Impact of Anakinra Treatment on Cytokine and Lymphocytes/ Monocytes Profile of an Erdheim-Chester Patient
Vliv anakinry na cytokinové profily a profily lymfocytů/ monocytů u pacienta s Erdheim-Chesterovou nemocí
Východiska:
Erdheim‑Chesterova nemoc (Erdheim‑Chester disease – ECD) je vzácná histiocytóza tzv. „nelangerhansových buněk“ spojená s intenzivní aktivací imunitního systému. Na našem klinickém pracovišti byl ECD pacient léčen anakinrou. Anakinra je antagonista receptoru pro interleukin‑1 (interleukin‑1 receptor antagonist – IL‑1RA). Tato léčba vedla ke zlepšení klinického stavu a výraznému snížení patologické únavy provázející toto onemocnění. Cílem této práce bylo analyzovat změny cytokinových profilů a změnu v zastoupení buněk imunitního systému pomocí průtokové cytometrie u ECD pacienta na počátku léčby anakinrou i po jejím skončení a srovnat je s profily zdravých dárců.
Metody:
Singleplex reakce 19 jednotlivých cytokinů ze séra ECD pacienta byly měřeny pomocí metody FACS array. Dále byla provedena analýza buněk z periferní krve pomocí průtokové cytometrie.
Výsledky:
Nejzajímavějším výsledkem bylo výrazné snížení hladiny IL‑6 bezprostředně po zahájení léčby anakinrou. Tyto výsledky naznačují významnou roli dráhy IL‑1 v patofyziologii ECD. Pomocí průtokové cytometrie jsme zaznamenali významné zvýšení počtu CD16+ monocytů před zahájením léčby, což je zcela nový poznatek.
Závěr:
Naše výsledky naznačují, že IL‑6 by se mohl stát markerem časné odpovědi na léčbu u ECD pacientů léčených anakinrou.
Klíčová slova:
Erdheim ‑ Chesterova nemoc – anakinra – cytokiny – průtoková cytometrie – FACS array
Práce byla podpořena grantem MŠMT ČR MSM 0021622434, granty IGA MZ ČR NT14575, NT12130, grantem MZ ČR – RVO (FNBr, 65269705) a grantem Masarykovy univerzity MUNI/A/0723/2012.
Autoři deklarují, že v souvislosti s předmětem studie nemají žádné komerční zájmy.
Redakční rada potvrzuje, že rukopis práce splnil ICMJE kritéria pro publikace zasílané do biomedicínských časopisů.
Obdrženo:
21. 2. 2014
Přijato:
10. 4. 2014
Authors:
S. Ševčíková 1,*; L. Kubiczková 1,*; L. Sedlaříková 1; L. Říhová 1; F. Kryukov 1; P. Szturz 2; R. Hájek 1; L. Pour 2; Z. Adam 2
Authors‘ workplace:
Babak Myeloma Group, Department of Pathological Physiology, Faculty of Medicine, Masaryk University, Brno, Czech Republic
1; Department of Internal Medicine – Hematooncology, University Hospital Brno, Czech Republic* These authors contributed equally.
2
Published in:
Klin Onkol 2014; 27(4): 276-282
Category:
Original Articles
Overview
Background:
Erdheim‑Chester disease (ECD) is a rare non‑Langerhans cells histiocytosis associated with intense immune activation. In our clinical center, an ECD patient was treated with anakinra, IL‑1RA (interleukin‑1 receptor antagonist), resulting in clinical improvement and major decrease of pathological fatigue. The aim of the study was to evaluate changes in cytokine profile and shift of immune cells estimated by flow cytometric analysis of ECD patient before, during initial stages of anakinra treatment as well as after treatment ceased in comparison to healthy donors.
Methods:
Singleplex reactions of 19 individual cytokines from serum of ECD patient were measured by FACS array. Flow cytometric analyses were performed on peripheral blood cells.
Results:
The most striking result is substantial decrease of IL‑6 immediately after anakinra treatment started suggesting a major role of IL‑1 pathway in ECD pathophysiology. As for flow cytometric analysis, increased number of CD16+ monocytes before treatment is a new finding.
