Teriparatide in the treatment of severe osteoporosis in elderly female patients: results of a multicenter prospective study
Teriparatid v liečbe ťažkej osteoporózy u starších pacientok: výsledky multicentrickej prospektívnej štúdie
Teriparatid (TPTD) je rekombinantný aminotermálny fragment (1-34) ľudského parathormónu (PTH), ktorý má najmä stimulujúci účinok na tvorbu kostí. Účinnosť liečby teriparatidom spočíva v znížení počtu zlomenín u žien s ťažkou osteoporózou. Uvádzame výsledky 18-mesačnej prospektívnej multicentrickej štúdie, v priebehu ktorej bolo 85 ženám s ťažkou osteoporózou denne podkožne aplikované 20 μg teriparatidu a bola sledovaná účinnosť, bezpečnosť a dodržiavanie liečby. Po 12 a 18 mesiacoch liečby sme sledovali nárast BMD v oblasti bedrovej chrbtice (9,2 % a 10,6 %; p = 0,001), konkrétne v oblasti krčka femoru (1,4 % a 4,6 %; p = 0,04), v proximálnom femoru (3,5 % a 3,7 %, p = 0,02) a taktiež nárast markerov kostného obratu po 6 a 18 mesiacoch (CTx 173% a 93%; p < 0,001 a osteokalcínu 134 %; p < 0,001). Typ predchádzajúcej antiosteoporotickej liečby nemal podľa ukazovateľov BMD a kostného obratu žiaden vplyv na účinnosť teriparatidu. Liečba bola dobre tolerovaná a neboli pozorované žiadne závažné nežiadúce účinky. U 5,3 % pacientok, ktoré užívali vyššie dávky vápnikových doplnkov, bola zistená hraničná hyperkalcémia, ktorá ale nemala klinický význam. Teriparatid je vysoko účinný osteoanabolický liečivý prípravok vhodný u starších žien s ťažkou osteoporózou a so zlomeninou stavca v anamnéze.
Klíčová slova:
BMD – osteoporóza – riziko zlomeniny – teriparatid
Authors:
Payer Juraj 1; Tomková Soňa 2; Jackuliak Peter 1; Kužma Martin 1; Vaňuga Peter 3; Letkovská Alexandra 4; Masaryk Pavol 4; Kmečová Zlata 5; Killinger Zdenko 1
Authors‘ workplace:
-5th Department of Internal Medicine, Medical Faculty of Comenius University, and University Hospital Bratislava, Hospital Ružinov, Bratislava, Slovakia
1; Osteocentrum, Hospital Košice – Šaca, Košice, Slovakia
2; National Institute of Endcrinology and Diabetology, Lubochňa, Slovakia
3; National Institute of Rheumatic Diseases, Piešťany, Slovakia
4; Osteocentrum of Faculty Hospital F. D. Roosevelt, Banská Bystrica, Slovakia
5
Published in:
Clinical Osteology 2018; 23(3): 88-93
Category:
Overview
Teriparatide (TPTD) is a recombinant aminoterminal fragment (1–34) of the human parathyroid hormone (PTH), which has a predominantly stimulating effect on bone formation. The effectiveness of the treatment with teriparatide consists in fracture reduction in females with severe osteoporosis. We report results of an 18-months prospective multicenter study with daily subcutaneous application of 20 μg of teriparatide in 85 elderly women with severe osteoporosis, the efficacy, safety and compliance of the treatment. We´ve observed an increase of BMD after 12 and 18 months of treatment in the lumbar spine region (9.2% and 10.6%, p = 0.001), respectively in femoral neck (1.4% and 4.6%, p = 0.04) and total hip (3.5% and 3.7%, p = 0.02) and an increase of markers of bone turnover after 6 and 18 months (CTx 173% and 93%, p < 0.001 and osteocalcin 134%, p < 0.001). The type of previous antiosteoporotic treatment had no influence on the effectiveness of teriparatide according to BMD and bone turnover markers. Treatment was well tolerated and no serious side effects were observed. Borderline hypercalcemia was present in 5.3% of patients with higher calcium supplements doses, but it has no clinical relevance. Teriparatide is a highly effective osteoanabolic drug in elderly women with severe osteoporosis with a preexisting vertebral fracture.
