to Eliminate Pediatric HIV? Reaching WHO Target Rates of Mother-to-Child HIV Transmission in Zimbabwe: A Model-Based Analysis. PLoS Med 9(1): e32767. doi:10.1371/journal.pmed.1001156
Category: Research Article
doi: https://doi.org/10.1371/journal.pmed.1001156Summary
Background:
The World Health Organization (WHO) has called for the “virtual elimination” of pediatric HIV: a mother-to-child HIV transmission (MTCT) risk of less than 5%. We investigated uptake of prevention of MTCT (PMTCT) services, infant feeding recommendations, and specific drug regimens necessary to achieve this goal in Zimbabwe.Methods and Findings:
We used a computer model to simulate a cohort of HIV-infected, pregnant/breastfeeding women (mean age, 24 y; mean CD4, 451/µl; breastfeeding duration, 12 mo). Three PMTCT regimens were evaluated: (1) single-dose nevirapine (sdNVP), (2) WHO 2010 guidelines' “Option A” (zidovudine in pregnancy, infant nevirapine throughout breastfeeding for women without advanced disease, lifelong combination antiretroviral therapy for women with advanced disease), and (3) WHO “Option B” (pregnancy/breastfeeding-limited combination antiretroviral drug regimens without advanced disease; lifelong antiretroviral therapy with advanced disease). We examined four levels of PMTCT uptake (proportion of pregnant women accessing and adhering to PMTCT services): reported rates in 2008 and 2009 (36% and 56%, respectively) and target goals in 2008 and 2009 (80% and 95%, respectively). The primary model outcome was MTCT risk at weaning.
The 2008 sdNVP-based National PMTCT Program led to a projected 12-mo MTCT risk of 20.3%. Improved uptake in 2009 reduced projected risk to 18.0%. If sdNVP were replaced by more effective regimens, with 2009 (56%) uptake, estimated MTCT risk would be 14.4% (Option A) or 13.4% (Option B). Even with 95% uptake of Option A or B, projected transmission risks (6.1%–7.7%) would exceed the WHO goal of less than 5%. Only if the lowest published transmission risks were used for each drug regimen, or breastfeeding duration were shortened, would MTCT risks at 95% uptake fall below 5%.Conclusions:
Implementation of the WHO PMTCT guidelines must be accompanied by efforts to improve access to PMTCT services, retain women in care, and support medication adherence throughout pregnancy and breastfeeding, to approach the “virtual elimination” of pediatric HIV in Zimbabwe.
: Please see later in the article for the Editors' SummaryIntroduction
When antiretroviral drugs (ARVs) are administered during pregnancy, and breastfeeding is avoided, mother-to-child HIV transmission (MTCT) may occur in fewer than 1% of pregnancies [1]. These highly effective prevention strategies have led the pediatric HIV epidemic to be nearly eliminated in the United States and Europe [1],[2]. In settings where breastfeeding is recommended for improved infant health [3], recent trials have demonstrated that the provision of ARVs to breastfeeding mothers or their infants can reduce total MTCT rates to 1%–5% at 6 mo of age [4]–[15]. Based on these encouraging results, in 2009, HIV prevention and treatment organizations emphasized a new goal: the “virtual elimination” of pediatric HIV infection, defined as the reduction of MTCT to less than 5% [16]–[20]. Toward this goal, the World Health Organization (WHO) released new guidelines for the prevention of MTCT (PMTCT) in 2010, based on combination antiretroviral therapy (ART) for women with advanced HIV disease, and country-level selection between two antiretroviral options during pregnancy and breastfeeding (“Option A” or “Option B”) for women with less advanced disease [21]. Option A includes zidovudine (ZDV) during pregnancy and single-dose nevirapine (sdNVP) at delivery, followed by daily nevirapine (NVP) syrup for infants throughout the duration of breastfeeding; Option B includes maternal triple-drug ARV regimens throughout pregnancy and breastfeeding (Table A in Text S1) [21].
However, opportunities for PMTCT may be lost at each step in a “cascade” of care, from presentation to antenatal care (ANC), through HIV testing and result receipt, to ARV adherence through the many months of pregnancy and breastfeeding (Figure 1; Figure A in Text S1 [22]–[25]. The WHO estimates that only 53% of pregnant women worldwide received any ARVs for PMTCT in 2009 [19]. As a result, more than 370,000 new perinatal HIV infections were estimated to have occurred in 2009; the majority of these were in sub-Saharan Africa, where access to pediatric HIV therapy is limited and infant mortality is extremely high [19],[26].
Fig. 1. Two dimensions for potential improvements in PMTCT in Zimbabwe. 
This figure shows the “two dimensions” in which PMTCT services can be improved. First, along the vertical arrow, PMTCT programs can transition to more intensive drug regimens (i.e., from sdNVP to Option A to Option B). Second, along the horizontal arrow, programs can undertake interventions to improve “uptake” of PMTCT services, defined as the proportion of pregnant, HIV-infected women who receive and adhere to ARVs for PMTCT, for example, from 36% in 2008 to 56% in 2009, and perhaps to 80% or 95% with future scale-up effort. Within the horizontal arrow are depicted the three “domains” of uptake examined in these analyses: care and testing, drug availability, or retention. sdNVP represents the current National PMTCT Program, based largely on sdNVP; “Option A” and “Option B” are the WHO 2010 PMTCT guideline-recommended regimens, as defined in the text and Text S1. Since 2002, the Zimbabwean national PMTCT program has provided ART to pregnant women with clinical evidence of WHO stage 3/4 disease, and sdNVP to all others [27]. As in many sub-Saharan African countries where prolonged breastfeeding is common, Zimbabwe is implementing the 2010 WHO guidelines with Option A. Because of the challenges of enrolling and retaining women in care at each step in the PMTCT cascade, it is not known how effective this strategy will be. We used a simulation model to project the level of PMTCT uptake in Zimbabwe, the PMTCT drug regimens, and the duration of breastfeeding that would be necessary to reach the WHO “virtual elimination” goal of an MTCT risk below 5%.
Methods
Analytic Overview
We expanded a validated computer simulation model of MTCT [28],[29] to include each step in the “cascade” of PMTCT-related care, from first presentation at ANC through 2 y postpartum (Figure A in Text S1). The MTCT model simulates a single pregnancy, delivery, and postpartum period for each mother–infant pair, and projects infant outcomes at birth. Additional clinical events for mothers and infants through 2 y after delivery are simulated using the CEPAC (Cost-Effectiveness of Preventing AIDS Complications) model (Figure B in Text S1) [30]–[32]. The primary outcome of the linked CEPAC and MTCT models is risk of infant HIV transmission at the time of weaning; secondary outcomes include HIV infection risk at 4–6 wk of age, 2-y pediatric survival, and 2-y HIV-free pediatric survival.
This analysis examined improvements along two dimensions of PMTCT care: more effective PMTCT regimens, and improved uptake of the PMTCT “cascade” (Figure 1). We examined three possible PMTCT regimens in Zimbabwe: (1) the 2002–2009 National PMTCT Program, based on sdNVP alone, (2) WHO 2010 guidelines' Option A, and (3) WHO 2010 guidelines' Option B. Infant outcomes were projected at four levels of PMTCT uptake (36%, 56%, 90%, and 95% of women/infants receiving medications by the time of infant delivery) [19],[23]. Key sensitivity analyses examined the impacts of duration of breastfeeding, maternal HIV disease stage, the range of published MTCT risks for each PMTCT regimen, and a “full” (100%) uptake scenario.
Population
The linked models were used to simulate two populations of pregnant and breastfeeding women in Zimbabwe, with mean age of 24 y (standard deviation [SD]: 5 y) [33]. Cohort 1 included women already HIV-infected at their first ANC visit (regardless of whether HIV status was known to patients or providers). Cohort 2 was composed of all women becoming pregnant each year in Zimbabwe, an estimated 392,460 women [34],[35], with HIV prevalence of 16% at first ANC visit and HIV incidence of 1%/year during late pregnancy and breastfeeding [36]. Cohort 1 was thus nested within Cohort 2. Cohort 1 was analyzed to project MTCT rates, and Cohort 2 to project the proportion and number of infants in an annual birth cohort anticipated to become HIV-infected by 18 mo of age. All women were assumed to breastfeed their infants for 12 mo in the base case, based on WHO infant feeding guidelines [3].
PMTCT Regimens and PMTCT Uptake Scenarios
The antenatal, intrapartum, and postpartum/neonatal components of each modeled PMTCT regimen are detailed in Table A in Text S1. In all modeled regimens, women identified as eligible for ART (CD4≤350/µl or WHO stage 3/4 disease) were referred for ART. The availability of ART after referral depended on the modeled PMTCT uptake scenario; the availability of CD4 assays was varied in sensitivity analyses. Following convention, we refer to combination antiretroviral therapy regimens as “ART” when intended for treatment of maternal HIV disease (also effective for PMTCT) and as “triple-drug ARV regimens” when offered to non-ART-eligible women for PMTCT.
To create the four uptake scenarios, the “cascade” of PMTCT services was categorized into three broad domains (Table 1; Figure 1): “care and testing,” “drug availability,” and “retention.” We then calculated a “product of participation” (POP), defined as the product of the proportions of women/infants receiving care in each of these three domains, both at delivery and at the end of the breastfeeding period [23]. Four primary scenarios of PMTCT uptake were simulated for each PMTCT regimen (Table 1). The first two scenarios reflected WHO and Zimbabwean Ministry of Health and Child Welfare estimates of uptake at each step in the PMTCT cascade for Zimbabwe in 2008 (total POP at delivery: 36%) and 2009 (total POP at delivery: 56%) [19],[23],[36],[37]. The “WHO target” scenario modeled the current WHO goal that 80% of pregnant women be HIV-tested and 80% of HIV-infected women receive PMTCT services [20],[37]. The “optimal” uptake scenario simulated 95% uptake of the complete PMTCT cascade through delivery, likely representing the best practically achievable outcomes and lowest MTCT risks for the evaluated PMTCT regimens. A scenario of full, 100% uptake was also examined in sensitivity analyses (Tables D–G in Text S1), to represent the maximum potential biologic efficacy of each regimen.
