-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
The Impact of Pyrethroid Resistance on the Efficacy of Insecticide-Treated Bed Nets against African Anopheline Mosquitoes: Systematic Review and Meta-Analysis
Background:
Pyrethroid insecticide-treated bed nets (ITNs) help contribute to reducing malaria deaths in Africa, but their efficacy is threatened by insecticide resistance in some malaria mosquito vectors. We therefore assessed the evidence that resistance is attenuating the effect of ITNs on entomological outcomes.Methods and Findings:
We included laboratory and field studies of African malaria vectors that measured resistance at the time of the study and used World Health Organization–recommended impregnation regimens. We reported mosquito mortality, blood feeding, induced exophily (premature exit of mosquitoes from the hut), deterrence, time to 50% or 95% knock-down, and percentage knock-down at 60 min. Publications were searched from 1 January 1980 to 31 December 2013 using MEDLINE, Cochrane Central Register of Controlled Trials, Science Citation Index Expanded, Social Sciences Citation Index, African Index Medicus, and CAB Abstracts. We stratified studies into three levels of insecticide resistance, and ITNs were compared with untreated bed nets (UTNs) using the risk difference (RD). Heterogeneity was explored visually and statistically. Included were 36 laboratory and 24 field studies, reported in 25 records. Studies tested and reported resistance inconsistently. Based on the meta-analytic results, the difference in mosquito mortality risk for ITNs compared to UTNs was lower in higher resistance categories. However, mortality risk was significantly higher for ITNs compared to UTNs regardless of resistance. For cone tests: low resistance, risk difference (RD) 0.86 (95% CI 0.72 to 1.01); moderate resistance, RD 0.71 (95% CI 0.53 to 0.88); high resistance, RD 0.56 (95% CI 0.17 to 0.95). For tunnel tests: low resistance, RD 0.74 (95% CI 0.61 to 0.87); moderate resistance, RD 0.50 (95% CI 0.40 to 0.60); high resistance, RD 0.39 (95% CI 0.24 to 0.54). For hut studies: low resistance, RD 0.56 (95% CI 0.43 to 0.68); moderate resistance, RD 0.39 (95% CI 0.16 to 0.61); high resistance, RD 0.35 (95% CI 0.27 to 0.43). However, with the exception of the moderate resistance category for tunnel tests, there was extremely high heterogeneity across studies in each resistance category (chi-squared test, p<0.00001, I2 varied from 95% to 100%).Conclusions:
This meta-analysis found that ITNs are more effective than UTNs regardless of resistance. There appears to be a relationship between resistance and the RD for mosquito mortality in laboratory and field studies. However, the substantive heterogeneity in the studies' results and design may mask the true relationship between resistance and the RD, and the results need to be interpreted with caution. Our analysis suggests the potential for cumulative meta-analysis in entomological trials, but further field research in this area will require specialists in the field to work together to improve the quality of trials, and to standardise designs, assessment, and reporting of both resistance and entomological outcomes.
Please see later in the article for the Editors' Summary
Published in the journal: The Impact of Pyrethroid Resistance on the Efficacy of Insecticide-Treated Bed Nets against African Anopheline Mosquitoes: Systematic Review and Meta-Analysis. PLoS Med 11(3): e32767. doi:10.1371/journal.pmed.1001619
Category: Research Article
doi: https://doi.org/10.1371/journal.pmed.1001619Summary
Background:
Pyrethroid insecticide-treated bed nets (ITNs) help contribute to reducing malaria deaths in Africa, but their efficacy is threatened by insecticide resistance in some malaria mosquito vectors. We therefore assessed the evidence that resistance is attenuating the effect of ITNs on entomological outcomes.Methods and Findings:
We included laboratory and field studies of African malaria vectors that measured resistance at the time of the study and used World Health Organization–recommended impregnation regimens. We reported mosquito mortality, blood feeding, induced exophily (premature exit of mosquitoes from the hut), deterrence, time to 50% or 95% knock-down, and percentage knock-down at 60 min. Publications were searched from 1 January 1980 to 31 December 2013 using MEDLINE, Cochrane Central Register of Controlled Trials, Science Citation Index Expanded, Social Sciences Citation Index, African Index Medicus, and CAB Abstracts. We stratified studies into three levels of insecticide resistance, and ITNs were compared with untreated bed nets (UTNs) using the risk difference (RD). Heterogeneity was explored visually and statistically. Included were 36 laboratory and 24 field studies, reported in 25 records. Studies tested and reported resistance inconsistently. Based on the meta-analytic results, the difference in mosquito mortality risk for ITNs compared to UTNs was lower in higher resistance categories. However, mortality risk was significantly higher for ITNs compared to UTNs regardless of resistance. For cone tests: low resistance, risk difference (RD) 0.86 (95% CI 0.72 to 1.01); moderate resistance, RD 0.71 (95% CI 0.53 to 0.88); high resistance, RD 0.56 (95% CI 0.17 to 0.95). For tunnel tests: low resistance, RD 0.74 (95% CI 0.61 to 0.87); moderate resistance, RD 0.50 (95% CI 0.40 to 0.60); high resistance, RD 0.39 (95% CI 0.24 to 0.54). For hut studies: low resistance, RD 0.56 (95% CI 0.43 to 0.68); moderate resistance, RD 0.39 (95% CI 0.16 to 0.61); high resistance, RD 0.35 (95% CI 0.27 to 0.43). However, with the exception of the moderate resistance category for tunnel tests, there was extremely high heterogeneity across studies in each resistance category (chi-squared test, p<0.00001, I2 varied from 95% to 100%).Conclusions:
This meta-analysis found that ITNs are more effective than UTNs regardless of resistance. There appears to be a relationship between resistance and the RD for mosquito mortality in laboratory and field studies. However, the substantive heterogeneity in the studies' results and design may mask the true relationship between resistance and the RD, and the results need to be interpreted with caution. Our analysis suggests the potential for cumulative meta-analysis in entomological trials, but further field research in this area will require specialists in the field to work together to improve the quality of trials, and to standardise designs, assessment, and reporting of both resistance and entomological outcomes.
Please see later in the article for the Editors' SummaryIntroduction
The World Health Organization (WHO) estimates that there were 655,000 malaria deaths in 2010, with 86% occurring in children under 5 y [1]. Malaria deaths are declining with the massive scaling up of control measures, of which insecticide-treated bed nets (ITNs) are a major component. ITNs reduce deaths in children [2] and provide personal protection to the user, and at scale they provide community-wide protection by reducing the number of infective mosquitoes in the vicinity where ITNs are used [3],[4]. Between 2008 and 2010, 254 million ITNs were supplied to countries in sub-Saharan Africa, and the proportion of African households in possession of a net rose from 3% in 2000 to 50% by 2010 [5]. Nets, when in good condition and used correctly, are effective, simple to use, easy to deliver to rural communities, and cost-effective when used in highly endemic malarious areas [6]. On account of their low mammalian toxicity, speed of action, and high insecticidal activity, pyrethroids [7] are the only insecticide class recommended by the WHO for use in ITNs [8]. ITNs are effective with the African vectors Anopheles gambiae s.s. and An. funestus in part because these species are endophagic (feed indoors) and endophilic (rest indoors after feeding). Aside from their insecticidal activity, pyrethroids also exert an excito-repellency effect, which can lead to fewer mosquitoes entering a home (deterrence) where ITNs are used, or can cause disrupted blood feeding and premature exit of mosquitoes from the home (induced exophily) [9]. Because of the excito-repellency property of ITNs, these nets retain their personal protection properties for users even after the nets become holed [10].
The emergence and spread of insecticide resistance to all four classes of public health insecticides (pyrethroids, organochlorines, organophosphates, and carbamates) threatens the effectiveness of ITNs and indoor residual house spraying. Currently, 27 countries in sub-Saharan Africa have reported pyrethroid resistance in Anopheles vectors [11]. The real figure could very well be higher, as a lack of in-country resistance monitoring prevents accurate assessment. Because of their pyrethroid dependency, ITNs are especially vulnerable to insecticide resistance, as unlike indoor residual house spraying there are no readily available alternative insecticides. To prevent amplifying pyrethroid resistance, the WHO recommends that pyrethroid insecticides should not be used for indoor residual house spraying in areas with high long-lasting insecticide-treated bed net (LLIN) coverage [1]. In a recent study the extensive deployment and use of LLINs was blamed in part for selecting resistance in Anopheles vectors in Senegal, where malaria morbidity also increased [12]. The threat of resistance has led the WHO and members of the Roll Back Malaria Partnership to produce the “Global Plan for Insecticide Resistance Management in Malaria Vectors”, which stresses the urgency with which this problem needs to be addressed [13].
Insecticide resistance takes multiple forms: target-site resistance, metabolic resistance, and cuticular resistance. Target-site resistance to pyrethroids in An. gambiae and An. arabiensis is underpinned by a non-silent point mutation (either L1014F or L1014S) in the sodium channel gene, which is referred to as the knock-down resistance (kdr) genotype [14],[15]. Target-site resistance prevents the successful binding of the insecticide molecule to sodium channels on the nerve membranes. Metabolic resistance is caused by the activity of three large multi - gene families (cytochrome P450s, glutathione transferases, and carboxylesterases) that are able to metabolise or sequester the insecticide, thereby preventing it from reaching its target [16]. It is becoming clear that the cytochrome P450s are responsible for the majority of cases of metabolic resistance, with a secondary role for the glutathione transferases [17]–[20]. There is also preliminary evidence that cuticular resistance may be a contributing factor, but this aspect requires further analysis [17],[18],[21]. As pyrethroids and the organochlorine insecticide DDT target the sodium channel protein, cross-resistance to both insecticides is common. There is evidence that phenotypic resistance and kdr frequency have increased following the introduction of ITNs in some areas [22],[23], which could nullify the effectiveness of ITNs [24].
Policy makers and researchers debate whether these various forms of resistance are having an impact on the effectiveness of ITNs in malaria control. We carried out a systematic review of all relevant studies on human outcomes, but it became clear very quickly that there was an almost total absence of evidence to draw any conclusions on the impact of pyrethroid resistance on the efficacy of nets in decreasing disease transmission. So we turned to entomological studies: evidence of an effect of resistance on mosquitoes could be indicative of resistance having an impac on disease transmission. Our objective is to assess the effects of insecticide resistance in African anopheline mosquitoes on ITNs in terms of entomological outcomes in precise laboratory assays (cone tests), in laboratory tests with animals (tunnel tests), and in field trials with human volunteers as the attractants.
Methods
Inclusion Criteria
Study design
We included laboratory tests (cone tests and tunnel tests) and field trials using experimental huts (see Box 1 for details of types of studies included).
Box 1. Types of Studies Included
Cone Test
Methods: Studies in the laboratory in which mosquitoes are placed inside a plastic cone that is attached to a net for three minutes; after net exposure the mosquitoes are placed in a holding container while entomological outcomes are measured [25].
Outcomes: Mosquito mortality after 24 h, percentage knock-down at 60 min, and time to 50% or 95% knock-down.
Advantages: Researchers can standardise confounding variables, such as mosquito species, sex, age, and blood feeding status. The number of mosquitoes used in the test is standardised.
Tunnel Test
Methods: Studies in a laboratory, using animal bait, such as a guinea pig, placed at one end of a specially constructed tunnel. A fixed number of mosquitoes are released at the other end of the tunnel, and they must pass through a holed ITN or UTN to reach the animal bait. The following morning, both live and dead mosquitoes, blood fed and non-blood fed, are collected and counted from both sides of the holed net. Live mosquitoes are monitored for a further 24 h to assess delayed mortality [25].
Outcomes: Deterrence (not passed through net), blood feeding, and mosquito mortality.
Advantages: As for cone test.
Field Trials
Methods: Studies in areas where mosquitoes breed. Volunteers sleep in experimental huts for a specific period under an ITN or an UTN, with one hut per person. The huts are identical in construction, and incorporate exit traps to catch wild mosquitoes entering and exiting the hut prematurely. Each morning of the trial, both live and dead mosquitoes, blood fed and non-blood fed, are collected and counted from both inside the hut and the exit traps. Live mosquitoes are monitored for a further 24 h to assess delayed mortality. Volunteers and nets are randomly allocated to huts at the start of the trial and are usually rotated to avoid bias. Often huts are cleaned between rotations to avoid cross-contamination of huts from the different treatment arms [25].
Outcomes: Deterrence, blood feeding, mosquito mortality, and induced exophily.
Advantages: Given that this method assesses the response of wild mosquitoes to human volunteers, it is a more realistic representation of how effective ITNs are in terms of entomological outcomes, compared with laboratory methods.
