Choroidal thickness in asymptomatic patients with carotid artery stenosis
Choroidální tloušťka u asymptomatických pacientů se stenózou karotidy
Cíl: Měřit choroidální tloušťku (ChT) metodou optické koherentní tomografie se zlepšeným hloubkovým zobrazováním (enhanced-depth imaging optic coherence tomography; EDI-OCT) u pacientů se stenózou a. carotis interna (ACI) a zkoumat vztah mezi ChT a stenózou ACI.
Materiál a metody: Do studie jsme zařadili 36 očí 25 asymptomatických pacientů s 50% nebo vyšší stenózou ACI a 36 očí 21 zdravých kontrol. ChT byla měřena metodou EDI-OCT z celkem 6 bodů u obou skupin. Výsledky byly statisticky porovnávány mezi skupinami.
Výsledky: Mezi pacienty s asymptomatickou stenózou ACI a zdravými jedinci bez stenózy nebyly signifikantní rozdíly v subfoveální ChT (p = 0,085), v 500 μm nasálně k fovee (p = 0,076), v 1 000 μm nasálně k fovee (p = 0,052), v 500 μm temporálně k fovee (p = 0,182), v 1 000 μm temporálně k fovee (p = 0,115), v 1 500 μm temporálně k fovee (p = 0,174). Navíc nebyl pozorován signifikantní rozdíl v hodnotách ChT naměřených z 6 bodů mezi stenotickou stranou a nestenotickou stranou u 14 pacientů s jednostrannou stenózou ACI (p > 0,05 pro všechny body).
Závěr: Choroidální tloušťka se nemusí měnit u asympromatické stenózy ACI v porovnání se zdravými jedinci bez stenózy. Jsou však zapotřebí další studie pro potvrzení našich výsledků.
Klíčová slova:
vnitřní karotida – cévnatka – optická koherentní tomografie – stenóza – tloušťka
Authors:
Ç. Öktem 1; E. Ö. Öktem 2; A. Kurt 3; R. Kilic 3; B. E. Sahin 4; A. Yetis 4; Y. Dadali 5
Authors‘ workplace:
Department of Ophthalmology, Alaaddin Keykubat University Alanya Education and Research Hospital, Antalya, Turkey
1; Department of Neurology, Alaaddin Keykubat University Alanya Education and Research Hospital, Antalya, Turkey
2; Department of Ophthalmology, Ahi Evran University Education and Research Hospital, Kirsehir, Turkey
3; Department of Neurology, Ahi Evran University Education and Research Hospital, Kirsehir, Turkey
4; Department of Radiology, Ahi Evran University Education and Research Hospital, Kirsehir, Turkey
5
Published in:
Cesk Slov Neurol N 2020; 83(1): 73-78
Category:
Original Paper
doi:
https://doi.org/10.14735/amcsnn202073
Overview
Aim: To measure the choroidal thickness (CT) with enhanced-depth imaging optic coherence tomography (EDI-OCT) in patients with internal carotid artery (ICA) stenosis and to investigate the relationship between the CT and ICA stenosis.
Material and methods: We included 36 eyes of 25 asymptomatic patients with 50% or higher ICA stenosis and 36 eyes of 21 healthy controls in the study. The CT was measured with EDI-OCT from a total of 6 points in both groups. The results were compared statistically between the groups.
Results: There were no significant differences between patients with asymptomatic ICA stenosis and non-stenotic healthy individuals at subfoveal CT (P = 0.085), at 500 μm nasal to the fovea (P = 0.076), at 1,000 μm nasal to the fovea (P = 0.052), at 500 μm temporal to the fovea (P = 0.182), at 1,000 μm temporal to the fovea (P = 0.115) and at 1,500 μm temporal to the fovea (P = 0.174). Additionally, no significant difference was observed in CT values measured from 6 points between the stenotic side and the non-stenotic side in 14 patients with unilateral ICA stenosis (P > 0.05 for all points).
Conclusion: The CT may not alter in asymptomatic ICA stenosis compared with healthy non-stenotic individuals. However, more studies are needed to corroborate our findings.