Tab. 1. PMTCT uptake scenarios. 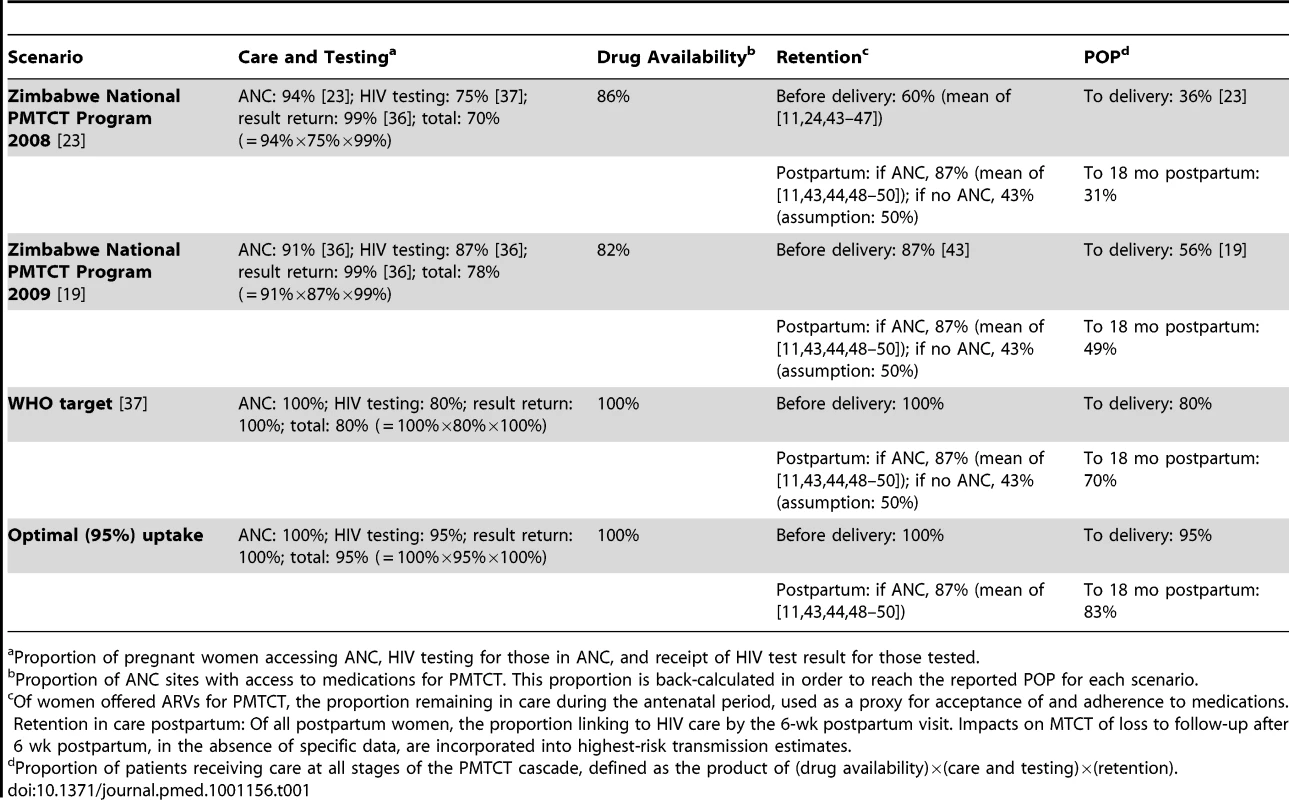
Proportion of pregnant women accessing ANC, HIV testing for those in ANC, and receipt of HIV test result for those tested. Model Structure
Antenatal and intrapartum outcomes: MTCT model
The MTCT model is a previously published, decision-analytic simulation of a cohort of pregnant women from the time of conception through delivery (TreeAgePro) [28],[29]. The model uses a decision-tree (deterministic) structure, including probabilities of the following: presentation to ANC; offer and acceptance of HIV testing; receipt of HIV test results; clinical assessment for ART eligibility; CD4 testing and receipt of results; offer of, acceptance of, and adherence to ARVs for PMTCT; maternal mortality during pregnancy; HIV testing in labor for women with unknown or negative HIV status; live birth; infant HIV infection by the time of delivery; and linkage to postnatal care and ART for mothers and infants (Figure A in Text S1).
Postnatal outcomes: CEPAC adult model
The CEPAC model of adult HIV infection is a computer simulation model of HIV disease in adults. Technical details of the CEPAC model are described in Text S1 [30],[38]; outcomes of the CEPAC model for postpartum women have been validated against published data (Text S1) [32]. For this analysis, the adult CEPAC model was used only to project maternal mortality risks during the first 2 y after delivery, in order to inform infant mortality rates and duration of breastfeeding in the infant model.
Postnatal outcomes: CEPAC infant model
A first-order, Monte Carlo simulation model of infant HIV infection and survival was added to the adult CEPAC model (Figure B in Text S1) [29]. Infants enter this model at birth and are assigned one of three HIV categories (HIV-unexposed, HIV-exposed but uninfected, or HIV-infected), as well as one of three maternal disease categories (HIV-uninfected, HIV-infected and “ART eligible,” or HIV-infected and “non-ART-eligible”). Over the first 2 y of life, modeled infants face a monthly probability of four key clinical events: (1) incident maternal HIV infection during breastfeeding, if mother was previously uninfected, causing infants to transition from “unexposed” to “exposed, uninfected”; (2) maternal death, with risks derived from the adult CEPAC model as above, after which infants are no longer at risk for HIV infection but are at higher risk of death due to orphanhood [39]–[42]; (3) infant HIV infection through breastfeeding, if infant was previously uninfected; and (4) infant death from any cause. Risks of infant mortality are stratified by infant HIV exposure and infection status, by receipt of ART if infected, and by maternal vital status.
The MTCT and CEPAC models were linked to allow a combined analysis in which each woman–infant pair is simulated together from the time of first presentation at ANC through pregnancy and delivery (the MTCT model), and then each woman and infant are simulated separately through the first 2 y postpartum (the CEPAC models). Details of the linkages between the CEPAC and MTCT models have been published previously [29] and are further described in Figures A and B in Text S1.
Loss to follow-up
During ANC in the MTCT model, women may be lost to follow-up between first ANC presentation and delivery [11],[24],[43]–[47], between delivery and 6 wk postpartum [11],[43],[44],[48]–[50], and after linkage to postnatal HIV care [43],[44],[51]–[56]. In the absence of data regarding rates of adherence to the Option A or Option B regimens during breastfeeding, the impacts of medication interruptions or discontinuations were assumed to be included in sensitivity analyses examining “highest risk” postnatal MTCT estimates.
Model Input Parameters
Baseline maternal characteristics reflected cohorts of pregnant women in Zimbabwe (Table 2). At first ANC visit, mean age was 24 y (SD: 5 y) [57], and the proportion with CD4≤350/µl was 36%, based on data from Zimbabwe [58]. In the base case, MTCT risks were derived as the average (mean or midpoint) of risks reported in PTMCT studies in Africa (Table 3; Text S1) [4]–[10],[12]–[15],[58]–[69]. To avoid underestimating early postpartum MTCT risks, we included only data from breastfed infants, necessarily excluding pivotal PMTCT studies among replacement-fed populations [8],[70]–. In the absence of reported postnatal transmission risks for infants older than 6 mo of age who continue to breastfeed with ongoing prophylaxis (Option A or B), we assumed a constant monthly risk based on data in younger children, as has been observed for postnatal transmission without prophylaxis [73]. Infant mortality rates (Table 4) were derived from Joint United Nations Programme on HIV/AIDS HIV-deleted mortality estimates (HIV-unexposed infants) [74], the ZVITAMBO study in Zimbabwe (HIV-exposed, uninfected infants) [75], and pooled analyses of several African cohorts (HIV-infected infants) [26],[76],[77].
Tab. 2. Model input parameters: maternal characteristics and uptake of PMTCT services. 
Age given as mean (SD) in years; CD4 counts given as mean (SD) in number/microliter. Maternal disease progression: details of the CEPAC model and data inputs describing maternal HIV disease progression with and without ART are provided in Text S1. Tab. 3. Model input parameters: mother-to-child transmission risks. 