Mosquito population
Included African malaria vectors were An. gambiae, An. arabiensis, or An. funestus. We included laboratory studies that used established laboratory-colonised strains of mosquitoes with known resistance phenotype or genotype. Experimental hut study trials were included if they measured the resistance status of the wild mosquito populations at the time of the study by bioassays with our without kdr genotyping.
Intervention
We included studies that compared an ITN (conventionally treated bed net [CTN] or a LLIN) versus an untreated bed net (UTN). The CTNs (which require dipping into insecticide and which also require retreatment at least once a year) must have been impregnated with a WHO-recommended pyrethroid with the recommended formulation and dose (see Table 1 for recommended impregnation regimens). The LLINs (which are factory-treated nets where the insecticide is incorporated within or bound around the net fibres) must have had either interim or full recommendation from the WHO (see Table 2 for recommended LLINs).
Tab. 1. WHO-recommended pyrethroids for treatment of CTNs for vector control. 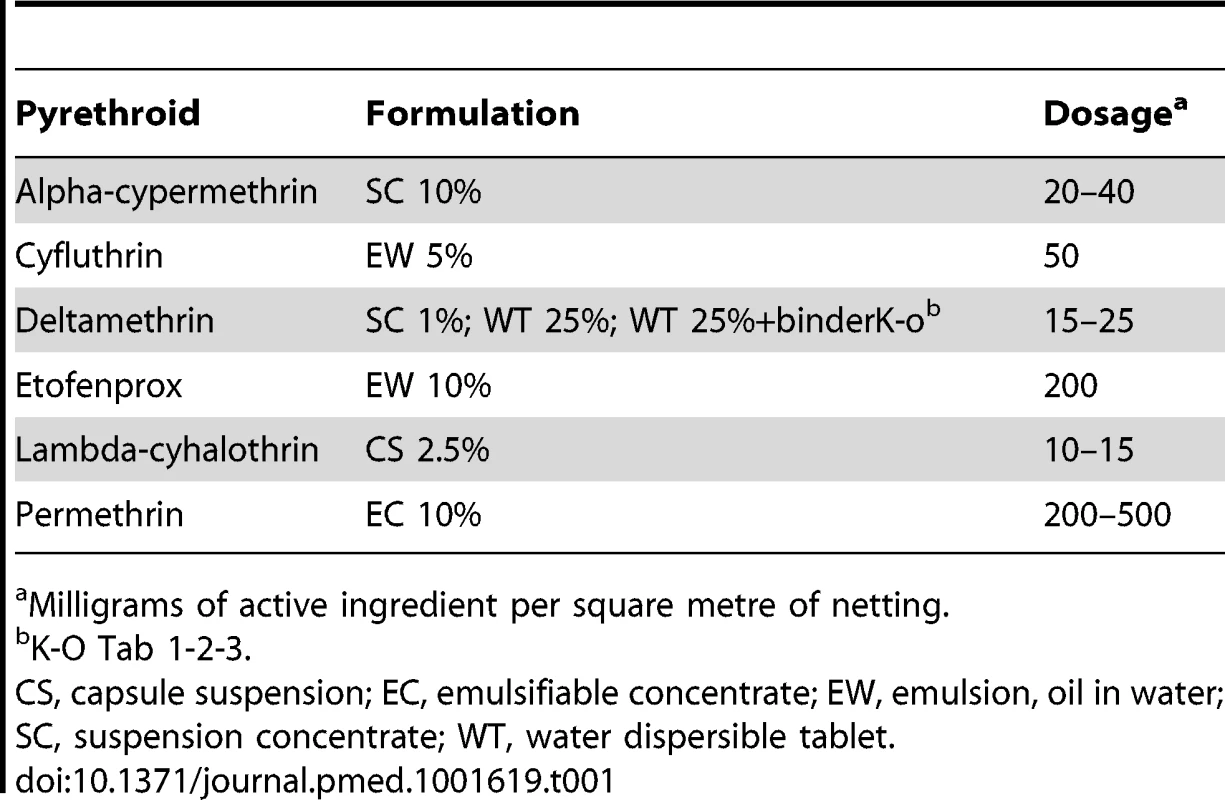
Milligrams of active ingredient per square metre of netting. Tab. 2. WHO-recommended LLINs for vector control. 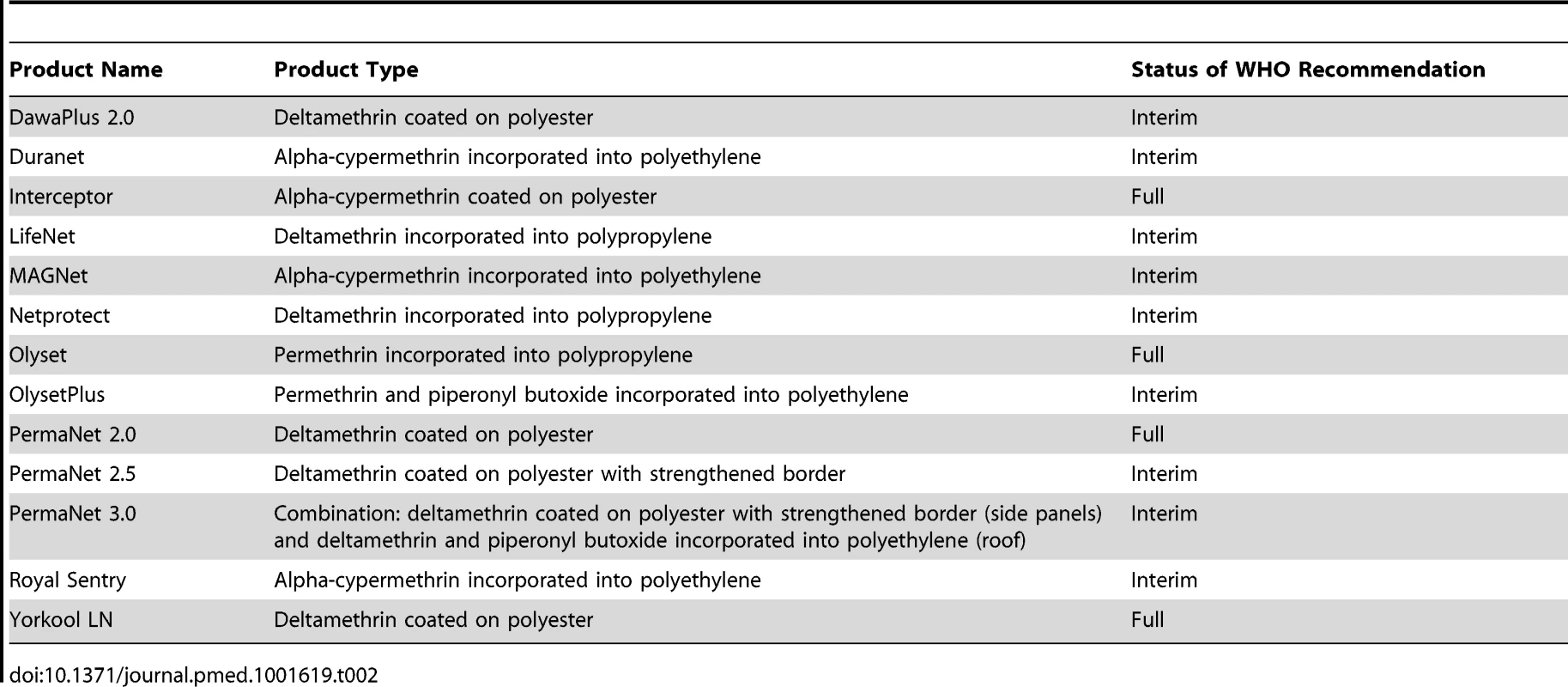
Outcomes
Included outcomes were blood feeding, mosquito mortality, deterrence (reduction in the number of mosquitoes found in experimental huts), induced exophily (number of mosquitoes found in the exit trap of experimental huts), not passed though net (measure of deterrence in tunnel test), percent knock-down at 60 min, time to 50% knock-down, and time to 95% knock-down [25] (Table 3).
Tab. 3. Measured outcomes appropriate for the different types of study. 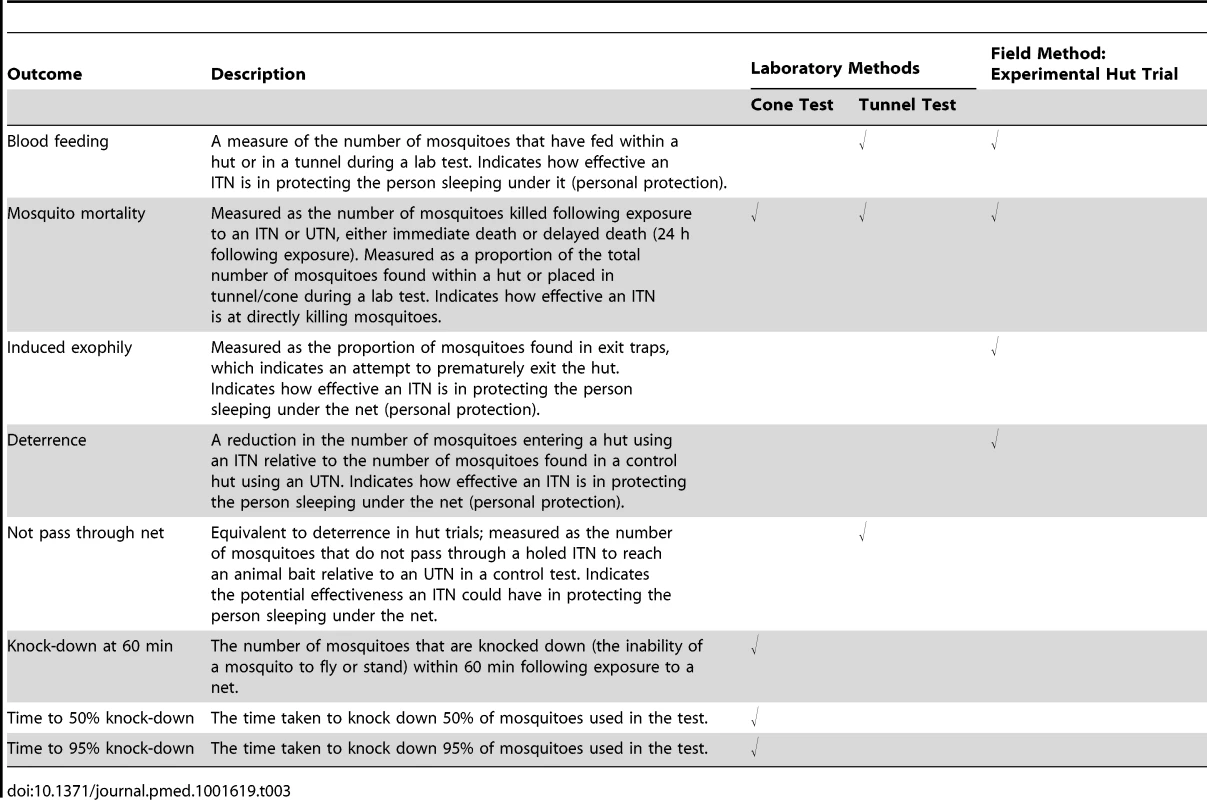
Search Strategy
The search period was from 1 January 1980 to 17 May 2013 or later. We searched the following databases for relevant studies: MEDLINE (from 1 January 1980 to 31 December 2013) and Cochrane Central Register of Controlled Trials, Science Citation Index Expanded, Social Sciences Citation Index, African Index Medicus, and CAB Abstracts (from 1 January 1980 to 17 May 2013). There was no language restriction (see Table S1 for the search terms used).
We also searched the following conference proceedings: First MIM Pan-African Malaria Conference, Senegal, 6–9 January 1997; Second MIM Pan-African Malaria Conference, South Africa, 15–19 March 1999; Third MIM Pan-African Malaria Conference, Tanzania, 17–22 November 2002; Fourth MIM Pan-African Malaria Conference, Cameroon, 13–18 November 2005; Fifth MIM Pan-African Malaria Conference, Nairobi, 2–6 November 2009; American Society of Tropical Medicine and Hygiene 59th Annual Meeting, Atlanta, Georgia, 3–7 November 2010; American Society of Tropical Medicine and Hygiene 60th Annual Meeting, Philadelphia, Pennsylvania, 4–8 December 2011; and American Society of Tropical Medicine and Hygiene 61st Annual Meeting, Atlanta, Georgia, 11–15 November 2012.
Study Selection
Two authors (C. S. and A. A. E.) independently screened the search results for potentially relevant studies and retrieved the corresponding full articles. C. S. and A. A. E. independently assessed the articles for eligibility using a standardised form (Table S2). Discrepancies between the eligibility results were resolved by discussion. Study investigators were contacted for clarification if the eligibility of a particular study was unclear. Multiple publications from the same study were identified, and if eligible, the original study was taken forward for inclusion.