Keywords:
internal carotid artery – Stenosis – choroid – optic coherence tomography – thickness
Introduction
Carotid artery disease (CAD) is a major cause of morbidity and mortality that usually develops due to atherosclerosis. It is characterized by internal carotid artery (ICA) stenosis or occlusion that can lead to cerebral or retinal ischaemia. CAD-related eye findings include amaurosis fugax, ischaemic optic neuropathy, ocular ischaemic syndrome (OIS), retinal embolism, retinal and iris neovascularization, venous stasis retinopathy and fundus cholesterol (Hollenhorst) plaques [1 – 3]. These ocular findings are thought to occur due to thromboembolic or haemodynamic mechanisms. The ICA becomes progressively narrower due to thromboembolic mechanism in atherosclerosis. The arterial thrombi on the atheromatous plaque break off and cause occlusion and ischaemia in the distal vessels. On the other hand, inadequate perfusion due to chronic ICA stenosis or occlusion may lead to retinal ischaemia due to the haemodynamic mechanism [1,2,4].
Orbital vascularization is mainly from the ophthalmic artery (OA), which is the first major branch of the ICA. The OA is the origin of the central retinal artery, posterior ciliary artery (PCA), lacrimal artery and muscular artery branches within the orbit. These branches make anastomoses, especially with the branches of the maxillary artery and other external system arteries. A small part of the orbital blood flow is provided by the orbital branch of the middle meningeal artery and the infraorbital artery originating from the external carotid artery [5,6].
The choroid, located between the retina and the sclera, has one of the highest blood flow rates in the human body, receiving 70% of all the blood flow of the eye [7,8]. This vascular structure consists mainly of the posterior ciliary branches of the OA and the plexus of these branches [7]. Short PCAs supply the posterior choroid and peripapillary region while the anterior parts of the choroid are supplied by the long PCAs and anterior ciliary arteries, which are the terminal branches of the muscular artery that also derives from the OA [9]. Therefore, the choroid receives approximately 85% of the OA blood [5,8]. According to these anatomical vascular relations, ICA stenosis may affect the choroidal perfusion due to hypoperfusion in branches of the OA, which originates from the ICA.
Until recently, the information available on choroidal thickness (CT) was based on histopathological studies conducted on cadavers. However, now it is possible to obtain in vivo sections of the choroid using the enhanced depth imaging optic coherence tomography (EDI-OCT), which was developed recently [10 – 12]. The aim of our study was to evaluate the CT in patients with asymptomatic ICA stenosis and to investigate the relationship between the degree of stenosis and the CT.
We hypothesized that developing ischaemia due to ICA stenosis might affect the perfusion of the choroid and alter the CT even in the asymptomatic stage of stenosis.
Material and Methods
In total of 36 eyes of 25 patients with ICA stenosis and 36 eyes of 21 healthy individuals with a similar mean age were included in the study.
Subjects who referred to Kirsehir Ahi Evran University Research and Training Hospital Ophthalmology Department with no previous history of stroke or transient ischaemic attack (TIA) underwent carotid duplex US. A detailed ophthalmological examination including visual acuity, intraocular pressure (IOP) measurement, anterior segment and fundus examination was performed in all subjects.
Inclusion Criteria
Subjects with a corrected distance visual acuity of 20/ 25 or above, – 1.5 to +1.5 dioptries of spherical refractive error and with an IOP of 21 mm Hg or less were included in the study. The study group consisted of asymptomatic patients with out history of stroke or TIA who had > 50% ICA stenosis at carotid US imaging. Age-matched subjects without history of stroke or TIA and with normal carotid Doppler imaging and ophthalmological examination were included in the control group.
Exclusion Criteria
The presence of refractive error > ±1.5 dio-ptres, choroidal neovascularization or any other macular/ retinal diseases that might affect the vision, intraocular inflammation and/ or infection, or a history of any type of intraocular surgery, trauma, serious eye disease (corneal disease, glaucoma, serious cataract), TIA or stroke and any systemic disease that might affect the eye (such as diabetes mellitus, arterial hypertension, vasculitis), smoking and alcohol use, coffee addiction or the use of vasoactive drugs were excluded
Internal Carotid Artery Imaging
Colour Doppler sonographic scanning was performed by an Aplio 500 apparatus and a 4 – 11-MHz linear array transducer (Toshiba, Tokyo, Japan). Plaque images were documented in the B-mode, colour mode and colour mode with a pulsed wave spectrum, reporting peak systolic velocity and end-diastolic velocity and plaque areas on longitudinal and transverse scans at the point of maximum stenosis for offline analysis and quantification (Fig. 1).