Data given as base-case value [references] (range for sensitivity analysis) [references]. Tab. 4. Model input parameters: infant mortality. 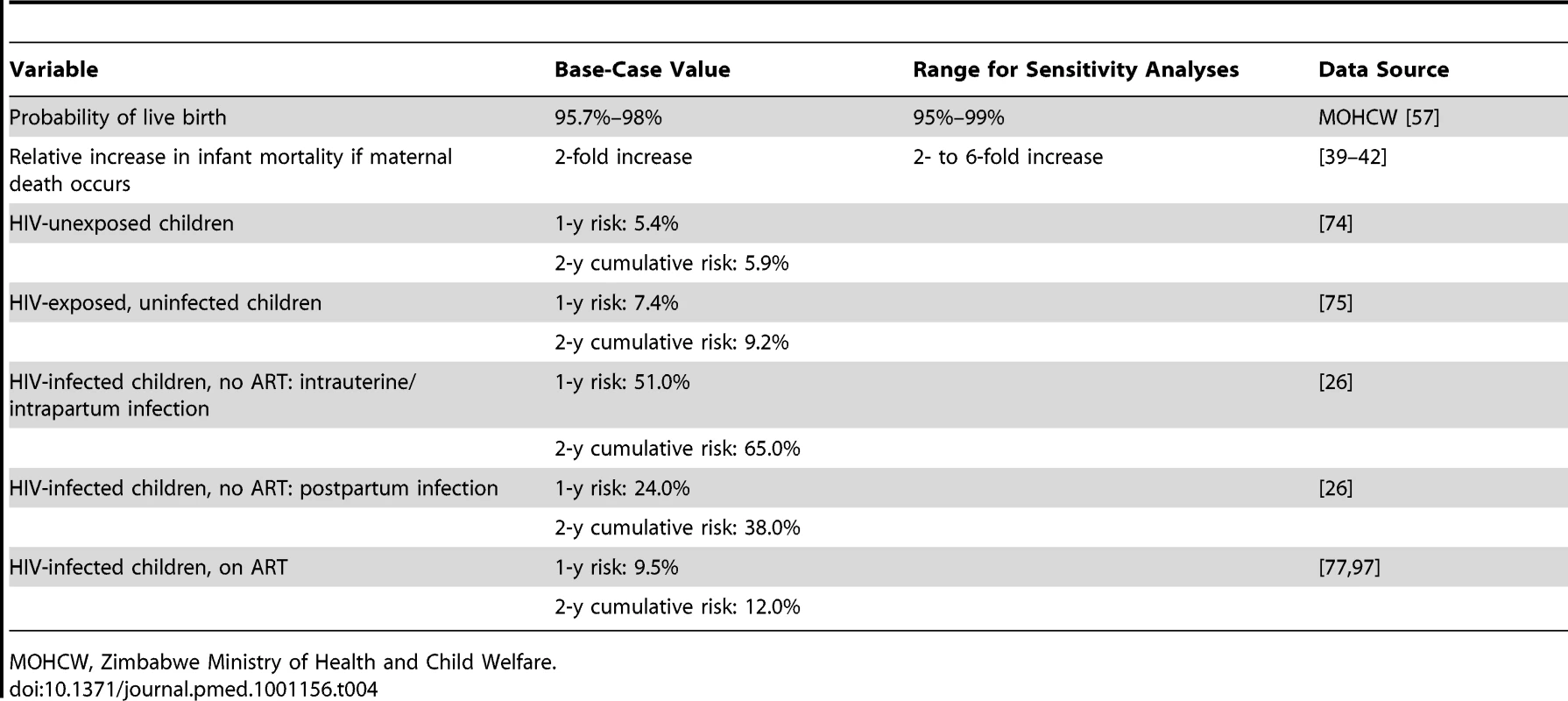
MOHCW, Zimbabwe Ministry of Health and Child Welfare. Model Validation and Sensitivity Analyses
Validation of model-derived MTCT and mortality risks against published data, and sensitivity analyses on many clinical and programmatic parameters, have been reported previously [29].
For this analysis, in addition to the impact of PMTCT uptake and regimen examined in the base-case analyses, we conducted univariate sensitivity analyses on three key factors influencing transmission risk. First, to examine the impact of the range of published MTCT risks for each regimen, we defined “highest risk” and “lowest risk” scenarios. The “lowest risk” scenarios use the lowest published MTCT risks for each drug regimen, reflecting the best reported field effectiveness or trial efficacy, and likely representing the most adherent study populations; the “highest risk” scenarios use the highest published MTCT risks for each drug regimen. Second, because the proportion of women with CD4≤350/µl or WHO stage 3/4 disease at first ANC visit has been reported to range widely (23%–68% [78],[79]), we varied this proportion in sensitivity analyses from 0% to 100%. Third, we investigated the contribution of prolonged breastfeeding by projecting MTCT risk at 4–6 wk of age (primarily reflecting transmission that occurs in the absence of breastfeeding), as well as after 18 mo of breastfeeding (the median duration reported in a cohort from Zimbabwe [58]), with ARV prophylaxis continued throughout breastfeeding.
We identified the parameters that exerted the greatest impact on MTCT risks by comparing the range of MTCT risks projected when each parameter was varied through clinically plausible ranges; the largest range of projected MTCT risks reflected parameters with the greatest influence on model results. We then performed multivariate sensitivity analyses to determine combinations of these factors necessary to reach MTCT risks less than 5%.
To further examine the multiplicative impact of individual components of the PMTCT cascade, we varied uptake at each step in the care pathway (access to ANC, HIV testing in ANC, receipt of HIV test results, medication availability, adherence to medications, and linkage to postnatal HIV-related care), as well as the availability of CD4 counts and ART for women with CD4≤350/µl.
Results
MTCT Risk among Infants Born to Women HIV-Infected Before Pregnancy (Cohort 1)
The modeled 12-mo MTCT risk in the sdNVP-based 2008 Zimbabwean National PMTCT Program was 20.3% (Table 5, top). Modeled improvements in PMTCT uptake in the 2009 National PMTCT Program are projected to have reduced this risk to 18.0%. If sdNVP could be replaced by WHO-recommended regimens at the current 56% uptake level, MTCT risk would be projected to fall to 14.4% with Option A, or 13.4% with Option B. The potential impacts of further improvements in coverage are highlighted in the “WHO target” and “optimal uptake” scenarios. If Option A were implemented at the WHO target level of 80%, MTCT risk would be projected to fall to 10.5%; “optimal” 95% uptake would reduce this further to 7.7%. With Option B, these risks are projected at 9.1% (80% uptake) and 6.1% (95% uptake). Similar impacts of PMTCT regimens and uptake scenarios are seen for the secondary outcomes of infant infection risk at 4–6 wk of age, 2-y pediatric survival, and 2-y pediatric HIV-free survival (Tables E–G in Text S1).
Tab. 5. Results of a model of PMTCT services in Zimbabwe: cumulative 12-mo infant HIV infection risks. 
Population-Level Results for All Women Becoming Pregnant Each Year in Zimbabwe (Cohort 2)
For an annual pregnancy cohort in Zimbabwe, incorporating HIV-negative women, chronically HIV-infected women, and women with incident HIV infection (Table 5, bottom), the overall modeled 12-mo risk of infant HIV infection under the 2008 National PMTCT Program was 3.5%, representing 13,910 HIV-infected infants. Improvements in uptake are modeled to have reduced this risk to 3.2% (12,440 infections) by 2009, averting 1,470 infections. Replacement of sdNVP by Option A or B at the current uptake level would be projected to reduce this risk to 2.4%–2.6% (9,560–10,200 infections), and improved levels of uptake would reduce this modeled risk further, to 1.3% (4,950 infections, a 60% reduction in infant infections compared to sdNVP at 56% uptake) with Option B at “optimal” 95% uptake.
Univariate Sensitivity Analyses
Univariate sensitivity analyses investigating the individual impacts of PMTCT uptake, lowest/highest risk scenarios, maternal CD4 count, breastfeeding duration, and PMTCT regimen among Cohort 1 are shown in Figure 2. When individual parameters were varied from the base-case scenario (Option A regimen, 12 mo of breastfeeding, 36% of mothers ART-eligible, and average of published MTCT risks for each regimen), PMTCT uptake was the most influential single parameter (Figure 2, widest bar). Projected transmission, with all other parameters held equal, ranged from 6.2% (100% uptake), through 14.4% (56% uptake, indicated by “base-case projection line”), to 17.9% (36% uptake). The second most influential parameter was the range of published MTCT risks for each regimen (ranges shown in Table 3): with Option A at 56% uptake, 12-mo MTCT risks ranged from 8.8% (“lowest risk”) to 18.5% (“highest risk”). The individual impacts of PMTCT uptake and lowest/highest risk scenarios, as well as of maternal CD4 count and breastfeeding duration, were greater than the impact of the choice of PMTCT regimen (Figure 2, narrowest bar). Additional univariate sensitivity analyses suggested that variations in the availability of HIV testing in labor and sdNVP for women identified as HIV-infected during labor also did not change the policy conclusions (Text S1). Overall, these univariate sensitivity analyses demonstrated that optimizing any single parameter shown in Figure 2 did not reduce modeled MTCT risk to less than 5%, underscoring the need for improvements in multiple parameters simultaneously to approach the “virtual elimination” of HIV in Zimbabwe.