Conclusion:
Our results suggest that IL‑6 may be a marker of early treatment response of ECD patients treated with anakinra.
Key words:
Erdheim ‑ Chester – anakinra – cytokines – flow cytometry – FACS array
Introduction
Erdheim‑Chester disease (ECD) is a rare non‑Langerhans cells histiocytosis that was first described by Dr. Erdheim’s student Dr. Chester in 1930 [1]. It is a heterogeneous systemic disease involving bones, lungs, skin, retro‑orbital tissues, central nervous system (CNS), pituitary gland, vessels, kidneys, retroperitoneum and heart. Clinical course of ECD depends on the extent and distribution of the disease, which may range from asymptomatic bone lesions to multisystemic, life ‑ threatening forms with poor prognosis, especially in the case of specific CNS or cardiovascular involvement [2].
ECD diagnosis is currently based on clinical, radiologic and pathologic features with infiltration of organs by CD68+/ CD1a – foamy histiocytes. ECD treatment includes steroids, cytotoxic agents, such as cladribine, interferon α (IFN ‑ α), recombinant human interleukin‑1 receptor antagonist (IL‑1RA), tyrosine kinase inhibitors, bisphosphonates and autologous hematopoietic stem cell transplantation but an optimal therapeutic strategy remains to be defined [3].
ECD is associated with intense systemic immune activation, mainly involving IFN ‑ α, interleukin 1 (IL‑1)/ IL‑1 receptor antagonist (IL‑RA), IL‑6, IL‑12, and monocyte chemoattractant protein 1 (MCP ‑ 1), consistent with systemic immune Th ‑ 1 - oriented disturbance associated with this disorder [3]. Increased concentrations of inflammatory cytokines increase also C ‑ reactive protein (CRP) and erythrocyte sedimentation. Increased production of inflammatory cytokines is responsible for crippling fatigue of these patients [4].
A report of an ECD patient treated in our center with anakinra (Kineret, Sobi), a human recombinant IL‑1RA, was previously published [4]. In brief, the ECD patient was initially treated with 2 - chlorodeoxyadenosine (cladribine) and later with lenalidomide with some improvement of clinical manifestation of the disease but no change in pathological fatigue. That is why anakinra treatment was used for three months. Anakinra was given to the patient once daily as s.c. injection of 100 mg, which led to improvement of clinical manifestations of the disease as well as significant decrease of pathological fatigue experienced by the patient.
Based on a paper published by Arnaud et al [2], which analyzed effect of IFN‑α treatment on a network of 23 serum cytokines of ECD patients, we profiled levels of chosen 19 serum cytokines of a single ECD patient treated with anakinra. Moreover, we performed flow cytometric analysis of lymphocytes and monocytes from peripheral blood of this patient. All samples were obtained before treatment, during initial treatment and after treatment.
To the best of our knowledge, we are the first to measure such a panel of cytokines as well as a flow cytometric panel during the initial phases of anakinra treatment of an ECD patient.
Material and methods
Patients and healthy controls
Serum and peripheral blood mononuclear cells (PBMC) samples were obtained from whole peripheral blood of a male ECD patient during treatment with human recombinant IL‑1RA (anakinra). As a control for cytokine profiles, two age ‑ matched healthy males were used. All samples were obtained at the University Hospital Brno, Czech Republic, only after obtaining informed consent approved by the Ethical committee of the Faculty Hospital Brno in accordance with the Helsinki Declaration of 1975. Altogether, 26 samples (serum for cytokines measurements) from the patient were obtained: 4 samples on day 4 and 3 before the treatment. Once anakinra treatment started, 3 serum samples were obtained each day (morning, noon and evening). Anakinra was given to the patient each day immediately after morning sampling. Then, samples were obtained 1,3 and 4 weeks after hospitalization and then 4 weeks after treatment ceased. At the same time, whole peripheral blood samples from the patient were obtained once per day for flow cytometric analysis during the entire sampling period.
