Use of medial femoral condyle flap and anterolateral thigh free flap in proxymal tibial posttraumatic non-union with multiple anastomosis - case report
Authors:
T. Kempný 1,2; M. Knoz 2,3,4; B. Lipový 2,3; J. Holoubek 2,3; Z. Dvořák 3,4
Authors‘ workplace:
Department of Plastic and Reconstructive Surgery, Klinikum Wels, Grieskirchen, Austria
1; Department of Burns and Reconstructive Surgery, University Hospital Brno, Czech Republic
2; Medical Faculty, Masaryk University Brno, Czech Republic
3; Department of Plastic and Aesthetic Surgery, St. Anne´s University Hospital Brno, Czech Republic
4
Published in:
ACTA CHIRURGIAE PLASTICAE, 61, 1-4, 2019, pp. 28-31
INTRODUCTION
Fractures of the proximal tibia are high energy fractures. Complications of treatment and injuries associated with this fracture led to several approaches.
The vascularized medial femoral condyle (MFC) corticoperiosteal bone pedicled flap was first introduced by Hertel and Masquelet in 19891. Use of this flap is still gaining in popularity in the last decades in the field of reconstructive surgery. Recent studies of different authors presented the use of this flap as a multicomponent unit in various clinical applications and situations, mostly in small bone defects emerging from posttraumatic septic or aseptic necrosis2,3,4.
Over the past decade, the use of MFC was described as a treatment of posttraumatic non-unions in the region of the upper extremity (ulna, humerus, clavicle, scaphoid) and the region of the facial skeleton (maxilla, mandibula)5,6,7,8.
The MFC multicomponent flap provides a highly vascularised osteogenic tissue that may be molded or wrapped in non-union sites of osseous tissue and defect in soft tissue coverage (Figure 1). It is considered to be a favourable vascularised periosteal flap because of the preservation of the critical osteogenic cambium layer, bone structure housed between the periosteum and the cortical bone. Cambium as a source of osteoprogenitor cells and osteoinductive molecules (bone morphogenetic proteins) is an essential element in the MFC flap defect surgical treatment9.
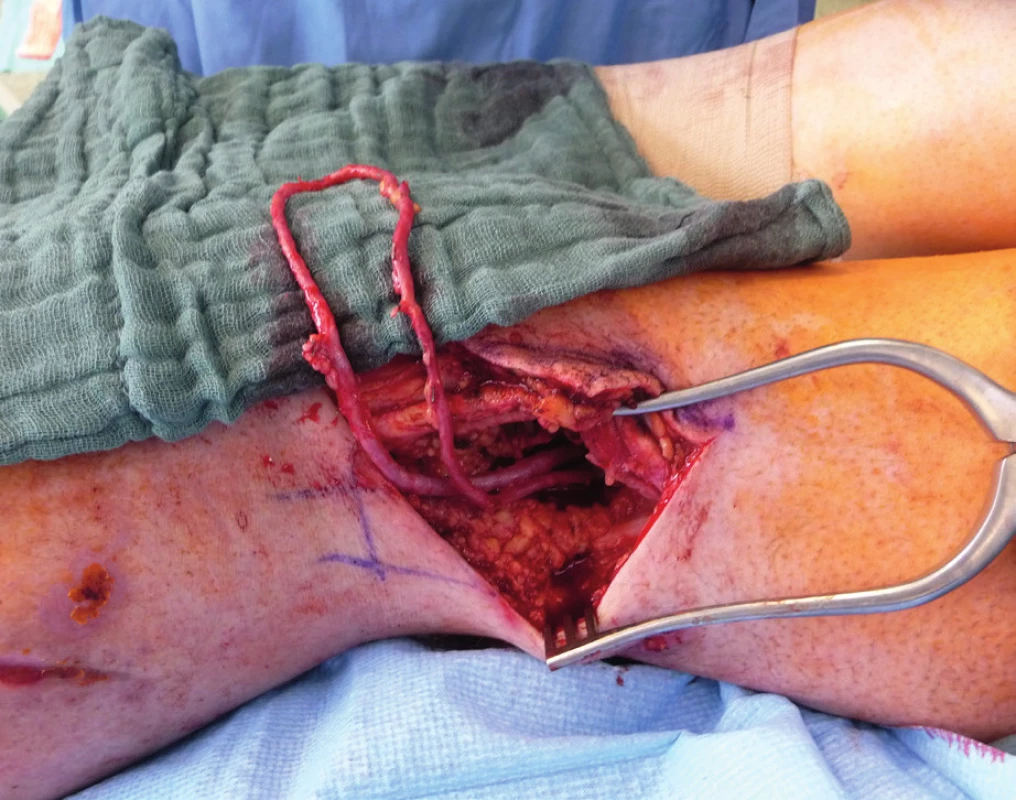
Only a few reports focus on the posttraumatic defects of the lower extremity. Some authors reported the treatment with MFC of the ankle and distal part of the lower extremity, but there is no evidence of proximal tibial non-union treatment.