Fig. 2. Key parameters determining MTCT risk. 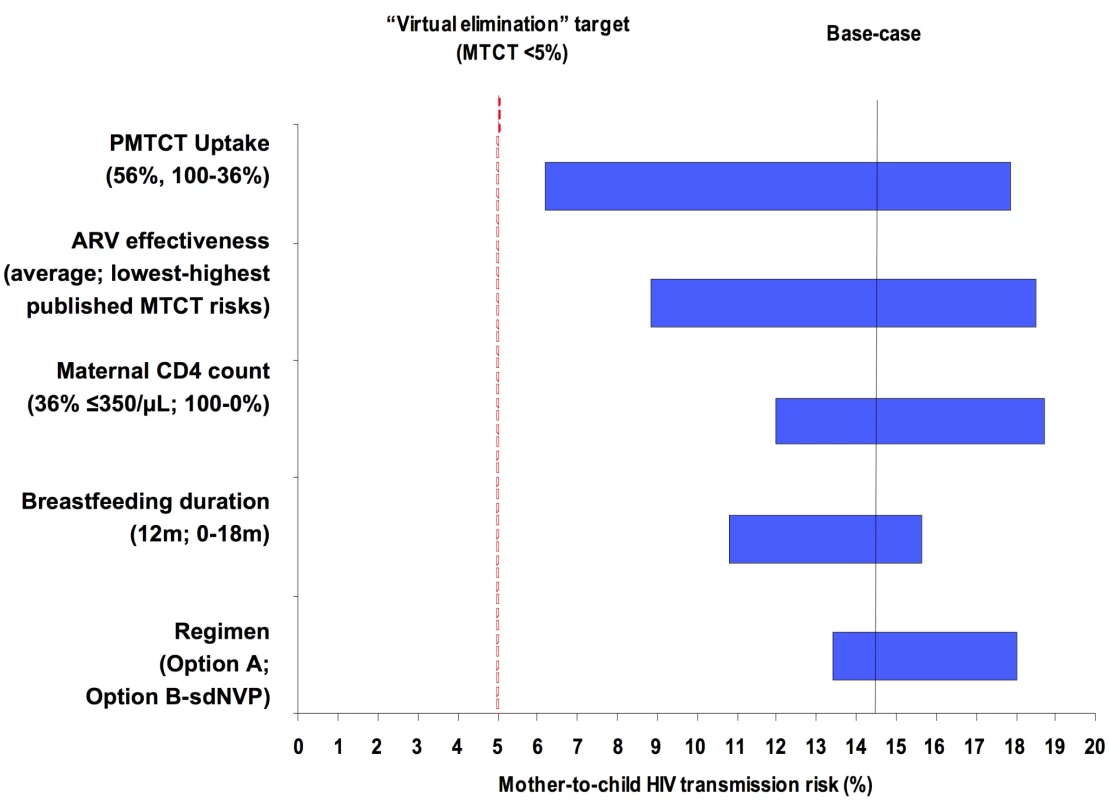
Tornado diagram summarizing the results of key one-way sensitivity analyses. Model parameters are on the vertical axis. For each parameter, the value used in the base-case analysis is listed in parentheses, followed by the range examined in sensitivity analysis. For example, the “regimen” provided for PMTCT is varied from Option B (lowest MTCT risk with all other parameters held constant), through Option A (base-case MTCT risk), to sdNVP (highest MTCT risk). The horizontal axis represents projected MTCT risk by the time of weaning. The solid vertical line represents transmission risk (14.4%) at the base-case set of parameters: 56% uptake, mean published MTCT risks, 36% of mothers with CD4<350/µl, breastfeeding duration of 12 mo, and the WHO “Option A” regimen. The dashed vertical line represents the 5% MTCT target of “virtual elimination” expressed by international HIV/AIDS agencies including WHO and the Joint United Nations Programme on HIV/AIDS. ARV prophylaxis in the Option A and Option B regimens is assumed to continue throughout the duration of breastfeeding. Multivariate Sensitivity Analyses
Multivariate sensitivity analyses highlighted the combinations of factors necessary to reduce MTCT among Cohort 1 to the WHO target of less than 5% (Figure 3, regions shaded in green). For example, at 80% uptake of Option A (middle panel), only if the lowest published MTCT risks and avoidance of breastfeeding were assumed was the <5% target projected to be achieved. With 80% uptake of Option B, a projected risk less than 5% still required assumption of the lowest published MTCT risks, but permitted breastfeeding durations up to 18 mo. With both the lowest published transmission risks and “optimal” (95%) uptake, 12-mo MTCT risks could decrease to 3.7% (Option A) or 1.9% (Option B).
Fig. 3. Combinations of parameters needed to achieve MTCT risks<5%, 5%–10%, and >10%. 
Each horizontal block represents results for a specific drug regimen: sdNVP (top), Option A (middle), and Option B (bottom). Within each block, four levels of uptake are depicted across the top horizontal axis: 56% uptake (current estimated uptake in Zimbabwe), 80% uptake (the WHO target), 95% uptake (reported in neighboring Botswana), and 100% uptake (to reflect maximum biologic efficacy of each regimen). The vertical axis illustrates three durations of breastfeeding (BF) for each modeled PMTCT regimen: 18 mo (median in Zimbabwe), 12 mo (concordant with 2010 WHO infant feeding guidelines), and no breastfeeding; ARV prophylaxis in the Option A and Option B regimens is assumed to continue throughout the duration of breastfeeding. The lower horizontal axis shows three categories of published MTCT risks for each drug regimen, including the lowest published risks, the average of published risks (the base-case parameters), and the highest published risks. The percentage in each cell reflects the MTCT risk associated with each set of parameters, and cells are color-coded to reflect broad categories of transmission. Red-colored cells indicate MTCT risks>10%, yellow-colored cells indicate MTCT risks between 5% and 10%, and green-colored cells indicate MTCT risks<5%. Impact of Overall Product of Participation (Cohort 1)
When overall uptake by the time of delivery was held constant at any given level, the specific step in the cascade at which uptake was varied did not affect 4–6-wk MTCT risk (Table 6). In contrast, the proportion of women–infant pairs linking to postnatal care had a substantial impact on 12-mo MTCT risk, especially with Options A and B (Table 7).
Tab. 6. Impact of uptake at key antenatal steps in the PMTCT cascade on MTCT at 4–6 wk of age. 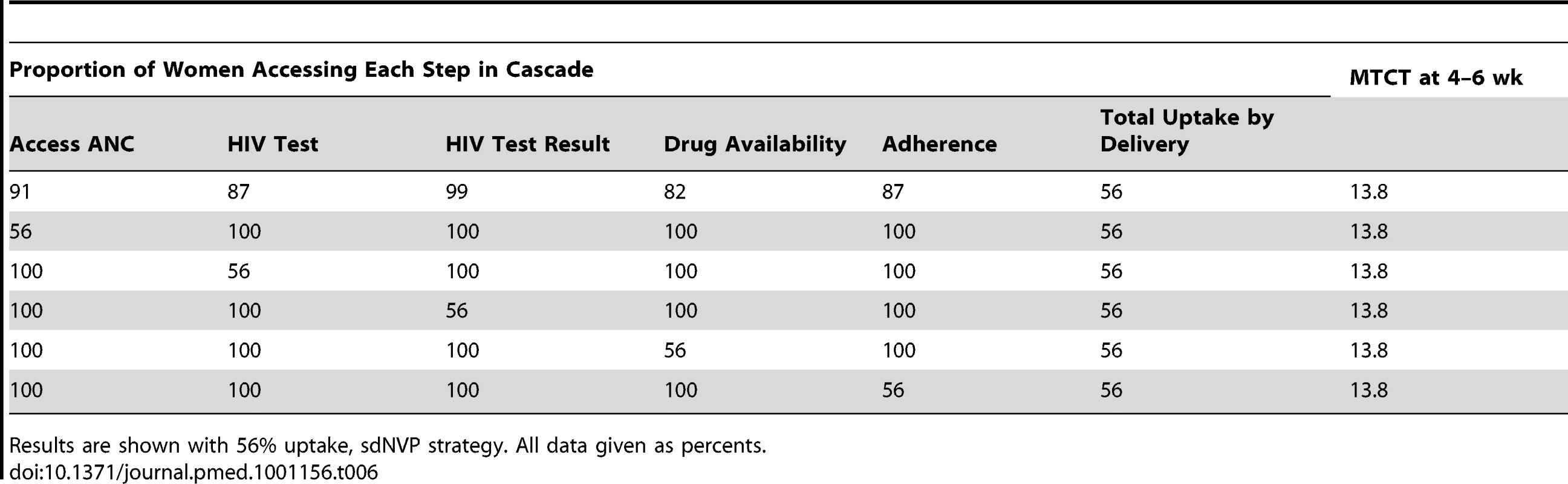
Results are shown with 56% uptake, sdNVP strategy. All data given as percents. Tab. 7. Impact of linkage to postnatal care by 6 wk postpartum, following 56% uptake at delivery. 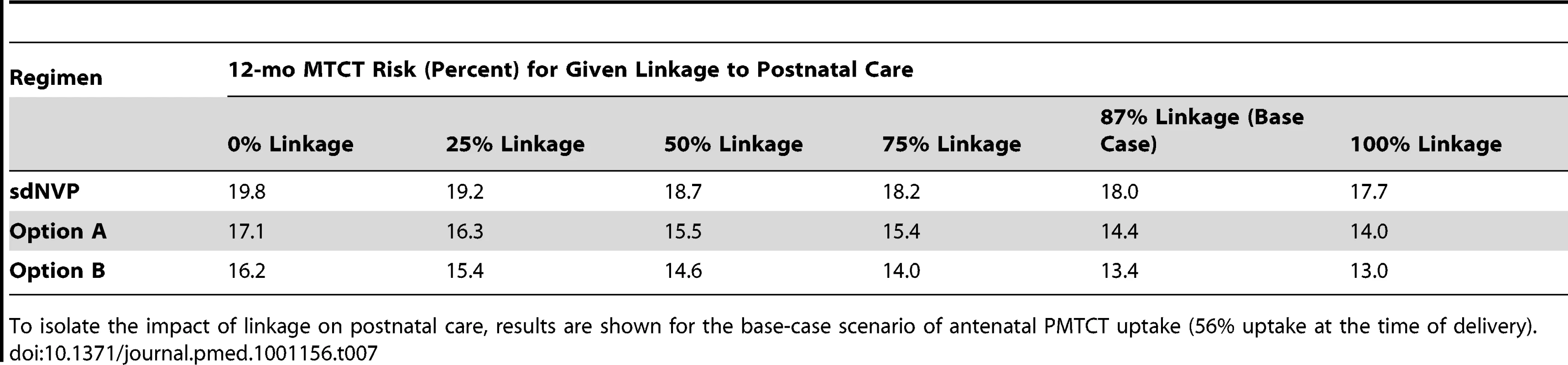
To isolate the impact of linkage on postnatal care, results are shown for the base-case scenario of antenatal PMTCT uptake (56% uptake at the time of delivery). Impact of Available CD4 Counts and ART for Women with CD4≤350/µl (Cohort 1)
In the base-case analysis, CD4 assays were assumed to be available only in the Option A and Option B strategies; for sdNVP, ART eligibility was assessed clinically, with a sensitivity of 36% for WHO stage 3/4 disease or CD4≤350/µl [80]. Furthermore, the proportion of women offered medications was assumed to be equal among identified ART-eligible women (offered ART) and all others (offered the sdNVP, Option A, or Option B regimens). Two modeled scenarios demonstrated the impact of improvements in CD4 and ART availability. Table 8 (top) depicts a scenario in which no CD4 assays were available, regardless of regimen. At 56% uptake of sdNVP or Option A, although 36% of the cohort was truly ART-eligible, only 7% of HIV-infected women were identified as ART-eligible and received ART. Table 8 (bottom) shows a scenario in which CD4 assays were available for all women identified as HIV-infected in ANC, and all women with CD4 values≤350/µl were offered ART. At 56% uptake of sdNVP or Option A, this scenario of CD4/ART availability led 25% of women to be identified as ART-eligible and to receive ART. Although the proportion of women receiving sdNVP or antenatal ZDV decreased from 49% to 31% (to maintain a total uptake of 56%), MTCT risks at 4–6 wk were substantially lower when CD4 assays were available. Notably, in the sdNVP strategy at 56% uptake, adding CD4 assays and ART for eligible women reduced 4–6-wk MTCT from 13.8% to 11.4%, a larger impact than that of replacing sdNVP with Option A without CD4/ART availability (4–6-wk MTCT risk of 12.0%). Under the Option B strategy, the effect of targeting ART to women with low CD4 counts was markedly smaller: at 56% uptake of Option B, prioritizing ART for women with CD4 values≤350/µl would reduce 4–6-wk MTCT from 9.9% to only 9.6%, by slightly shifting the distribution of women on three-drug ARVs from non-ART-eligible to ART-eligible women.