Keywords:
osteoporosis – BMD – teriparatide
Introduction
Osteoporosis is a disorder with both medical and socio-economical dimensions and is the most severe disorder of the locomotor system due to its high incidence and mortality [1]. The most frequent locations of osteoporotic fractures are the forearm, vertebrae and the hip [2]. The lifelong risk of any osteoporotic fracture in a 50-year old woman represents approximately 39% and approximately 13% in a man. Hip fracture is the most serious complication of osteoporosis and is associated with high morbidity and mortality. An increase in the number of these fractures from 1.6 million in 1990 to 6.26 million in 2050 can be expected worldwide. The 5-year survival is only 82% of the total population. The highest mortality after hip-fracture is in the first 6 months [3].
The epidemiology of vertebral fractures is less understood due to the lack of generally accepted diagnostic criteria and high proportion of asymptomatic fractures (Figure 1) [4]. The prevalence of radiologically detected fractures in people over 50 years represents 8–25% (depending on the definition) and vertebral fractures are associated with a similar increase in 5-year mortality as hip fractures. A present vertebral fracture is the most important risk factor of recurrent vertebral fractures. Treatment of these high-risk patients as is the most difficult challenge [3,4].
![Vertebral fractures semi-quantitative grading. Modified according to [16]](https://www.prelekara.sk/media/cache/resolve/media_object_image_small/media/image/60db98284fe3ca4c18d3fff947bb20bc.png)
The aim of our 18-months study was to evaluate the effect of teriparatide on bone density, levels of bone turnover markers, compliance and safety of the treatment in a high-risk group of patients with severe postmenopausal osteoporosis.
Design and participants
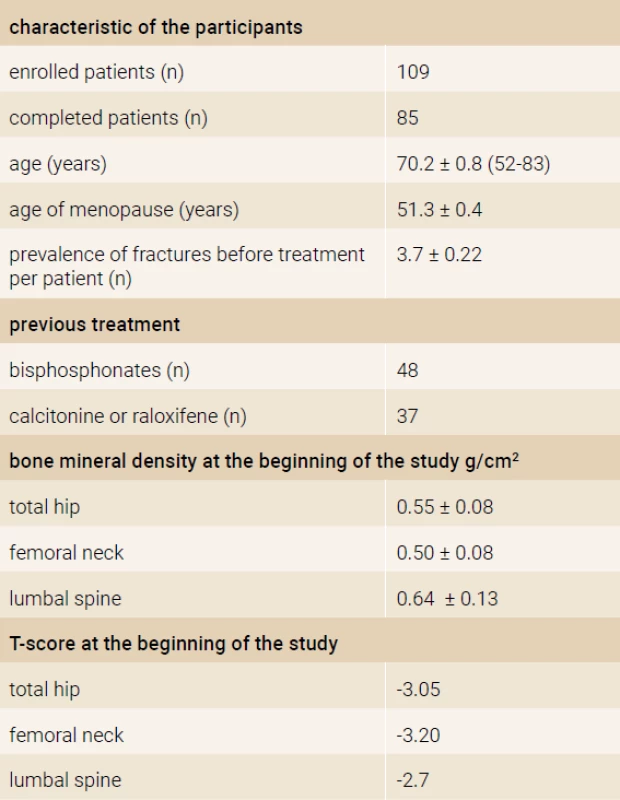
The prospective, open label, non-randomized, single-armed 18-months study was performed in 5 centers in the Slovak republic. The inclusion criteria were: (1) bone mineral density (BMD) T-score < -2.9 in the region of the femoral neck, or hip and 2 or more vertebral fractures, (2) the failure of previous antiresorptive treatment. The failure of treatment was defined as a new osteoporotic fracture after 2 or more years of antiosteoporotic treatment or BMD loss in the femoral neck or hip region more than 6% after 2 or more years of treatment. The definition of vertebral fractures was < 20% of the regular height of vertebrae, according to the Genant criteria. We enrolled 109 women in the study, with mean age of 70.2 ± 0.8 years (52–83 years). All patients were daily applicated subcutaneously 20 micrograms of recombinant humane parathyroide hormone (1–34) – teriparatide (Forsteo®, Eli Lilly and Company, Nederland BV). All enrolled women received daily supplements of 500 to 1 000 mg of Calcium and 400 to 800 IU of vitamin D. Characteristic of the participants is summarized in Table 1.
Outcomes
The primary outcomes were defined as changes from basal/basic BMD of femoral neck, total hip and lumbar spine after 12 and 18 months.