Data Extraction
C. S. and A. A. E. independently extracted data from all included studies into a data extraction form. Missing or unclear outcome data were requested from the study investigators. For dichotomous outcomes for the ITN and UTN groups, the number of mosquitoes experiencing the outcome and the total number of mosquitoes were extracted (Tables S3–S5). For continuous outcomes, we extracted the mean and standard deviation when possible. For deterrence, the total number of mosquitoes was extracted for the ITN and UTN groups. A sub-sample of 10% of the studies was randomly selected to assess the performance of the duplicate extraction processes by C. S. and A. A. E. Differences between the two extraction processes were examined, and no serious discrepancies were found. The data extracted by C. S. were used in all analyses.
Stratification of Resistance
The WHO classifies mosquitoes as susceptible to insecticides if, after exposure to a diagnostic dose, there is ≥98% mortality, and as resistant to insecticides if there is ≤90% mortality; mortality between 97% and 90% requires the confirmation of resistance genes for mosquitoes to be classified as resistant [26]. Characterisation of resistance across studies was not consistent, as some studies used bioassays, others used kdr genotyping, and some used a combination of both. We therefore developed a composite classification system to allow us to categorise the insecticide resistance status of mosquitoes in three broad groups (low, moderate, and high), based on phenotypic resistance measured using bioassay mortality data and/or kdr frequency (Table 4). The alleles for kdr are presented as a frequency or percentage.
Tab. 4. Stratification of mosquito resistance constructed for this study based on either percent mortality from WHO bioassay data and/or <i>kdr</i> frequency. 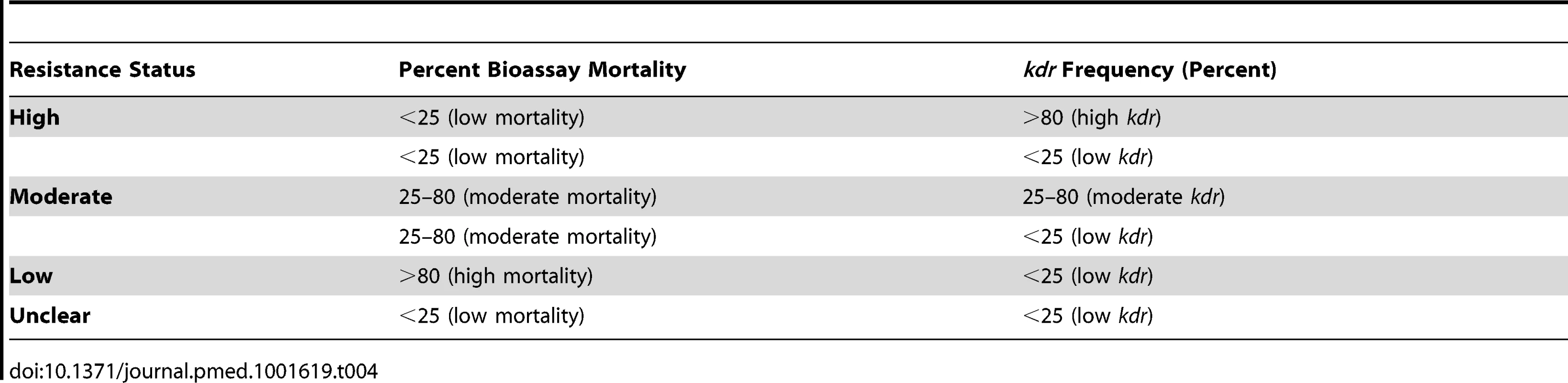
Risk of Bias Assessment
C. S. assessed the risk of bias of each included study. We developed a quality assessment tool that used four criteria for tunnel and cone tests: (1) comparability of mosquitoes in ITN and UTN groups (all female, age matched, and non-blood fed), (2) observers blinded, (3) complete outcome data, and (4) raw data reported for ITN and UTN groups.
For experimental hut trials we developed seven criteria: (1) comparability of mosquitoes in ITN and UTN huts, (2) collectors blinded, (3) sleepers blinded, (4) raw data reported for ITN and UTN groups, (5) ITNs randomly allocated to huts, (6) ITNs rotated, and (7) sleepers rotated. For all criteria, we made a judgement of high, low, or unclear risk of bias.
For hut trials, we generated an additional set of variables to assess variability in the design and execution of the studies, called “rigor of implementation”. The criteria assessed included (1) nets being washed according to WHO protocol, (2) cleaning of huts before the trial and between rotations to avoid cross-contamination of huts from the different treatment arms and to remove any insects that may have been missed during collections, (3) whether ITNs were tested either chemically or using bioassays to assess the insecticide impregnation efficacy and residual activity (applicable to CTNs), and (4) whether male mosquitoes were excluded from the analysis. We also reported how each study measured resistance in the wild mosquito populations: whether phenotypic resistance was measured by bioassays and/or kdr genotyping (and the number of mosquito screened for kdr), and whether metabolic resistance was measured.
Data Analysis
Analyses were carried out in Review Manager 5. We stratified the analyses by study design and the resistance status of the mosquito population (Table 4). Dichotomous outcomes were summarised using the RD; therefore, results are generalisable only to situations where the UTN group event rate is comparable to those observed here. When the same study compared multiple ITNs, the event rate in the UTN group was split to ensure each mosquito was included in the analysis only once.
The results of studies were pooled using meta-analysis when possible. DerSimonian and Laird random effects models were used when heterogeneity was detected; otherwise, a fixed effects Mantel-Haenszel method was applied. It is worth noting that a random effects meta-analysis awards more weight to smaller studies than a fixed effects meta-analysis, and the weights for each study tend to equality as the between-trial variance increases.
Assessment of Heterogeneity
Data that could not be presented in forest plots were tabulated. Heterogeneity was assessed by visually inspecting the forest plots to detect overlapping confidence intervals, applying the chi-squared test with a p-value of 0.10 used to indicate statistical significance, and implementing the I2 test statistic, with a value of 50% indicating a moderate level of heterogeneity. Of course, such assessments of heterogeneity are influenced by the number of included studies and should be interpreted with caution.
Heterogeneity was substantive and common in all the analyses, and we sought explanations through a variety of pre-specified subgroup analyses. Subgroups included net type, type and concentration of insecticide, and whether the net was washed or not. We carried out sensitivity analyses by examining the effects when analyses were restricted to hut trials that had a low risk of bias (i.e., ITNs randomly allocated to huts, ITNs rotated, sleepers rotated). Reporting biases were explored using funnel plots. We calculated the confidence intervals for the I2 statistic using the method described in [27].
Results
Search Results
Figure 1 displays the review profile. Database searches recovered 1,107 records, from which three duplicates were removed. Searching other sources did not yield any potentially relevant records. After screening the 1,104 records, 914 records were excluded. Of the remaining 73 records, 55 records were excluded (see Figure 1 for exclusion reasons). The remaining 25 records [4],[6],[9],[28]–[49] described 60 separate studies (a study is defined as a comparison that has a distinct control UTN arm). Results of 53 of the 60 studies were combined in a meta-analysis; the results of five studies are described in Tables 5 and 6; and two studies did not report useable data.
Fig. 1. Flow diagram of the study selection process. 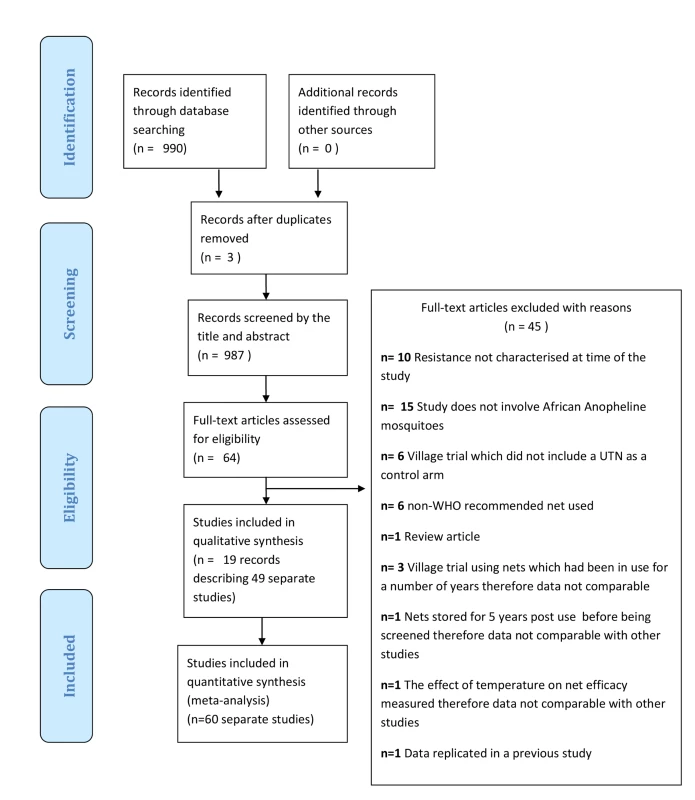
Tab. 5. Results from cone tests comparing LLIN or CTN versus UTN for time to 50% and 95% knock-down. 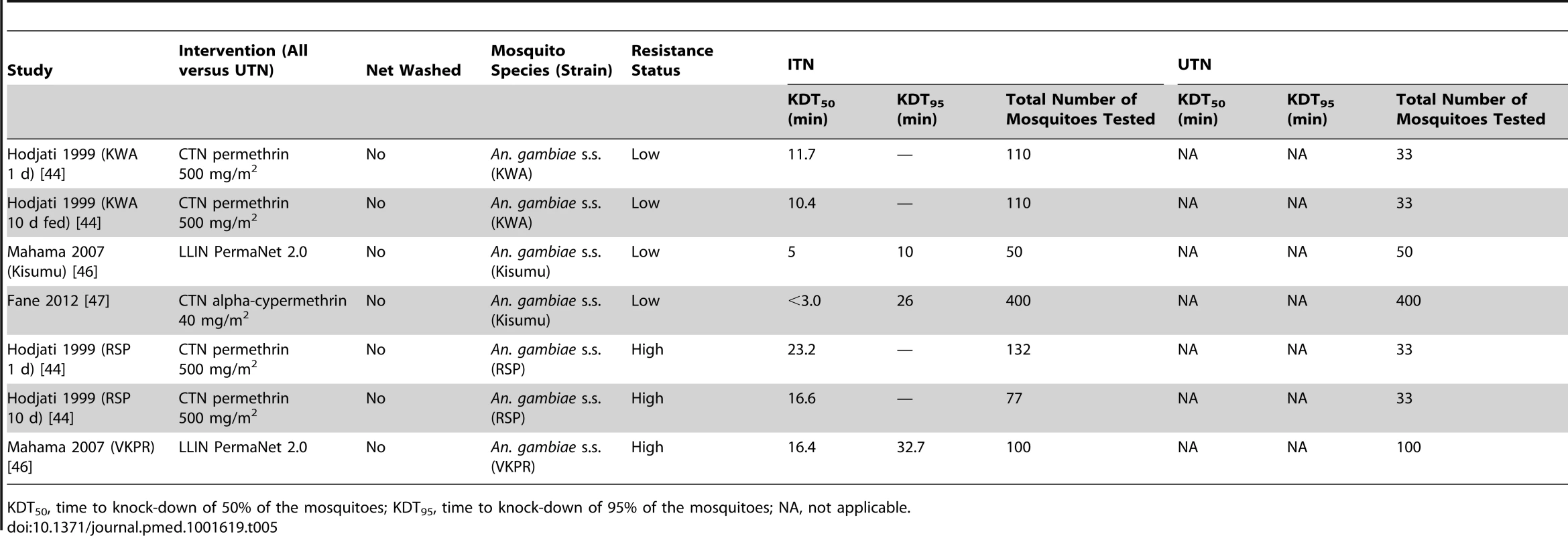
KDT50, time to knock-down of 50% of the mosquitoes; KDT95, time to knock-down of 95% of the mosquitoes; NA, not applicable. Tab. 6. Results from tunnel tests comparing CTN versus UTN for mosquito mortality, blood feeding, and not passed though net. 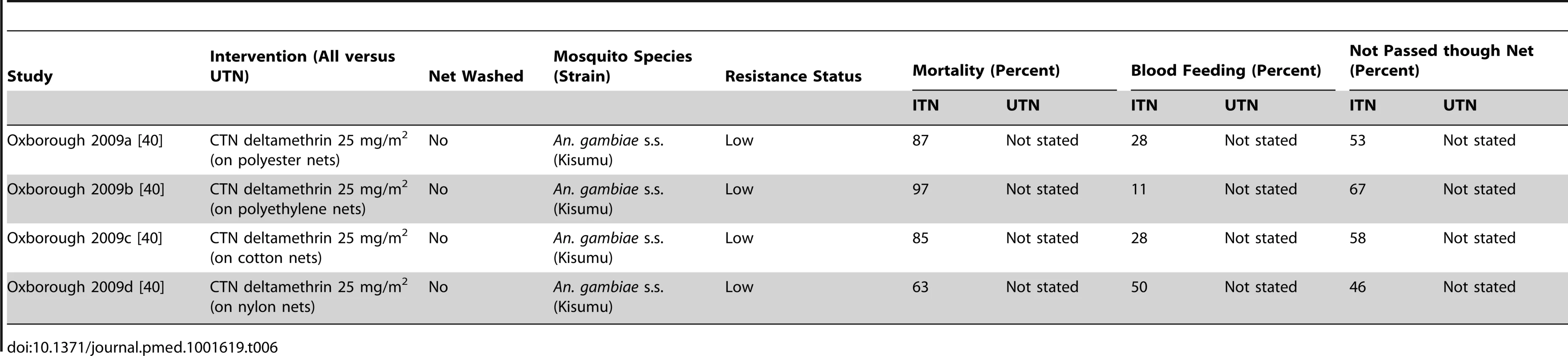
The updated MEDLINE search (May–December 2013) recovered 291 records, of which two records were assessed for eligibility. They were subsequently excluded for not meeting the study design inclusion criteria and for not characterising resistance in the mosquito populations at the time of the study.