Tab. 8. Impact of availability of CD4 assays and ART for women with CD4≤350/µl. 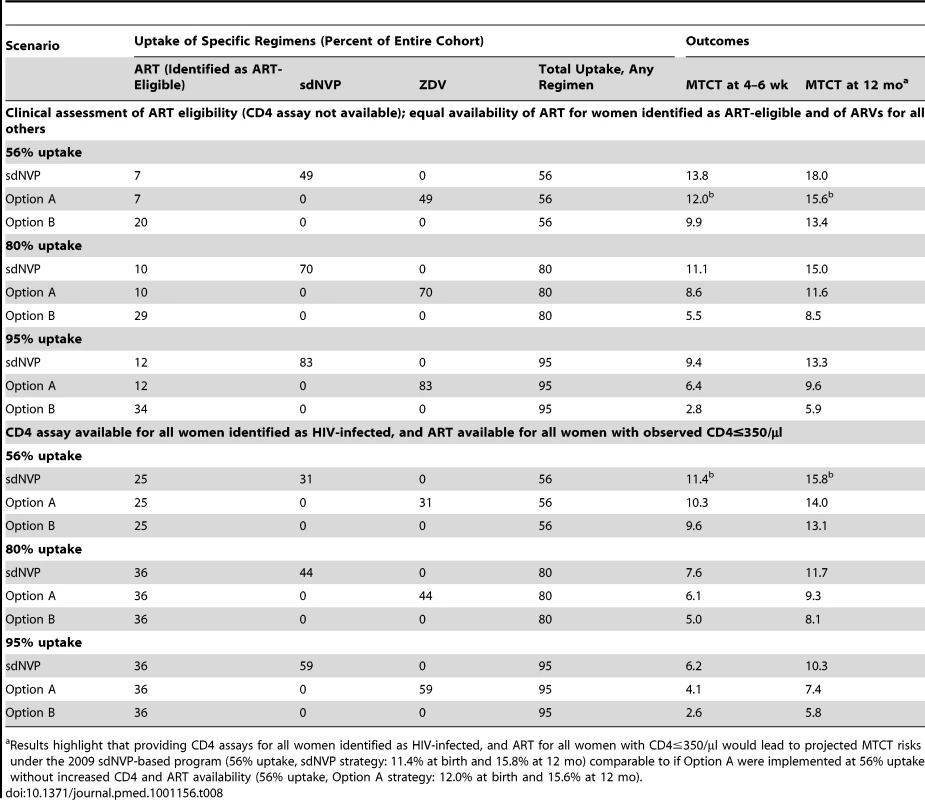
Results highlight that providing CD4 assays for all women identified as HIV-infected, and ART for all women with CD4≤350/µl would lead to projected MTCT risks under the 2009 sdNVP-based program (56% uptake, sdNVP strategy: 11.4% at birth and 15.8% at 12 mo) comparable to if Option A were implemented at 56% uptake without increased CD4 and ART availability (56% uptake, Option A strategy: 12.0% at birth and 15.6% at 12 mo). Discussion
We present results from a validated computer simulation model of MTCT in Zimbabwe, a setting where high HIV prevalence, prolonged breastfeeding, and limited resources make the WHO 2010 PMTCT guidelines both critical and challenging to implement. These analyses enumerate the potential benefits of improved PMTCT services along two dimensions: implementation of the WHO-recommended Option A or B regimens, and improved uptake of any given regimen at each step in the PMTCT cascade (Figure 1). Our results are similar to those from prior analyses using different modeling methodologies, including the analyses from which the initial targets were generated; consistent findings suggest that “virtual elimination” will require massive scale-up of PMTCT services [22],[81]. Although the WHO target may therefore seem difficult to reach, the WHO's “3 by 5” campaign (to deliver ART to 3 million HIV-infected patients by 2005) demonstrated the role that such ambitious public health goals can play in catalyzing efforts to dramatically expand HIV/AIDS services [82]. In addition, the analyses presented in this article simultaneously examine PMTCT uptake, breastfeeding duration, the range of MTCT risks published for each regimen, and both Options A and B from the 2010 WHO PMTCT guidelines, to identify combinations of factors that will facilitate achieving MTCT risks of less than 5% and the “virtual elimination” of pediatric HIV.
Most importantly, we find that improvements in PMTCT uptake are critical to approaching the goal of virtual elimination of MTCT. For example, a marked increase in PMTCT uptake occurred in Zimbabwe between 2008 (36%) and 2009 (56%), despite a period of economic hyperinflation [83]; as a result, projected MTCT risk was reduced from 20.3% to 18.0%, and an estimated 1,470 additional new infections were averted in a single year. Furthermore, once sdNVP is replaced by either WHO-recommended regimen, the level of PMTCT uptake exerts a far greater impact on MTCT rates than does the specific choice between Option A and Option B. Building from the 2009 National PMTCT Program in Zimbabwe, replacing sdNVP with Option A at 56% uptake (MTCT risk, 14.4%) would be more effective than expanding sdNVP uptake to 80% (MTCT risk, 15.4%). However, once the program reaches 56% uptake of Option A, improving uptake of Option A to 80% (MTCT risk, 10.5%) is projected to result in better outcomes than would replacing Option A with Option B at current uptake (MTCT risk, 13.4%). Notably, even with 95%–100% uptake, there are very few scenarios in which MTCT risk is estimated to fall below the 5% “virtual elimination” target threshold set by the WHO (Figure 3). With 12 mo of breastfeeding and average published MTCT risks for each regimen, nearly 100% uptake of Option B would be required to reach MTCT risks of less than 5%.
The results of this analysis depend critically on the modeled effectiveness of each ARV regimen, leading to two key conclusions. First, Option B may not be superior to Option A in preventing MTCT. The ranges of published MTCT risks for Options A and B are wide and overlapping (Table 3), and no randomized comparison of Option A with Option B has yet been reported. While data from available trials are limited by differences in populations and interventions [4]–[15],[67],[84], they suggest that Options A and B might have similar efficacy among women with CD4>350/µl, supporting WHO's equally strong recommendation of both regimens [21]. Results of a multi-national clinical trial comparing these regimens in women with CD4>350/µl will critically inform policy decisions around Options A and B [85].
Second, the lowest published MTCT risks for each regimen likely reflect an additional contribution of medication adherence, beyond that explicitly included in our MTCT model. For example, the lowest reported MTCT risks with maternal triple-drug ARV regimens in breastfeeding suggest 0% transmission by 6 mo of age, even among women with CD4<200/µl [9], which is lower than other reported risks of 0.5%–2.9% [6],[7],[10]–[13],[15]. Zero transmission was observed in a trial in which 92%–99% of women achieved viral suppression (HIV RNA<400 copies/ml) at delivery and 6 mo postpartum, suggesting that sustained adherence might have been greater in this trial than in trials reporting higher MTCT rates without such suppression data [9]. Among mother–infant pairs attempting to complete the Option A or Option B regimens beyond 6 mo of breastfeeding, adherence has not yet been reported. Based on high reported loss to follow-up rates among HIV-infected adults on ART, particularly postpartum women, as well as anecdotal evidence of the challenges of administering daily medications to otherwise healthy infants, it might be anticipated that the adherence that is observed when Options A and B are implemented in treatment programs will be lower than the trial-based adherence rates that are reflected in our base-case and “lowest risk” scenarios [43],[86].
In addition to the range of published MTCT risks for each regimen, reduced duration of breastfeeding is a key parameter that might permit the risk of MTCT to approach 5%. In settings where replacement feeding is the norm, MTCT risks have been reduced to 0%–2% [1],[2]. However, in Zimbabwe, as in many other resource-limited settings, infant formula milk may not be available, affordable, socially acceptable, or safe (due to lack of clean water or facilities for clean formula preparation) [3]. Replacement feeding, while reducing HIV transmission risk, therefore confers high risks of diarrheal disease, malnutrition, and mortality [3],[8]. Interventions to safely reduce breastfeeding duration in such settings, such as improved societal infrastructure for safe water systems, would likely confer tremendous health benefits, extending far beyond the infants of HIV-infected women, but will be costly and challenging to implement [87]. In the interim, our results suggest that promotion of high levels of access to postnatal care and adherence to ARV prophylaxis during breastfeeding will be critical to reduce the risk of MTCT to less than 5%.