We analysed also BMD changes in respect of previous treatment (bisphophonates, calcitonin or raloxifene). Another primary outcome was defined as an analysis of serum level changes of bone turnover markers after 6 and 18 months.
Methods
The bone mineral density of lumbar spine (L1–4), femoral neck and total hip was measured before treatment (base line) and at 12 and 18 months using dual-energy X-ray (DXA Hologic). We measured the concentration of C-telopeptide of collagen (CTx) and osteocalcin (OC) at base line and at 6 and 18 months. These markers were measured in serum using electro-chemoluminiscent immuno-assay analysis (Elecsys, Roche, Basel, Sschwitzerland). Statistical analyses were performed according to the intention-to-treat principle. T-test was used to determine the changes in percentage of BMD and levels of serum markers in 6, 12 and 18 months compared to baseline.
Results
From 109 women enrolled in this study 85 (77.9%) completed the protocol. From the 24 excluded patients 9 patients due to difficulties with the subcutaneous application of teriparatide without further specification, one patient died on cardiovascular event (stroke), but not in relationship with the treatment. 2 women had mild adverse events (headache after 2 months of treatment) and whole body pain (myalgia and arthralgia) after 5 months of treatment. In 3 participants no effect of treatment was observed (no increase of BMD and osteocalcin) due to non-compliance. The remaining 9 participants were excluded due to technical difficulties (we lost contact to these patients).
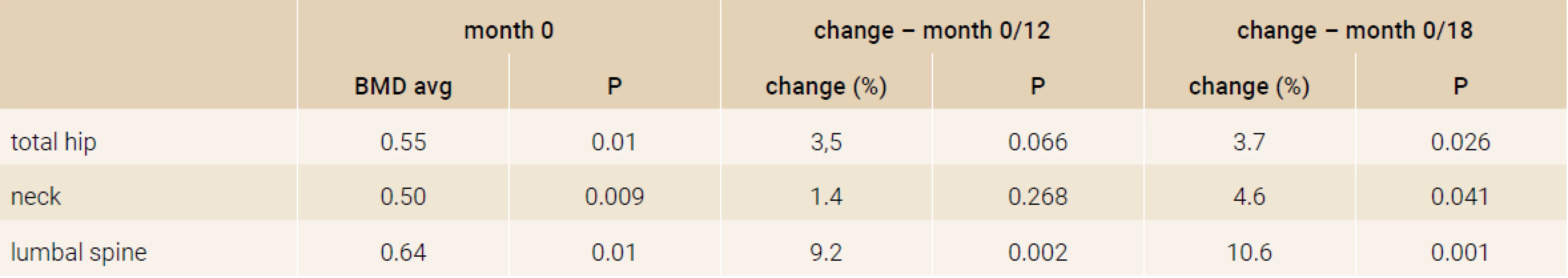
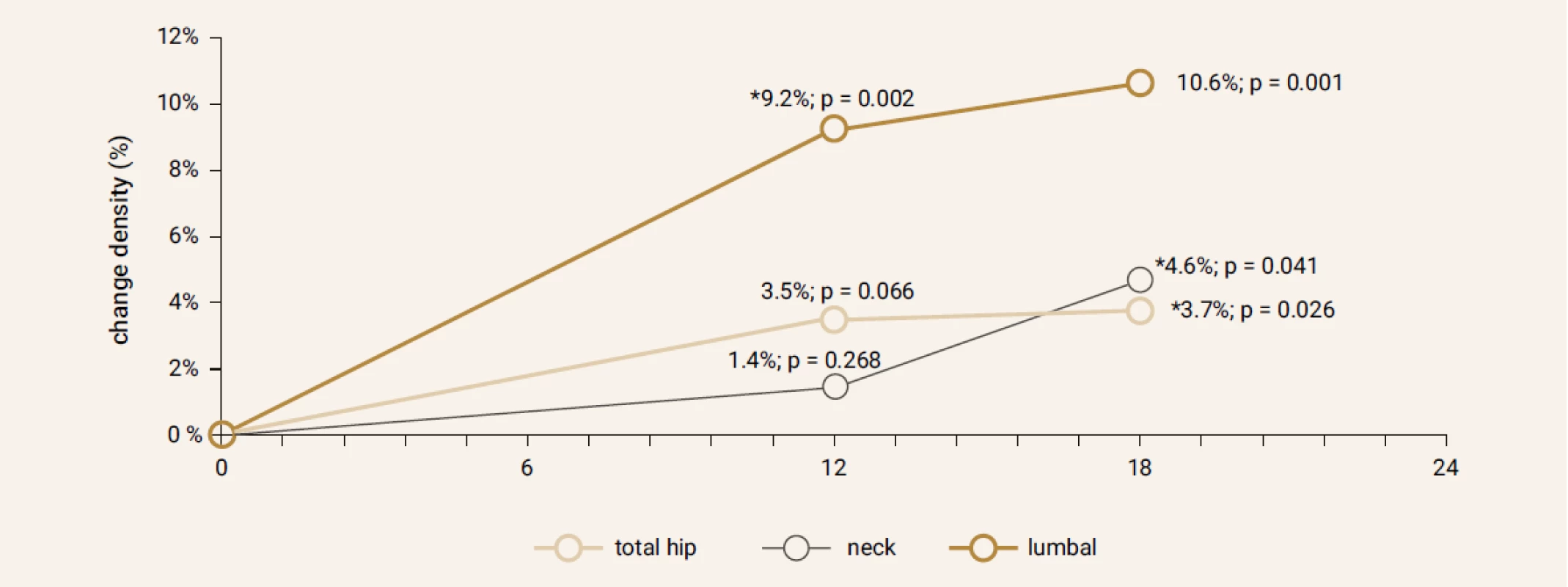
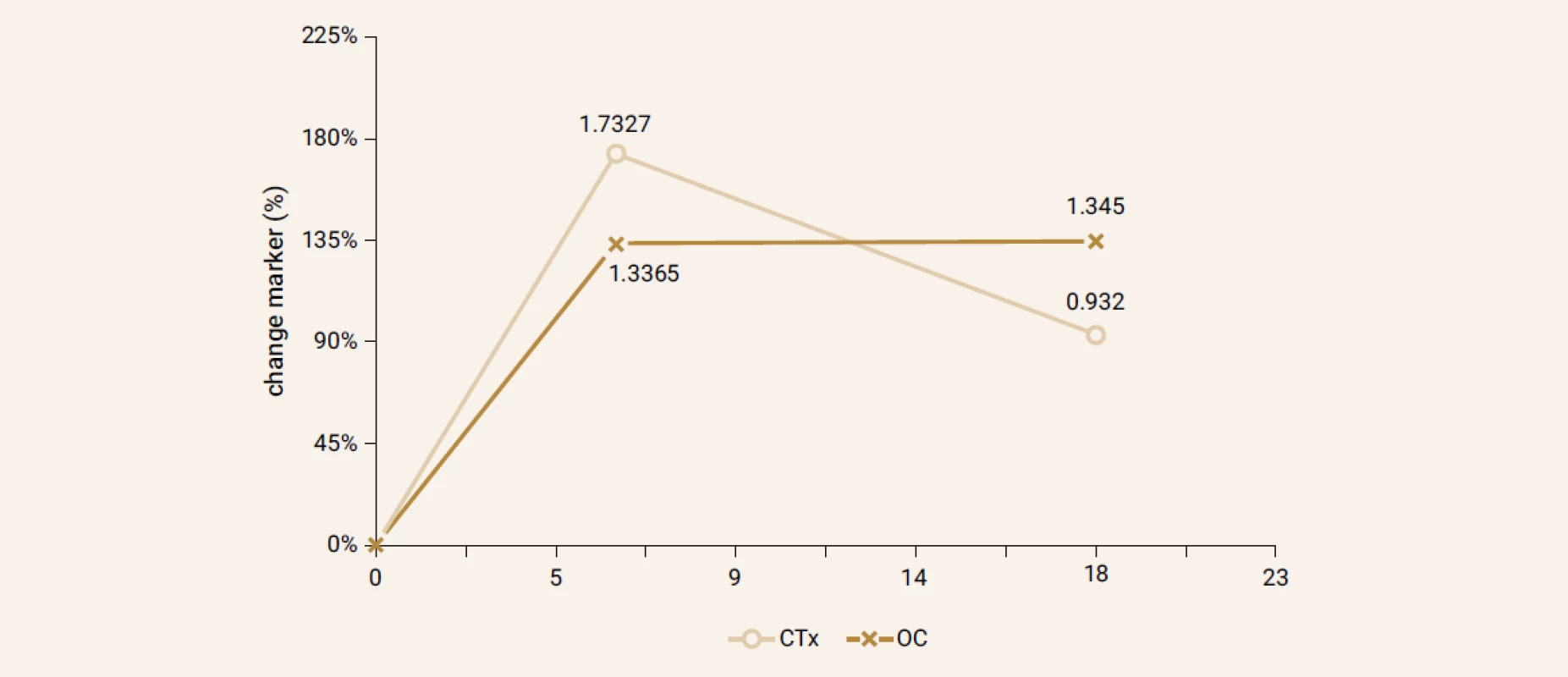
