The use of optical coherence tomography in chiasmal compression
Authors:
P. Póczoš 1,2; J. Kremláček 3; T. Česák 1; M. Macháčková 4; N. Jirásková 4
Authors‘ workplace:
Neurochirurgická klinika, Fakultní nemocnice Hradec Králové, Sokolská 581, Hradec Králové, 500 05 přednosta: prof. MUDr. Svatopluk Řehák, CSc.
1; Ústav anatomie, Lékařská fakulta v Hradci Králové, Univerzita Karlova, Šimkova 870, Hradec Králové, 500 03 přednosta: MUDr. Petr Hájek, Ph. D.
2; Ústav patologické fyziologie, Lékařská fakulta v Hradci Králové, Univerzita Karlova, Šimkova 870, Hradec Králové, 500 03 přednosta: prof. MUDr. Miroslav Kuba, CSc.
3; Oční klinika Fakultní nemocnice Hradec Králové, Sokolská 581, Hradec Králové, 500 05 přednostka: prof. MUDr. Naďa Jirásková, Ph. D., FEBO
4
Published in:
Čes. a slov. Oftal., 75, 2019, No. 3, p. 120-127
Category:
Original Article
doi:
https://doi.org/10.31348/2019/3/2
Overview
The objective of this prospective study is to explore the benefits of optical coherence tomography (OCT) in cases of optic chiasm (OC) compression by measuring the thickness of the Retinal Nerve Fiber Layer (RNFL) and the Ganglion Cell Layer (GCL).
Material and methods: 16 patients (32 eyes) with chiasmal compression were included in the study. They presented no other pathology of the visual pathway or of the eye globe. The second inclusion criterion was a subsequent indication of decompressive surgery, either by transcranial or transnasal approach. Measurements of visual acuity, visual field, RNFL and GCL were performed once preoperatively and three times postoperatively (one week, 3 and 6 months postoperatively). The observed peripapillary and perifoveal regions were divided into quadrants. The degree (grade 0-5) of chiasmal compression was determined on preoperative brain MRI (magnetic resonance imaging). In need of some data analysis, participants were split into a group with no or minimal (grade 0–1) and with substantial pressure (grade 2–4) on OC.
Results: The median global peripapillary RNFL was 87 μm, the perifoveal nasal GCL 41,2 µm and the temporal 44,2 µm. There was a pronounced preoperative RNFL thinning in nasal (63,5 μm) and temporal (65 μm) quadrant, in comparison to the age-matched normative database for RNFL thickness in the OCT protocol. There is a statistically important connection between bitemporal hemianopia and RNFL, resp. GCL. Larger perimetric outages is related to important OC compression, seen on MRI.
Conclusion: There exists a correlation between the thickness of the peripapillary RNFL, resp. perifoveal GCL, and visual field defects in chiasmal compression. Thinner preoperative RNFL does not present a statistically important limiting factor for better functional outcomes after surgical decompression. Horizontal asymmetry of perifoveal GCL is an indicator of compressive ophthalmopathy. The grade of preoperative OC compression presents another important prognostic factor.
Keywords:
resection – Pituitary adenoma – optic chiasm – compression – optical coherence tomography – sellar mass
INTRODUCTION
In the treatment of pathologies of the sellar region, which compress the optic chiasm (OC), it is of absolutely fundamental importance to achieve an improvement of visual complaints. This is accomplished in most cases by surgical decompression (3). Compression of the OC has an influence mostly on the transmission of impulses especially in the crossed fibres of the visual pathway, and is most often diagnosed through the presence of a defect in the temporal part of the visual field (VF) (21, 31, 32). Examination of the VF is used in particular for objectification of visual complaints, although this may not always unequivocally detect the pathology (4). In recent decades optical coherence tomography (OCT) has been used more frequently in the diagnosis of compressive lesions of the parts of the visual pathway formed by fibres of ganglion cells. OCT is capable of detecting the thickness of the individual layers of the retina (1, 23, 28). In the case of compression of the OC, the thickness of the retinal nerve fibre layer (RNFL) is significantly thinner in the peripapillary region temporally and nasally. By contrast, in the case of glaucoma there is a typical loss of thickness of the RNFL in the upper and lower segment (7). Further studies indicate the expediency of measuring the RNFL (24, 27) or the thickness of the ganglion cell layer (GCL) (2, 16, 19, 29, 30, 34) in the macular region. There is also a study which, in addition to finding a significant reduction of the above-described parameters, also found a parallel thickening of the inner nuclear layer (INL) (25).
In summary it can be stated that studies with short-term and long-term observation of patients with compression of the OC of various etiology have demonstrated the capacity of examination by OCT to predict the postoperative development of visual acuity and the visual field (8, 15, 18, 23, 33).