CASE REPORT
A 30-year-old male suffered an intraarticular fracture of proximal tibia and fibula in a motorbike accident, classified as 4C3 AO (Arbeitsgemeinschaft für Osteosynthesefragen classification) after the initial assessment and X-ray examination. The patient suffered from no co-morbidities or chronical diseases. Shortly after admission to the department of trauma surgery, an acute pain and paleness of lower extremity appeared. An angiography with contrast proved an obstruction of the distal part of popliteal artery caused by fracture bone fragments external compression. An acute intervention was performed with an introduction of an intravascular self-expanding stent (Astron–Biotronic, Germany). Tube to tube external fixator was applied along with reduction of bone fragments.
An opened fixation with locking compression plate (LCP) on proximal tibia (DePuy, Synthes) was performed after seven days following the admission to the department of traumatology, with release of the compression of popliteal artery. Shortly after the surgery, primary symptoms of compartment syndrome appeared in the region of the distal lower limb, which was confirmed by intramuscular pressure monitoring. Due to the clinical status, a septal fasciotomy was performed.
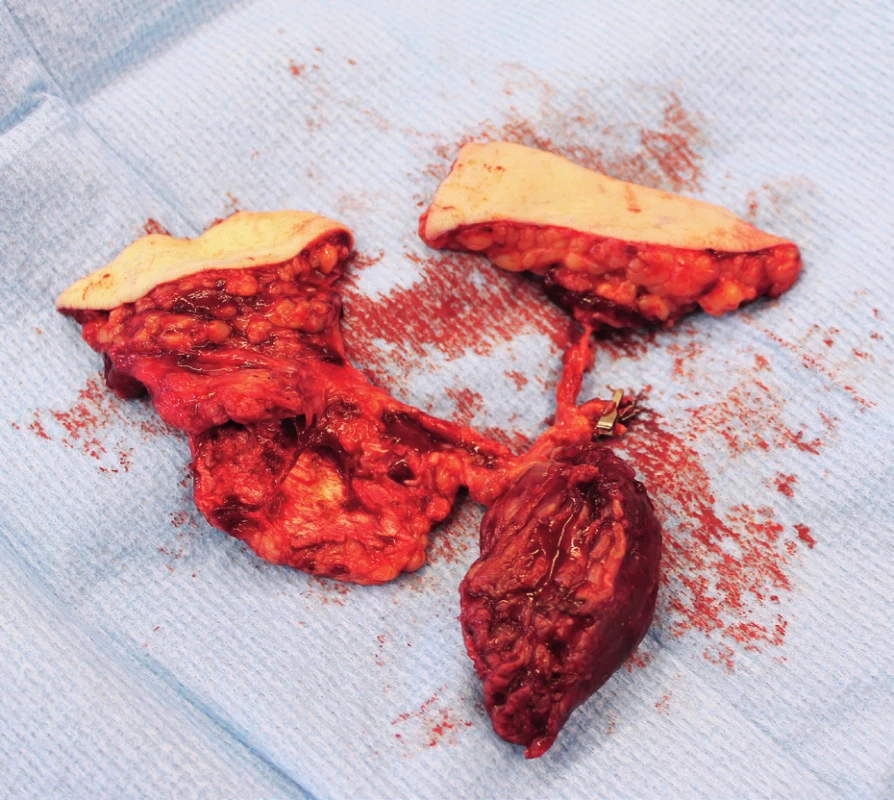
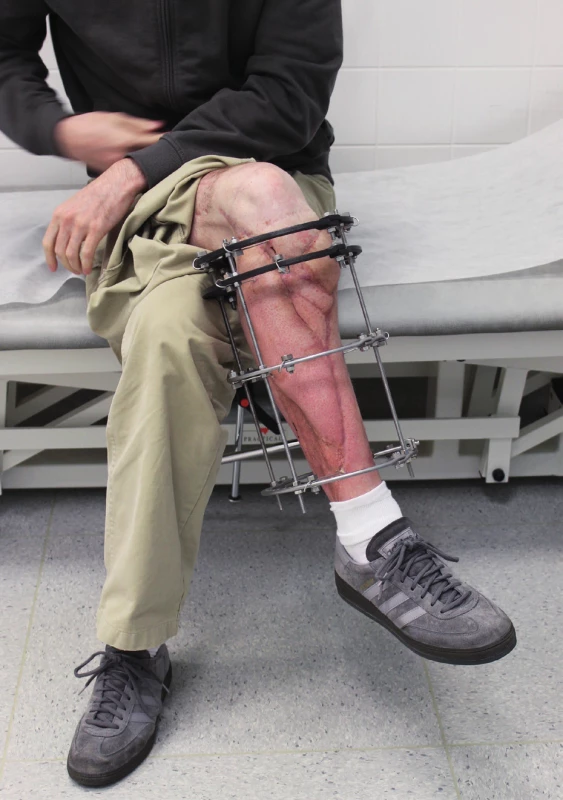
Two weeks after fasciotomy and stabilisation of the patient’s clinical status, a reconstructive surgeon was asked for review to consider the closure of tibial post-fasciotomy defect with presence of nude plate fixation and tibia bone. Because of the advantage of satisfactory soft tissue on the thigh, the anterolateral thigh flap (ALT) was chosen. It was harvested as a two-component flap consisting of muscle and skin parts. The size of the ALT flap was 43 x 16 cm covering the entire length of the exposed tibia and tibia fixation plate (Figure 2) Due to the previous intravascular procedure, we harvested the great saphenous vein for the Corlett loop12 Corlett loop is a temporary arterial-venous shunt performed via a. poplitea above Astron stent and v. poplitea end to side, prolonging source blood flow. Final microanastomosis to ALT pedicle was performed in end to end (Figure 3). The skin