Although PMTCT uptake in low - and middle-income countries has risen from 10% in 2004 to 53% in 2009 [19],[37], greater access to care and medications for HIV-infected pregnant women is still needed. The PMTCT “cascade” of care comprises multiple steps at which pregnant and breastfeeding women and their infants can be lost from care, and overall uptake is reduced multiplicatively at each step [22],[24],[25],[88]. For example, considering eight steps (access to ANC, HIV testing, receipt of test result, CD4 testing, receipt of CD4 result, adherence/retention before delivery, presentation to postnatal care, and adherence to breastfeeding prophylaxis regimens), each with the 92% average uptake observed in the PEARL multi-country study [24], the proportion of women completing care may be as low as 0.926 = 61% by delivery, or 0.928 = 51% by weaning. Notably, we find that the specific step in the antenatal PMTCT cascade at which improvements are made does not impact the risk of MTCT, if uptake is improved equally among ART-eligible and non-ART-eligible women. However, large reductions in the risk of MTCT are projected to occur if PMTCT uptake is increased by improving the proportion of ART-eligible women who receive ART (for example, through greater availability of CD4 testing and timely CD4 result return, or through selection of the Option B strategy). In addition, interventions to improve linkage to postnatal maternal HIV-related care (and ART for ART-eligible breastfeeding mothers) may substantially reduce postnatal HIV transmission. A focused operational research agenda is critical to identify, implement, and evaluate interventions that improve uptake and retention at each step in the PMTCT cascade, with a particular focus on availability of CD4 assays and ART for women with low CD4 counts [89].
There are three main limitations to this analysis. First, computer models simplify complex biologic and operational processes. Second, data from multiple sources were necessarily combined. However, sensitivity analyses were conducted to examine the effects of variation in key simplifying assumptions, including CD4 assay availability and the availability of HIV testing and sdNVP during labor, as well as wide ranges for each model input parameter. We found that these assumptions and data inputs had no substantive impact on policy conclusions, except where highlighted in the Results and Discussion. Finally, we report only short-term pediatric outcomes, and exclude potential impacts on maternal or community health resulting from expansion of PMTCT programs. In addition to a reduction in infant HIV infections, many other benefits may result from the planned transition from sdNVP to Option A or B, including the avoidance of exposure to sdNVP and the associated risk of drug-resistant virus for both mothers and infants [90],[91], as well as long-term maternal and pediatric health benefits associated with the monitoring and treatment infrastructure required for implementation of more complex PMTCT regimens. The potential health benefits and costs of these “indirect effects” will comprise important areas of future research.
Conclusions
In Zimbabwe, the planned implementation of the 2010 WHO PMTCT guidelines with Option A is projected to substantially reduce infant HIV infection risk compared to the 2009 national program. In order to approach the goal of “virtual elimination” of pediatric HIV (MTCT risk less than 5%), a national program based on either Option A or Option B will also need to include strategies to improve PMTCT uptake to nearly 100% throughout pregnancy and breastfeeding, to safely reduce the duration of breastfeeding, and to support medication adherence (thereby maximizing the effectiveness of each ARV regimen) during both pregnancy and breastfeeding.
Supporting Information
Zdroje
1. TubianaRLe ChenadecJRouziouxCMandelbrotLHamreneK 2010 Factors associated with mother-to-child transmission of HIV-1 despite a maternal viral load<500 copies/ml at delivery: a case-control study nested in the French perinatal cohort (EPF-ANRS CO1). Clin Infect Dis 50 585 596
2. United States Centers for Disease Control and Prevention 2010 Diagnoses of HIV infection by age. Available: http://www.cdc.gov/hiv/topics/surveillance/basic.htm#hivaidsage. Accessed 20 July 2011
3. World Health Organization 2010 Guidelines on HIV and infant feeding 2010: principles and recommendations for infant feeding in the context of HIV and a summary of evidence. Available: http://www.who.int/child_adolescent_health/documents/9789241599535/en/index.html. Accessed 20 July 2011
4. MofensonLTahaTLiQKumwendaJKafulafulaG 2009 Infant extended antiretroviral (ARV) prophylaxis is effective in preventing postnatal mother-to-child HIV transmission (MTCT) at all maternal CD4 counts [abstract]. 5th IAS Conference on HIV Pathogenesis; Treatment and Prevention; 19–22 July 2009; Cape Town, South Africa. Available: http://www.ias2009.org/pag/Abstracts.aspx?AID=1251. Accessed 20 July 2011
5. VyankandonderaJLuchtersSHassinkE 2003 Reducing risk of HIV-1 transmission from mother to infant through breastfeeding using antiretroviral prophylaxis in infants (SIMBA-study) [abstract]. 2nd IAS Conference on HIV Pathogenesis and Treatment; 13–16 July 2003; Paris, France
6. KilewoCKarlssonKNgarinaMMassaweALyamuyaE 2009 Prevention of mother-to-child transmission of HIV-1 through breastfeeding by treating mothers with triple antiretroviral therapy in Dar es Salaam, Tanzania: the Mitra Plus study. J Acquir Immune Defic Syndr 52 406 416
7. Kesho Bora Study Group 2010 Eighteen-month follow-up of HIV-1-infected mothers and their children enrolled in the Kesho Bora study observational cohorts. J Acquir Immune Defic Syndr 54 533 541
8. ThiorILockmanSSmeatonLMShapiroRLWesterC 2006 Breastfeeding plus infant zidovudine prophylaxis for 6 months vs formula feeding plus infant zidovudine for 1 month to reduce mother-to-child HIV transmission in Botswana: a randomized trial: the Mashi Study. JAMA 296 794 805
9. ShapiroRLHughesMDOgwuAKitchDLockmanS 2010 Antiretroviral regimens in pregnancy and breast-feeding in Botswana. N Engl J Med 362 2282 2294
10. ChaselaCSHudgensMGJamiesonDJKayiraDHosseinipourMC 2010 Maternal or infant antiretroviral drugs to reduce HIV-1 transmission. N Engl J Med 362 2271 2281
11. PeltierCANdayisabaGFLepagePvan GriensvenJLeroyV 2009 Breastfeeding with maternal antiretroviral therapy or formula feeding to prevent HIV postnatal mother-to-child transmission in Rwanda. AIDS 23 2415 2423
12. PalombiLMarazziMCVoetbergAMagidNA 2007 Treatment acceleration program and the experience of the DREAM program in prevention of mother-to-child transmission of HIV. AIDS 21 Suppl 4 S65 S71
13. Tonwe-GoldBEkoueviDKVihoIAmani-BosseCToureS 2007 Antiretroviral treatment and prevention of peripartum and postnatal HIV transmission in West Africa: evaluation of a two-tiered approach. PLoS Med 4 e257 doi:10.1371/journal.pmed.0040257
14. KumwendaNIHooverDRMofensonLMThigpenMCKafulafulaG 2008 Extended antiretroviral prophylaxis to reduce breast-milk HIV-1 transmission. N Engl J Med 359 119 129
15. ThomasTKMasabaRBorkowfCBNdivoRZehC 2011 Triple-antiretroviral prophylaxis to prevent mother-to-child HIV transmission through breastfeeding–the Kisumu Breastfeeding Study, Kenya: a clinical trial. PLoS Med 8 e1001015 doi:10.1371/journal.pmed.1001015
16. MoszynskiP 2010 Global elimination of mother to child HIV transmission is now achievable, say agencies. BMJ 341 c5152
17. Global AIDS Alliance 2010 Campaign to End Pediatric HIV/AIDS. Available: http://www.endpediatricaids.net/index.php/1032. Accessed 20 July 2011
18. PR Newswire 2010 Elizabeth Glaser Pediatric AIDS Foundation releases toolkit for implementing new WHO recommendations on PMTCT and ARV treatment. Available: http://www.prnewswire.com/news-releases/elizabeth-glaser-pediatric-aids-foundation-releases-toolkit-for-implementing-new-who-recommendations-on-pmtct-and-arv-treatment-99104624.html. Accessed 20 July 2011
19. World Health Organization 2010 Towards universal access: scaling up priority HIV/AIDS interventions in the health sector. Progress report 2010. Available: http://www.who.int/hiv/pub/2010progressreport/report/en/index.html. Accessed 20 July 2011
20. Joint United Nations Programme on HIV/AIDS 2009 Annual report 2009. Available: http://data.unaids.org/pub/Report/2010/2009_annual_report_en.pdf. Accessed February 21, 2011
21. World Health Organization 2010 Antiretroviral drugs for treating pregnant women and preventing HIV infection in infants: recommendations for a public health approach. Available: http://whqlibdoc.who.int/publications/2010/9789241599818_eng.pdf. Accessed 20 July 2011
22. BarkerPMMphatsweWRollinsN 2010 Antiretroviral drugs in the cupboard are not enough: the impact of health systems' performance on mother-to-child transmission of HIV. J Acquir Immune Defic Syndr 56 e45 e48
23. Joint United Nations Programme on HIV/AIDS, United Nations Children's Fund, World Health Organization 2009 Children and AIDS: fourth stocktaking report, 2009. Available: http://www.unicef.org/publications/files/Children_and_AIDS_Fourth_Stocktaking_Report_EN_110609.pdf. Accessed 6 December 2011