In this paper we inform about the results of our prospective study, which was targeted in particular at measuring the thickness of the peripapillary RNFL and perifoveal GCL in patients with compression of the OC.
COHORT AND METHOD
16 patients (32 eyes) who formed the study cohort signed an informed consent form. The method of examination and observation of the patients was approved by the ethical commission of the University Hospital Hradec Králové (UH HK). The condition for inclusion of a patient in the study was the presence of a tumour in the sellar region and subsequent indication for its surgical removal. The patients included in the study had undergone a surgical procedure in the period from October 2016 to August 2018 at the Department of Neurosurgery at UH HK. The exclusion criterion was another serious pathology of the eyes (especially the retina) or visual pathway. The examinations took place within the framework of a single preoperative and three postoperative sessions (after one week, then 3 and 6 months). In the case of six patients the complete set of all examinations was not performed. In one case this was for technical reasons, and in the remainder the patients did not report for the examination. As a result, in a number of analyses we had to base our results on a smaller total number of patients or eyes. Visual acuity was tested with the aid of Landolt C charts. The visual field was examined on the instrument Humphrey Field Analyser II (Carl Zeiss, Meditec, Dublin, CA, USA; test: SITA – FAT strategy, test central 30-2 Threshold Test) and OCT on the instrument Spectralis OCT system (Heidelberg Engineering, Heidelberg, Germany; software Heidelberg Eye Explorer 1.10.2). Thickness of the RNFL with centration of the optic nerve head (ONH) was measured along a circle with a diameter of 3.4 mm, and the result was related to the norm for the given age. The average value of