Selective expanded polytetrafluorethylene patch angioplasty vs. primary closure after carotid endarterectomy – a 13-year experience
Selektivní plastika pomocí záplaty z expandovaného polytetrafluorethylenu vs. primární sutura při karotické endarterektomii – 13letá zkušenost
Cíl: Dle současné evidence rutinní uzávěr arteriotomie pomocí záplaty („patch“ angioplastika; PA) může snižovat výskyt komplikací karotické endarterektomie (carotid endarterectomy; CEA) ve srovnání s primární suturou (primary closure; PC). Cílem práce bylo srovnání výsledků PA a PC indikovaných na základě intraoperačního zhodnocení průměru a. carotis interna (ACI) a kvality okrajů arteriotomie. Soubor a metodika: Do retrospektivní studie byli zařazeni všichni konsekutivní pacienti, kteří podstoupili na našem pracovišti CEA v letech 2006 až 2018. Všichni pacienti byli po CEA pravidelně sledováni pomocí UZ i klinicky. Definované cíle byly následně srovnány mezi skupinami pacientů s PA a PC. Výsledky: Do studie bylo zařazeno celkem 607 nemocných (451 mužů, průměrný věk 64,4 ± 8,5 let), kteří podstoupili 654 CEA (587 PC a 67 PA). Třistadvacetpět (49,7 %) CEA bylo provedeno pro symptomatickou stenózu. Nebyl zjištěn žádný rozdíl ve výskytu ipsilateralního iktu (1,5 vs. 2,2 %, p = 1,000; 1,5 vs. 1,4 %, p = 1,000), ipsilateralního ischemického iktu (1,5 vs. 1,9 %, p = 1,000; 1,5 vs. 1,4 %, p = 1,000), jakéhokoliv iktu (1,5 vs. 2,7 %, p = 1,000; 4,5 vs. 4,3 %, p = 1,000), perioperační trombózy ACI (3,0 vs. 2,6 %; p = 0,690) a restenózy ACI ≥ 70 % (3,0 vs. 4,1 %; p = 1,000) mezi skupiami PA a PC skupinou během 30denního perioperačního období, respektive během dlouhodobého sledování (medián 46 měsíců). U symptomatických stenóz byla během dlouhodobého sledování zjištěna nižší mortalita ve skupině s PC (9,7 vs. 25,9 %; p = 0.022); nicméně většina (95,1 %) úmrtí měla jinou příčinu než iktus. Závěr: U PA indikované na základě intraoperačních nálezů nebyl prokázán významný rozdíl ve výskytu komplikací a hemodynamicky významné restenózy nebo perioperační trombózy ACI po CEA ve srovnání s PC.
Klíčová slova:
vnitřní krkavice – karotická endarterektomie – primární sutura – ePTFE patch angioplastika – CMP
Authors:
P. Dráč 1; D. Šaňák 2; P. Utíkal 1; M. Köcher 3; M. Král 2; M. Černá 3; J. Zapletalová 4; R. P. Thomas 5
Authors‘ workplace:
Department of Surgery II., Vascular and, Transplantation Surgery, Palacký, University Medical School and Univesity, Hospital Olomouc, Czech Republic
1; Comprehensive Stroke Center, Department of Neurology, Palacký University, Medical School and University, Hospital Olomouc, Czech Republic
2; Department of Radiology, Palacký University, Medical School and University, Hospital Olomouc, Czech Republic
3; Department of Medical Biophysics and, Statistics, Palacký University Medical, School, Olomouc, Czech Republic
4; Department of Diagnostic and Interventional, Radiology, UKGM University, Hospital Marburg, Philipps University, Marburg, Germany
5
Published in:
Cesk Slov Neurol N 2021; 84/117(3): 257-262
Category:
Original Paper
doi:
https://doi.org/10.48095/cccsnn2021257
Overview
Aim: Current evidence suggests that routine carotid patch angioplasty (PA) may reduce the complications of carotid endarterectomy (CEA) when compared to primary closure (PC). We aimed to compare the results of PA and PC choosen according to the intraoperative internal carotid artery (ICA) diameter and the quality of arteriotomy margins. Patients and methods: All consecutive patients who underwent elective CEA between years 2006 and 2018 in our center were enrolled in the retrospective study. All patients underwent regular ultrasound and clinical controls after CEA during the follow-up and defined endpoints were compared between PA and PC patients. Results: In total, 607 patients (451 males, mean age 64.4 ± 8.5 years) who underwent 654 CEAs (587 PC and 67 PA) were enrolled. Three hundred twenty-five (49.7%) CEAs were done for symptomatic stenosis. No difference was found in the rates of ipsilateral stroke (1.5 vs. 2.2%, P = 1.000; 1.5 vs. 1.4%, P = 1.000), ipsilateral ischemic stroke (1.5 vs. 1.9%, P = 1.000; 1.5 vs. 1.4%, P = 1.000), any stroke (1.5 vs 2.7%, P = 1.000; 4.5 vs. 4.3%, P = 1.000), perioperative ICA thrombosis (3.0 vs. 2.6%; P = 0.690) and ICA restenosis ≥ 70% (3.0 vs. 4.1%; P = 1.000) between PA and PC groups during a 30-day perioperative period and a long-term follow-up (median 46 months), respectively. In symptomatic stenosis, lower mortality was found in PC group during the follow-up (9.7 vs. 25.9%; P = 0.022); however, most (95.1%) deaths were not stroke-related. Conclusion: PA used according to the intraoperative findings showed no difference in the rates of complications and significant restenosis or perioperative ICA thrombosis after CEA when compared to PC.