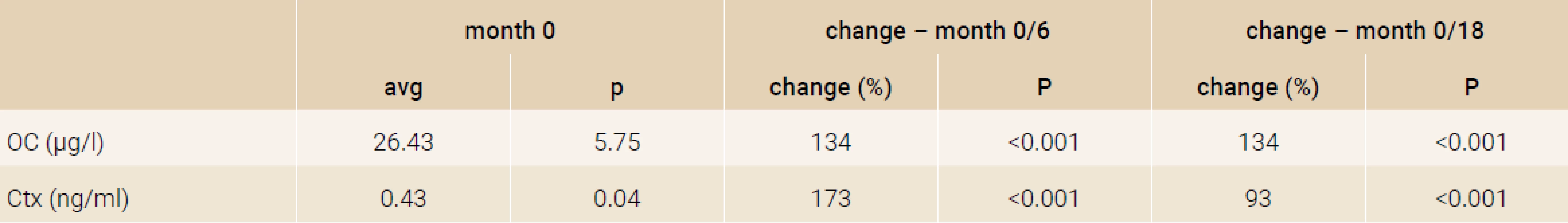
Treatment with TPTD resulted in increase of bone mineral density of the total hip during 12 months (+3.5%, p = 0.066) and significant increase of BMD in 18 months of treatment (+3.7%, p = 0.026). The BMD changes in the femoral neck region were also significant, we observed an increase of BMD + 4.6% (p = 0.041) in 18 months. The most significant increase of BMD was found in the lumbar spine region, +9.2% (p = 0.002) during 12 months of treatment and an increase of 10.6% after 18 months of treatment with TPT (p = 0.001), Figure 2. The changes in serum levels of markers of bone turnover are shown in Figure 3. Over 18 months of teriparatide therapy led to significant increase of CTx and osteocalcin. The increase of CTx was 173% after 6 months and 93% after 18 months (p < 0.001). As for osteocalcin, the increase of serum level represented 134% after 6 and also 18 months of therapy (p < 0.001).
The increase in bone mineral density was found in all groups of patients, there was found no significant difference in the effect of TPTD on BMD and bone turnover markers according to the previous medication (Table 2 and Table 3).
Discussion
The main goal of treatment of osteoporosis is the reduction of fractures [4]. In all drugs generally used in treatment, this efficacy has been proven in prospective placebo controlled long term trials. The majority of these drugs inhibit the bone turnover, both osteoresorption and osteoformation (bisphosphonates, estrogens, SERMs, calcitonine), strontium ranelate has a dual mechanism of action (inhibition of resorbtion and stimulation of osteoformation) [5,6]. Parathormone and its active recombinant aminoterminal fragment (1–34) teriparatide are the only pure osteoanabolic drugs [7].