the RNFL along the entire circle is hereinafter referred to as the “global RNFL”. For further data processing this thickness was divided into the upper, temporal, lower and nasal sector (Fig. 1). We quantified the thickness of the ganglion cell layer (GCL) in a rectangle of 3.45 and 4.15 mm, centred onto the fovea. From the selected region the central part (1.73 x 2.08 mm) was excluded, and the remaining area was divided into two symmetrical fields: nasal and temporal (Fig. 2). On the coronary sequence T1 of the balanced images of the preoperative MRI of the brain, the degree of compression of the OC (“grade”) was determined according to the criteria proposed by Fujimoto et al. (12). In grade 0 there was no contact between the tumour and the OC. In grade 1 there was minimal contact without disfiguring of the upper surface of the OC. In grade 2 there was presence of disfiguring, although the suprachiasmatic cistern was perceptible. Grade 3 was characterised by a defunct cistern without disfiguring of the brain parenchyma. Contact of the tumour with the OC to such a degree that it also caused disfiguring of the adjacent brain tissue was indicated as grade 4. All decompressive procedures were performed at the Department of Neurosurgery at UH HK by the same operating team.
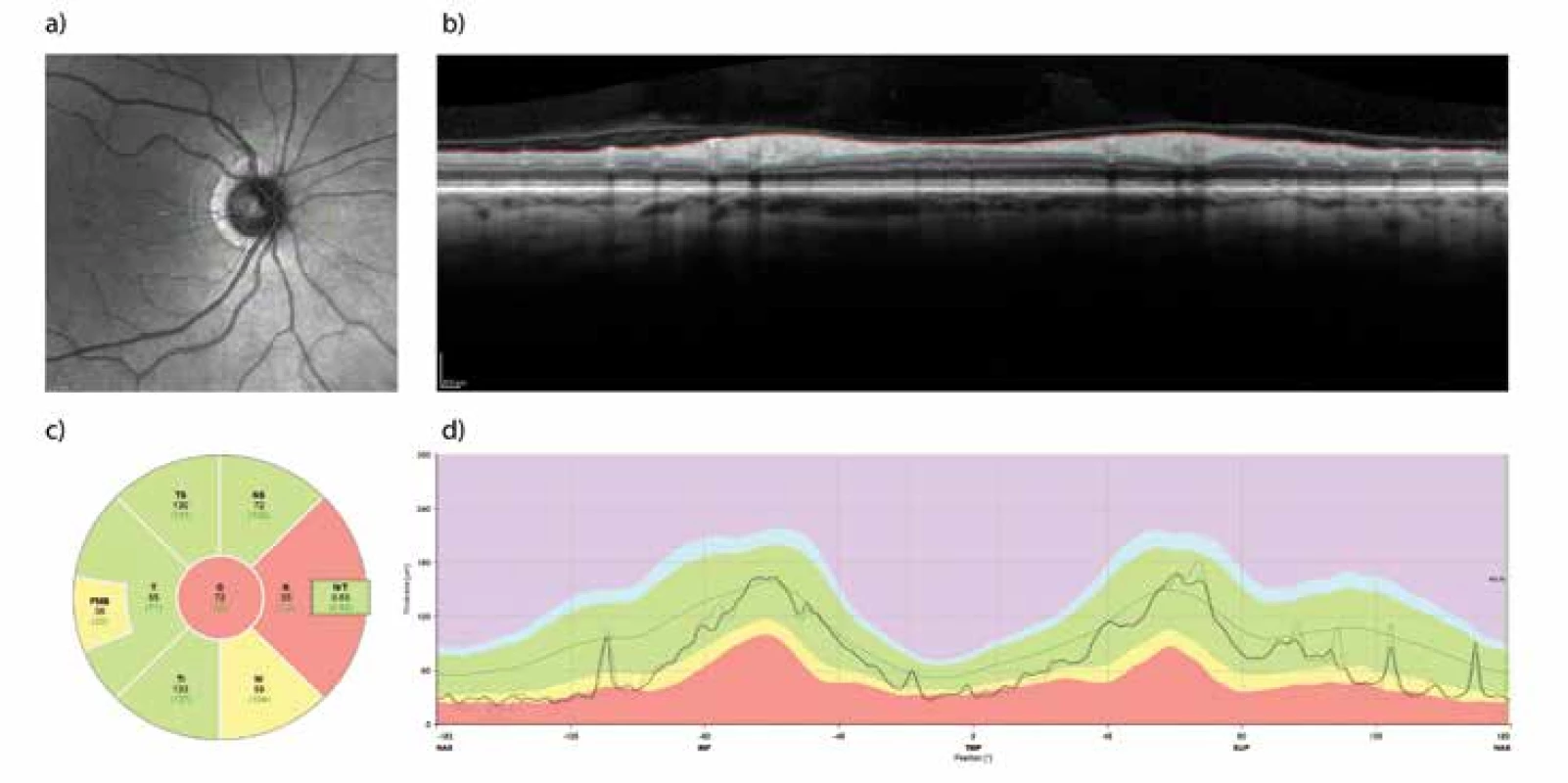
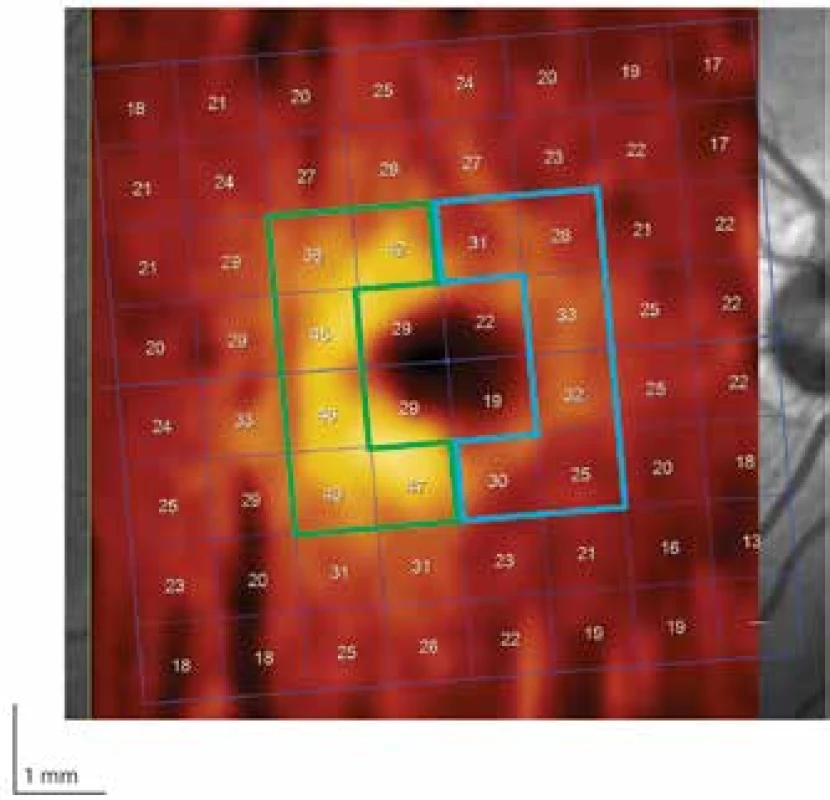
Descriptive statistics was used for interpretation of the data, parametric or non-parametric tests were used for pair and inter-group comparison, dependencies between the parameters were evaluated by a Pearson or Spearman correlation coefficient. For post-hoc analyses of the results the cohort of patients was divided into a group with no or minimal pressure on the OC (grade 0-1) and unequivocal pressure on the OC (grade 2-4), and an analysis of variability was conducted. The analysis was conducted in the program environment R version 3.5.1.