Keywords:
internal carotid artery – carotid endarterectomy – primary closure – ePTFE patch angioplasty – stroke
Introduction
A type of arterial wall closure used after carotid endarterectomy (CEA) usually depends on the individual preference of a performing surgeon or on the tradition of an individual surgical department. Some surgeons tend to use a patch angioplasty (PA) routinely, others use it only selectively and some do not use a patch at all [1]. Primary suture closure (PC) remains to be favoured due to shorter carotid clamp time, which leads to shortening of the total operation time [2]. On the contrary, the use of patch could reduce the risk of artificial stenosis secondary to the primary suture itself [3]. Current European Society for Vascular Surgery (ESVS) guidelines report the benefit of routine PA after CEA when compared to routine primary suture [1]. This statement is supported by the results of the largest meta-analysis of randomized controlled trials (RCTs) [4].
At our department, we prefer the type of arteriotomy closure according to the intraoperative assessment of the internal carotid artery (ICA) diameter and quality of the arteriotomy margins after CEA. Patch closure is performed if ICA diameter is narrow or if arteriotomy margins after CEA are of poor quality and there is need to run the suture far from the arteriotomy edge. An expanded polytetrafluorethylene (ePTFE) material is used for a patch.
In the current ESVS guidelines [1], there is no recommendation for selective patching; thus, we aimed to assess whether selective PA based on the intraoperative diameter and quality of the arteriotomy margins after CEA may lead to complications and outcomes similar to those of PC.
Patients and methods
Patients
All consecutive patients who underwent an elective CEA of ICA for symptomatic or asymptomatic stenosis between 2006 and 2018 at our department were enrolled in this retrospective single center study. The indication for CEA included both symptomatic and asymptomatic stenosis of ICA according to the guidelines followed at the time. The degree of symptomatic stenosis indicated for CEA was 50–99%. During a study period of 13 years, the guidelines developed slight discrepancies in the indications of CEA for asymptomatic stenosis. In our study, the asymptomatic stenosis of a grade of 70–99% was indicated for CEA in patients with life expectancy of more than 5 years and with the presence of following risk factors: a combination of diabetes mellitus, hyperlipidemia and arterial hypertension, silent stroke in vascular teritory of stenotic ICA documented on brain imaging, rapid stenosis progression in time, as well as ultrasound signs of unstable carotid plaque. The North American Symptomatic Carotid Endarterectomy (NASCET) criterion was used for measurement of both symptomatic and asymptomatic stenoses [5].
In all patients, demographic and baseline clinical characteristics were recorded, including the presence of traditional vascular risk factors. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) cohort reporting guidelines were used for the preparation of the manuscript [6].
Surgery procedure
Carotid endarterectomy was performed under locoregional (LRA) or general anesthesia (GA). In patients operated under LRA, an intraluminal shunt was inserted selectively, if a neurological deficit appeared. In patients under GA, a shunt was used routinely.
Before the closure of the arterial wall after CEA, the ICA diameter and the quality of the arteriotomy margins were assessed intraoperatively by a certified vascular surgeon. In case of a narrow ICA diameter or poor quality of arteriotomy margins after CEA, PA was performed after subjective evaluation of the involved surgeon. No precise measurement of ICA diameter or quality of arteriotomy margins (thickness of the arterial wall) was performed. In all other cases, PC was performed. In all patients, preventive doses of broad-spectrum antibiotics and intravenous unfractionated heparin (5,000–7,500 IU) were administered during the CEA procedure and 4–5 mL of protamine was used at the end of the procedure.
Follow-up protocol
All patients received antiplatelet monotherapy with daily doses of 100 mg of acetylsalicylic acid or 75 mg of clopidogrel during a follow - up (FUP) period. All patients underwent a standardized FUP protocol, which included regular ultrasound and clinical controls at 1, 3, 6 and 12 months after CEA and, thereafter, annually. The neurologists performing FUP ultrasound and clinical controls were blinded to the type of the arterial closure used. For the study analyses, the FUP was ended in December 2019 or by the last medical record of the patients in our hospital medical database.
Outcomes and endpoints
The following outcome parameters and defined endpoints were analyzed and compared between PC and PA groups of patients in the perioperative (defined as the first 30 days after CEA) and long-term FUP: the incidence of ipsilateral stroke, ipsilateral ischemic stroke, any stroke, death, stroke or death, perioperative thrombosis of ICA and restenosis ≥ 70%. The endpoints of ipsilateral stroke included both haemorrhagic and ischemic strokes, including fatal as well as non-fatal ones. The endpoints of any stroke included fatal, non-fatal, ipsilateral, contralateral or brainstem infarct and hemorrhagic stroke. Separate sub-analyses were performed in patients with CEA for symptomatic and asymptomatic ICA stenosis.
Statistical analysis
Statistical software SPSS Version 22 (IBM, Armonk, NY, USA) was used for the data processing and statistical analyses. The comparison of age between the groups was evaluated using the Student t-test, the comparison of the defined endpoints and outcomes was performed using the Chi-square test and the Fisher exact test. The age in the groups was verified using the Shapiro-Wilk test of normality. All tests used an α-level of 0.05 for significance.