24. StringerEMEkoueviDKCoetzeeDTihPMCreekTL 2010 Coverage of nevirapine-based services to prevent mother-to-child HIV transmission in 4 African countries. JAMA 304 293 302
25. StringerEMChiBHChintuNCreekTLEkoueviDK 2008 Monitoring effectiveness of programmes to prevent mother-to-child HIV transmission in lower-income countries. Bull World Health Organ 86 57 62
26. MarstonMBecquetRZabaBMoultonLHGrayG 2011 Net survival of perinatally and postnatally HIV-infected children: a pooled analysis of individual data from sub-Saharan Africa. Int J Epidemiol 40 385 396
27. PerezFOrne-GliemannJMukotekwaTMillerAGlenshawM 2004 Prevention of mother to child transmission of HIV: evaluation of a pilot programme in a district hospital in rural Zimbabwe. BMJ 329 1147 1150
28. CiaranelloALSeageGRFreedbergKAWeinsteinMCLockmanS 2008 Antiretroviral drugs for preventing mother-to-child transmission of HIV in sub-Saharan Africa: balancing efficacy and infant toxicity. AIDS 22 2359 2369
29. CiaranelloALPerezFMaruvaMChuJEnglesmannB 2011 WHO 2010 guidelines for prevention of mother-to-child HIV transmission in Zimbabwe: modeling clinical outcomes in infants and mothers. PLoS ONE 6 e20224 doi:10.1371/journal.pone.0020224
30. GoldieSJYazdanpanahYLosinaEWeinsteinMCAnglaretX 2006 Cost-effectiveness of HIV treatment in resource-poor settings—the case of Côte d'Ivoire. N Engl J Med 355 1141 1153
31. WalenskyRPWolfLLWoodRFofanaMOFreedbergKA 2009 When to start antiretroviral therapy in resource-limited settings. Ann Intern Med 151 157 166
32. CiaranelloALockmanSFreedbergKAHughesMChuJ 2011 First-line antiretroviral therapy after single-dose nevirapine exposure in South Africa: a cost-effectiveness analysis of the OCTANE trial. AIDS 25 479 492
33. National Drug and Therapeutics Policy Advisory Committee & AIDS and TB Unit 2009 Guidelines for antiretroviral therapy in Zimbabwe Harare Zimbabwe Ministry of Health and Child Welfare
34. World Bank 2011 Population, total. Available: http://data.worldbank.org/indicator/SP.POP.TOTL. Accessed 20 July 2011
35. Index Mundi 2011 Zimbabwe birth rate. Available: http://www.indexmundi.com/zimbabwe/birth_rate.html. Accessed 20 July 2011
36. Zimbabwe Ministry of Health and Child Welfare 2009 National HIV estimates Harare Zimbabwe Ministry of Health and Child Welfare
37. World Health Organization 2009 Towards universal access: scaling up priority HIV/AIDS interventions in the health sector. Progress report. Available: http://www.who.int/hiv/pub/tuapr_2009_en.pdf. Accessed 20 July 2011
38. WalenskyRPWoodRCiaranelloALPaltielADLorenzanaSB 2010 Scaling up the 2010 World Health Organization HIV treatment guidelines in resource-limited settings: a model-based analysis. PLoS Med 7 e1000382 doi:10.1371/journal.pmed.1000382
39. NewellMLCoovadiaHCortina-BorjaMRollinsNGaillardP 2004 Mortality of infected and uninfected infants born to HIV-infected mothers in Africa: a pooled analysis. Lancet 364 1236 1243
40. CrampinACFloydSGlynnJRMadiseNNyondoA 2003 The long-term impact of HIV and orphanhood on the mortality and physical well-being of children in rural Malawi. AIDS 17 389 397
41. NakiyingiJSBracherMWhitworthJARuberantwariABusingyeJ 2003 Child survival in relation to mother's HIV infection and survival: evidence from a Ugandan cohort study. AIDS 17 1827 1834
42. ZabaBWhitworthJMarstonMNakiyingiJRuberantwariA 2005 HIV and mortality of mothers and children: evidence from cohort studies in Uganda, Tanzania, and Malawi. Epidemiology 16 275 280
43. AhouaLAyikoruHGnauckKOdaruGOdarE 2010 Evaluation of a 5-year programme to prevent mother-to-child transmission of HIV infection in Northern Uganda. J Trop Pediatr 56 43 52
44. ManziMZachariahRTeckRBuhendwaLKazimaJ 2005 High acceptability of voluntary counselling and HIV-testing but unacceptable loss to follow up in a prevention of mother-to-child HIV transmission programme in rural Malawi: scaling-up requires a different way of acting. Trop Med Int Health 10 1242 1250
45. RamdhialRRamkissoonA 2009 Integration of HAART into public sector antenatal care services in a high prevalence HIV setting in South Africa [abstract]. 5th IAS Conference on HIV Pathogenesis and Treatment; 19–22 July 2009; Cape Town, South Africa. Available: http://www.iasociety.org/Default.aspx?pageId=12&abstractId=200722054. Accessed 20 July 2011
46. SrikewalJMoodleyDMsweliL 2009 The impact of an integrated health system on the delivery of PMTCT services in Kwazulu Natal [abstract]. 5th IAS Conference on HIV Pathogenesis; Treatment and Prevention; 19–22 July 2009; Cape Town, South Africa. Available: http://www.ias2009.org/pag/Abstracts.aspx?AID=3432. Accessed 20 July 2011
47. ChiBHChintuNLeeAStringerEMSinkalaM 2007 Expanded services for the prevention of mother-to-child HIV transmission: field acceptability of a pilot program in Lusaka, Zambia. J Acquir Immune Defic Syndr 45 125 127
48. KaplanROrrellCZwaneEBekkerLGWoodR 2008 Loss to follow-up and mortality among pregnant women referred to a community clinic for antiretroviral treatment. AIDS 22 1679 1681
49. StinsonKBoulleACoetzeeDAbramsEJMyerL 2010 Initiation of highly active antiretroviral therapy among pregnant women in Cape Town, South Africa. Trop Med Int Health 15 825 832
50. KumwendaJMatayaRKumwendaNKafulafulaGLiQ 2009 Coverage of highly active antiretroviral therapy (HAART) among postpartum women in Malawi [abstract]. 5th IAS Conference on HIV Pathogenesis; Treatment and Prevention; 19–22 July 2009; Cape Town, South Africa. Available: http://www.ias2009.org/pag/Abstracts.aspx?AID=1938. Accessed 20 July 2011
51. BrinkhofMWDabisFMyerLBangsbergDRBoulleA 2008 Early loss of HIV-infected patients on potent antiretroviral therapy programmes in lower-income countries. Bull World Health Organ 86 559 567
52. GengEHBangsbergDRMusinguziNEmenyonuNBwanaMB 2010 Understanding reasons for and outcomes of patients lost to follow-up in antiretroviral therapy programs in Africa through a sampling-based approach. J Acquir Immune Defic Syndr 53 405 411
53. AmuronBNamaraGBirungiJNabiryoCLevinJ 2009 Mortality and loss-to-follow-up during the pre-treatment period in an antiretroviral therapy programme under normal health service conditions in Uganda. BMC Public Health 9 290
54. MyerLCarterRJKatyalMToroPEl-SadrWM 2010 Impact of antiretroviral therapy on incidence of pregnancy among HIV-infected women in Sub-Saharan Africa: a cohort study. PLoS Med 7 e1000229 doi:10.1371/journal.pmed.1000229
55. RosenSFoxMPGillCJ 2007 Patient retention in antiretroviral therapy programs in sub-Saharan Africa: a systematic review. PLoS Med 4 e298 doi:10.1371/journal.pmed.0040298
56. ToroPLKatyalMCarterRJMyerLEl-SadrWM 2010 Initiation of antiretroviral therapy among pregnant women in resource-limited countries: CD4+ cell count response and program retention. AIDS 24 515 524
57. Ministry of Health and Child Welfare 2008 Maternal and Perinatal Mortality Study 2007 Harare Zimbabwe Ministry of Health and Child Welfare
58. IliffPJPiwozEGTavengwaNVZunguzaCDMarindaET 2005 Early exclusive breastfeeding reduces the risk of postnatal HIV-1 transmission and increases HIV-free survival. AIDS 19 699 708
59. FawziWMsamangaGSpiegelmanDRenjifoBBangH 2002 Transmission of HIV-1 through breastfeeding among women in Dar es Salaam, Tanzania. J Acquir Immune Defic Syndr 31 331 338
60. ChigwederePSeageGRLeeTHEssexM 2008 Efficacy of antiretroviral drugs in reducing mother-to-child transmission of HIV in Africa: a meta-analysis of published clinical trials. AIDS Res Hum Retroviruses 24 827 837
61. CoutsoudisAPillayKKuhnLSpoonerETsaiWY 2001 Method of feeding and transmission of HIV-1 from mothers to children by 15 months of age: prospective cohort study from Durban, South Africa. AIDS 15 379 387
62. DabisFBequetLEkoueviDKVihoIRouetF 2005 Field efficacy of zidovudine, lamivudine and single-dose nevirapine to prevent peripartum HIV transmission. AIDS 19 309 318
63. NduatiRJohnGMbori-NgachaDRichardsonBOverbaughJ 2000 Effect of breastfeeding and formula feeding on transmission of HIV-1: a randomized clinical trial. JAMA 283 1167 1174
64. Petra Study Team 2002 Efficacy of three short-course regimens of zidovudine and lamivudine in preventing early and late transmission of HIV-1 from mother to child in Tanzania, South Africa, and Uganda (Petra study): a randomised, double-blind, placebo-controlled trial. Lancet 359 1178 1186
65. GuayLAMusokePFlemingTBagendaDAllenM 1999 Intrapartum and neonatal single-dose nevirapine compared with zidovudine for prevention of mother-to-child transmission of HIV-1 in Kampala, Uganda: HIVNET 012 randomised trial. Lancet 354 795 802
66. ThistlePSpitzerRFGlazierRHPilonRArbessG 2007 A randomized, double-blind, placebo-controlled trial of combined nevirapine and zidovudine compared with nevirapine alone in the prevention of perinatal transmission of HIV in Zimbabwe. Clin Infect Dis 44 111 119
67. Kesho Bora Study Group 2011 Triple antiretroviral compared with zidovudine and single-dose nevirapine prophylaxis during pregnancy and breastfeeding for prevention of mother-to-child transmission of HIV-1 (Kesho Bora study): a randomised controlled trial. Lancet Infect Dis 11 171 180