RESULTS
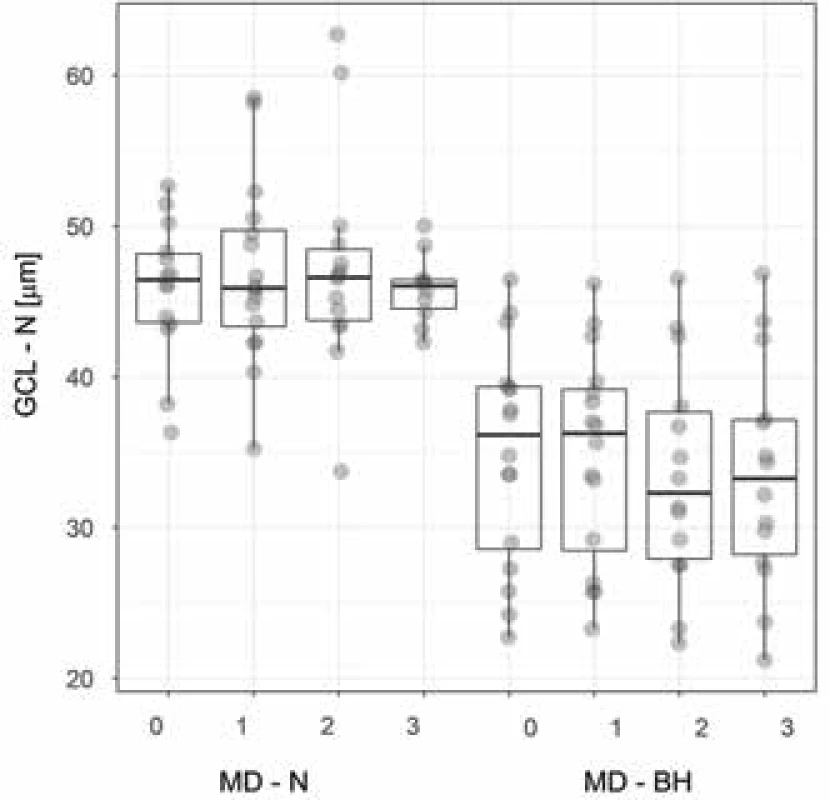
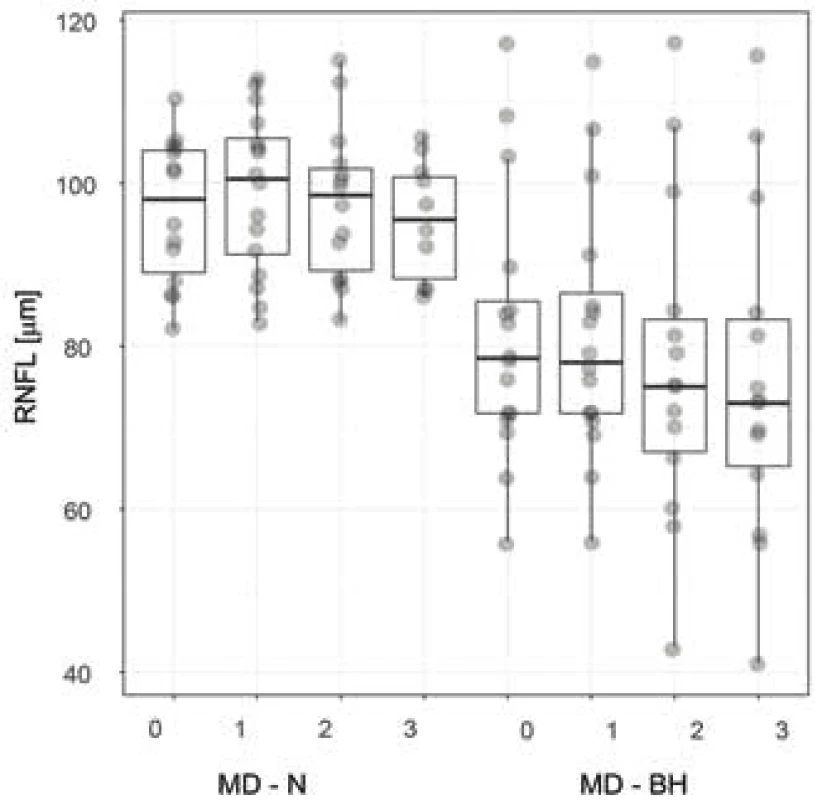
The median age of the entire cohort, comprising 8 women and 8 men, was 54 years, with an interquartile range (IQR) of 45 to 63 years. According to the preoperative MRI, in two patients (12.5%) there was no presence of compression of the OC, while three patients were in the group of grade 1 (18.75%) and 3 patients in grade 2 (18.75%). In two cases (12.5%) grade 3 was determined, and in six (37.5%) grade 4. In all cases this concerned symmetrical compression of the OC. A surgical procedure by a transcranial approach was selected for two patients (12.5%), in whom the histopathological examination confirmed a preoperative working diagnosis of meningioma. In the remaining 14 surgical procedures, decompression was achieved by a transnasal transsphenoidal approach, in which a microscope was used for 5 patients and an endoscope for 9 patients. The pathologist recorded a non-functional adenoma in 5 cases (31.25%), a somatotropic adenoma in one case (6.25%), somatotropin and prolactin producing an adenoma in two cases (12.5%), and finally in one case each an adrenocortical adenoma (6.25%), a tumour from the granular cells of the infundibulum (6.25%) and a spindle cell oncocytoma (6.25%). A post-infection cyst (6.25%), tension arachnoid cyst (6.25%) and post-apoplexy state probably in a non-functional adenoma (6.25%) were also present in one case each. The preoperative and postoperative median values of visual acuity and the mean deviation (MD) across the tested region by the perimeter are presented in table 1. The median preoperative thickness of the peripapillary RNFL in the cohort of all eyes was 87 µm (IQR 78.6-102.8 µm). At the third postoperative follow-up examination the measured RNFL thickness was statistically significantly lower (86.5; 72.3 – 98.5 µm; p = 0.005). Before surgery the median (IQR) thickness of the perifoveal GCL for the temporal region was 44.2 (39.6-45.5) µm, and for the corresponding nasal part 41.4 (35.2-46.4) µm. After the third postoperative follow-up examination this value recorded a statistically significant decrease to 43.8 (40.1-46.4) µm temporally and to 42.4 (31.7-46) µm nasally (p < 0.01) (Table 2). In 8 patients (50%) with bitemporal hemianopia there was statistically significantly lower thickness of the GCL in the nasal half at all 4 examinations in comparison with the other half of the patients without bitemporal hemianopia (p < 0.001) (Fig. 3). The mean values of thickness of global RNFL were also statistically significantly lower in patients with bitemporal hemianopia (at all 4 examinations p < 0.019) (Fig. 4).