Results
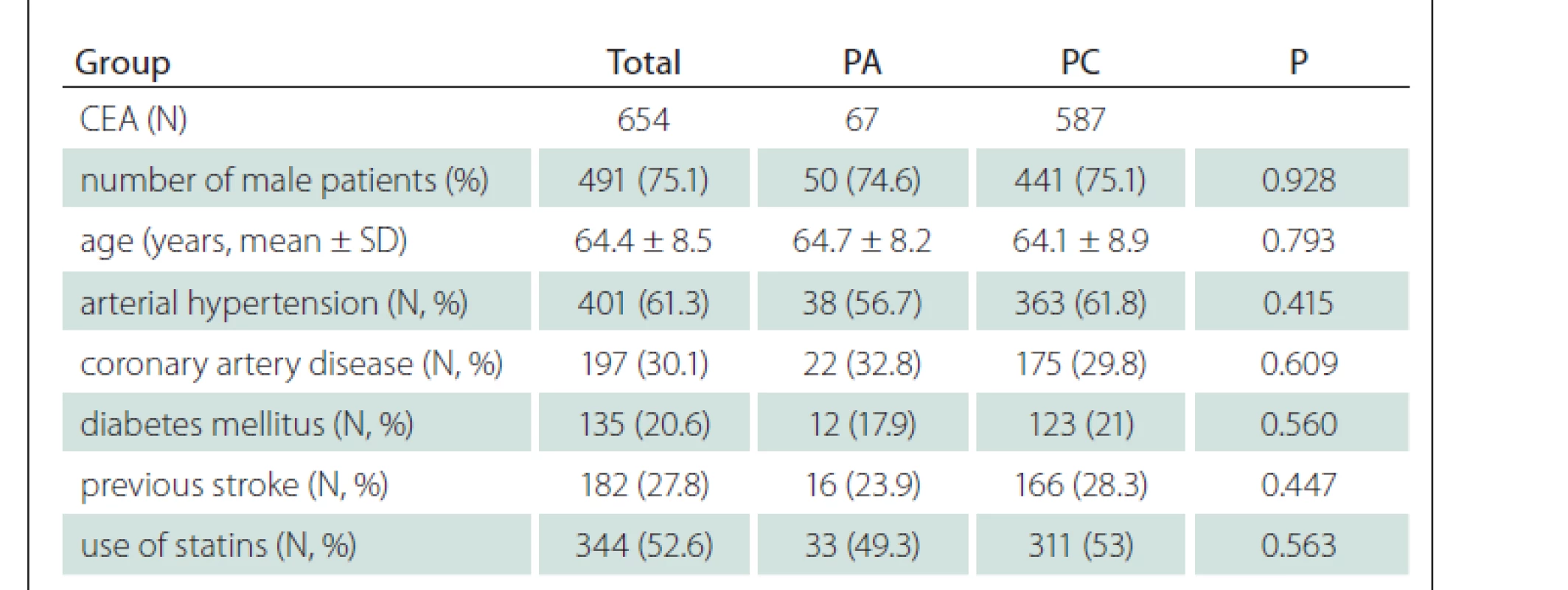
In total, 607 patients (451 males, mean age 64.4 ± 8.5 years) were enrolled and underwent 654 CEAs; 587 (89.8%) CEA were performed with PC and 67 (10.2%) with PA. Forty-seven (7.7%) patients underwent bilateral CEA and 325 (49.7%) CEAs were done for symptomatic stenosis. Baseline demographic and clinical parameters of the enrolled patients are shown in Tab. 1 and no difference was found between PC and PA groups of patients. The median time of FUP was 46 months (range: 1–163 months).
Six hundred twenty-one CEAs (558 in PC group and 63 in PA group) were performed under LRA. The remaining 33 CEAs (29 in PC group and 4 PA group) were performed under GA. In 28 cases (24 in PC group and 4 in PA group), GA was used primarily. In five CEAs in PC group, a conversion from LRA to GA was performed due to patient‘s intolerance of LRA. In total, a shunt was used in 85 CEAs; in 75 (12.8%) CEAs in PC group and in 10 (14.9%) CEAs in PA group (P = 0.620). In CEAs performed under LRA, a shunt was used in 46 (8.2%) cases in PC group and in 6 (9.5%) cases in PA group (P = 0.728). A shunt was used in all CEAs performed in GA (29 CEAs in PC group and 4 CEAs in PA group).