Sample preparation and cytokine measurement
Blood samples were collected and processed immediately. After centrifugation at 2 000 g for 10 min, serum samples were stored at – 80 °C and thawed only once for cytokine measurement using Human FlowCytomix Simplex Kits (TNF‑α, IL‑1β, IL‑1RA, IL‑2, IL‑4, IL‑5, IL‑6, IL‑8, IL‑10, IL‑12p70, IL‑13, IL‑17A, IFN ‑ α, IFN ‑ γ, IP ‑ 10, MCP ‑ 1, MIG, MIP‑1α, MIP‑β, all e ‑ Bioscience, San Diego, USA). Samples were measured according to manufacturers’ recommendations by two‑step incubation assay: first incubation with Biotin‑Conjugate mixture and second incubation with Streptavidin‑PE solution. For all cytokines, standard dilution curves were prepared, and all samples were measured in triplicates on BD FACS Array using BD FACS Array System Software and further analyzed by FlowCytomix Pro, v3.0. Data were analyzed using standard descriptive statistics (min–max); for analysis of continuous parameters relationship, the Spearman correlation coefficient was adopted.
Flow cytometry
Anticoagulated peripheral blood samples were incubated with the following monoclonal antibodies: CD38 - Pacific Blue (Exbio), CD45 - Krome Orange (Beckman Coulter), CD3 - FITC/ CD16+56 - PE (Beckman Coulter), CD19 - PC7 (Beckman Coulter), CD8 - PerCP (Exbio), CD4 - APC (Exbio),HLA‑DR ‑ APC ‑ eFluor780 (eBioscience) and lysed with ammonium chloride. Analyses were done by flow cytometer FACS Canto II (BD Biosciences) equipped with 3 lasers in 8 - color setting. About 0.25 – 0.3 × 106 cells per sample were analyzed. Relative counts of lymphocytes, monocytes (according to CD45 vs complexity) and especially CD56+ monocytes, were assessed as well as CD3+ T, CD3+CD4+ Th, CD3+CD8+ Tc, CD19+ B, CD3 – CD16+CD56+ NK, CD3+CD16+CD56+ NKT lymphocytes and subpopulations of CD3+HLA-DR++ and CD8+CD38+ activated T lymphocytes were determined.