In the group of grade 0-1 (5 patients, 10 eyes), the median preoperative values of global RNFL (104 µm) and GCL (45.1 µm) were statistically significantly higher than in the group of grade 2-4 (84 µm, respectively 40 µm). Fig. 5 illustrates this difference in the case of all 4 examinations for global RNFL. As regards GCL, the most marked differences were again in the nasal half.

A correlation analysis demonstrated a clear negative dependency between preoperative thickness of the RNFL and an improvement of visual acuity already at the first follow-up examination. There is also a correlation between low preoperative RNFL and a greater improvement of MD by the perimeter. This was more marked at the third follow-up examination.
DISCUSSION
The most common pathological processes diagnosed in the sellar region are adenomas of the hypophysis, craniopharyngioma, meningioma, aneurysm, apoplexy, cystic formations and metastases.
Tumours of the hypophysis, representing 12-15% of all intracranial tumours, are a frequent cause of compression of the OC (10). Literary sources state that sight is afflicted to a certain degree in as many as 68% of patients (11). From practice it is known that surgical solution is not indicated immediately in the case of a chance finding of tumours in the region of the hypophysis, and the tumour is observed for a certain period. However, defects of the VF (recently demonstrated in the patient) are considered an element contributing to an indication for a surgical solution of adenomas and other lesions (20) (with the exception of prolactinoma, where medicamentous therapy is applied in first place under the supervision of an endocrinologist). Nevertheless, it is necessary to keep in mind the factor of subjectivity upon examination of the VF. Its result may be influenced by worse co-operation on the part of the patient. Cases have even been documented in which standard automatic perimetric examinations lack sufficient sensitivity in order to identify especially residual blind spots in the visual field (14, 30).
The pathophysiology of compressive lesions of the visual pathway is composed of several phases. First of all a block appears in the transmission of the action potential, which leads to a deterioration of vision. If decompression is achieved in this phase, the result is removal of the block with subsequent restoration of the correct transmission of the signal. Clinical improvement is attained usually within a matter of weeks (6, 8). In the opposite case, there is a progression of reversible or irreversible axonal damage (26). If decompression is performed, there follows a probable improvement of visual functions, and within the range of several months also remyelination and reconstruction distally from the location of compression. In the final phase, progressive losses of GCL cells occur (8, 26).