Characteristics of Included Studies
The 60 included studies included cone tests (n = 25), tunnel tests (n = 11), and experimental hut trials (n = 24).
Cone tests
The 25 included cone test studies made 60 comparisons. Characteristics for each comparison are given in Table 7. UTNs were compared against unwashed and washed CTNs and LLINs.
Tab. 7. Study characteristics of the included cone tests. 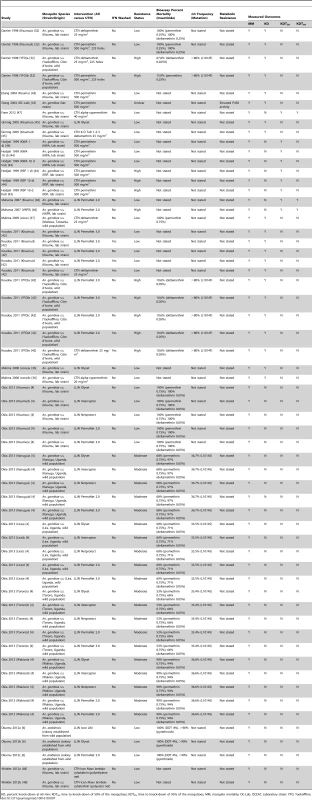
KD, percent knock-down at 60 min; KDT50, time to knock-down of 50% of the mosquitoes; KDT95, time to knock-down of 95% of the mosquitoes; MM, mosquito mortality; OC-Lab, OCEAC Laboratory strain; YFO, Yaokoffikro. Fifty-seven comparisons used An. gambiae s.s. mosquitoes, whilst three were of An. arabiensis. Overall, 29 comparisons used laboratory-reared mosquito strains (Kisumu, VKPR, OC-Lab, KWA, and RSP strains), and 28 comparisons used wild field-caught mosquitoes from Yaokoffikro (Côte d'Ivoire), Muheza (Tanzania), and localities in Uganda. Three comparisons used recently colonised An. arabiensis mosquitoes that were originally collected from the Ulanga District of Tanzania.
Based on the reported WHO bioassay percent mortalities and kdr frequencies, 28 comparisons were carried out with mosquitoes with low resistance, 20 comparisons with moderately resistant mosquitoes, and 11 comparisons with highly resistant mosquitoes; resistance was unclear for one comparison. Only one comparison measured metabolic resistance.
For the risk of bias assessment, all comparisons reported comparability of ITN and UTN mosquito groups, but it was unclear in all studies whether observers were blinded (Table S6). No comparison reported incomplete outcome data. Fifteen comparisons reported raw data for ITN and UTN groups, the remaining 45 did not.
Tunnel tests
The 11 included tunnel test studies made 20 comparisons. UTNs were compared against unwashed CTNs and LLINs. Characteristics for each comparison are given in Table 8. All comparisons used An. gambiae mosquitoes (the number of mosquitoes used varied from 200 to 592). Three comparisons used wild field-caught mosquitoes from Yaokoffikro (Côte d'Ivoire) and Muheza (Tanzania) in their assessment, whilst 17 comparisons used laboratory-reared mosquito strains (Kisumu, VKPR, Kisumu/VKPR hybrids, Tola, and Kou strains). Based on the reported WHO bioassay percent mortalities and kdr frequencies, 12 comparisons were carried out with mosquitoes with low resistance, six comparisons used highly resistant mosquitoes, and resistance was moderate for two comparisons. No comparison measured metabolic resistance.
Tab. 8. Study characteristics of the included tunnel tests. 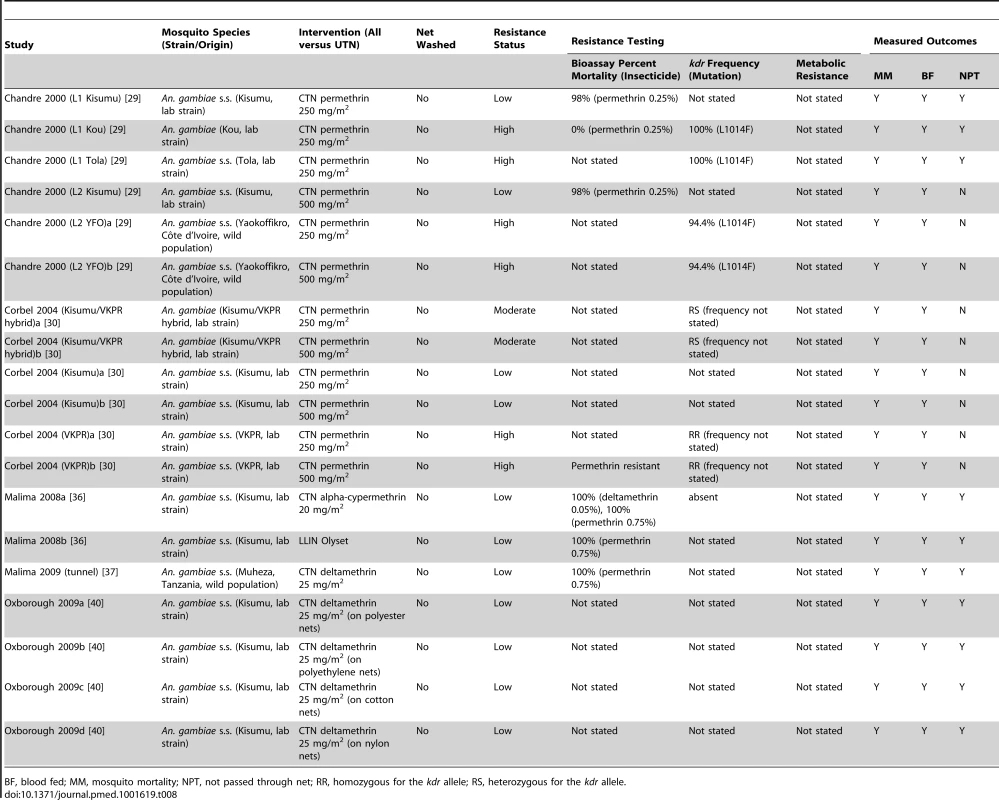
BF, blood fed; MM, mosquito mortality; NPT, not passed through net; RR, homozygous for the kdr allele; RS, heterozygous for the kdr allele. For the risk of bias assessment, 16 comparisons reported comparability of ITN and UTN mosquito groups, whilst comparability was unclear in four comparisons (Table S7). It was unclear in all studies whether observers were blinded. No comparison reported incomplete outcome data. Sixteen comparisons reported raw data for ITN and UTN groups, the remaining four did not.
Experimental hut field trials
The 24 included hut studies made 56 comparisons (Table 9). 20 comparisons used field sites in Côte D'Ivoire, 14 in Tanzania, 11 in Benin, six in Burkina Faso, and five in Cameroon. Most comparisons (41 of 56) were of An. gambiae mosquitoes, 12 were of An. arabiensis, and three were of An. funestus. Two comparisons used laboratory-reared strains (Kisumu). Based on the reported WHO bioassay percent mortalities and kdr frequencies, 26 comparisons were carried out with mosquitoes with low resistance, 21 comparisons used highly resistant mosquitoes, and resistance was moderate for nine comparisons. Two comparisons measured metabolic resistance.
Tab. 9. Study characteristics of the included experimental hut trials. 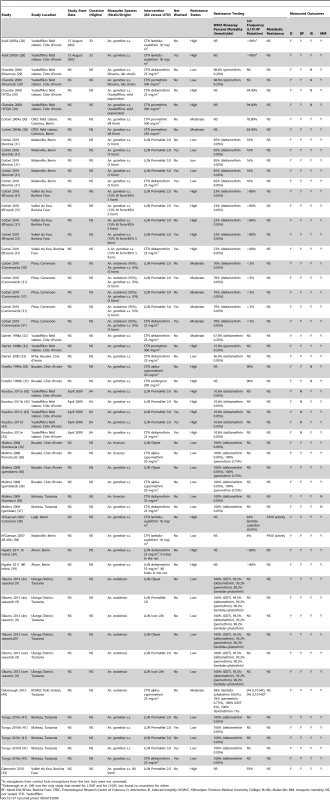
In mosquitoes from control huts (mosquitoes from the test huts were not screened). For the risk of bias assessment, no comparisons reported comparability of ITN and UTN mosquito groups or blinded collectors of mosquitoes or the sleepers (Table S8). Forty-eight of the 56 comparisons reported raw data for ITN and UTN groups. It was unclear in 16 comparisons as to whether nets were randomly allocated to huts at the start of the study. Overall, 41 comparisons rotated ITNs, eight did not, and seven did not report rotation. Fifty comparisons rotated sleepers, whilst it was unclear as to whether the remaining comparisons rotated the sleepers between huts.
Table 10 displays the rigor of implementation assessment of each hut trial in terms of particular study design characteristics. Standardisation across studies both in terms of the experimental design and reporting was not consistent. Of the 16 comparisons that compared a washed net, 12 washed the net in accordance with the WHO protocol, one did not wash the net using WHO procedures, and it was unclear whether the remaining three had followed WHO procedures. Seven of the 56 comparisons cleaned the huts before the study, whereas 25 comparisons cleaned the huts after each rotation; the remaining comparisons were unclear regarding when the huts were cleaned. Overall, 38 of the 56 comparisons tested the ITNs before the study, 32 comparisons tested the ITNs on completion of the study, and 22 comparisons tested the nets chemically; the remaining comparisons did not test the nets. Outcomes were not measured on male mosquitoes in 30 of the 56 comparisons, but were measured in the remaining 26 comparisons.
Tab. 10. Assessment of “rigor” for experimental hut trials. 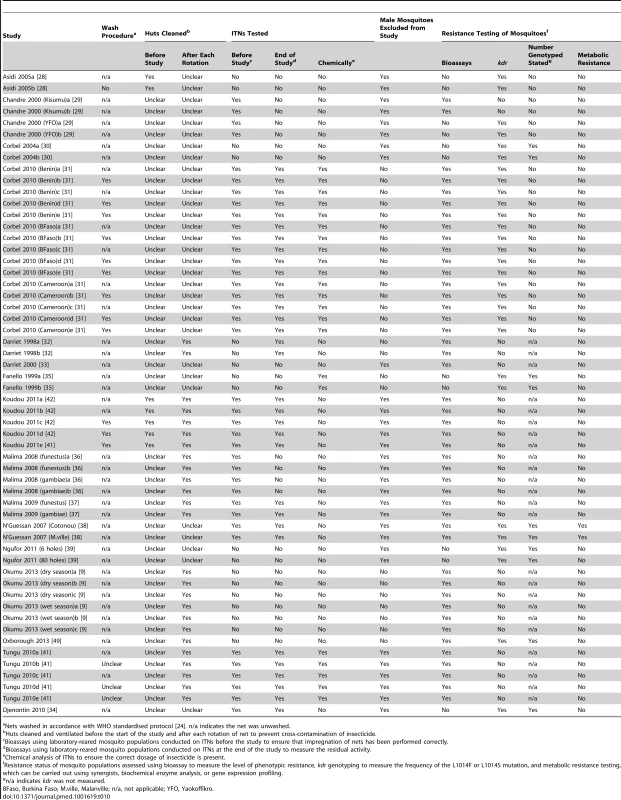
Nets washed in accordance with WHO standardised protocol [24]. n/a indicates the net was unwashed. Characterisation of resistance was not consistent across studies. Seventeen comparisons measured phenotypic resistance using bioassays complemented with kdr genotyping in the mosquito populations under investigation. Bioassays on their own were used in 27 comparisons, whilst 11 comparisons were performed on mosquitoes for which only kdr genotyping was used. Characterisation of metabolic resistance was reported in just two studies, where the authors also measured phenotypic resistance and kdr. For those studies which screened for kdr, ten stated the number of mosquitoes that had been genotyped.