Obr. 1. Zobrazování vnitřní karotidy. UZ zobrazení stupnice šedi těžké (80%) stenózy
vnitřní karotidy (a, b). Barevné dopplerovské UZ zobrazení těžké (80%) stenózy vnitřní
karotidy (c, d).

Optic Coherence Tomography Protocol and Choroidal Thickness Measurement
The EDI-OCT method has been described previously [13] and integrated as software into spectral domain OCT (SD-OCT) devices. We used a Heidelberg SD-OCT (Heidelberg Engineering, Heidelberg, Germany) and software version 6.3.3.0. The instrument contained an 870-nm wavelength superluminescent diode. It could acquire 40,000 A-scans per second at an axial resolution of 7 μm and a transverse resolution of 14 μm. We obtained two high-quality horizontal single line scans through the fovea within a 1 × 30 degree foveal area and averaged 100 scans for each section. The automatic real-time averaging mode was used to maximize the signal-to-noise ratio and to ensure high-quality images. The CT was accepted as the distance between the outer reflective retinal pigment epithelium layer and the inner choroid-sclera border and measured manually using the caliper tool. Measurements were made horizontally across the fovea at 500 μm intervals. The measurements were performed from a total of 6 points: subfoveal (SF), 500 μ (N1) and 1,000 µ (N2) nasal to the fovea, and 500 μ (T1), 1,000 μ (T2) and 1,500 μ (T3) temporal to the fovea (Fig. 2).
Obr. 2. Měření choroidální tloušťky v 6 bodech s intervaly 500 μ na řezech z optické koherentní tomografie.

Choroidal thickness measurements were performed at the same time (10 : 00 – 12 : 00) every day by two ophthalmologists (CO, AK). The mean value of the measurements was calculated and recorded. In the event of a discrepancy, another measurement was performed by both ophthalmologists.
Statistical Analysis
The SPSS 20.0.0 (IBM, Armonk, NY, USA) software program was used in the analysis of the data. The measured data were described as the arithmetic mean ± standard deviation whereas the categorical data were described as percentages (%). Normal distribution of the measured data was evaluated with the Kolmogorov-Smirnov test. Student’s t-test was used to compare the groups if the data were normally distributed. The Mann-Whitney U test was used if the data were not normally distributed. The relationship between the OCT values and the severity of the carotid stenosis was evaluated using the Spearman correlation analysis.
A post-hoc power analysis was performed using G Power 3.1.9.2 software for Windows (Heinrich-Heine-Universität Düsseldorf, Düsseldorf, Germany). According to this test, our study had 99.9% power within 0.05 alpha [13].
Sample Size Calculation