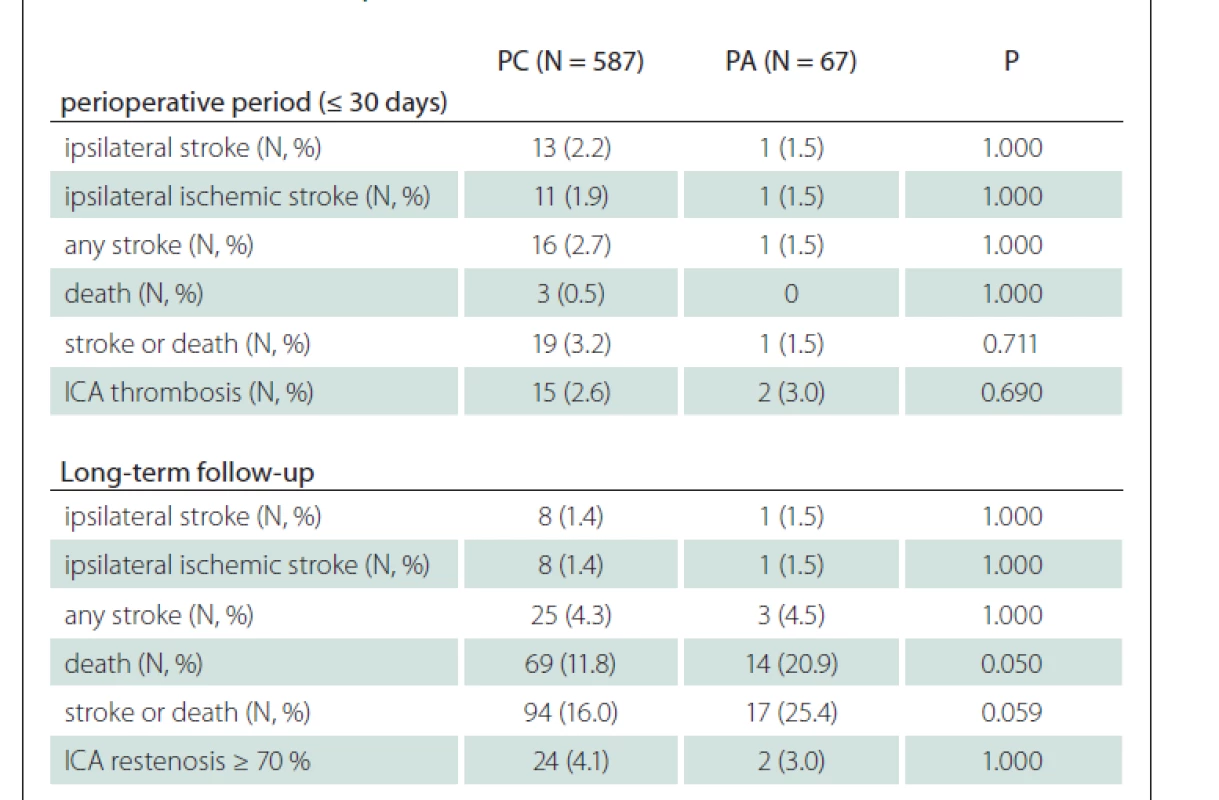
In the perioperative period, no difference was found in all investigated outcome parameters and defined endpoints between patients with PA and PC (when symptomatic and asymptomatic stenoses were evaluated together). Similarly, in the long-term FUP, no significant difference was found between PA and PC groups of patients (when symptomatic and asymptomatic stenoses were evaluated together), however, a trend of more frequent deaths was observed in patients with PA (Tab. 2).
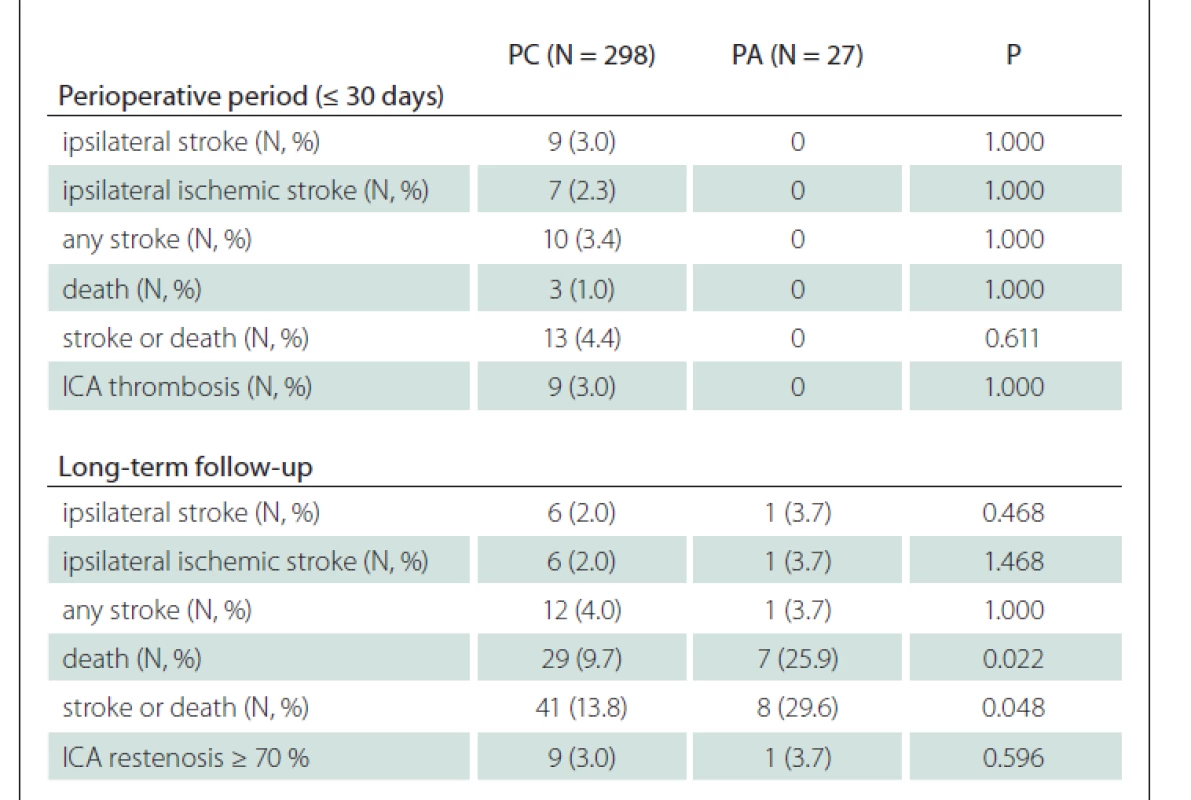
In the subgroup analysis of patients with CEA for symptomatic stenosis, a higher rate of deaths in PA group was observed (25.9 vs. 9.7%; P = 0.022) during the FUP, and 95.1% of deaths were of non-stroke origin (Tab. 3). Similarly, a higher rate of deaths in PA group with a comparable rate of any stroke in both groups (3.7 vs. 4.0%; P = 1.000) led to a higher rate of the composit endpoint of stroke or death in PA group (29.6 vs. 13.8%; P = 0.048) during the FUP in these patients.