Relationship between Resistance and Entomological Outcomes
Cone tests
Forty-seven cone test comparisons reported mosquito mortality (21 low, 20 moderate, and five high resistance and one unclear) (Figure S1). Mortality was very low in the untreated net group, and the risk of mosquito mortality is much higher using ITNs as compared with UTNs regardless of resistance. The study-specific RDs showed huge variability within all three categories of resistance. The meta-analytic results showed that the difference in mortality risk using ITNs as compared with UTNs decreased as resistance increased. Nevertheless, mortality risk was significantly higher for ITNs compared to UTNs regardless of resistance: with low resistance, the difference in risk of mortality is 0.86 (95% CI 0.72 to 1.01; 4,626 mosquitoes, 21 comparisons; I2 = 100%, 95% CI 100% to 100%); in the case of moderate resistance the difference in risk is 0.71 (95% CI 0.53 to 0.88; 5,760 mosquitoes, 20 comparisons; I2 = 100%, 95% CI 100% to 100%); with high resistance, the difference in risk is 0.56 (95% CI 0.17 to 0.95; 784 mosquitoes, five comparisons; I2 = 99%, 95% CI 99% to 100%). The test for subgroup differences did not demonstrate a difference in the RD between high, medium, and low resistance subgroups (p = 0.12, I2 = 49%, 95% CI 23% to 66%). A further 12 comparisons (seven low resistance, five high) presented data that could not be combined in meta-analysis (Table 11).
Tab. 11. Results from cone tests comparing LLIN or CTN versus UTN for mosquito mortality and knock-down at 60 min. 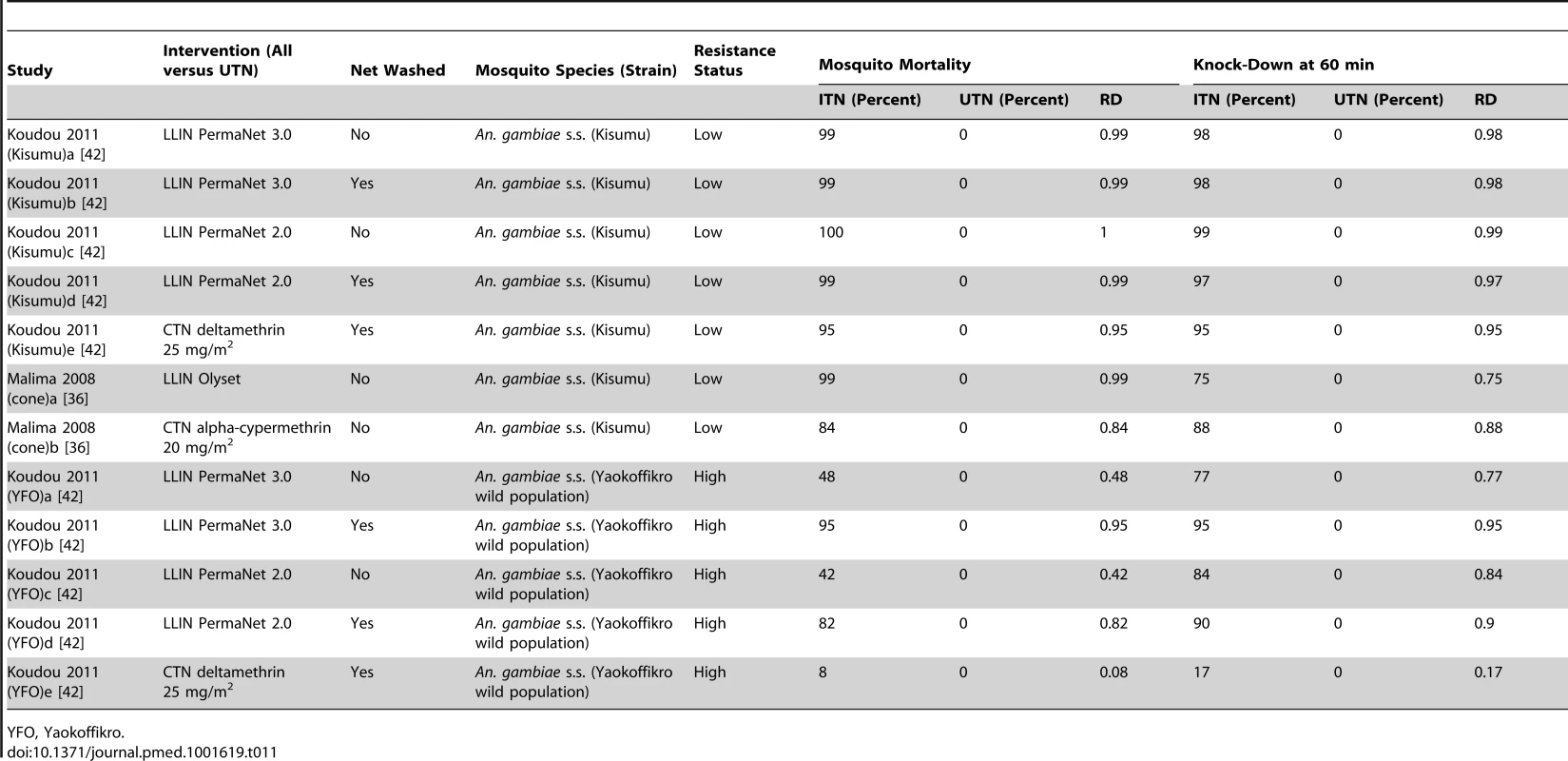
YFO, Yaokoffikro. Nine comparisons reported percentage knock-down at 60 min (six low resistance, two high, one unclear; Figure S2). In mosquitoes with low resistance, the risk of being knocked down is significantly higher using ITNs as compared with UTNs, but with high resistance, there is no difference between ITNs and UTNs. A significant difference is detected between the meta-analytic results for mosquitoes with low, unclear, and high resistance (p<0.00001, I2 = 98.8%, 95% CI 98.3% to 99.2%).
The majority of studies show that the risk of knock-down is higher using ITNs than using UTNs, regardless of resistance. In mosquitoes with low resistance, the difference in risk of knock-down is 0.87 (95% CI 0.69 to 1.05; 3,440 mosquitoes, 17 comparisons; I2 = 100%, 95% CI 100% to 100%); with high resistance, the difference in risk is 0.09 (95% CI −0.03 to 0.21; 309 mosquitoes, two comparisons; I2 = 87%, 95% CI 94% to 97%). There is high variability between the results from studies within the same resistance category, although all comparisons tend to favour ITNs. A further 12 comparisons (seven low resistance, five high) presented data that could not be combined in meta-analysis (Table 11).
Seven cone test comparisons reported time to 50% knock-down (four low resistance, three high), and two comparisons presented time to 95% knock-down (one low, one high). By visual inspection of Table 5, the knock-down times tend to be longer in studies of mosquitoes with high resistance than in studies of mosquitoes with low resistance. However, this comparison is made across trials and may be subject to confounding.
Tunnel tests
Fourteen tunnel test comparisons reported feeding (eight low resistance, two moderate, six high) (Figure S3). The higher the resistance, the lower the effectiveness of ITNs (as compared with UTNs). A significant difference is detected between the meta-analytic results for mosquitoes with low, moderate, and high resistance (p = 0.001, I2 = 85.1%, 95% CI 68.7% to 92.9%). A lower risk of blood feeding is apparent when using ITNs as compared with UTNs, regardless of resistance. For mosquitoes with low resistance, the difference in the risk of blood feeding is −0.66 (95% CI −0.77 to −0.55; 2,177 mosquitoes, eight comparisons; I2 = 92%, 95% CI 87% to 95%); for mosquitoes with moderate resistance, the difference in risk is −0.53 (95% CI −0.63 to −0.42; 300 mosquitoes, two comparisons; I2 = 0% 95% CI not estimable); and for mosquitoes with high resistance, the difference in risk is −0.27 (95% CI −0.45 to −0.09; 2,472 mosquitoes, six comparisons; I2 = 97%, 95% CI 94% to 98%). There is high variability among the results from studies of mosquitoes with low resistance and also among those from studies of mosquitoes with high resistance, although most comparisons significantly favour ITNs. Four additional comparisons (low resistance) presented data that could not be combined in meta-analysis (Table 6).
Sixteen tunnel test comparisons reported mosquito mortality (eight low resistance, two moderate, six high) (Figure S4). The risk of mortality is significantly higher using ITNs as compared with UTNs, regardless of resistance. The meta-analytic results showed that the difference in mortality risk using ITNs as compared with UTNs decreased as resistance increased. The test for subgroup differences showed significant variability between the meta-analytic results from low, moderate, and high resistance subgroups (p = 0.001, I2 = 84.7%, 95% CI 67.9% to 92.7%). For mosquitoes with low resistance, the difference in risk is 0.74 (95% CI 0.61 to 0.87; 2,177 mosquitoes, eight comparisons; I2 = 96%, 95% CI 94% to 97%); for mosquitoes with moderate resistance, the difference in risk is 0.50 (95% CI 0.40 to 0.60; 300 mosquitoes, two comparisons; I2 = 11%, 95% CI not estimable); and for mosquitoes with high resistance, the difference in risk is 0.39 (95% CI 0.24 to 0.54; 2,472 mosquitoes, six comparisons; I2 = 95%, 95% CI 94% to 98%). There is high variability among the results from studies of mosquitoes with low resistance and also among those from studies of mosquitoes with high resistance, yet almost all comparisons significantly favour ITNs. Table 6 shows the results of additional comparisons (low resistance) that could not be combined in meta-analysis.
Six tunnel test comparisons reported whether mosquitoes could not pass through the net (four low resistance, two high) (Figure S5). Results show that the higher the resistance, the lower the effectiveness of ITNs (as compared with UTNs). The observed trend could be caused by differences in characteristics (other than resistance) between the studies of low resistance mosquitoes and those of high resistance mosquitoes. A significant difference is detected between the meta-analytic results for low and high resistance mosquitoes (p<0.00001, I2 = 98.4%, 95% CI 97.1% to 99.1%).
The risk of not passing though the net is significantly higher when using ITNs than when using UTNs, regardless of mosquito resistance. In mosquitoes with low resistance, the difference in risk is 0.68 (95% CI 0.62 to 0.75; 1,140 mosquitoes, four comparisons; I2 = 61%, 95% CI 0% to 87%), and in mosquitoes with high resistance, the difference in risk is 0.36 (95% CI 0.31 to 0.41; 1,309 mosquitoes, two comparisons; I2 = 0%, 95% CI not estimable). There is variability among the results from studies of mosquitoes with low resistance, yet all comparisons significantly favour ITNs. Four additional comparisons (low resistance) presented data that could not be combined in meta-analysis (Table 6).
Experimental hut trials
Overall, 44 hut trial comparisons reported blood feeding (20 low resistance, nine moderate, 15 high) (Figure 2). There is no clear relationship between resistance and the effectiveness of ITNs. A significant difference is not detected between the meta-analytic results for low, moderate, and high resistance groups (p = 0.84, I2 = 0%, 95% CI 0% to 35%).