Some studies point out that a preoperative mean thickness of the RNFL of less than 70-85 µm is a prognostically adverse sign for postoperative improvement of sight (8, 22). Other authors dispute this assertion (17), referring to the possibility of distortion of results attained by older models of OCT, known as TD-OCT (Time Domain). Our results support the view that a lower preoperative RNFL does not limit a desirable postoperative development. If there is no compression, RNFL is within the norm and there is no sight deficit, it is not possible to expect an improvement within the visual area following decompression. This fact is probably the reason why this correlation has been found. For an accurate assessment of the effect of the thickness of the RNFL or GCL on the postoperative development, it would be necessary to examine a group of patients with the same functional affliction. A slight correlation exists between the preoperative examination and the 1st follow-up examination. Upon a comparison with the 3rd follow-up examination there is a larger correlation. We were unable to confirm similar findings, such as in the publication by Danesh-Meyer et al. (8), because the mean value of RNFL in our observation was 89 µm, whereas in their study this value was 66 µm. The largest improvement of the perimeter (by 2.71 dB, median value of improvement) took place between the preoperative measurement and the 2nd follow-up examination (see table 1). We did not demonstrate statistically significant asymmetry between the nasal and temporal sector of the RNFL. The explanation may be a less systematic “projection of the viewed object” into the topic of the nerve fibres than in the case of the GCL cells. However, a significant thinning of the RNFL was present in the nasal and temporal sector (in comparison with the upper and lower quadrant), as is the case in other publications dealing with compressive lesions of the visual pathway (19, 34).
Because ideas have proliferated which cast doubt on the expediency of measurement of the RNFL, more recent studies have accentuated the role of the GCL, or GCC (Ganglion Cell layer Complex) in the objectification of compression of the OC. More precisely speaking, binasal thinning of the GCC frequently corresponds with a bitemporal blind spot in the visual field (16, 19, 25, 26, 29, 30). In the stated publications, the term “macular” GCL or GCC is used. As regards our results, we give preference to the term “perifoveal”, which is more precise from the anatomical perspective. Jørstad et al. (18), on the basis of the fact that a greater thickness of the GCC from the nasal half of the macular retina is a precondition of so-called horizontal asymmetry in a healthy patient (5), assert that loss of this asymmetry is an indicator of chiasmal compression. It is necessary to emphasise the differences in the definition of the GCC layer in the case of individual OCT instruments and authors. The last presented study incorporated into the thickness of the GCC also the RNFL, which, as is known, thickens nasally from the fovea (31). In our study, we evaluated the GCL layer without the RNFL or inner plexiform layer. Thus horizontal asymmetry, which was statistically insignificant (p = 0.065) preoperatively, is, in the case of our study, a pathological finding (see table 2). Statistically significant, but opposite to physiological, asymmetry (p = 0.028) was present only at the third follow-up examination, when there was a significant decrease of the GCL nasally. The explanation may be the continuing process of degeneration of nerve fibres, rescpectively the death of GCL cells, at the expense of preoperative compression of the OC or at the expense, even if minimal, of manipulation of the optic nerve upon resection of the tumour.
According to the studies by Lee et al. (19) and Tieger et al. (30), there was a stronger correlation with preoperative blind spots in the visual field in the case of the GCC layer in comparison with the RNFL. Yoneoka et al. (33) paradoxically presented a result of stronger correlation with the RNFL. An analysis of our results shows a more significant accordance between the GCL with p = 2.5 x 10-6 (especially nasally, p = 2.4 x 10-10) and a disorder in the visual field (see fig. 3).
A further question arises as to why, in the majority of the cited articles, thinning of the RNFL and GCL, or even progression of thinning, as also documented by our data (see tables 1 and 2) persisted after adjustment of the visual field. Perhaps these results are due to the relatively short postoperative observations, and the answer will be provided by future studies with a markedly longer observation of patients. As already noted above, a further explanation may be the lower sensitivity of the perimetric examination methods used as standard (14 ,30) or the completion of the process of degeneration of the nerve fibres. It is always necessary to keep in mind the influence of the interindividual differences and not to consider the obtained results of the perimeter or OCT to be unequivocal prognostic parameters. Another such parameter is the degree of compression of the OC. In our group of 16 pairs of eyes, the mean thickness of the RNFL (see fig. 5) as well as GCL was statistically significantly larger in grade 0-1 than in grade 2-4. On the other side of the argument axis is, for example, the publication by Cennamo et al. (4). Upon a clear absence of pressure of hypophyseal adenomas they measured pathological values of the RNFL and GCC attesting to compression of the OC in individuals who had no other pathology of the visual apparatus. A factor that cannot be overlooked which influences the functional result of the entire therapeutic process of compressive lesions of the visual pathway is not only the duration of the compression, but also the consistency of the tumour and the actual surgical procedure itself. Meningiomas represent more rigid tumours in comparison with sometimes even semi-fluid adenomas (13). Upon the selection of a transnasal approach, the decompressive procedure presents a lesser risk of perioperative injury to the optic nerve and chiasm (3, 9). From our own experience it is possible to state that there are cases (e.g. meningiomas) in which, despite the fact that an endoscopic approach offers itself in the sagittal corridor, the neurosurgeon selects a transcranial approach with respect to the expected harder consistency.