Results
Flow cytometric analysis
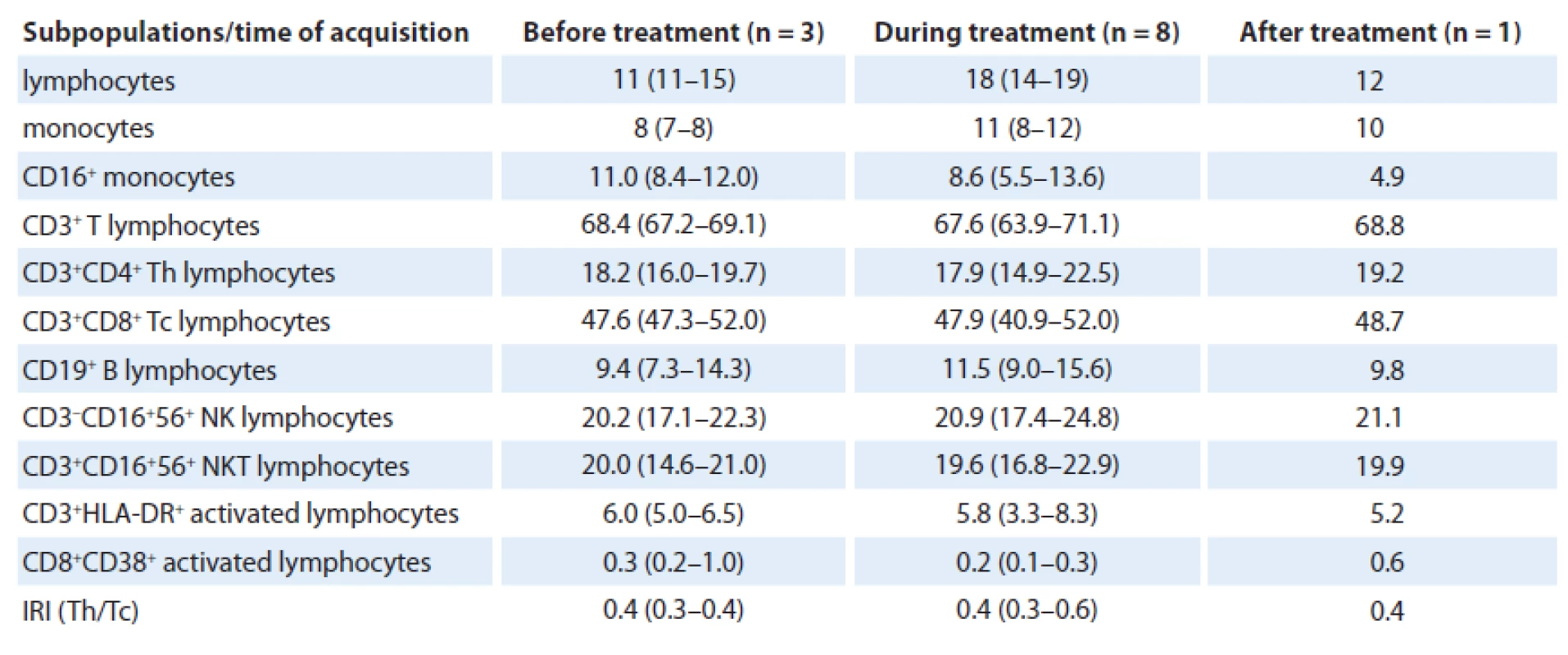
Total of 12 analyses were performed during the patient’s follow-up with no significant changes in relative numbers of selected subpopulations when data before (three analyses), during (eight analyses) and after the treatment (one analysis) were compared. In great detail, relative number of lymphocytes increased during treatment (median 18% (range 14 – 19)) and after anakinra withdrawal decreased again to similar level (12%) as before treatment (11% (11 – 15)). Number of monocytes slightly increased during the treatment as well, from 8% (7 – 8) to 11% (8 – 12), but more interesting is reduction of slightly increased amount of CD16+ monocytes from 11.0% (8.4 – 12.0) to 8.6% (5.5 – 13.6) during the treatment to only 4.9% after anakinra withdrawal (Fig. 1). The reduction is also observed when compared with healthy controls (median 9.8% (4.4 – 14.4)).

As for T cells, number of CD3+ T lymphocytes was physiological during the whole time; however, percentage of CD4+ Th lymphocytes was reduced when compared with physiological number (18.2% (16.0 – 19.7) vs 17.9% (14.9 – 22.5) vs 19.2%) and percentage of CD8+ Tc lymphocytes was increased (47.6% (47.3 – 52.0) vs 47.9% (40.9 – 52.0) vs 48.7%). Hence, immunoregulatory index (IRI), the ratio of CD4+ Th/ /CD8+ Tc lymphocytes (0.4 (0.3 – 0.4) vs 0.4 (0.3 – 0.6) vs 0.4) was inversed. Concerning B cells, their number was normal, as well as numbers of activated CD3+HLA-DR and CD8+CD38+ T lymphocytes. Further, number of NK cells (20.2% (17.1 – 22.3) vs 20.9% (17.4 – 24.8) vs 21.1%) as well as NKT lymphocytes (20.0% (14.6 – 21.0) vs 19.6% (16.8 – 22.9) vs 19.9%) was slightly higher than normal. We assume that there was no considerable effect of treatment on relative count of analyzed immunocompetent cells (Tab. 1).