Since similar studies were published, to be able to determine the adequate sample size, according to the groups in the previous study by Wang et al [13] we tried to predict the effect size using the descriptive statistics in the “central choroidal thickness” variable, which was the same term with subfoveal thickness. We calculated a total of 54 subjects, 27 subjects in each group, to be able to statistically detect 17.70 units of difference between the groups in terms of SF CT under the conditions of 80% power and 5% type I error.
Results
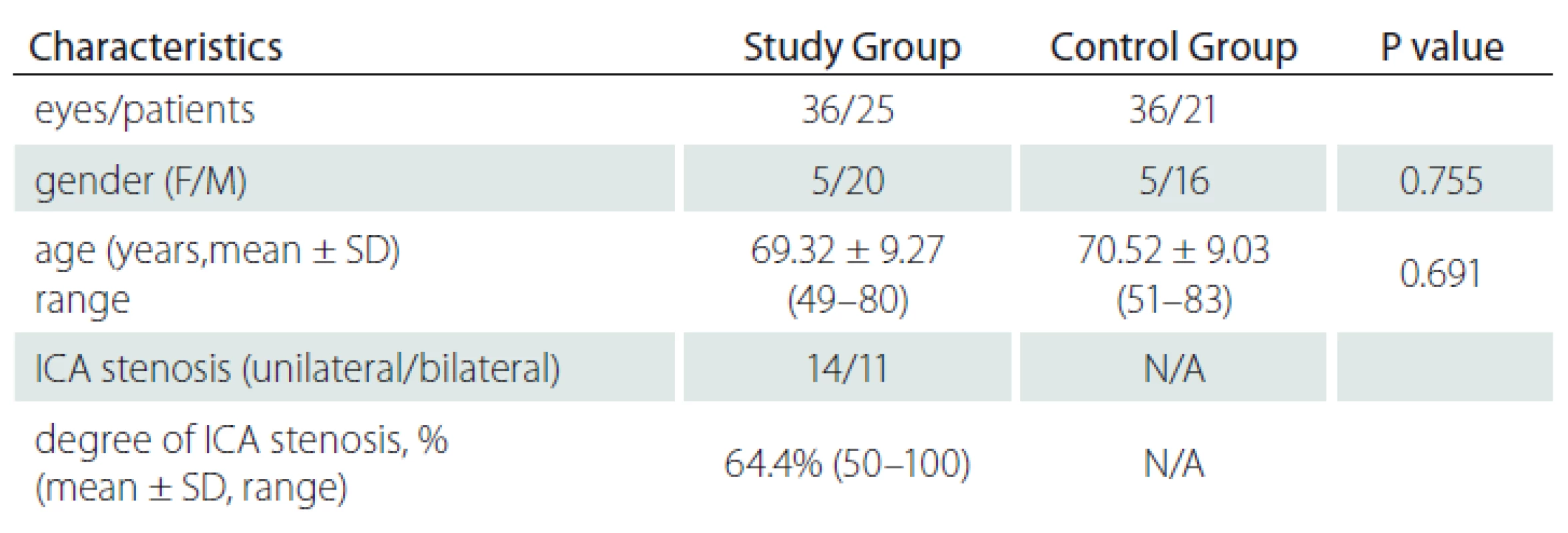
The study group (group 1) included 36 eyes (16 right, 20 left) of 25 patients and the control group (group 2), 36 eyes (19 right, 17 left) of 21 subjects. The mean age was 69.32 ± 9.27 (49 – 80) years in group 1 and 70.52 ± 9.03 (51 – 83) years in group 2; there was no significant difference between the mean ages of both groups (P = 0.691). The study group included 5 women and 20 men and the control group 5 women and 16 men (P = 0.755). Eleven patients had bilateral and 14 patients had unilateral ICA stenosis. The degree of ICA stenosis was 50 – 70% in 23 patients and ≥ 70% in 13 patients. Two patients had total occlusion. The total mean degree of ICA stenosis was 64.4% (50 – 100) (Tab. 1).
The SF CT was 219 μm in group 1 and 242.9 µm in group 2 (P = 0.085). Also, measurements of the CT from extrafoveal points were not significantly different between both groups (Tab. 2).

The relationship between the degree of stenosis and the CT was also investigated and no statistically significant correlation was found (SF [P = 0.589], N1 [P = 0.424], N2 [P = 0.288], T1 [P = 0.345], T2 [P = 0.611], T3 [P = 0.916]).
In 14 patients with unilateral ICA stenosis, no significant difference was observed in the CT values measured from 6 points between the stenotic side and the non-stenotic side (P > 0.05, for all points) (Tab. 3).

Discussion
In the present study, we evaluated the CT in asymptomatic ICA stenosis using EDI-OCT. This study showed that there was no significant difference in CT between patients with asymptomatic ICA stenosis and healthy individuals without ICA stenosis who were age - and gender-matched. Additionally, in patients with unilateral stenosis, no significant difference was observed in the CT at the side with ICA stenosis compared to the non-stenotic side.
The choroid is one of the most vascularized tissues in the body and receives the highest blood volume among the ocular tissues [8]. With the advances in OCT technology and updated software in recent years, evaluation of the choroid has become possible [10 – 14]. The relationship between the CT and gender, age and circadian rhythm has been investigated in certain studies on factors affecting CT and haemodynamics. CT has been reported to be higher in males and in young people. It has also been suggested to be higher at night and to be related to systolic blood pressure changes [15 – 17].