Fig. 2. Forest plot for experimental hut trials comparing LLIN or CTN versus UTN for blood feeding. 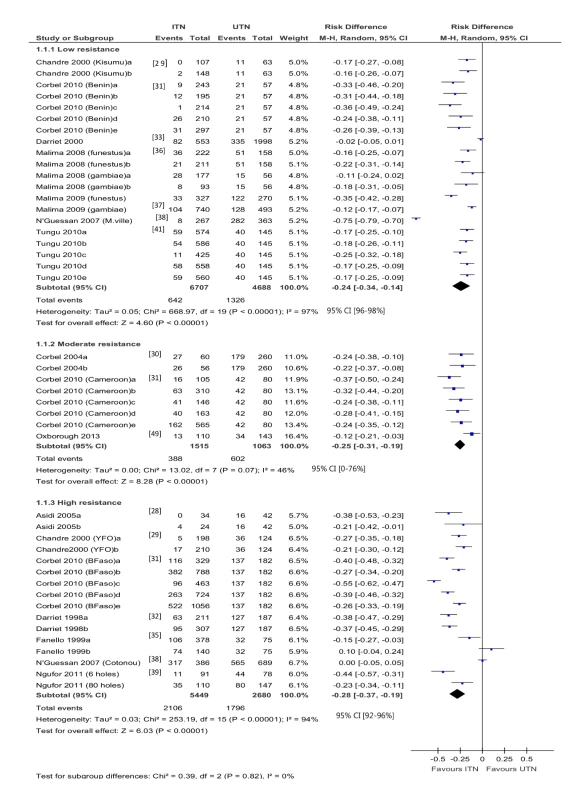
BFaso, Burkina Faso; M-H, Mantel-Haenszel; M.ville, Malanville (Benin); YFO, Yaokoffikro, (Côte d'Ivoire). Blood feeding was reduced when using ITNs as compared with UTNs, regardless of resistance. In mosquitoes with low resistance, the difference in the risk of blood feeding is −0.24 (95% CI −0.34 to −0.14; 11,395 mosquitoes, 20 comparisons; I2 = 97%, 95% CI 96% to 98%); in mosquitoes with moderate resistance, the difference in risk is −0.25 (95% CI −0.31 to −0.19; 2,578 mosquitoes, eight comparisons; I2 = 46%, 95% CI 0% to 76%); and in mosquitoes with high resistance, the difference in risk is −0.28 (95% CI −0.37 to −0.19; 8,129 mosquitoes, 16 comparisons; I2 = 94%, 95% CI 92% to 96%). There is particularly high variability among the results from studies of mosquitoes with low resistance and among those from studies of mosquitoes with high resistance, although most comparisons significantly favour ITNs. One comparison [22], with high resistance, reported 38% and 68% blood feeding (figures estimated from graph) in the ITN and UTN groups, respectively (RD = 0.3).
Fifty-three hut trial comparisons reported mosquito mortality (24 low resistance, eight moderate, 20 high) (Figure 3). There is high heterogeneity across study-specific results with each category of resistance. In addition, one study [9] appears to show no evidence of an effect of ITNs in low resistance mosquitoes. The authors also report on the bioassay, which shows 90%–100% susceptibility to insecticides. However, mortality risk was higher for ITNs compared to UTNs irrespective of the resistance category. In mosquitoes with low resistance, the difference in risk is 0.56 (95% CI 0.43 to 0.68; 67,610 mosquitoes, 24 comparisons; I2 = 100%, 95% CI 100% to 100%); in mosquitoes with moderate resistance, the difference in risk is 0. 39 (95% CI 0.16 to 0.61; 2,578 mosquitoes, eight comparisons; I2 = 98%, 95% CI 97% to 98%); and with high resistance, the difference in risk is 0.35 (95% CI 0.27 to 0.43; 10,417 mosquitoes, 21 comparisons; I2 = 96%, 95% CI 95% to 97%). The meta-analytic results showed that the difference in mortality risk using ITNs as compared with UTNs modestly decreased as resistance increased, and the test for subgroup differences demonstrated a difference in the RD between high, medium, and low resistance subgroups (p = 0.03, I2 = 72.0%, 95% CI 58.7% to 81.0%).
Fig. 3. Forest plot for experimental hut trials comparing LLIN or CTN versus UTN for mosquito mortality. 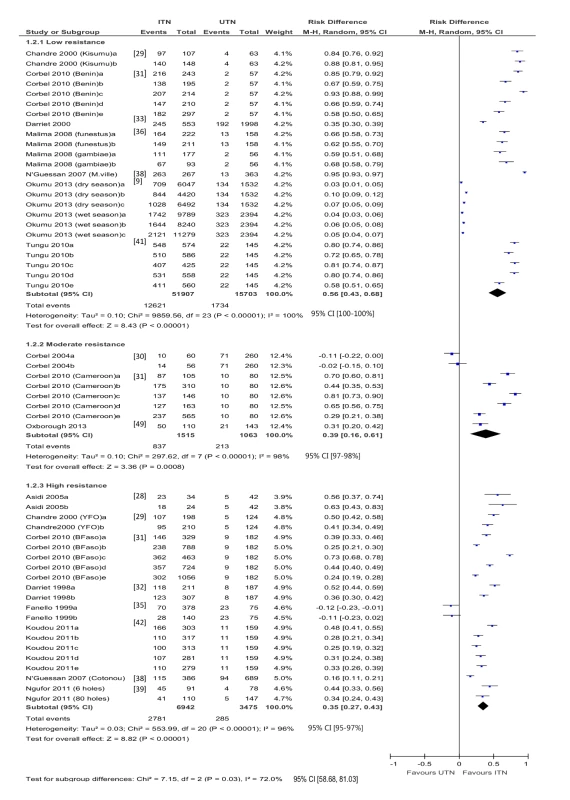
BFaso, Burkina Faso; M-H, Mantel-Haenszel; M.ville, Malanville (Benin); YFO, Yaokoffikro, (Côte d'Ivoire). One comparison [22], with high resistance mosquitoes, reported 42% and 2% mortality (figures estimated from graph) in the ITN and UTN groups, respectively (RD = 0.4).
Forty-three trial hut comparisons reported results for induced exophily (18 low resistance, nine moderate, 16 high) (Figure 4). There is no clear relationship between resistance and the effectiveness of ITNs in relation to this outcome. A significant difference is detected between the meta-analytic results for low, moderate, and high resistance (p = 0.0002, I2 = 88.2%, 95% CI 81.6% to 92.3%).
Fig. 4. Forest plot for experimental hut trials comparing LLIN or CTN versus UTN for induced exophily. 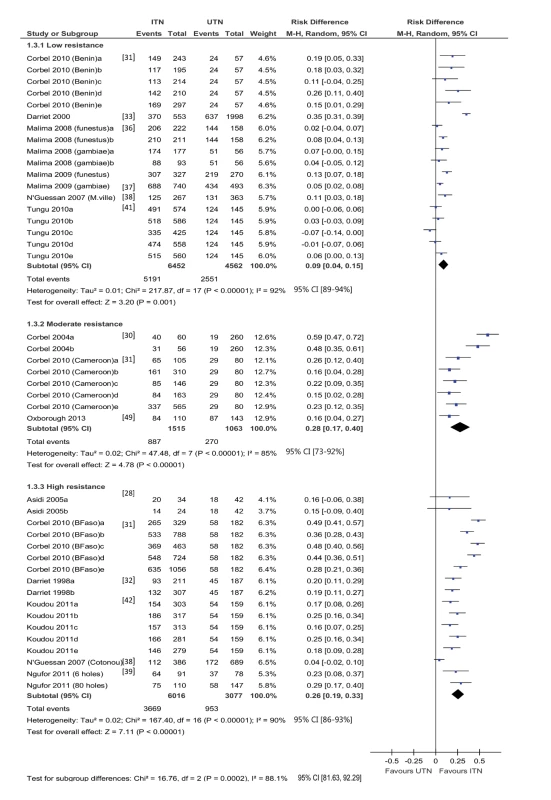
BFaso, Burkina Faso; M-H, Mantel-Haenszel; M.ville, Malanville (Benin); YFO, Yaokoffikro, (Côte d'Ivoire). Generally, the risk of exiting the hut is higher using ITNs than using UTNs, regardless of resistance. For mosquitoes with low resistance, the difference in risk is 0.09 (95% CI 0.04 to 0.15; 11,014 mosquitoes, 18 comparisons; I2 = 92%, 95% CI 89% to 94%); for mosquitoes with moderate resistance, the difference in risk is 0.28 (95% CI 0.17 to 0.40; 2,578 mosquitoes, eight comparisons; I2 = 85%, 95% CI 73% to 92%); and for mosquitoes with high resistance, the difference in risk is 0.26 (95% CI 0.19 to 0.33; 8,695 mosquitoes, 16 comparisons; I2 = 90%, 95% CI 86% to 93%). There is substantive heterogeneity within and across resistance groups, but most comparisons significantly favour ITNs. One comparison [22], with high resistance mosquitoes, reported 80% and 20% induced exophily (figures estimated from graph) in the ITN and UTN groups, respectively (RD = 0.6).
Fifty-five comparisons reported on deterrence (21 low resistance, 13 moderate, 21 high) (Table 12). There is no clear relationship between resistance status and deterrence based on a visual inspection of the results.
Tab. 12. Results from experimental hut trials comparing LLIN or CTN versus UTN for deterrence. 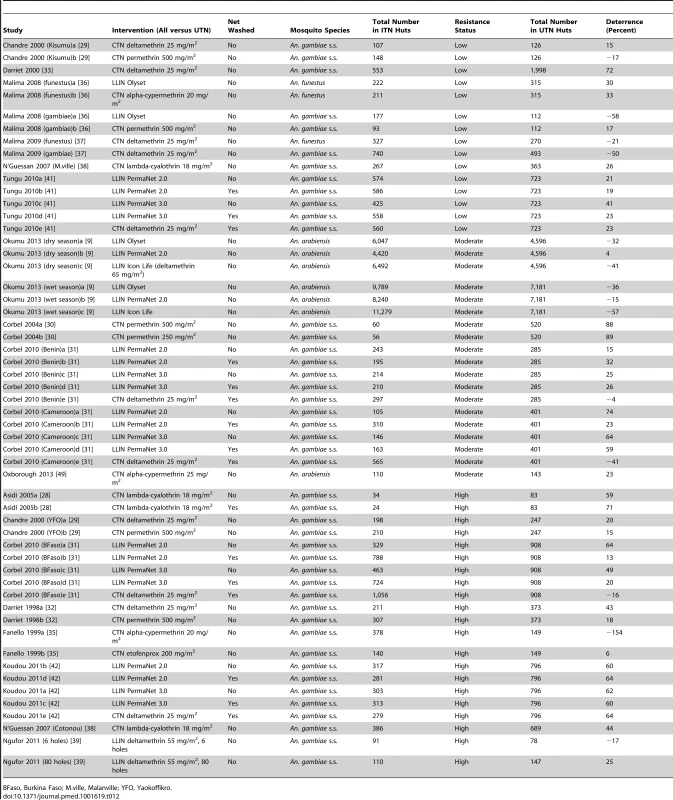
BFaso, Burkina Faso; M.ville, Malanville; YFO, Yaokoffikro. Results of Subgroup Analyses, Sensitivity Analyses, and Funnel Plots
Considerable heterogeneity was found across all studies, therefore sources of heterogeneity were explored using subgroup analyses. We carried out subgroup analyses by net type, insecticide used, the concentration of insecticide, and whether nets were washed or not. Because of the wide variation between studies in relation to these factors, these plots were numerous. We carried out analyses grouping in different ways, but these analyses did not provide any explanation of the heterogeneity between studies. The funnel plots do not resemble symmetric funnels; this may be because of the high level of variability between studies and the low quality of the evidence (see Figures S6–S13). For experimental hut trials, similar conclusions are drawn from the sensitivity analyses and primary analyses (Table S9; Figures S14–S20).
Discussion
The study set out to determine whether mosquito resistance to insecticides is having an impact on entomological outcomes in ITNs compared to UTNs in three experimental settings: highly controlled cone studies, laboratory tunnel studies with animal bait, and field trials in huts with humans as the attractant. Cone tests for mosquito knock-down showed reduced levels of knock-down associated with higher levels of resistance. Laboratory tunnel test results demonstrated a reduced effect of ITNs in mosquitoes with higher levels of resistance in terms of blood feeding, mosquito mortality, and passage through the nets.
In experimental hut trials the RD for mortality for ITNs compared to UTNs showed that ITNs continued to have an effect in all categories of resistance. The meta-analytic results showed that the difference in mortality risk using ITNs as compared with UTNs modestly decreased as resistance increased, and the test for subgroup differences demonstrated a difference in the RD between high, medium, and low resistance subgroups. The substantive heterogeneity in the studies' results and design may mask the true relationship between resistance and the RD, and the results need to be interpreted with caution.
What is clear from the results is that ITNs continue to have a substantive effect compared to UTNs in many studies, and that despite best efforts, explaining the heterogeneity between studies has been problematic, with field studies showing quite varied results. Sometimes there are quite unexpected and inconsistent findings such as in the study by Okumu et al. [9], which shows no evidence of a benefit of insecticide despite bioassays indicating “sensitivity”. Studies overall are very poor in characterising the resistance pattern of the mosquitoes, and the classification systems are unclear and lack uniformity.