flap was designed and divided into two parts with relevant perforator sources of perfusion. The rest of the defect distally was covered with temporary synthetic skin cover (Epigard, Consept GmbH, Weibaden, Germany), later after regression of postoperative swelling, covered with skin mesh. The patient was discharged after three weeks of clinical observation and immediate rehabilitation from the department of trauma surgery with satisfactory improvements in lower extremity movement without obvious limitation in the range of motion. In two-week intervals for the first two months of the follow up, clinical and X-ray examination was performed. An X-ray examination performed six months after the initial reconstructive surgery has shown signs of non-union located in the previous fracture line along with a fistula opening on the skin surface. The culture from fistula was positive for Staphylococcus aureus. The decision was made to give the patient systematic antibiotic treatment combined with surgical management. The LCP in proximal tibia was removed and a radical debridement was performed after marking all fistula folds with patent blue marker (Figure 4). Gentamicin globules were introduced locally into the defect in osseous and soft tissues along with VAC therapy. After seven days, a reconstructive surgery was performed to fill the defect. The MFC flap was selected due to its favourable properties. As a three-component flap, the MFC flap consists of all three required tissue types to cover the defect, and in addition, the osseous part of the flap includes highly vascularised osteogenic tissue in cambium layer, muscle tissue resistant to infection and skin. After harvesting the MFC flap, the skin part of the flap was divided in two parts on their own perforators to cover the skin defect in proper design. We performed the microsurgical anastomosis of the MFC flap (superomedial geniculate vessels) with cutaneous perforator of the medial part of skin part of ALT flap end to site with excellent flow. The periosteal part of the MFC flap was fixed and stabilised in the tibia defect with Ilisarov fixator10. Six weeks after the final reconstructive procedure, the patient was capable of full range of motion in the knee.