In our study, we hypothesized that ICA stenosis might affect the choroidal perfusion and CT due to vascular mechanism. Several studies showed that the choroid was sensitive to blood pressure changes and was affected by blood flow and perfusion pressure [6]. Congestion-related SF CT increase has been reported in a case with carotid cavernous fistula (CCF) with the value decreasing after treatment with embolization [18]. The SF CT of a 47-year-old female diagnosed with CCF was found to be significantly higher on the fistula side in a similar case report. Once the fistula was embolized through the OA, the asymmetry disappeared and the subfoveal CT became equal on both sides. The authors reported that OCT could be used as an auxiliary test in the diagnosis of CCF [19]. Lareyre et al retrospectively evaluated SF CT changes following carotid endarterectomy (CEA) in patients with severe chronic carotid stenosis. They found that SF CT increased bilaterally and more prominently on the ipsilateral side following CEA [20]. The correlation between ocular pulse amplitude (OPA), subfoveal CT and ICA Doppler US findings was investigated in another study. OPA was found to provide useful information on intraocular blood flow and to be an indirect indication of choroidal perfusion. The results were reported to indicate a moderately positive correlation between OPA and SF CT [21]. Despite these reports [17 – 21] that have investigated changes in ocular blood flow and perfusion, there is still limited information in the literature regarding the CT of patients with ICA stenosis. Kang et al reported that the CT as measured with OCT was lower in the eye on the stenotic side than on the healthy side in 3 cases with severe ICA stenosis [22]. Unlike this previous report, in our study, no statistically significant difference was observed between the CT values on the stenotic and the non-stenotic side in unilateral ICA stenosis. In a cross-sectional study performed by Sayin et al, a decrease in the CT central and paracentral to the fovea was shown in patients with ICA stenosis, but no significant correlation could be shown between the degree of stenosis and the CT value [23]. Similarly to the literature, in our study there was no statistically significant correlation between the degree of ICA stenosis and the CT.
Recent studies have investigated the relationship between ICA stenosis and OIS. The CT and choroidal volume values were compared with the affected and healthy eyes of 19 OIS patients in one study and the SF CT and choroidal volume were shown to have decreased in these eyes. The interpretation was that ICA stenosis decreases choroidal circulation [24].
In a retrospectively designed study performed by Wang et al, the CT values were lower in patients with severe ICA stenosis and they suggested that choroidal thinning might occur before retinal changes in OIS patients and evaluation of the CT may therefore be useful in choosing the optimal therapeutic timing for patients with ICA stenosis [13]. About 30% of patients with symptomatic ICA occlusion were reported to have asymptomatic retinal vascular changes and 1.5% of these were to become symptomatic within 1 year in another study [25]. The degree of stenosis, presence of collateral vessels, duration of CAD, presence of bilateral or unilateral stenosis and, presence of systemic vascular disease determines the severity of OIS [26]. A comparison of patients with a mean stenosis of 74 and 47.5% showed SF CT values of 231.9 μm and 216.2 μm, resp., in a study evaluating the relationship between ICA stenosis and SF CT in the elderly population. The authors stated that a compensatory increase in SF CT could be seen with an ICA stenosis of 70% and higher [27].
Our study has several limitations. First, the degree of ICA stenosis was less than 70% (mean 64.4%) in the majority of our patients. Second, the sample size of the study was relatively small. The lack of a statistical significance could be explained by the low degree of ICA stenosis and a small sample size. Third, the axial length was not measured in our patients. However, we excluded patients with myopia and hypermetropia with a spherical equivalent of ±1.5 dioptries or higher to minimize the effect of the axial length. Finally, despite the advances in OCT technology, the choroidal borders are still defined by manual measurement, which is also encountered as a limitation in all studies in this field.
Conclusion
The present study showed that there was no difference in the CT of asymptomatic ICA stenosis patients compared with non-stenotic individuals. However, more studies with a larger sample size are needed to evaluate the effects of ICA stenosis and corroborate these findings.
Ethical Principles
The study was approved by the Local Ethics Committee and followed the principles of Helsinki Declaration of 1975 (as revised in 2004 and 2008). The participants were informed about the study and written consent was obtained from all subjects.
Disclosures
The authors declare they have no potential conflicts of interest concerning drugs, products, or services used in the study.
The Editorial Board declares that the manuscript met the ICMJE “uniform requirements” for biomedical papers.
Accepted for review: 22. 5. 2019
Accepted for print: 18. 11. 2019
Çağlar Öktem, MD
Department of Ophthalmology
Alaaddin Keykubat University
Alanya Education and Research Hospital
074 00 Antalya
Turkey
e-mail: cglroktm@gmail.com
Sources
1. Fisher CM. Transient monocular blindness associated with hemiplegia. AMA Arch Ophthalmol 1952; 47(2): 167 – 203. doi: 10.1001/ archopht.1952.01700030174005.
2. Hollenhorst RW. Vascular status of patients who have cholesterol emboli in the retina. Am J Ophthalmol 1966; 61 (5 Pt 2): 1159 – 1165. doi: 10.1016/ 0002-9394(66)90238-8.