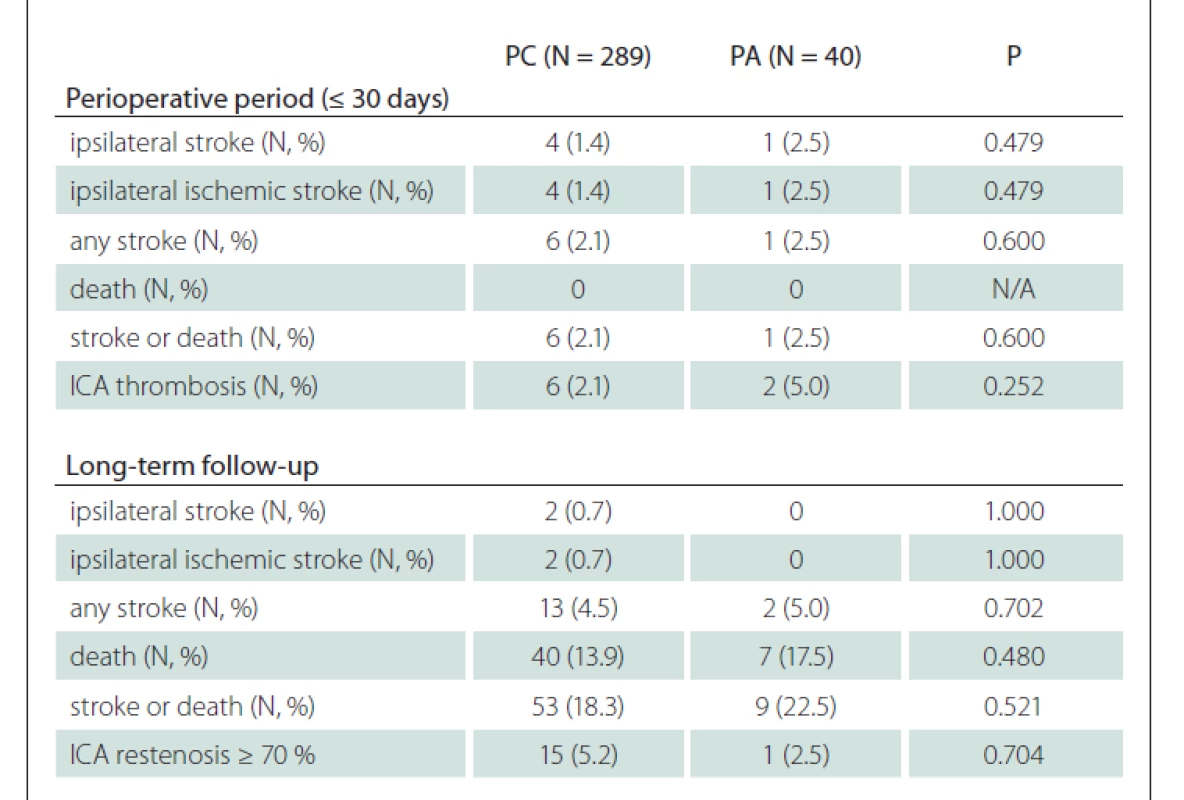
No difference was observed in the subgroup analysis of patients with CEA for asymptomatic stenosis (Tab. 4).
In total, 17 (2.6%) perioperative strokes (16 in PC group and 1 in PA group) were observed and 14 (2.1%) of them were ipsilateral (13 in PC group vs. 1 in PA group; P = 1.000) (Tab. 2). Eleven (84.6%) of 13 ipsilateral strokes in PC group were of ischemic origin and the only ipsilateral stroke in the PA group was also ischemic (Tab. 2).
Eight (1.3%) patients suffered from perioperative ipsilateral ischemic stroke due to ICA thrombosis. Seven (87.5%) of them were in PC group. Six (75%) patients underwent ICA thrombectomy for acute ICA occlusion. Three (0.5%) patients suffered from perioperative non-ipsilateral ischemic stroke, two of them were in the teritory of contralateral ICA and the remaing one in the brainstem.
All other recorded complications related to performed CEA are shown in Tab. 5. The most frequent complication was cranial nerve injury (3.1%) followed by major bleeding requiring surgery (2.4%), acute myocardial infarction (0.8%), hemorrhagic stroke (0.5%) and pneumonia (0.3%).