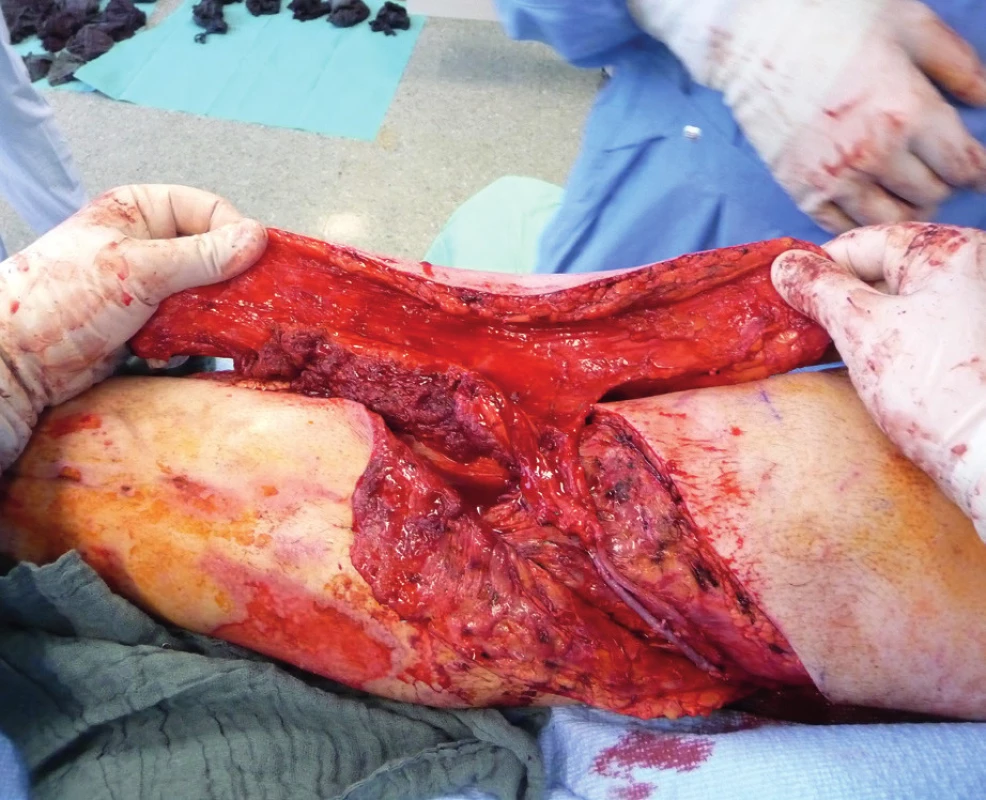
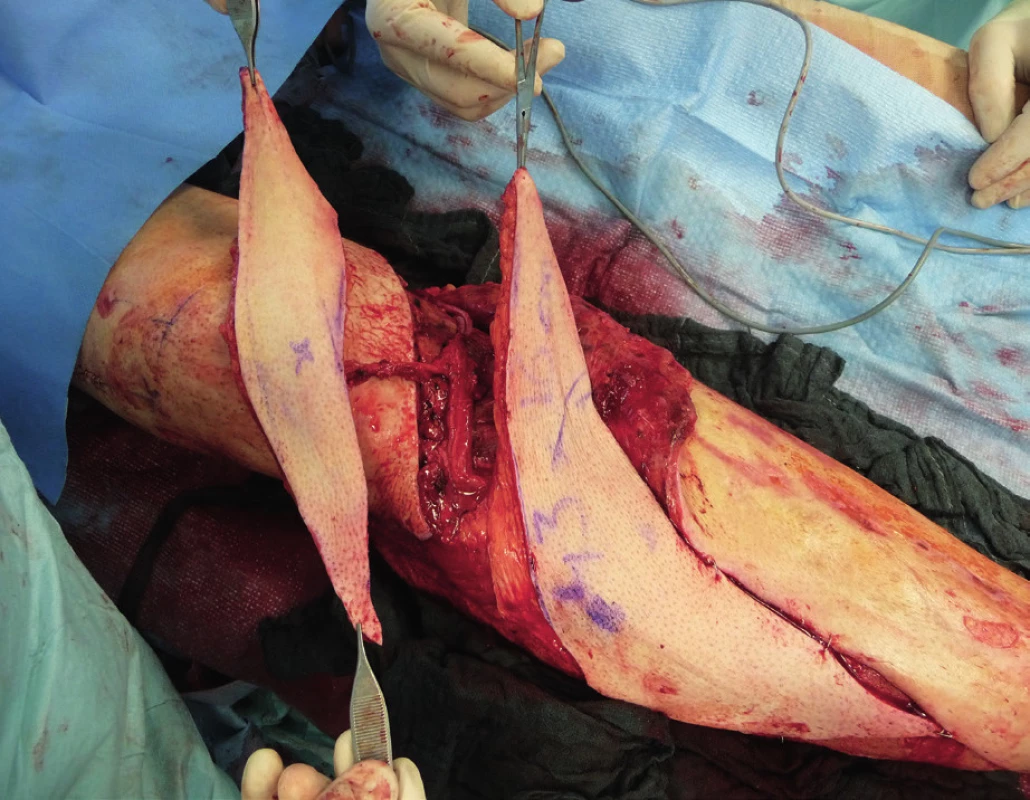
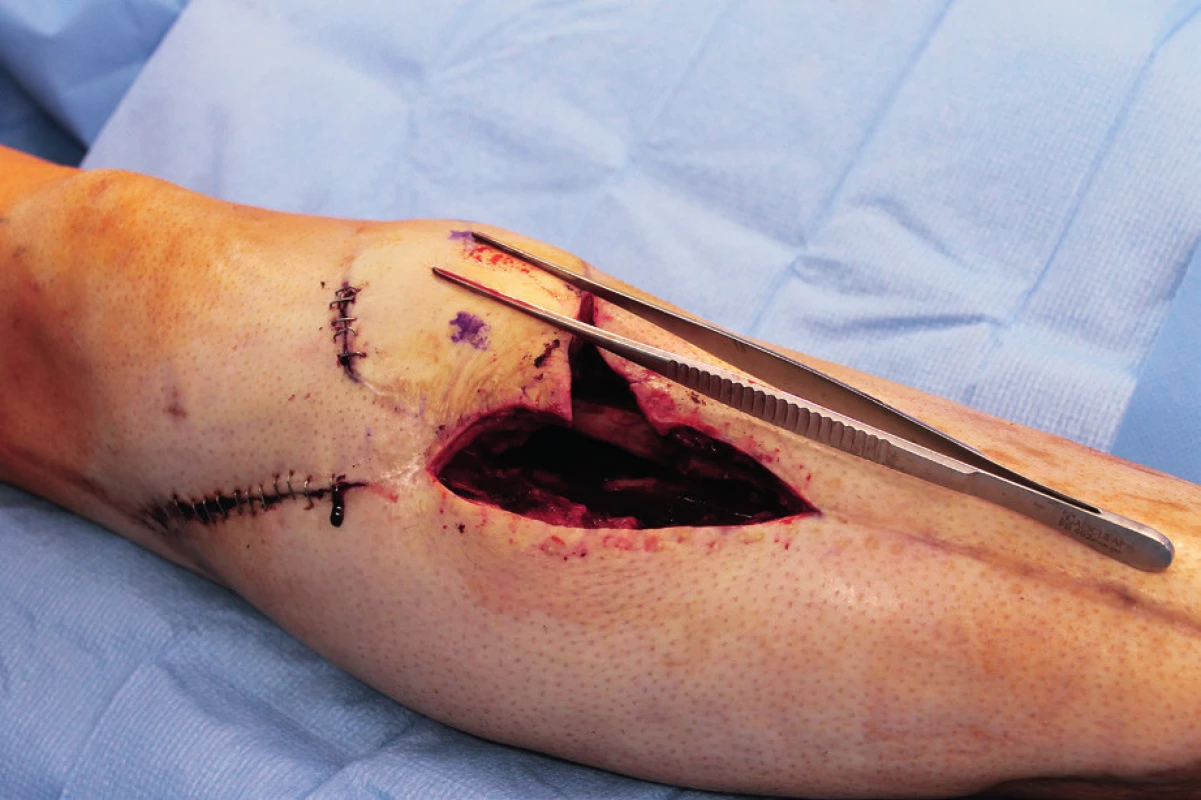
DISCUSSION
Intra-articular fractures of the tibia pilon are known as high energy injuries. The location of the injury entails the risk of associated soft tissue or neurovascular structures injury. The most common method of choice in the treatment of these injuries is the usage of LCP plates according to predefined procedures of AO manuals. This approach to the treatment of fractures is advantageous because of the possibility of early fracture stabilization and osteosynthesis with the possibility of early rehabilitation leading to restoration of functionality. The AO plating systems need more fracture dissection and soft tissue dissection, thus carrying the risk of bleeding, infection and soft tissue healing problems. Although the soft tissue healing problem can be avoided with a hybrid fixator, it carries a risk of malunion, pin track infection and non-union. Thus, the chosen approach carries the potential of postoperative complications, very often compartment syndrome affecting the vitality of the limb. Invasive surgical treatment with septal incisions then carries the risk of wound infection and subsequent formation of defects that cannot be closed by a simple suture. According to the available literature, about 3–4% of all fractures heals problematically with non-union or osteomyelitis11. Thus, the defect of bone tissue with associated defects of surrounding soft tissues requires unique reconstructive solutions. In our case, we were facing numerous complications of intra-articular fracture of the proximal tibia with dislocation compressing popliteal blood vessels. Endovascular stenting during contrast X-ray examination, together with