68. KuhnLSinkalaMKankasaCSemrauKKasondeP 2007 High uptake of exclusive breastfeeding and reduced early post-natal HIV transmission. PLoS ONE 2 e1363 doi:10.1371/journal.pone.0001363
69. LeroyVNewellMLDabisFPeckhamCVan de PerreP 1998 International multicentre pooled analysis of late postnatal mother-to-child transmission of HIV-1 infection. Ghent International Working Group on Mother-to-Child Transmission of HIV. Lancet 352 597 600
70. LallemantMJourdainGLe CoeurSMaryJYNgo-Giang-HuongN 2004 Single-dose perinatal nevirapine plus standard zidovudine to prevent mother-to-child transmission of HIV-1 in Thailand. N Engl J Med 351 217 228
71. CooperERCharuratMMofensonLHansonICPittJ 2002 Combination antiretroviral strategies for the treatment of pregnant HIV-1-infected women and prevention of perinatal HIV-1 transmission. J Acquir Immune Defic Syndr 29 484 494
72. DorenbaumACunninghamCKGelberRDCulnaneMMofensonL 2002 Two-dose intrapartum/newborn nevirapine and standard antiretroviral therapy to reduce perinatal HIV transmission: a randomized trial. JAMA 288 189 198
73. CoutsoudisADabisFFawziWGaillardPHaverkampG 2004 Late postnatal transmission of HIV-1 in breast-fed children: an individual patient data meta-analysis. J Infect Dis 189 2154 2166
74. Department of Economic and Social Affairs 2009 World population prospects: the 2008 revision New York United Nations Available: http://www.un.org/esa/population/publications/wpp2008/wpp2008_highlights.pdf. Accessed 20 July 2011
75. MarindaEHumphreyJHIliffPJMutasaKNathooKJ 2007 Child mortality according to maternal and infant HIV status in Zimbabwe. Pediatr Infect Dis J 26 519 526
76. Kids' ART-LINC Collaboration 2008 Low risk of death, but substantial program attrition, in pediatric HIV treatment cohorts in sub-Saharan Africa. J Acquir Immune Defic Syndr 49 523 531
77. SutcliffeCGvan DijkJHBoltonCPersaudDMossWJ 2008 Effectiveness of antiretroviral therapy among HIV-infected children in sub-Saharan Africa. Lancet Infect Dis 8 477 489
78. LeroyVSakarovitchCCortina-BorjaMMcIntyreJCoovadiaH 2005 Is there a difference in the efficacy of peripartum antiretroviral regimens in reducing mother-to-child transmission of HIV in Africa? AIDS 19 1865 1875
79. KuhnLAldrovandiGMSinkalaMKankasaCMwiyaM 2010 Potential impact of new WHO criteria for antiretroviral treatment for prevention of mother-to - child HIV transmission. AIDS 24 1374 1377
80. CarterRJDuganKEl-SadrWMMyerLOtienoJ 2010 CD4+ cell count testing more effective than HIV disease clinical staging in identifying pregnant and postpartum women eligible for antiretroviral therapy in resource-limited settings. J Acquir Immune Defic Syndr 55 404 410
81. MahyMStoverJKiraguKHayashiCAkwaraP 2010 What will it take to achieve virtual elimination of mother-to-child transmission of HIV? An assessment of current progress and future needs. Sex Transm Infect 86 Suppl 2 ii48 ii55
82. World Health Organization 2005 The 3 by 5 initiative. Available: http://www.who.int/3by5/en/. Accessed 20 July 2011
83. KramarenkoVEngstromLVerdierGFernandezGOppersSE 2010 Zimbabwe: challenges and policy options after hyperinflation Washington (District of Columbia) International Monetary Fund Available: http://www.imf.org/external/pubs/ft/dp/2010/afr1003.pdf. Accessed 20 July 2011
84. BedriAGudettaBIsehakAKumbiSLulsegedS 2008 Extended-dose nevirapine to 6 weeks of age for infants to prevent HIV transmission via breastfeeding in Ethiopia, India, and Uganda: an analysis of three randomised controlled trials. Lancet 372 300 313
85. National Institutes of Health IMPAACT Trial Network 2010 IMPAACT P1077: the PROMISE Study (Promoting Maternal and Infant Survival Everywhere)—synopsis. Available: http://www.docstoc.com/docs/42000343/IMPAACT-P1077_-PROMISE. Accessed 20 July 2011
86. GengEHEmenyonuNBwanaMBGliddenDVMartinJN 2008 Sampling-based approach to determining outcomes of patients lost to follow-up in antiretroviral therapy scale-up programs in Africa. JAMA 300 506 507
87. HuttonGBartramJ 2008 Global costs of attaining the Millennium Development Goal for water supply and sanitation. Bull World Health Organ 86 13 19
88. MofensonLM 2010 Prevention in neglected subpopulations: prevention of mother-to-child transmission of HIV infection. Clin Infect Dis 50 Suppl 3 S130 S148
89. YounglesonMSNkurunzizaPJenningsKArendseJMateKS 2011 Improving a mother to child HIV transmission programme through health system redesign: quality improvement, protocol adjustment and resource addition. PLoS ONE 5 e13891 doi:10.1371/journal.pone.0013891
90. LockmanSHughesMDMcIntyreJZhengYChipatoT 2010 Antiretroviral therapies in women after single-dose nevirapine exposure. N Engl J Med 363 1499 1509
91. PalumboPLindseyJCHughesMDCottonMFBobatR 2010 Antiretroviral treatment for children with peripartum nevirapine exposure. N Engl J Med 363 1510 1520
92. MellorsJWMunozAGiorgiJVMargolickJBTassoniCJ 1997 Plasma viral load and CD4+ lymphocytes as prognostic markers of HIV-1 infection. Ann Intern Med 126 946 954
93. FawziWWMsamangaGIHunterDRenjifoBAntelmanG 2002 Randomized trial of vitamin supplements in relation to transmission of HIV-1 through breastfeeding and early child mortality. AIDS 16 1935 1944
94. LeroyVKaronJMAlioumAEkpiniERMedaN 2002 Twenty-four month efficacy of a maternal short-course zidovudine regimen to prevent mother-to-child transmission of HIV-1 in West Africa. AIDS 16 631 641
95. ThistlePGottesmanMPilonRGlazierRHArbessG 2004 A randomized control trial of an ultra-short zidovudine regimen in the prevention of perinatal HIV transmission in rural Zimbabwe. Cent Afr J Med 50 79 84
96. ConnorEMSperlingRSGelberRKiselevPScottG 1994 Reduction of maternal-infant transmission of human immunodeficiency virus type 1 with zidovudine treatment. Pediatric AIDS Clinical Trials Group Protocol 076 Study Group. N Engl J Med 331 1173 1180
97. FassinouPElengaNRouetFLaguideRKouakoussuiKA 2004 Highly active antiretroviral therapies among HIV-1-infected children in Abidjan, Côte d'Ivoire. AIDS 18 1905 1913
Štítky
Interné lekárstvo
Článek Trends in Compulsory Licensing of Pharmaceuticals Since the Doha Declaration: A Database AnalysisČlánek Effect of Sanitation on Soil-Transmitted Helminth Infection: Systematic Review and Meta-AnalysisČlánek A New Year at : Maintaining a Focus on the World's Health Priorities and Identifying the Gaps
Článok vyšiel v časopisePLOS Medicine
Najčítanejšie tento týždeň
2012 Číslo 1- Parazitičtí červi v terapii Crohnovy choroby a dalších zánětlivých autoimunitních onemocnění
- Intermitentní hladovění v prevenci a léčbě chorob
- Statinová intolerance
- Co dělat při intoleranci statinů?
- Metabolit živočišné stravy produkovaný střevní mikroflórou zvyšuje riziko závažných kardiovaskulárních příhod
-
Všetky články tohto čísla
- What Will It Take to Eliminate Pediatric HIV? Reaching WHO Target Rates of Mother-to-Child HIV Transmission in Zimbabwe: A Model-Based Analysis
- Challenging Medical Ghostwriting in US Courts
- The Inadequate Treatment of Pain: Collateral Damage from the War on Drugs
- A United Nations General Assembly Special Session for Mental, Neurological, and Substance Use Disorders:
- Trends in Compulsory Licensing of Pharmaceuticals Since the Doha Declaration: A Database Analysis
- Monitoring the Introduction of Pneumococcal Conjugate Vaccines into West Africa: Design and Implementation of a Population-Based Surveillance System
- The Role of Health Systems Factors in Facilitating Access to Psychotropic Medicines: A Cross-Sectional Analysis of the WHO-AIMS in 63 Low- and Middle-Income Countries
- Trends in Resource Utilization by Children with Neurological Impairment in the United States Inpatient Health Care System: A Repeat Cross-Sectional Study
- Hitting Hotspots: Spatial Targeting of Malaria for Control and Elimination
- Effect of Sanitation on Soil-Transmitted Helminth Infection: Systematic Review and Meta-Analysis
- Effects of Two Commercial Electronic Prescribing Systems on Prescribing Error Rates in Hospital In-Patients: A Before and After Study
- A New Year at : Maintaining a Focus on the World's Health Priorities and Identifying the Gaps
- Long-Term Survival in a Large Cohort of Patients with Venous Thrombosis: Incidence and Predictors
- Ensemble Modeling of the Likely Public Health Impact of a Pre-Erythrocytic Malaria Vaccine
- Adult Mortality Attributable to Preventable Risk Factors for Non-Communicable Diseases and Injuries in Japan: A Comparative Risk Assessment
- PLOS Medicine
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- Ensemble Modeling of the Likely Public Health Impact of a Pre-Erythrocytic Malaria Vaccine
- Adult Mortality Attributable to Preventable Risk Factors for Non-Communicable Diseases and Injuries in Japan: A Comparative Risk Assessment
- What Will It Take to Eliminate Pediatric HIV? Reaching WHO Target Rates of Mother-to-Child HIV Transmission in Zimbabwe: A Model-Based Analysis
- Challenging Medical Ghostwriting in US Courts
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