Cytokine profile during anakinra treatment
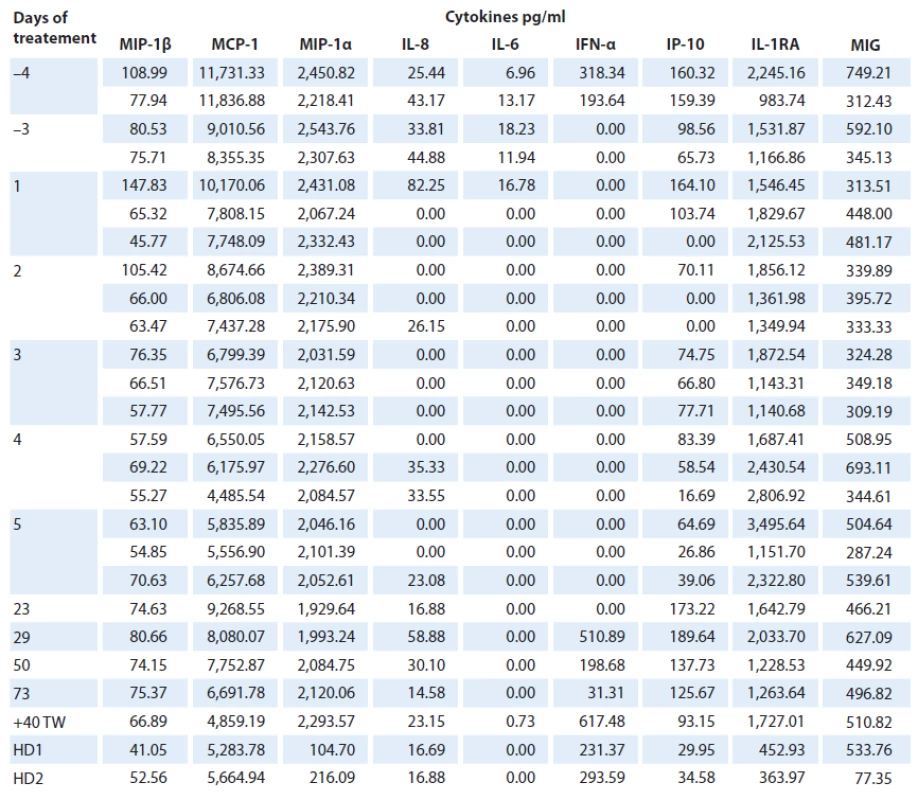
Total of 26 analyses of 19 cytokines were performed on serum samples of the ECD patient as well as two age ‑ matched healthy controls (Fig. 2). Levels of macrophage inflammatory protein 1α and β (MIP‑1α, MIP‑1β), monocyte chemoattractant protein 1 (MCP ‑ 1), interferon ‑ γ ‑ inducible protein‑10 (IP ‑ 10), IL‑1 receptor antagonist (IL‑1RA), monokine induced by interferon-γ (MIG), IL‑6, IL‑8 and interferon ‑ α (IFN ‑ α) were detectable in patient’s follow‑up serum samples and in healthy controls. However, levels of IL‑17A, IL‑2, IL‑10, IL‑4, IL‑1β were present in patient’s samples only at three (3/ 5) time points before the treatment (IL‑17A: 23.6 – 33.1 pg/ ml, IL‑2 : 186.1 – 252.7 pg/ ml, IL‑10 : 0.41 – 11.48 pg/ ml, 54.7 – 102.9 pg/ ml, 2.75 – 25.1 pg/ ml). Levels of IL‑13, IL‑5, IL12 - p70, IFN ‑ γ and tumor necrosis factor α (TNF‑α) were not detectable at all, either in patient or healthy control samples and were excluded from further analyses.

IL‑6, IL‑8 and IP ‑ 10 were found to be reduced in samples obtained during the treatment and increased after treatment withdrawal. There was a decrease of IL‑6 in the samples taken during the treatment when compared to the samples taken prior to treatment (0 pg/ ml vs 18.23 – 6.96 pg/ ml resp.), and levels of IL‑6 were higher in patient’s untreated samples than in healthy controls (18.23 – 6.96 pg/ ml vs 0 pg/ ml). IL‑8 was present in patient’s serum prior to treatment (25.4 – 82.3 pg/ ml); during the treatment, its levels fluctuated between detectable and undetectable levels, and after first five doses of anakinra, its level was stable (14.6 – 58.9 pg/ ml). As for IP ‑ 10, there was an increased level of this cytokine in untreated samples (65.7 – 164.1 pg/ ml) when compared with healthy controls (30.0 – 34.6 pg/ ml). IP ‑ 10 was further reduced during the first five days of treatment (0 – 83.4 pg/ ml); however, after first five days of therapy, it increased considerably (125.7 – 189.6 pg/ ml); one month after treatment withdrawal, it was comparable to levels before the treatment (93.2 pg/ ml). Furthermore, IFN ‑ α level was detectable only in two (2/ 5) samples before treatment (193.6 – 318.3 pg/ ml), it decreased to undetectable level prior the treatment, continued to be undetectable during the treatment and then reappeared again after five doses of treatment (31.3 – 617.5 pg/ ml).