We observed a large amount of heterogeneity and bias across studies, which was particularly acute in the field studies. Variations in the wild mosquito populations—such as their resistance levels, age, blood feeding and mating status (factors that themselves could influence resistance levels and host-seeking behaviour)—and also the local environment cannot be controlled for across studies. In addition, the execution of the field trials was not uniform across the studies, e.g., washing of nets, rotation of nets/sleepers, season in which the trial took place, length of the trial, decontamination of huts, and exclusion of male mosquitoes from the analysis. Only one field trial conducted a direct comparison of susceptible versus resistant mosquitoes [29]. Deterrence could not be measured because the mosquitoes were directly placed inside the huts. For the remaining studies we conducted indirect comparisons between trials of nets in areas of high or moderate resistance and those in low resistance areas. Blinding of mosquito collectors, observers, and sleepers was not addressed in any of the studies.
One area of concern is that assessment of resistance of mosquito populations is not optimised across studies, and hence misclassification of resistance is likely to occur, adding to the high levels of heterogeneity. It is possible that target-site and metabolic resistance exert a differential impact on LLIN effectiveness, but most studies fail to accurately assess the presence of metabolic resistance. Insecticide resistance profiling of mosquito populations was varied across all studies, with just under half of the field studies measuring phenotypic resistance or kdr frequency, two out of the 14 studies measuring both, and only one measuring phenotypic resistance, kdr, and metabolic resistance [50]. Phenotypic resistance, as measured by bioassays, is regarded as the first step in identifying resistance [51]. It is prudent to always carry out bioassays to establish resistance levels before implementing mechanistic studies (e.g., genotyping for target-site and metabolic resistance and biochemical assays). It is unwise to assume that kdr alone is solely responsible for the resistant phenotype [52],[53]; mosquitoes could still harbour metabolic resistance, for example. Based on this, we were reluctant to label mosquito populations with no or low kdr frequency as “susceptible” (low resistance).
It is becoming increasingly clear that metabolic resistance often underpins pyrethroid resistance in mosquitoes, as demonstrated by both gene expression studies of resistant populations [11],[17],[18],[19],[20],[54] and enzyme characterisation studies [55],[56]. To date, resistance has been directly implicated in operational control failure of pyrethroids only in An. funestus in South Africa [57]. Metabolic resistance is the underlying mechanism [54],[58],[59], and therefore this mosquito species offers a unique opportunity to measure the impact of resistance on ITN efficacy. Unfortunately, none of the included studies have included the resistant form of this species.
A large number of studies were excluded because the insecticide resistance status of the wild mosquito populations was not characterised at the time of the study, but rather relied upon retrospective data. Mosquito populations are dynamic, and although a kdr frequency of >0.90, which is close to fixation, is unlikely to revert rapidly, we cannot rule out the migration of mosquito populations or other confounding factors that could dramatically influence mosquito populations and/or resistance profiles over time.
In terms of interpreting the patterns, this has to be done with care, given the variability of the results. Reduced killing of mosquitoes with increasing resistance in tunnel and hut studies raises concerns. Feeding preferences of mosquitoes can be plastic [60], and there is evidence that anthropogenic species such as An. gambiae and An. funestus can switch to feeding on cattle to obtain a blood meal in the presence of pyrethroid-treated materials [61],[62]. So, although the personal protection properties of ITNs (i.e., prevention of blood feeding and induced exophily) are still maintained, there is still the risk that if different hosts are available, mosquitoes could adapt their feeding preferences and thereby maintain large population sizes. If LLIN coverage is lowered, nets become badly damaged, are inappropriately used, are sold on, or are used less over time (all of which are realistic scenarios) [63], the reduced killing of resistant mosquitoes, which may have obtained a blood meal elsewhere, could be a cause for concern.
Inconsistency between studies in relation to study design, execution, and reporting format across all experimental hut trials is an obstacle in addressing the relationship between resistance and ITN efficacy confidently. There are no clear guidelines for measuring ITN efficacy against resistant mosquitoes. As a consequence, the studies do not easily lend themselves to meta-analysis, and so it is difficult to generate a consensus. It is likely that the effects of resistance on some outcomes may be moderate or small, but the lack of standardisation means the methodological differences between studies obscure any detection or coherent synthesis between studies. So, if this field of research aims to identify generalisable findings, then researchers need to consider how best to measure the dependent and independent variables so that the results are more comparable. Our concern with this lack of transparency and standardisation, and the need for improved reporting, echoes recent calls [64] for research to be better planned, co-ordinated, and of higher quality. With such gaps and lack of standardisation in the primary studies, it could be argued that current research represents inefficient use of scarce resources of the scientific community as a whole.
Based on the studies included in this meta-analysis, ITNs remain at least somewhat effective against African anopheline mosquitoes even when resistance has developed. However, whether ITNs remain effective against resistant mosquitoes cannot be definitively addressed whilst the execution and reporting of field studies and the profiling of resistance in mosquito populations is inadequate and inconsistent. Ideally, phenotypic resistance, target-site resistance, and metabolic resistance testing should all be applied to mosquito populations in the vicinity of the hut trial. If this is not feasible, then a combination of either phenotypic and target-site resistance testing or target-site and metabolic resistance testing should be performed. Authors should make it clear in their reporting if they have omitted to test for any of the three categories of resistance highlighted above. It is also imperative that resistance is measured at the time of the study rather than relying on retrospective data. International agreement is needed for standardised methods for measuring the impact of resistance on ITNs before conclusive statements about the effect of resistance can be made. In order to initiate dialogue about the standardisation of methods and reporting we have generated a list of criteria that need to be addressed based on the experience of this review (Box 2). It is important that policy makers and non-governmental organizations plan vector control strategies and purchase ITNs based on the best available data.
Box 2. Considerations for Experimental Hut Study Design and Reporting
Resistance Testing of Mosquito Populations: Reporting Information Required
-
Phenotypic resistance: doses of insecticide tested, exposure times to insecticide, total number of mosquitoes tested, total number of mosquitoes killed
-
Target-site resistance: type of mutation screened for (i.e., L1014F or L104S), associated kdr allele frequencies
-
Metabolic resistance: identification of genes or enzyme class implicated in conferring resistance
Study Design Reporting Criteria: Reporting Requirement
-
Study start date: date
-
Study duration: number of nights
-
Mosquito species present at location: species name and molecular form
-
Nets randomly allocated to huts at start of trial: yes or no
-
Nets rotated between huts during trial: yes or no
-
Sleepers rotated between huts during trial: yes or no
-
Washing of nets: wash procedure provided
-
Huts cleaned between rotations: yes or no
-
Observers collecting mosquitoes blinded to intervention: yes or no
-
Sleepers blinded to intervention: yes or no
-
Male mosquitoes used in the analysis: excluded or included
-
Raw data for measured outcomes: provided
-
Raw data for UTNs: provided
Supporting Information
Zdroje
1. World Health Organization (2011) World malaria report 2011. Geneva: World Health Organization.
2. LengelerC (2004) Insecticide-treated bed nets and curtains for preventing malaria. Cochrane Database Syst Rev 2004: CD000363.
3. JonesCM, SanouA, GuelbeogoWM, SagnonN, JohnsonPC, et al. (2012) Aging partially restores the efficacy of malaria vector control in insecticide-resistant populations of Anopheles gambiae s.l. from Burkina Faso. Malar J 11 : 24.
4. OkiaM, NdyomugyenyiR, KirundaJ, ByaruhangaA, AdibakuS, et al. (2013) Bioefficacy of long-lasting insecticidal nets against pyrethroid-resistant populations of Anopheles gambiae s.s. from different malaria transmission zones in Uganda. Parasit Vectors 6 : 130.
5. World Health Organization (2010) World malaria report 2010. Geneva: World Health Organization.
6. OkumuFO, ChipwazaB, MadumlaEP, MbeyelaE, LingambaG, et al. (2012) Implications of bio-efficacy and persistence of insecticides when indoor residual spraying and long-lasting insecticide nets are combined for malaria prevention. Malar J 11 : 378.
7. BrietOJ, PennyMA, HardyD, AwololaTS, Van BortelW, et al. (2013) Effects of pyrethroid resistance on the cost effectiveness of a mass distribution of long-lasting insecticidal nets: a modelling study. Malar J 12 : 77.
8. HougardJM, DuchonS, DarrietF, ZaimM, RogierC, et al. (2003) Comparative performances, under laboratory conditions, of seven pyrethroid insecticides used for impregnation of mosquito nets. Bull World Health Organ 81 : 324–333.
9. OkumuFO, MbeyelaE, LingambaG, MooreJ, NtamatungiroAJ, et al. (2013) Comparative field evaluation of combinations of long-lasting insecticide treated nets and indoor residual spraying, relative to either method alone, for malaria prevention in an area where the main vector is Anopheles arabiensis. Parasit Vectors 6 : 46.
10. Darriet F, Robert V, Tho Vien N, Carnevale P (1984) Evaluation of the efficacy of permethrin-impregnated intact and perforated mosquito nets against vectors of malaria. WHO/VBC/84.899. Geneva: World Health Organization.
11. RansonH, N'GuessanR, LinesJ, MoirouxN, NkuniZ, et al. (2011) Pyrethroid resistance in African anopheline mosquitoes: what are the implications for malaria control? Trends Parasitol 27 : 91–98.
12. TrapeJF, TallA, DiagneN, NdiathO, LyAB, et al. (2011) Malaria morbidity and pyrethroid resistance after the introduction of insecticide-treated bednets and artemisinin-based combination therapies: a longitudinal study. Lancet Infect Dis 11 : 925–932.
13. World Health Organization (2012) Global plan for insecticide resistance management in malaria vectors. Geneva: World Health Organization.
14. Martinez-TorresD, ChandreF, WilliamsonMS, DarrietF, BergeJB, et al. (1998) Molecular characterization of pyrethroid knockdown resistance (kdr) in the major malaria vector Anopheles gambiae s.s. Insect Mol Biol 7 : 179–184.
15. RansonH, JensenB, VululeJM, WangX, HemingwayJ, et al. (2000) Identification of a point mutation in the voltage-gated sodium channel gene of Kenyan Anopheles gambiae associated with resistance to DDT and pyrethroids. Insect Mol Biol 9 : 491–497.
16. HemingwayJ, HawkesNJ, McCarrollL, RansonH (2004) The molecular basis of insecticide resistance in mosquitoes. Insect Biochem Mol Biol 34 : 653–665.
17. DjouakaRF, BakareAA, CoulibalyON, AkogbetoMC, RansonH, et al. (2008) Expression of the cytochrome P450s, CYP6P3 and CYP6M2 are significantly elevated in multiple pyrethroid resistant populations of Anopheles gambiae s.s. from Southern Benin and Nigeria. BMC Genomics 9 : 538.
18. AwololaTS, OduolaOA, StrodeC, KoekemoerLL, BrookeB, et al. (2009) Evidence of multiple pyrethroid resistance mechanisms in the malaria vector Anopheles gambiae sensu stricto from Nigeria. Trans R Soc Trop Med Hyg 103 : 1139–1145.
19. MullerP, WarrE, StevensonBJ, PignatelliPM, MorganJC, et al. (2008) Field-caught permethrin-resistant Anopheles gambiae overexpress CYP6P3, a P450 that metabolises pyrethroids. PLoS Genet 4: e1000286.
20. MitchellSN, StevensonBJ, MullerP, WildingCS, Egyir-YawsonA, et al. (2012) Identification and validation of a gene causing cross-resistance between insecticide classes in Anopheles gambiae from Ghana. Proc Natl Acad Sci U S A 109 : 6147–6152.
21. WoodO, HanrahanS, CoetzeeM, KoekemoerL, BrookeB (2010) Cuticle thickening associated with pyrethroid resistance in the major malaria vector Anopheles funestus. Parasit Vectors 3 : 67.
22. NdiathMO, SougoufaraS, GayeA, MazenotC, KonateL, et al. (2012) Resistance to DDT and pyrethroids and increased kdr mutation frequency in An. gambiae after the implementation of permethrin-treated nets in Senegal. PLoS ONE 7: e31943.
23. NorrisLC, NorrisDE (2011) Insecticide resistance in Culex quinquefasciatus mosquitoes after the introduction of insecticide-treated bed nets in Macha, Zambia. J Vector Ecol 36 : 411–420.
24. RansonH, AbdallahH, BadoloA, GuelbeogoWM, Kerah-HinzoumbeC, et al. (2009) Insecticide resistance in Anopheles gambiae: data from the first year of a multi-country study highlight the extent of the problem. Malar J 8 : 299.
25. World Health Organization (2005) Guidelines for laboratory and field testing of long-lasting insecticidal mosquito nets. WHO/CDS/WHOPES/GCDPP/2005.11. Geneva: World Health Organization.
26. World Health Organization (2013) Test procedures for insecticide resistance monitoring in malaria vector mosquitoes. Geneva: World Health Organization.