As every study, ours also has its limiting factors. We cannot eliminate the possibility of false positive and negative results. A set of 32 eyes represents a relatively small cohort. Furthermore, in certain patients there was a lack of a complete series of all 3 postoperative follow-up examinations. Another factor is the different consistency, in other words solidity, of individual pathologies exerting pressure on the OC.
The question of the trajectory and topics of the centripetally directing axons of the retinal ganglion cells, through the optic nerves up to the chiasm, would welcome new data with regard to the topography of fibres of the RNFL, especially in the nasal quadrant of the peripapillary region. In addition, the pathophysiology of the chiasmal syndrome is not entirely unequivocal. Its further in vivo examination is virtually impossible to conduct for ethical reasons, and so methods using computer models remain as backup.
CONCLUSION
The results of this prospective study confirm a correlation between the thickness of the peripapillary RNFL or respectively perifoveal GCL, and defects in the visual field as a consequence of compression of the optic chiasm. Nevertheless, no precise correlation was performed between the topics of locations with a reduced RNFL around the optic nerve head and the individual types of blind spots in the visual field. Lesser preoperative thickness of the RNFL was not a significant limiting factor in improvement of the functional results following surgical decompression. Horizontal asymmetry in the thickness of the perifoveal GCL is an indicator of a compressive lesion of the visual pathway. The degree of compression of the OC, which can be evaluated on preoperative magnetic resonance imaging, is a further prognostic factor. From our observation and from the available literary sources, it is evident that it is necessary to view perimetric and OCT examinations as complementary methods providing the clinician with the necessary information about the morphological and functional condition of the visual pathway. It is clear that the morphological data on the condition of the retina has greater potential for prediction of the postoperative development.
The authors of the study declare that no conflict of interest exists in the compilation, theme and subsequent publication of this professional communication, and that it is not supported by any pharmaceuticals company.
MUDr. Pavel Póczoš, Ph.D.
Neurochirurgická klinika, FN Hradec Králové,
Sokolská 581, 500 05 Hradec Králové
Received: 26. 2. 2019
Accepted: 27. 3. 2019
Available on-line: 19. 10. 2019
Sources
1. Banc, A., Stan, C., Florian, IS.: Optical coherence tomography impacts the evaluation of visual pathway tumors. Neurosurg Rev, 41(2); 2018 : 415-426.
2. Blanch, RJ., Micieli, JA., Oyesiku, NM. et al.: Optical coherence tomography retinal ganglion cell complex analysis for the detection of early chiasmal compression. Pituitary, 21(5); 2018 : 515-523.
3. Castinetti, F., Dufour, H., Gaillard, S. et al.: Non-functioning pituitary adenoma: when and how to operate? What pathologic criteria for typing? Ann Endocrinol, 76; 2015 : 220-227.
4. Cennamo, G., Auriemma, RS., Cardone, D. et al.: Evaluation of the retinal nerve fibre layer and ganglion cell complex thickness in pituitary macroadenomas without optic chiasmal compression. Eye (Lond), 29(6); 2015 : 797-802.
5. Curcio, CA., Allen, KA.: Topography of ganglion cells in human retina. J Comp Neurol, 300(1); 1990 : 5-25.
6. Danesh-Meyer, HV., Carroll, SC., Foroozan, R. et al.: Relationship between retinal nerve fiber layer and visual field sensitivity as measured by optical coherence tomography in chiasmal compression. Invest Ophthalmol Vis Sci, 47(11); 2006 : 4827-4835.
7. Danesh-Meyer, HV., Yap, J., Frampton, C., Savino, PJ.: Differentiation of compressive from glaucomatous optic neuropathy with spectral-domain optical coherence tomography. Ophthalmology, 121(8); 2014 : 1516-1523.
8. Danesh-Meyer, HV., Wong, A., Papchenko, T.: Optical coherence tomography predicts visual outcome for pituitary tumors. J Clin Neurosci, 22(7); 2015 : 1098-1104.