MIP‑1α and MIG levels in patient’s samples did not display any visible change between the initial and the follow‑up evaluation; however, MIP‑1α levels were increased when compared with healthy controls (1,929.6 – 2,543.8 pg/ ml vs 104.7 – 216.1 pg/ ml), but no change between patient and healthy controls was observed for MIG Although MIP‑1β, MCP ‑ 1 and IL‑1RA fluctuated during the follow‑up (MIP‑1β: 54.8 – 145.8 pg/ ml; MCP ‑ 1 : 4,485.5 – 11,836.9 pg/ ml; IL‑1RA: 983.7 – 3 495.6 pg/ ml), there was no considerable difference in the cytokines levels connected to the treatment; as for MIP‑1β and MCP ‑ 1, there was no difference when compared with healthy controls (MIP‑1β: 41.1 – 52.6 pg/ ml; MCP ‑ 1 : 5,283.8 – 5,664.9 pg/ ml); however, IL‑1RA levels were reduced in healthy controls (364.0 – 452.9 pg/ ml) (Tab. 2).
Markers of inflammation, such as CRP and ferritin, were increased before the treatment (CRP: 19.7 – 22.1 mg/ l, ferritin: 33.3 µg/ l) and then decreased during anakinra treatment (CRP: 0.0 – 14.4 mg/ l, ferritin: 33.3 – 11.3 µg/ l) and increased again after treatment withdrawal (CRP: 12.8 mg/ l, ferritin 20.2 µg/ l) [4]. We found a significant positive correlation between CRP or ferritin and some of the cytokines. Levels of CRP were associated with levels of MIP‑1α (p = 0.01, rs = 0.733) and levels of ferritin with levels of IL1RA (p = 0.016, rs = 0.847).
Discussion
Erdheim‑Chester is a rare disease that is hard to treat although currently there are several treatment options. None of them work long‑term, and although IFN ‑ α treatment showed some promise, it cannot be used for all patients. There are some studies suggesting a major role of IL‑1 in ECD. As IFN ‑ α induces IL‑1RA, the natural negative regulator of IL‑1, it seems to suggest that IFN ‑ α therapy modulates the IL‑1 pathway. Thus, therapy with human recombinant form of IL‑1RA has been reported to be effective and safe in several auto ‑ inflammatory diseases, such as cold auto ‑ inflammatory periodic fever and familial Mediterranean fever, in which IL‑1 has a pivotal role [5].
Anakinra is a recombinant antagonist of IL‑1R. IL‑1 is a major inducer of a cascade of inflammatory cytokines. In ECD, after IL‑1R is blocked by anakinra, IL‑1 produced by histiocytes cannot induce production of more inflammatory cytokines. Thus, anakinra can significantly decrease systemic inflammatory reaction of these patients as well as major fatigue that is often crippling for these patients [4].
So far, there are only three case reports of using anakinra for ECD patients, including the report from our clinical center. In the first study, Aouba et al [5] showed marked clinical improvement in two ECD patients connected to decrease in CRP and decrease of mean fluo-rescence intensity of IL‑1α on monocytes. A recent study by Killu et al [6] used anakinra on an ECD patient with cardiac involvement but reported only clinical improvement of the patient.
In our clinical center, an ECD patient was treated with anakinra with major decrease of fatigue of the patient as well as clinical manifestations of the disease; decrease in CRP was also noted [4]. As a follow-up of the clinical study, we analyzed cytokine profile of this ECD patient. Unlike Aouba et al [5] who concentrated only on several cytokines, we decided to analyze serum levels of cytokines based on a study by Arnaud et al [2]. That study is the largest study of ECD patients – in total, 37 ECD patients treated with IFN ‑ α were included in this study. Cytokine levels before and after treatment and in comparison to healthy controls were analyzed in that study.