the application of the tube to tube external fixation and reposition of fracture is an elegant and effective method leading to reperfusion of the lower extremity. Following stabilization and fixation with a LCP plate, it is an evidence-based procedure leading to early rehabilitation and restoration of functionality. The subsequent emergence of compartment syndrome with denudation of the LCP plate is an unpleasant complication leading to soft tissue defect formation and the risk of local infectious complications11. The closure of the defect with an ALT flap in the case of our patient was advantageous not only because of the early closure of the defect with muscle component of the flap but also with a fasciocutaneous component, reaching a satisfactory size of 43 x 16 cm, suitable for alteration according to the shape of the defect of considerable size. The pedicle of the ALT flap and stent placement in popliteal artery justify the use Corlett loop12 (Figure 5). Subsequent non-union and chronic fistula in a young patient presents a significant problem. Extraction of osteosynthetic material, systematic and local antibiotic therapy, along with methods of vacuum assisted therapy do not guarantee the success of treatment. In our experience, the method of choice is radical debridement followed by free flap reconstruction. The MFC flap provides benefits of a multicomponent unit and especially the presence of osteogenic cambium layer. Available publications provide data about the use of MFC flap in solving minor osseous defects13,14. In our case, the size of defect was 4 x 5x 6 cm and the defect localization was in a supporting bone, which is rare in the available resources. Connection of the MFC pedicle flap to a skin perforator of the ALT flap results from the length