3. Carter JE. Chronic ocular ischemia and carotid vascular disease. Stroke 1985; 16(4): 721 – 728. doi: 10.1161/ 01.str.16.4.721.
4. Kerty E, Eide N, Horven I. Ocular hemodynamic changes in patients with high-grade carotid occlusive disease and development of chronic ocular ischaemia. II. Clinical findings. Acta Ophthalmol Scand 1995; 73(1): 72 – 76. doi: 10.1111/ j.1600-0420.1995.tb00017.x.
5. Hayreh SS. Orbital vascular anatomy. Eye (Lond) 2006; 20(10): 1130 – 1144. doi: 10.1038/ sj.eye.6702377.
6. Cioffi GA, Granstam E, Alm A. Ocular circulation. In: Kaufman PL, Alm A (eds). Adler‘s physiology of the eye: clinical application. 10th ed. St Louis, USA: Mosby 2003 : 747 – 784.
7. Roh S, Weiter JJ. Retinal and choroidal circulation. In: Bavbek T (ed). Yanoff and Duker ophthalmology. 2nd ed. Istanbul, Turkey: Hayat Tıp 2007 : 779 – 782.
8. Nickla DL, Wallman J. The multifunctional choroid. Prog Retin Eye Res 2010; 29(2): 144 – 168. doi: 10.1016/ j.preteyeres.2009.12.002.
9. Ehrlich R, Harris A, Wentz SM et al. Anatomy and regulation of the optic nerve blood flow. In: Stein JP (ed). Reference module in neuroscience and biobehavioral psychology. Amsterdam: Elsevier 2016.
10. Spaide RF, Koizumi H, Pozzoni MC. Enhanced depth imaging spectral-domain optical coherence tomography. Am J Ophthalmol 2008; 146(4): 496 – 500. doi: 10.1016/ j.ajo.2008.05.032.
11. Margolis R, Spaide RF. A pilot study of enhanced depth imaging optical coherence tomography of the choroid in normal eyes. Am J Ophthalmol 2009; 147(5): 811 – 815. doi: 10.1016/ j.ajo.2008.12.008.
12. Manjunath V, Taha M, Fujimoto JG et al. Choroidal thickness in normal eyes measured using Cirrus-HD optical coherence tomography. Am J Ophthalmol 2010; 150(3): 325 – 329. doi: 10.1016/ j.ajo.2010.04.018.
13. Wang H, Wang YL, Li HY. Subfoveal choroidal thickness and volume in severe internal carotid artery stenosis patients. Int J Ophthalmol 2017; 10(12): 1870 – 1876. doi: 10.18240/ ijo.2017.12.13.
14. Laviers H, Zambarakji H. Enhanced depth imaging-OCT of the choroid: a review of the current literature. Graefes Arch Clin Exp Ophtalmol 2014; 252(12): 1871 – 1883. doi: 10.1007/ s00417-014-2840-y.
15. Li XQ, Larsen M, Munch IC. Subfoveal choroidal thickness in relation to sex and axial length in 93 Danish university students. Invest Ophtalmol Vis Sci 2011; 52(11): 8438 – 8441. doi: 10.1167/ iovs.11-8108.
16. Usui S, Ikuno Y, Akiba M et al. Circadian changes in subfoveal choroidal thickness and the relationship with circulatory factors in healthy subjects. Invest Ophtalmol Vis Sci 2012; 53(4): 2300 – 2307. doi: 10.1167/ iovs.11-8383.
17. Chakraborty R, Read SA, Read SA. Diurnal variations in axial length, choroidal thickness, intraocular pressure, and ocular biometrics. Invest Ophthalmol Vis Sci 2011; 52(8): 5121 – 5129. doi: 10.1167/ iovs.11-7364.
18. Shinohara Y, Kashima T, Akiyama H et al. Alteration of choroidal thickness in a case of carotid cavernous fistula: a case report and a review of the literature. BMC Ophthalmol 2013; 13 : 75. doi: 10.1186/ 1471-2415-13-75.
19. González Martín-Moro J, Sales-Sanz M, Oblanca-Llamazares N et al. Choroidal thickening in a case of carotid cavernous fistula. Orbit 2018; 37(4): 306 – 308.