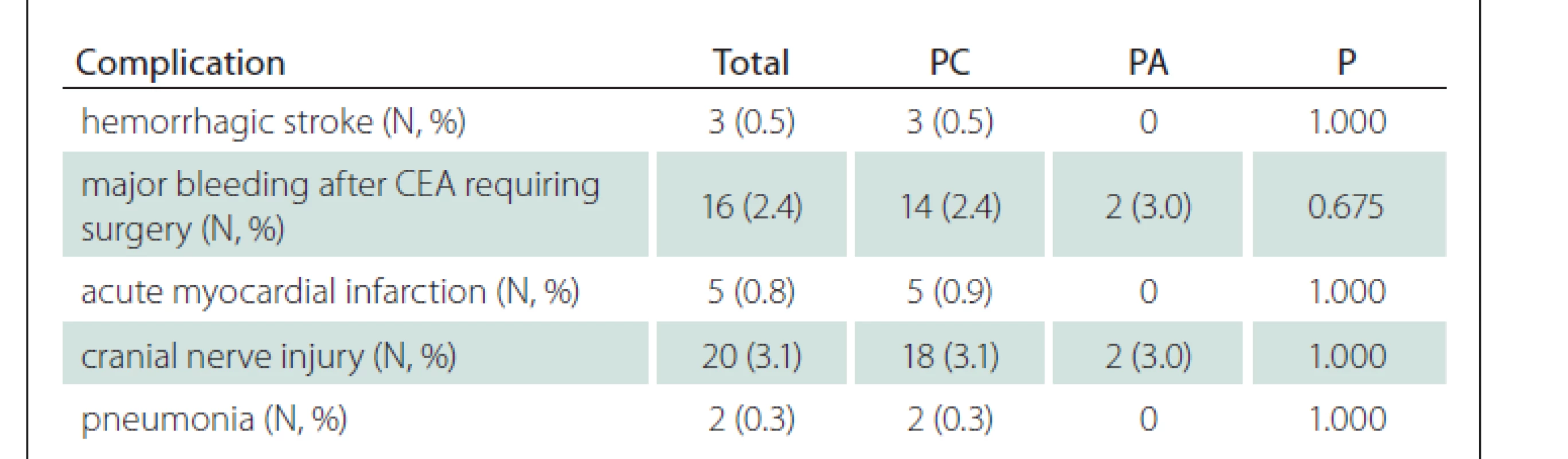
Three (0.5%) patients died within the first 30 days after CEA. All these patients underwent CEA for symptomatic stenosis and had PC. During the FUP, a total of 83 (13.7%) patients died and only four (0.7%) of them due to stroke. Other 37 (6.1%) patients died of cardiac or respiratory failure, 26 (4.3%) of malignant diseases, seven (1.2%) of sepsis, three (0.5%) of abdominal aortic aneurysm rupture, and the remaining five (0.8%) of other reasons of non-vascular etiology.
Discussion
The results of our study showed no significant difference in the rate of complications including significant restenosis/ perioperative ICA thrombosis between the use of PA and PC in CEA for severe stenosis of ICA based on the intraoperative findings.
Current ESVS guidelines [1] and Society for Vascular Surgery (SVS) guidelines [7] recommend routine PA rather than routine PC (Class I, Level of Evidence A). Both the guidelines are mainly based on the meta-analysis of ten RCTs (including 1,967 patients with 2,157 CEAs), which showed a benefit of routine PA in the reduction of early and late complications of CEA when compared to PC [4]; nevertheless, the authors of this meta-analysis later pointed on the overestimation of the reported results and on the reliability of small RCTs included. In the updated meta-analysis, the benefit of patching was less obvious [8]. Another reported meta-analysis of 13 RCTs also showed no significant difference in perioperative or late mortality rates, incidence of stroke and restenosis as well as in operation time when using venous patch and synthetic patch material or ePTFE patch versus polyester patch material during CEA [9]. Several recent studies comparing PC with PA also question the superiority of PA with regard to the poor quality of the included studies performed over 20 years ago and with regard to relative small sample sizes and significant loss of FUP.
Maertens et al reported no difference in stroke and death rates within one month after CEA when performing selective PA in their retrospective study, in which symptomatic and asymptomatic patients were evaluated together [10]. PC was performed in ICA diameter above 5 mm in cases of high carotid bifurcation or contralateral ICA occlusion. However, they found significantly longer clamping time in PA [10]. Similarly, Huizing et al reported no difference in the incidence of stroke, death and other complications within 1 month afer CEA and also in the rate of restenosis at 6 weeks and one year in a retrospective study of symptomatic patients [11].
Avgerinos et al retrospectively analysed a consecutive cohort of 1,737 CEA cases, in which a choice of PA, PC or eversion technique depended on surgeon’s personal preference. Symptomatic and asymptomatic patients were evaluated together [12]. They found no difference between PA and PC in stroke and death rates and of restenosis in a 5 - and 10-year FUP [11].
To our knowledge, Huizing et al recently performed the largest and most updated (2019) systematic review which included 9 randomized and 20 nonrandomized studies with a total of 12,696 patients (13,219 CEAs) [13]. The overall stroke risk within 1 month afer CEA was significantly lower in PA group; however, this benefit lost its statistical significance after exclusion of nonrandomized studies [13]. The restenosis was significantly lower in the PA group. Remarkably, this review included studies, in which the symptoms of the patients and the reasons for PA (diameter of ICA or other anatomical conditions, physician’s personal preference or both) were not taken into consideration.
In our study, PA was used depending on the subjective evaluation of the morphological parameters of ICA: the intra-operative ICA diameter and the quality of arteriotomy margins after CEA. In three RCTs included in the above-mentioned meta - -analysis, patients were excluded if their ICAs were deemed to be too narrow for PC (ICA diameter ≤ 3.5 or 4 or 5 mm) [2]. A different diameter for ICA exclusion reflects the fact that the minimum diameter for a necessary PA remains still unclear. Similarly, other RCTs also reported conversion from one type of arteriotomy closure to another after randomization in a few patients [4]. In a randomized study of Myers et al, patients with ICA diameter under 5 mm were excluded from the enrollement [14]. No significant difference was found among the three closure groups in any of the observed variables including perioperative and late neurological events, perioperative death and restenoses (i.e. for randomised groups between PC and vein patch as well as for the third nonrandomized group with obligatory vein patch) [14].
Our results are in line with the findings of recent nonrandomized studies [10–12], which showed no difference in the outcomes and in the rates of complications between both groups. In our study, the only difference was found in the rates of death and composit endpoints of stroke or death in PA group during a long-term FUP in the subanalysis of symptomatic stenoses (Tab. 3), but most deaths were of non-stroke origin. The rates of occurred restenosis ≥ 70% during the FUP in our study were similar to those reported in recent clinical trials [12].