of the pedicle of the MFC flap. Despite the high frequency of anastomosis in our case, we observed no rheological discrepancy or failure of patency. Final healing then occurs without infection or other complications. According to X-ray examination, the healing of bone defect reconstructed by a MFC flap was satisfactory. Examination of movements after early rehabilitation showed a satisfactory range of motion (Figure 6).
CONCLUSION
The use of MFC flap is a well described method in non-union and post-traumatic osteomyelitis complicated by chronic infection in cases where poor result is assumed when using non-vascularised grafts. Many authors cite examples of MFC flap reconstruction for disorders of small bones. Our case shows the reconstruction of a long bone. The reconstruction of primary defect was accomplished with an ALT flap. Consequently, an MFC flap was used due to local infectious chronic condition. MFC flap pedicle was microsutured to ALT flap perforator. Despite the number of anastomoses and pedicle extension, the selected method of reconstruction seems to be safe and with an overall long-lasting benefit for the patient.
Conflict of interest: All authors declare that they have no conflict of interest.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Compliance with ethical requirements: Informed consent was obtained from all patients for being included in the study.
Martin Knoz, MD
Department of Plastic and Aesthetic Surgery
St. Anne´s University Hospital
Berkova 34
612 00 Brno
Czech Republic
E-mail: martin.knoz@fnusa.cz
Sources
1. Masquelet A. Free vascularized corticoperiosteal grafts. Plast Reconstr Surg. 1991, 88 : 1106.
2. Doi K, Hattori Y. Vascularized bone graft from the supracondylar region of the femur. Microsurgery. 2009, 29 : 379–84.