Teriparatide affects the metabolism of calcium and phosphates in several ways – stimulation of the release of calcium and phosphate from the bone, stimulation of the reabsorption of calcium from the glomerular filtrate and loss of phosphate to urine and stimulation of the renal synthesis of 1,25-(OH)2-vitamin D3 and so the absorption of calcium and phosphate from the gastrointestinal tract. The greatest impact on the bone microarchitecture is achieved in therapeutic doses. Teriparatide increases periosteal and endocortical bone formation – total bone area, cortical area and bone strength [8,9].
The teriparatide treatment first stimulates bone formation and later on the bone resorption too, with a positive formation – resorption ratio. Daily dose of 20 µg of teriparatide stimulates the production of growth factors - IGF-1 and TGF-β in osteoblasts without reduction of osteoprotegerin [10]. Although, in this study the increase in CTx during first months of treatment was observed, the results are borderline significant.
Neer and colleagues documented in a group of postmenopausal females with prevalent vertebral fracture a decrease in risk of vertebral and non-vertebral fractures in an 18 months prospective study. The treatment was associated with significant improvement of life quality [11].
In this 18-months prospective multicenter study we applicated the same treatment protocol as in Neer´s trial. But our study patients were suffering from more severe osteoporosis, with lower T-score of total hip and more vertebral fractures (3.7 in our study vs 2.3 fractures in Neer’s trial).