Unlike Arnaud, we decided to frequently monitor initial cytokine fluctuations using singleplex measurement of each cytokine. Four serum samples were obtained before treatment. Then, while the patient was hospitalized, in the initial phase of treatment, the samples were obtained three times a day (at 8 am, 12 pm and 6 pm) for seven days, then at several time points after hospitalization and after treatment withdrawal.
In our cytokine analysis of the ECD patient, we were not able to show the ECD cytokine signature as published by Arnaud et al [2]. In their study, they suggested the existence of a five cytokine profile (IFN ‑ α, IL‑12, MCP ‑ 1, IL‑4 and IL‑7) that distinguishes ECD patients from healthy donors. We were not able to detect IL‑12p70 in our samples at all, and IL‑4 was detectable only at 3/ 5 time points before treatment. The original analysis of Arnaud excluded 4 ECD samples as they did not have this signature [2]; so it is possible that our patient would fall into this category as well.
Of the measured cytokines, the most interesting findings are undetectable levels of IL‑6 during the treatment suggesting that anakinra is able to decrease the intense immune system activation of this patient. The major role of IL‑1 pathway in ECD seems to hold true even in the case of our ECD patient. Clinical findings of the patient are in concordance with our measurements, as the patient reported decreased fatigue on the second day of anakinra treatment.
In general, the cytokine profiles follow clinical findings of the patient that had major improvement in quality of life as the pathological fatigue disappeared during anakinra treatment.
As IL‑1 family cytokines are essentially produced by monocytes and macrophages, we analyzed monocytes in detail. Unlike Myra et al [7], we did not find any monocytosis. On the other hand, we found slightly increased presence of CD16+ monocytes persisting in the ECD patient before treatment – a fact that has not been published yet. Population of CD16+ monocytes is usually increased during acute inflammatory illness as well as during chronic inflammatory illnesses such as rheumatoid arthritis and HIV infections [8]. Reduced number of these cells in the 1st week of treatment, was followed by their increased number, so the effect of anakinra on this population seems to be only temporary, and this subpopulation is probably not involved in clinical manifestation of ECD. Relative numbers of selected lymphocyte subpopulations, including activated forms, were not affected by anakinra treatment, so we found no effect of treatment on immunocompetent cells.
Fluctuation of cytokines found in this study mirrors the disappearance of clinical manifestations of the disease; however, this change is only temporary connected to anakinra administration.
Thus, based on this paper as well as the previous clinical study, we believe that anakinra treatment may be a viable option for treatment of ECD patients. While anakinra does not cure the disease, it suppresses ECD consequences and markedly improves the quality of life of ECD patients. In our opinion, anakinra is showing a great potential for eliminating at least some symptoms of ECD.
This study was supported by grants of the Czech Ministry of Education, Youth and Sport MSM0021622434, grants of Internal Grant Agency of the Czech Ministry of Health NT14575, NT12130, grant of the Czech Ministry of Health – conceptual development of research organization (FNBr, 65269705), and grant of Masaryk University MUNI/A/0723/2012.
Práce byla podpořena grantem MŠMT ČR MSM 0021622434, granty IGA MZ ČR NT14575, NT12130, grantem MZ ČR – RVO (FNBr, 65269705) a grantem Masarykovy univerzity MUNI/A/0723/2012.
The authors declare they have no potential conflicts of interest concerning drugs, products, or services used in the study.
The Editorial Board declares that the manuscript met the ICMJE “uniform requirements” for biomedical papers.
RNDr. Sabina Ševčíková, Ph.D.
Babak Myeloma Group
Department of Pathological Physiology
Faculty of Medicine
Masaryk University
Kamenice 5, A4
625 00 Brno
Czech Republic
e-mail: sevcik@med.muni.cz
Submitted: 21. 2. 2014
Accepted: 10. 4. 2014
Sources
1. Chester W. Über Lipoidgranulomatose. Virchows archiv für pathologische anatomie und physiologie und für klinische medizin 1930; 279(2): 561 – 602.