3. Rodríguez-Vegas JM, Delgado-Serrano PJ. Corticoperiosteal flap in the treatment of nonunions and small bone gaps: technical details and expanding possibilities. J Plast Reconstr Aesthet Surg. 2011, 64 : 515–27.
4. del Piñal F, Innocenti M. Evolving concepts in the management of the bone gap in the upper limb. Long and small defects. J Plast Reconstr Aesthet Surg. 2007, 60 : 776–92.
5. del Piñal F, García-Bernal FJ, Regalado J, et al. Vascularised corticoperiosteal grafts from the medial femoral condyle for difficult non-unions of the upper limb. J Hand Surg. 2007, 32 : 135–42.
6. Pelzer M, Reichenberger M, Germann G. Osteo-periostealcutaneous flaps of the medial femoral condyle: a valuable modification for selected clinical situations. J Reconstr Microsurg. 2010, 26 : 291–4.
7. Doi K, Hattori Y. The use of free vascularized corticoperiosteal grafts from the femur in the treatment of scaphoid nonunion. Orthop Clin North Am. 2007, 38 : 87–94.
8. Yoshida A, Yajima H, Murata K, et al. Pedicled vascularized bone graft from the medial supracondylar region of the femur for treatment of femur nonunion. J Reconstr Microsurg. 2009, 25 : 165–70.
9. Giannoudis P, Dinopoulos H. Autologous bone graft: when shall we add growth factors? Orthop Clin North Am. 2010, 41 : 85–94.
10. Paley D, Lamm BM, Katsenis D, Bhave A, Herzenberg JE. Treatment of malunion and nonunion at the site of an ankle fusion with the Ilizarov apparatus: surgical technique. J Bone Joint Surg. 2006, 88 : 119–34.
11. Rosen H. Treatment of nonunion: general principles. In: Chapman WM, editor. Operative Orthopaedics. Philadelphia: Lippincott Raven; 1988; p. 489–509.
12. Ethunandan M, Cole R, Flood TR. Corlett loop for microvascular reconstruction in a neck depleted of vessels. Br J Oral Maxillofac Surg. 2007, 45 : 493–5.
13. Haddock NT, Alosh H, Easley ME, Levin LS, Wapner KL. Applications of the medial femoral condyle free flap for foot and ankle reconstruction. Foot Ankle Int. 2013, 34 : 1395–1402.
14. Teeny SW, Wiss DA. Open reduction and internal fixation of tibial plafond fractures: variables contributing to poor results and complications. Clin Orthop. 1993, 292 : 108–117.
Labels
Plastic surgery Orthopaedics Burns medicine TraumatologyArticle was published in
Acta chirurgiae plasticae

2019 Issue 1-4
- Possibilities of Using Metamizole in the Treatment of Acute Primary Headaches
- Metamizole at a Glance and in Practice – Effective Non-Opioid Analgesic for All Ages
- Metamizole vs. Tramadol in Postoperative Analgesia
- Spasmolytic Effect of Metamizole
- Metamizole in perioperative treatment in children under 14 years – results of a questionnaire survey from practice
-
All articles in this issue
- České souhrny
- Bilobed flap in facial reconstruction
- Free-flap monitoring: review and clinical approach
- Giant basal carcinoma on the forehead and why should prevent them - case report
- Use of medial femoral condyle flap and anterolateral thigh free flap in proxymal tibial posttraumatic non-union with multiple anastomosis - case report
- The impact of aesthetic plastic surgery on body image, body satifaction and self-esteem
- Reconstruction of deep head burn with a free flap - a case study
- Acknowledgemets to reviewers
- Acta chirurgiae plasticae
- Journal archive
- Current issue
- About the journal
Most read in this issue
- The impact of aesthetic plastic surgery on body image, body satifaction and self-esteem
- Free-flap monitoring: review and clinical approach
- Bilobed flap in facial reconstruction
- Use of medial femoral condyle flap and anterolateral thigh free flap in proxymal tibial posttraumatic non-union with multiple anastomosis - case report