We have found significant increase of BMD of the hip and lumbar spine in our whole group. The highest increase was present in the lumbar spine region. The anabolic effect on bone turnover was significant during the whole period of treatment. Our data are very similar to the results from other studies with teriparatide. For example our results are similar to data from FPT (Fracture Prevention trial), the most significant changes in BMD were observed in the lumbar spine region (+9.7%) at the end of treatment (after 18 months) unlike the total hip (+2.6%) and femoral neck (+2.8%) [12].
The evaluation of the effect of teriparatide on fracture risk reduction was not the purpose of our study. Only two clinical fractures were proved during the 18-months trial period (one fracture of forearm after 16 months of treatment and one fracture of femoral neck after 2 months of treatment). We suppose much higher occurrence of fractures when using other treatment modalities due to a very high risk of fractures in our population.
There are existing data on dependence of the effect of teriparatide treatment on previous antiresorptive treatment [13,14]. Ettinger et al did show that increase of BMD and bone turnover was delayed and of lower magnitude in patients pretreated with alendronate compared to patients treated with raloxifene [15].
We didn´t notice any influence of the type of preexisting antiosteoporotic treatment on the effect of teriparatide in our study.
The drug was well tolerated, without any several adverse events, and we observed a high adherence to the long-term treatment. Osteosarcoma as an adverse event did not occur in our group of patients. Safety of the treatment was proved by monitoring normal blood (serum) and urine calcium levels. We found no case of clinically significant hypercalcemia. Only 10 patients had increased range of serum level after 6 month of treatment, but after 12 month levels remained normal.
Conclusion
In conclusion, osteoanabolic treatment using teriparatide was effective, well tolerated and safe. According to or results parathormone could be a drug of choice in postmenopausal women with severe osteoporosis.
Disclosure statement
The authors confirm that there are no conflicts of interests (both personal and institutional) regarding specific financial interests of Eli Lilly Company, that are relevant to the work conducted or reported in this manuscript.
Acknowledgments
The authors confirm that all the research meets the ethical guidelines, including adherence to the legal requirements of Slovakia. We assert that there are no conflicts of interest (both personal and institutional) regarding specific financial interests that are relevant to the work conducted or reported in this manuscript.
Received | Doručeno do redakce | Doručené do redakcie 28. 9. 2018
Accepted | Přijato po recenzi | Prijaté po recenzii 18. 10. 2018
MUDr. Martin Kužma, PhD.
Sources
- Rovensky J, Payer J (eds). Dictionary of Rheumatology. Springer-Verlag, Wien 2009. 230 p. ISBN 978–3-211–68584–6.
- Vestergaard P. Bone diseases. Incident fractures during treatment for osteoporosis. Nat Rev Rheumatol 2013; 9(9): 508–510. Available on DOI: <http://doi: 10.1038/nrrheum.2013.122>.
- Ballane G, Cauley JA, Luckey MM, EL-Hajj Fuleihan G. Worldwide prevalence and incidence of osteoporotic vertebral fractures. Osteoporos Int 2017; 28(5): 1531–1542. Available on DOI: <http://doi: 10.1007/s00198–017–3909–3>.
- McCarthy J, Davis A. Diagnosis and management of vertebral compression fractures. Am Fam Physician 2016; 94(1): 44–50.
- Adler RA, EL-Hajj Fuleihan G, Bauer DC et al. Managing osteoporosis in patients on long-term bisphosphonate treatment: report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res 2016; 31(1): 16–35. Available on DOI: <http://doi: 10.1002/jbmr.2708>.
- Tella SH, Gallagher JC. Prevention and treatment of postmenopausal osteoporosis. J Steroid Biochem Mol Biol 2014; 142 : 155–170. Available on DOI: <http://doi: 10.1016/j.jsbmb.2013.09.008>.
- Oswald AJ, Berg J, Milne G, Ralston SH. Teriparatide treatment of severe osteoporosis reduces the risk of vertebral fractures compared with standard care in routine clinical practice. Calcif Tissue Int 2014; 94(2): 176–182. Available on DOI: <http://doi: 10.1007/s00223–013–9788–5>.
- Nardi A, Ventura L, Cozzi L et al. The bone anabolic therapy. Aging Clin Exp Res 2013; 25(Suppl 1): 121–124. Available on DOI: <http://doi: 10.1007/s40520–013–0133–7>.