27. ThorlundK, ImbergerG, JohnstonBC, WalshM, AwadT, et al. (2012) Evolution of heterogeneity (I2) estimates and their 95% confidence intervals in large meta-analyses. PLoS ONE 7: e39471.
28. AsidiAN, N'GuessanR, KoffiAA, CurtisCF, HougardJM, et al. (2005) Experimental hut evaluation of bednets treated with an organophosphate (chlorpyrifos-methyl) or a pyrethroid (lambdacyhalothrin) alone and in combination against insecticide-resistant Anopheles gambiae and Culex quinquefasciatus mosquitoes. Malar J 4 : 25.
29. ChandreF, DarrietF, DuchonS, FinotL, ManguinS, et al. (2000) Modifications of pyrethroid effects associated with kdr mutation in Anopheles gambiae. Med Vet Entomol 14 : 81–88.
30. CorbelV, ChandreF, BrenguesC, AkogbetoM, LardeuxF, et al. (2004) Dosage-dependent effects of permethrin-treated nets on the behaviour of Anopheles gambiae and the selection of pyrethroid resistance. Malar J 3 : 22.
31. CorbelV, ChabiJ, DabireRK, EtangJ, NwaneP, et al. (2010) Field efficacy of a new mosaic long-lasting mosquito net (PermaNet 3.0) against pyrethroid-resistant malaria vectors: a multi centre study in western and central Africa. Malar J 9 : 113.
32. DarrietF, GuilletP, N'GuessanR, DoannioJM, KoffiA, et al. (1998) [Impact of resistance of Anopheles gambiae s.s. to permethrin and deltamethrin on the efficacy of impregnated mosquito nets.]. Med Trop (Mars) 58 : 349–354.
33. DarrietF, N'GuessanR, KoffiAA, KonanL, DoannioJMC, et al. (2000) Impact of the resistance to pyrethroids on the efficacy of impregnated bednets used as a means of prevention against malaria: results of the evaluation carried out with deltamethrin SC in experimental huts. Bull Soc Pathol Exot 93 : 131–134.
34. DjenontinA, ChandreF, DabireKR, ChabiJ, N'GuessanR, et al. (2010) Indoor use of plastic sheeting impregnated with carbamate combined with long-lasting insecticidal mosquito nets for the control of pyrethroid-resistant malaria vectors. Am J Trop Med Hyg 83 : 266–270.
35. FanelloC, KolaczinskiJH, ConwayDJ, CarnevaleP, CurtisCF (1999) The kdr pyrethroid resistance gene in Anopheles gambiae: tests of non-pyrethroid insecticides and a new detection method for the gene. Parassitologia 41 : 323–326.
36. MalimaRC, MagesaSM, TunguPK, MwingiraV, MagogoFS, et al. (2008) An experimental hut evaluation of Olyset (R) nets against anopheline mosquitoes after seven years use in Tanzanian villages. Malaria Journal 7 : 38.
37. MalimaRC, OxboroughRM, TunguPK, MaxwellC, LyimoI, et al. (2009) Behavioural and insecticidal effects of organophosphate-, carbamate - and pyrethroid-treated mosquito nets against African malaria vectors. Med Vet Entomol 23 : 317–325.
38. N'GuessanR, BokoP, OdjoA, AkogbetoM, YatesA, et al. (2007) Chlorfenapyr: a pyrrole insecticide for the control of pyrethroid or DDT resistant Anopheles gambiae (Diptera: Culicidae) mosquitoes. Acta Trop 102 : 69–78.
39. NguforC, N'GuessanR, BokoP, OdjoA, VigninouE, et al. (2011) Combining indoor residual spraying with chlorfenapyr and long-lasting insecticidal bed nets for improved control of pyrethroid-resistant Anopheles gambiae: an experimental hut trial in Benin. Malar J 10 : 343.
40. OxboroughRM, WeirV, IrishS, KaurH, N'GuessanR, et al. (2009) Is K-O Tab 1-2-3((R)) long lasting on non-polyester mosquito nets? Acta Trop 112 : 49–53.
41. TunguP, MagesaS, MaxwellC, MalimaR, MasueD, et al. (2010) Evaluation of PermaNet 3.0 a deltamethrin-PBO combination net against Anopheles gambiae and pyrethroid resistant Culex quinquefasciatus mosquitoes: an experimental hut trial in Tanzania. Malar J 9 : 21.
42. KoudouBG, KoffiAA, MaloneD, HemingwayJ (2011) Efficacy of PermaNet(R) 2.0 and PermaNet(R) 3.0 against insecticide-resistant Anopheles gambiae in experimental huts in Cote d'Ivoire. Malar J 10 : 172.
43. EtangJ, ChandreF, GuilletP, MangaL (2004) Reduced bio-efficacy of permethrin EC impregnated bednets against an Anopheles gambiae strain with oxidase-based pyrethroid tolerance. Malar J 3 : 46.
44. HodjatiMH, CurtisCF (1999) Evaluation of the effect of mosquito age and prior exposure to insecticide on pyrethroid tolerance in Anopheles mosquitoes (Diptera: Culicidae). Bull Entomol Res 89 : 329–337.
45. GimnigJE, LindbladeKA, MountDL, AtieliFK, CrawfordS, et al. (2005) Laboratory wash resistance of long-lasting insecticidal nets. Trop Med Int Health 10 : 1022–1029.
46. MahamaT, DesireeEJ, PierreC, FabriceC (2007) Effectiveness of permanet in Cote d'Ivoire rural areas and residual activity on a knockdown-resistant strain of Anopheles gambiae. J Med Entomol 44 : 498–502.
47. FaneM, CisseO, TraoreCS, SabatierP (2012) Anopheles gambiae resistance to pyrethroid-treated nets in cotton versus rice areas in Mali. Acta Trop 122 : 1–6.
48. WinklerMS, TchicayaE, KoudouBG, DonzeJ, NsanzabanaC, et al. (2012) Efficacy of ICON(R) Maxx in the laboratory and against insecticide-resistant Anopheles gambiae in central Cote d'Ivoire. Malar J 11 : 167.
49. OxboroughRM, KitauJ, MatowoJ, FestonE, MndemeR, et al. (2013) ITN mixtures of chlorfenapyr (pyrrole) and alphacypermethrin (pyrethroid) for control of pyrethroid resistant Anopheles arabiensis and Culex quinquefasciatus. PLoS ONE 8: e55781.
50. N'GuessanR, CorbelV, AkogbetoM, RowlandM (2007) Reduced efficacy of insecticide-treated nets and indoor residual spraying for malaria control in pyrethroid resistance area, Benin. Emerg Infect Dis 13 : 199–206.
51. World Health Organization (1998) Test procedures for insecticide resistance monitoring in malaria vectors, bio-efficacy and persistence of insecticide on treated surfaces. Geneva: World Health Organization.
52. BrookeBD (2008) kdr: can a single mutation produce an entire insecticide resistance phenotype? Trans R Soc Trop Med Hyg 102 : 524–525.
53. DonnellyMJ, CorbelV, WeetmanD, WildingCS, WilliamsonMS, et al. (2009) Does kdr genotype predict insecticide-resistance phenotype in mosquitoes? Trends Parasitol 25 : 213–219.
54. IrvingH, RiveronJM, IbrahimSS, LoboNF, WondjiCS (2012) Positional cloning of rp2 QTL associates the P450 genes CYP6Z1, CYP6Z3 and CYP6M7 with pyrethroid resistance in the malaria vector Anopheles funestus. Heredity (Edinb) 109 : 383–392.
55. StevensonBJ, PignatelliP, NikouD, PaineMJ (2012) Pinpointing P450s associated with pyrethroid metabolism in the dengue vector, Aedes aegypti: developing new tools to combat insecticide resistance. PLoS Negl Trop Dis 6: e1595.
56. StevensonBJ, BibbyJ, PignatelliP, MuangnoicharoenS, O'NeillPM, et al. (2011) Cytochrome P450 6M2 from the malaria vector Anopheles gambiae metabolizes pyrethroids: sequential metabolism of deltamethrin revealed. Insect Biochem Mol Biol 41 : 492–502.
57. HargreavesK, KoekemoerLL, BrookeBD, HuntRH, MthembuJ, et al. (2000) Anopheles funestus resistant to pyrethroid insecticides in South Africa. Med Vet Entomol 14 : 181–189.
58. WondjiCS, IrvingH, MorganJ, LoboNF, CollinsFH, et al. (2009) Two duplicated P450 genes are associated with pyrethroid resistance in Anopheles funestus, a major malaria vector. Genome Res 19 : 452–459.
59. AmenyaDA, NaguranR, LoTC, RansonH, SpillingsBL, et al. (2008) Over expression of a cytochrome P450 (CYP6P9) in a major African malaria vector, Anopheles Funestus, resistant to pyrethroids. Insect Mol Biol 17 : 19–25.
60. BonizzoniM, AfraneY, BaliraineFN, AmenyaDA, GithekoAK, et al. (2009) Genetic structure of Plasmodium falciparum populations between lowland and highland sites and antimalarial drug resistance in Western Kenya. Infect Genet Evol 9 : 806–812.
61. DabireRK, DiabateA, BaldetT, Pare-ToeL, GuiguemdeRT, et al. (2006) Personal protection of long lasting insecticide-treated nets in areas of Anopheles gambiae s.s. resistance to pyrethroids. Malar J 5 : 12.
62. GithekoAK, AdungoNI, KaranjaDM, HawleyWA, VululeJM, et al. (1996) Some observations on the biting behavior of Anopheles gambiae s.s., Anopheles arabiensis, and Anopheles funestus and their implications for malaria control. Exp Parasitol 82 : 306–315.
63. World Health Organization (2007) Insecticide-treated nets: a WHO position statement. Geneva: World Health Organization.
64. IoanndisJP, GreenlandS, HlatkyMA, KhouryMJ, MacleodMR, et al. (2014) Increasing value and reducing waste in research design, conduct, and analysis. Lancet 383 : 166–175 doi:10.1016/S0140-6736(13)62227-8
Štítky
Interné lekárstvo
Článok vyšiel v časopisePLOS Medicine
Najčítanejšie tento týždeň
2014 Číslo 3- Parazitičtí červi v terapii Crohnovy choroby a dalších zánětlivých autoimunitních onemocnění
- Intermitentní hladovění v prevenci a léčbě chorob
- Statinová intolerance
- Co dělat při intoleranci statinů?
- Metabolit živočišné stravy produkovaný střevní mikroflórou zvyšuje riziko závažných kardiovaskulárních příhod
-
Všetky články tohto čísla
- How Can Journals Respond to Threats of Libel Litigation?
- and Water, Sanitation, and Hygiene: A Committed Relationship
- Representation and Misrepresentation of Scientific Evidence in Contemporary Tobacco Regulation: A Review of Tobacco Industry Submissions to the UK Government Consultation on Standardised Packaging
- Building Research Capacity in Africa: Equity and Global Health Collaborations
- Associations between Intimate Partner Violence and Health among Men Who Have Sex with Men: A Systematic Review and Meta-Analysis
- Active or Passive Exposure to Tobacco Smoking and Allergic Rhinitis, Allergic Dermatitis, and Food Allergy in Adults and Children: A Systematic Review and Meta-Analysis
- The Effectiveness of Community Action in Reducing Risky Alcohol Consumption and Harm: A Cluster Randomised Controlled Trial
- Assessing Causality in the Association between Child Adiposity and Physical Activity Levels: A Mendelian Randomization Analysis
- The Impact of Pyrethroid Resistance on the Efficacy of Insecticide-Treated Bed Nets against African Anopheline Mosquitoes: Systematic Review and Meta-Analysis
- Lung Function and Incidence of Chronic Obstructive Pulmonary Disease after Improved Cooking Fuels and Kitchen Ventilation: A 9-Year Prospective Cohort Study
- Early Experiences Implementing Pre-exposure Prophylaxis (PrEP) for HIV Prevention in San Francisco
- The Role of Viral Introductions in Sustaining Community-Based HIV Epidemics in Rural Uganda: Evidence from Spatial Clustering, Phylogenetics, and Egocentric Transmission Models
- PLOS Medicine
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- and Water, Sanitation, and Hygiene: A Committed Relationship
- Representation and Misrepresentation of Scientific Evidence in Contemporary Tobacco Regulation: A Review of Tobacco Industry Submissions to the UK Government Consultation on Standardised Packaging
- The Impact of Pyrethroid Resistance on the Efficacy of Insecticide-Treated Bed Nets against African Anopheline Mosquitoes: Systematic Review and Meta-Analysis
- The Role of Viral Introductions in Sustaining Community-Based HIV Epidemics in Rural Uganda: Evidence from Spatial Clustering, Phylogenetics, and Egocentric Transmission Models
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