20. Lareyre F, Nguyen E, Raffort J et al. Changes in ocular subfoveal choroidal thickness after carotid endarterectomy using enhanced depth imaging optical coherence tomography: a pilot study. Angiology 2018; 69(7): 574 – 581. doi: 10.1177/ 0003319717737223.
21. Demirok G, Topalak Y, Başaran MM et al. Correlation of ocular pulse amplitude, choroidal thickness, and internal carotid artery doppler ultrasound findings in normal eyes. Semin Ophthalmol 2017; 32(5): 620 – 624. doi: 10.3109/ 08820538.2016.1141223.
22. Kang HM, Lee CS, Lee SC. Thinner subfoveal choroidal thickness in eyes with ocular ischemic syndrome than in unaffected contralateral eyes. Graefes Arch Clin Exp Ophthalmol 2014; 252(5): 851 – 852. doi: 10.1007/ s00417-014-2609-3.
23. Sayin N, Kara N, Uzun F et al. A quantitative evaluation of the posterior segment of the eye using spectral domain OCT in carotid artery disease: a pilot study. Ophtalmic Surg Lasers Imaging Retina 2015; 46(2): 180 – 185. doi: 10.3928/ 23258160-20150213-20.
24. Kim DY, Joe SG, Lee JY et al. Choroidal thickness in eyes with unilateral ocular ischemic syndrome. J Opthalmol 2015; 2015 : 620372. doi: 10.1155/ 2015/ 620372.
25. Mizener JB, Podhajsky P, Hayreh SS. Ocular ischemic syndrome. Ophthalmology 1997; 104(5): 859 – 864. doi: 10.1016/ s0161-6420(97)30221-8.
26. Klijn CJ, Kappelle LJ, van Schooneveld MJ et al. Venous stasis retinopathy in symptomatic carotid artery occlusion: prevalence, cause, and outcome. Stroke 2002; 33(3): 695 – 701. doi: 10.1161/ hs0302.104619.
27. Akçay Bİ, Kardeş E, Maçin S et al. Evaluation of subfoveal choroidal thickness in internal carotid artery stenosis. J Ophthalmol 2016; 2016 : 5296048. doi: 10.1155/ 2016/ 5296048.
Labels
Paediatric neurology Neurosurgery NeurologyArticle was published in
Czech and Slovak Neurology and Neurosurgery

2020 Issue 1
- Advances in the Treatment of Myasthenia Gravis on the Horizon
- Memantine in Dementia Therapy – Current Findings and Possible Future Applications
- Memantine Eases Daily Life for Patients and Caregivers
-
All articles in this issue
- Poděkování recenzentům
- The role of inflammation in etiopathogenesis of pharmacoresistant epilepsy and refractory status epilepticus
- Cognitive rehabilitation in patients with Parkinson’s disease
- Copper homeostasis as a therapeutic goal in amyotrophic lateral sclerosis with a mutation in superoxide dismutase 1 and CuATSM molecule
- Possible prevention of Alzheimer’s disease
- Dominant (Kjer’s) optic atrophy associated with mutations in OPA1 gene
- Methodology of cognitive impairment assessment in depressive disorder
- Neonatal seizures – current view of the issue
- Polysomnographic findings in individuals over 50 years of age lacking subjective signs of sleep disturbanc
- Neuropsychiatric symptoms as early manifestation of Alzheimer’s disease
- Effectiveness of natalizumab extended interval dosing in multiple sclerosis patients
- Atherosclerotic plaque characteristics and the risk of brain ischemia during internal carotid artery stenting
- A randomized controlled study of the effect of balance disorder therapy using audiovisual feedback on senior citizens
- The primary Non-Hodgkin’s central nervous system lymphoma
- Analýza dat v neurologii
- Vzpomínka na prof. Pavla Petrovického
- Prof. MUDr. Rudolf Malec, CSc. (1924–2019)
- Recenze monografie
- Comorbidities of Alzheimer‘s disease – results of a multicentric observational COSMOS study in the Slovak Republic
- Choroidal thickness in asymptomatic patients with carotid artery stenosis
- Czech and Slovak Neurology and Neurosurgery
- Journal archive
- Current issue
- About the journal
Most read in this issue
- Neonatal seizures – current view of the issue
- Possible prevention of Alzheimer’s disease
- The primary Non-Hodgkin’s central nervous system lymphoma
- Neuropsychiatric symptoms as early manifestation of Alzheimer’s disease