- Graeff C, Chevalier Y, Charlebois M et al. Improvements in Vertebral Body Strength Under Teriparatide Treatment Assesses in Vivo by Finite Element Analysis: Results From the EUROFORS Study. J Bone Miner Res 2009; 24(10): 1672–1680. Available on DOI: <http://doi: 10.1359/jbmr.090416>.
- Ljunggren O, Barrett A, Stoykov I et al. Effective osteoporosis treatment with teriparatide is associated with enhanced quality of life in postmenopausal women with osteoporosis: the European Forsteo Observational Study. BMC Musculoskelet Disord 2013; 14 : 251 Available on DOI: <http://doi: 10.1186/1471–2474–14–251>.
- Neer RM, Arnaud CD, Zanchetta JR et al. Effect of Parathyroid Hormone (1–34) on Fractures and Bone Mineral Density in Postmenopausal Women with Osteoporosis. N Engl J Med 2001; 344(19): 1431–1441. Available on DOI: <http://doi: 10.1056/NEJM200105103441904>.
- Paschalis EP, Glass EV, Donley DW et al. Bone mineral and collagen quality in iliac crest biopsies of patients given teriparatide: new results from the Fracture Prevention Trial. J Clin Endocrinol Metab 2005; 90(8): 4644–4649. Available on DOI: <http://doi: 10.1210/jc.2004–2489>.
- Boonen S, Marin F, Obermayer-Pietsch B et al. Effects of Previous Antiresorptive Therapy on the Bone Mineral Density Response to Two Years of Teriparatide Treatment in Postmenopausal Women with Osteoporosis. J Clin Endocrinol Metab 2008; 93(3): 852–860. Available on DOI: <http://doi: 10.1210/jc.2007–0711>.
- Obermayer-Pietsch B., Marin F., V McCloskey E. et al. Effects of Two Years of Daily Teriparatide Treatment on BMD in Postmenopausal Women with Severe Osteoporosis With and Without Prior Antiresorptive Treatment. J Bone Miner Res 2008; 23(10): 1591–1600. Available on DOI: <http://doi.org/10.1359/jbmr.080506>.
- Ettinger B, San Martin J, Crans G et al. Differential effects of teriparatide on BMD after treatment with raloxifene or alendronate. J Bone Miner Res 2004; 19(5): 745–751. Available on DOI: <http://doi: 10.1359/JBMR.040117>.
- Genant HK, Wu CY, van Kuijk C et al. Vertebral fracture assessment using a semiquantitative technique. J Bone Miner Res. 1993; 8(9): 1137–1142. Available on DOI: <http://doi: 10.1002/jbmr.5650080915>.
Labels
Clinical biochemistry Paediatric gynaecology Paediatric radiology Paediatric rheumatology Endocrinology Gynaecology and obstetrics Internal medicine Orthopaedics General practitioner for adults Radiodiagnostics Rehabilitation Rheumatology Traumatology OsteologyArticle was published in
Clinical Osteology

2018 Issue 3
- Advances in the Treatment of Myasthenia Gravis on the Horizon
- Memantine in Dementia Therapy – Current Findings and Possible Future Applications
- Possibilities of Using Metamizole in the Treatment of Acute Primary Headaches
- Memantine Eases Daily Life for Patients and Caregivers
-
All articles in this issue
- Odešel pan prof. MUDr. Jaroslav Blahoš, DrSc. (30. 6. 1930 – 27. 11. 2018)
- Comparison of patient mortality after proximal femoral fracture in the periods 1995–2002 and 2003–2010
- Prevention and treatment of osteoporosis in postmenopausal women with estrogen receptor-positive breast cancer receiving aromatase inhibitors
- Vertebral compression fractures in pediatric patients with Crohn´s disease: case reports
- Examination methods in sarcopenia
- Teriparatide in the treatment of severe osteoporosis in elderly female patients: results of a multicenter prospective study
- Clinical Osteology
- Journal archive
- Current issue
- About the journal
Most read in this issue
- Teriparatide in the treatment of severe osteoporosis in elderly female patients: results of a multicenter prospective study
- Comparison of patient mortality after proximal femoral fracture in the periods 1995–2002 and 2003–2010
- Prevention and treatment of osteoporosis in postmenopausal women with estrogen receptor-positive breast cancer receiving aromatase inhibitors
- Vertebral compression fractures in pediatric patients with Crohn´s disease: case reports