The overall rates of periprocedural complications after CEA in our patients did not differ from the results published previously (Tab. 2–4) [15]. Also, in patients with symptomatic ICA stenosis, the rates of complications were similar to the results reported from recent studies (Tab. 3) [15,16].
Eversion CEA may be considered a promising alternative to traditional CEA with PC or PA, which may reduce perioperative and long-term complications. Previously published meta-analysis showed no significant differences between eversion and traditional CEA and between eversion and CEA with PA in the rates of perioperative and late complications [17]. Eversion CEA reached only a borderline superiority in the rate of restenosis > 50% during the FUP when compared to traditional CEA. A more recent and greater meta-analysis, which included seven randomized and 14 nonrandomized studies with 16,251 performed CEAs, showed a significantly lower incidence of perioperative and late complications in eversion CEA vs. traditional CEA. The benefit of eversion CEA was also demonstrated in comparison to PA only [18]. Current ESVS guidelines as well as SVS guidelines recommend PA or eversion CEA over routine PC [1,7]. Current national clinical guideline for the secondary prevention of ischemic stroke / transient ischemic attack do not recommend or prefer any type of arterial wall closure after CEA for symptomatic stenosis of ICA [19].
There are several limitations of our study. A nonrandomized retrospective single center design was used. Precise measurement of ICA diameter or the quality of arteriotomy margins after CEA was not performed, because no reliable method of measurement was available; thus, the diameter of ICA and the quality of arteriotomy margins were evaluated by individual surgeons subjectively and this fact might cause a bias in the selection of PA or PC. Substantially unequal numbers of patients with PC and PA may affect the results; however, most previous nonrandomized studies presented similar imbalance [10,11]. We did not perform a subanalysis in females; however, a smaller ICA diameter in women was reported in some previous studies [20] and a greater benefit of PA in women was stated in previous guidelines [7]. Nevertheless, current ESVS guidelines do not refer to this topic [1] and the studies focusing on sex-based outcome diferences mostly showed more frequent use of PA in females when compared to males only [21,22].
In conclusion, the use of PA based on the intraoperative findings showed no significant difference in the rate of complications endarterecand significant restenosis or perioperative ICA thrombosis after CEA for severe stenosis of ICA, when compared to PC in our study. Our finding may suggest that selective PA might be an alternative instead of routine PA. A multicentric RCT is needed to validate the role of selective PA in the prevention of complications of CEA.
Ethical principles
The entire study was conducted in accordance with the Helsinki Declaration of 1975 (as revised in 2004 and 2008).
Conflict of interest
The authors declare they have no potential conflicts of interest concerning drugs, products, or services used in the study.
The Editorial Board declares that the manuscript met the ICMJE “uniform requirements” for biomedical papers.
Redakční rada potvrzuje, že rukopis práce splnil ICMJE kritéria pro publikace zasílané do biomedicínských časopisů.
Prof. Daniel Šaňák, MD, PhD, FESO
Comprehensive Stroke Center Department of Neurology Palacký University Medical School and University Hospital Olomouc I. P. Pavlova 6, 775 20 Olomouc Czech Republic
e-mail: daniel.sanak@fnol.cz
Accepted for review: 4. 2. 2021
Accepted for print: 21. 4. 2021
Sources
1. Naylor AR, Ricco JB, de Borst GJ et al. Management of atherosclerotic carotid and vertebral artery disease: 2017 clinical practice guidelines of the European Society for Vascular Surgery. Eur J Vasc Endovasc Surg 2018; 55(1): 3–81. doi: 10.1016/ j.ejvs.2017.06.021.
2. Rerkasem K, Rothwell PM. Systematic review of randomized controlled trials of patch angioplasty versus primary closure and different types of patch materials during carotid endarterectomy. Asian J Surg 2011; 34(1): 32–40. doi: 10.1016/ S1015-9584.
3. Ott DA, Cooley DA, Chapa L et al. Carotid endarterectomy without temporary intraluminal shunt. Study of 309 consecutive operations. Ann Surg 1980; 191(6): 708 – 714. doi: 10.1097/ 00000658-198006000-00008.
4. Rerkasem K, Rothwell PM. Patch angioplasty versus primary closure for carotid endarterectomy. Coch rane Database Syst Rev 2009; 2009(4): CD000160. doi: 10.1002/ 14651858.CD000160.pub3.
5. Barnett HJ, Taylor DW, Haynes RB et al. Beneficial effect of carotid endarterectomy in symptomatic patients with high grade carotid stenosis. N Engl J Med 1991; 325(7): 445–453.
6. von Elm E, Altman DG, Egger M et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Int J Surg 2014; 12(12): 1495–1499. doi: 10.1016/ j.ijsu.2014.07.013.
7. Ricotta JJ, AbuRahma A, Ascher E et al. Updated Society for Vascular Surgery guidelines for management of extracranial carotid disease. J Vacs Surg 2011; 54(3): e1 – e31. doi: 10.1016/ j.jvs.2011.07.031.
8. Rerkasem K, Rothwell PM. Meta-analysis of small randomized controlled trials in surgery may be unreliable. Br J Surg 2010; 97(4): 466–469. doi: 10.1002/ bjs.6988.
9. Ren S, Li X, Wen J, Zhang W et al. Systematic review of randomized controlled trials of different types of patch materials during carotid endarterectomy. PLoS One 2013; 8(1): e55050. doi: 10.1371/ journal.pone.0055050.