2. Arnaud L, Gorochov G, Charlotte F et al. Systemic perturbation of cytokine and chemokine networks in Erdheim ‑ Chester disease: a single‑center series of 37 patients. Blood 2011; 117(10): 2783 – 2790. doi: 10.1182/ blood ‑ 2010 ‑ 10 ‑ 313510.
3. Haroche J, Arnaud L, Cohen ‑ Aubart F et al. Erdheim ‑ chester disease. Rheum Dis Clin North Am 2013; 39(2): 299 – 311. doi: 10.1016/ j.rdc.2013.02.011.
4. Adam Z, Szturz P, Bučková P et al. Interleukin‑1 receptor blockade with anakinra provided cessation of fatigue, reduction in inflammation markers and regression of retroperitoneal fibrosis in a patient with Erdheim ‑ Chester disease – case study and a review of literature. Vnitr Lek 2012; 58(4): 313 – 318.
5. Aouba A, Georgin‑Lavialle S, Pagnoux C et al. Rationale and efficacy of interleukin‑1 targeting in Erdheim ‑ Chester disease. Blood 2010; 116 : 4070 – 4076. doi: 10.1182/ blood ‑ 2010 ‑ 04 ‑ 279240.
6. Killu AM, Liang JJ, Jaffe AS. Erdheim ‑ Chester disease with cardiac involvement successfully treated with anakinra. Int J Cardiol 2013; 167(5): e115 – e117. doi: 10.1016/ j.ijcard.2013.04.057.
7. Myra C, Sloper L, Tighe PJ et al. Treatment of Erdheim ‑ Chester disease with cladribine: a rational approach. Br J Ophthalmol 2004; 88(6): 844 – 847.
8. Grip O, Bredberg A, Lindgren S et al. Increased subpopulations of CD16(+) and CD56(+) blood monocytes in patients with active Crohn’s disease. Inflamm Bowel Dis 2007; 13(5): 566–572.
Labels
Paediatric clinical oncology Surgery Clinical oncologyArticle was published in
Clinical Oncology

2014 Issue 4
- Possibilities of Using Metamizole in the Treatment of Acute Primary Headaches
- Metamizole at a Glance and in Practice – Effective Non-Opioid Analgesic for All Ages
- Metamizole vs. Tramadol in Postoperative Analgesia
- Spasmolytic Effect of Metamizole
- Metamizole in perioperative treatment in children under 14 years – results of a questionnaire survey from practice
-
All articles in this issue
- Paraneoplastic Vasculitis in a Patient with Cervical Cancer
- Acupuncture in the Treatment of Symptoms of Oncological Diseases in the Western World
- Dendritic Cell Vaccines Against Non‑ small Cell Lung Cancer – an Emerging Therapeutic Alternative
- Pacient s Cowdenovým syndromem způsobeným mutací v genu PTEN (archiv 2. LF UK a FN v Motole)
- Anticipated Efficacy of HPV Vaccination in Prophylaxis Against Nongenital Cancers
- The Cost Study of First- line Treatment of Metastatic Colorectal Carcinoma with Bevacizumab- containing Regimen in the Czech Republic
- Screening of Malnutrition Risk Versus Indicators of Nutritional Status and Systemic Inflammatory Response in Newly Diagnosed Lung Cancer Patients
- Relation between Carbonic Anhydrase IX Serum Level, Hypoxia and Radiation Resistance of Head and Neck Cancers
- Brazilian Story of the R337H p53 Mutation
- Impact of Anakinra Treatment on Cytokine and Lymphocytes/ Monocytes Profile of an Erdheim-Chester Patient
- Positron Emission Tomography Combined with Computed Tomography for Diagnosis of Synchronous Tumors
- Clinical Oncology
- Journal archive
- Current issue
- About the journal
Most read in this issue
- Brazilian Story of the R337H p53 Mutation
- Acupuncture in the Treatment of Symptoms of Oncological Diseases in the Western World
- Paraneoplastic Vasculitis in a Patient with Cervical Cancer
- Screening of Malnutrition Risk Versus Indicators of Nutritional Status and Systemic Inflammatory Response in Newly Diagnosed Lung Cancer Patients