10. Maertens V, Maertens H, Kint M et al. Complication rate after carotid endarterectomy comparing patch angioplasty and primary closure. Ann Vasc Surg. 2016; 30 : 248–252. doi: 10.1016/ j.avsg.2015.07.045.
11. Huizing E, Vos CG, Hulsebos RG et al. Patch angioplasty or primary closure following carotid endarterectomy for symptomatic carotid artery stenosis. Surg J (NY) 2018; 4(2): e96–e101. doi: 10.1055/ s-0038-1655757.
12. Avgerinos ED, Chaer RA, Naddaf A et al. Primary closure after carotid endarterectomy is not inferior to other closure techniques. J Vasc Surg 2016; 64(3): 678–683. doi: 10.1016/ j.jvs.2016.03.415.
13. Huizing E, Vos CG, van den Akker PJ et al. A systematic review of patch angioplasty versus primary closure for caoritd endarterectomy. J Vasc Surg 2019; 69(6): 1962 – 1974. doi: 10.1016/ j.jvs.2018.10.096.
14. Myers SI, Valentine RJ, Chervu A et al. Saphenous vein patch versus primary closure for carotid endarterecand tomy: long term assesment of a randomized prospective study. J Vasc Surg 1994; 19(1): 15–22 doi: 10.1016/ s0741 - 5214(94)70116-4.
15. Paraskevas KI, Kalmykov EL, Naylor AR. Stroke/ death rates following carotid artery stenting and carotid endarterectomy in contemporary administrative dataset registries: a systematic review. Eur J Vasc Endovasc Surg 2016; 51(1): 3–12. doi: 10.1016/ j.ejvs.2015.07.032.
16. Guňka I, Krajíčková D, Leško M et al. Bezpečnost karotické endarterektomie s ohledem na její načasování po ischemické cévní mozkové příhodě. Cesk Slov Neurol N 2020; 83/ 116(4): 394–399. doi: 10.14735/ amcsnn2020 394.
17. Cao P, de Rango P, Zannetti S. Eversion vs conventional carotid endarterectomy: a systematic review. Eur J Vasc Endovasc Surg 2002; 23(3): 195–201. doi: 10.1053/ ejvs.2001.1560.
18. Antonopoulos CN, Kakisis JD, Sergentanis TN et al. Eversion versus conventional carotid endarterectomy: a metaanalysis of randomised and non-randomised studies. Eur J Vasc Endovasc Surg 2011; 42(6): 751–765. doi: 10.1016/ j.ejvs.2011.08.012.
19. Škoda O, Herzig R, Mikulík R et al. Klinický standard pro diagnostiku a léčbu pacientů s ischemickou cévní mozkovou příhodou a s tranzitorní ischemickou atakou – verze 2016. Cesk Slov Neurol N 2016; 79/ 112(3): 351–363. doi: 10.14735/ amcsnn2016351.
20. Krejza J, Arkuszewski M, Kasner SE et al. Carotid artery diameter in men and women and the relation to body and neck size. Stroke 2006; 37(4): 1103–1105. doi: 10.1161/ 01.STR.0000206440.48756.
21. Schneider JR, Droste JS, Golan JF. Carotid endarterectomy in women versus men: patient characteristics and outcomes J Vasc Surg 1997; 25(5): 890–898. doi: 10.1016/ s0741-5214(97)70219-2.
22. Djedović M, Imširović B, Djedović S et al. Carotid endarterectomy in women versus man: patient characteristics and perioperative complication (<30 day) open access maced. J Med Sci 2018; 6(3): 463–466. doi: 10.3889/ oamjms.2018.109.
Labels
Paediatric neurology Neurosurgery NeurologyArticle was published in
Czech and Slovak Neurology and Neurosurgery

2021 Issue 3
- Advances in the Treatment of Myasthenia Gravis on the Horizon
- Memantine in Dementia Therapy – Current Findings and Possible Future Applications
- Memantine Eases Daily Life for Patients and Caregivers
-
All articles in this issue
- Ventilation treatment in patients with amyotrophic lateral sclerosis
- Developmental dysphasia – functional and structural correlations
- Proteomics of cerebrospinal fluid in pediatric oncology patients
- Surgical treatment possibilities of drug-resistant Ménière‘s disease
- Malignant prolactinoma – can a long-term survival be affected?
- Surgery for idiopatic glossopharyngeal neuralgia – case report and overview of therapeutic modalities
- Ethylenglycol poisoning
- Guidelines on intravenous thrombolysis in the treatment of acute cerebral infarction – 2021 version
- Aktualita z kongresu AAN 2021
- Comparison of MRI findings of glioblastoma and gliosarcoma – can conventional MRI provide beneficial differences for diagnosis?
- Selective expanded polytetrafluorethylene patch angioplasty vs. primary closure after carotid endarterectomy – a 13-year experience
- Age-dependent specifics of epidural hematoma
- Multifocal, metaphyseal osteonecrosis of knee due to pulse steroid treatment after cessation of fingolimod treatment in a 19-week-pregnant patient
- Polymorphous low-grade neuroepithelial tumor of the young
- Treatment of a large supraclinoid aneurysm via clipping with a prophylactic low-flow bypass
- Czech and Slovak Neurology and Neurosurgery
- Journal archive
- Current issue
- About the journal
Most read in this issue
- Developmental dysphasia – functional and structural correlations
- Guidelines on intravenous thrombolysis in the treatment of acute cerebral infarction – 2021 version
- Ethylenglycol poisoning
- Surgical treatment possibilities of drug-resistant Ménière‘s disease