9. Dubourg, J., Jouanneau, E., Messerer, M.: Pituitary surgery: legacies from the past. Acta Neurochir, 153(12); 2011 : 2397-2402.
10. Ezzat, S., Asa, SL., Couldwell, WT. et al.: The prevalence of pituitary adenomas : a systematic review. Cancer, 101(3), 2004 : 613-619.
11. Ferrante, E., Ferraroni, M., Castrignanò, T. et al.: Non-functioning pituitary adenoma database : a useful resource to improve the clinical management of pituitary tumors. Eur J Endocrinol, 155(6); 2006 : 823-829.
12. Fujimoto, N., Saeki, N., Miyauchi, O. et al.: Criteria for early detection of temporal hemianopia in asymptomatic pituitary tumor. Eye (Lond), 16(6); 2002 : 731-738.
13. Greenberg, MS (ED): Handbook of Neurosurgery, New York, Thieme, 2016, 690-744 p.
14. Horton, JC.: Invited commentary: ganglion cell complex measurement in compressive optic neuropathy. J Neuroophthalmol, 37(1); 2017 : 13-15.
15. Jacob, M., Raverot, G., Jouanneau, E. et al: Predicting visual outcome after treatment of pituitary adenomas with optical coherence tomography. Am J Ophthalmol, 147(1); 2009 : 64-70.
16. Jeong, AR., Kim, EY., Kim, NR.: Preferential ganglion cell loss in the nasal hemiretina in patients with pituitary tumor. J Neuroophthalmol, 36(2); 2016 : 152-155.
17. Johansson, C., Lindblom, B.: The role of optical coherence tomography in the detection of pituitary adenoma. Acta Ophthalmol, 87(7); 2009 : 776-779.
18. Jørstad, ØK., Wigers, AR., Marthinsen, PB. et al.: Loss of horizontal macular ganglion cell complex asymmetry: an optical coherence tomography indicator of chiasmal compression. BMJ Open Ophthalmol, 3(1); 2018: e000195. doi: 10.1136/bmjophth-2018-000195. eCollection 2018.
19. Lee, EJ., Yang, HK., Kim, TW. et al.: Comparison of the pattern of retinal ganglion cell damage between patients with compressive and glaucomatous optic neuropathies. Invest Ophthalmol Vis Sci, 56(12); 2015 : 7012-7020.
20. Lešták, J., Vladyková J., Houšťava L.: Útlakové léze chiazmatické krajiny z pohledu oftalmologa. Čs Oftal, 51(3); 1995 : 171-176.
21. Lešták, J., Houšťava L.: Rozsah poškození zorného pole u chiazmatických lézí – příspěvek k volbě programu vyšetření zorného pole. Čs Oftal, 51(3); 1995 : 165-170.
22. Loo, JL., Tian, J., Miller, NR. et al.: Use of optical coherence tomography in predicting post-treatment visual outcome in anterior visual pathway meningiomas. Br J Ophthalmol, 97(11); 2013 : 1455-1458.
23. Monteiro, ML., Leal, BC., Rosa, AA. et al.: Optical coherence tomography analysis of axonal loss in band atrophy of the optic nerve. Br J Ophthalmol, 88(7); 2004 : 896-899.
24. Monteiro, ML., Costa-Cunha, LV., Cunha, LP. et al.: Correlation between macular and retinal nerve fibre layer Fourier-domain OCT measurements and visual field loss in chiasmal compression. Eye (Lond), 24(8); 2010 : 1382-1390.
25. Monteiro, ML., Hokazono, K., Fernandes, DB. et al.: Evaluation of inner retinal layers in eyes with temporal hemianopic visual loss from chiasmal compression using optical coherence tomography. Invest Ophthalmol Vis Sci, 55(5); 2014 : 3328-3336.
26. Moon, CH., Hwang, SC., Ohn ,YH. et al.: The time course of visual field recovery and changes of retinal ganglion cells after optic chiasmal compression. Invest Ophthalmol Vis Sci, 52(11); 2011 : 7966-7973.
27. Moura, FC., Costa-Cunha, LV., Malta, RF. et al.: Relationship between visual field sensitivity loss and quadrantic macular thickness measured with Stratus-Optical coherence tomography in patients with chiasmal syndrome. Arq Bras Oftalmol, 73(5); 2010 : 409-413.
28. Němec, P.: Interpretace OCT skenu – OCT anatomie. In Němec, P., Kousal, B., Löfflerová, V. (EDs): Optická koherenční tomografie – klinický atlas sítnicových patologií. Praha, Mladá fronta, 2017, s. 17-39.
29. Ohkubo, S., Higashide, T., Takeda, H. et al.: Relationship between macular ganglion cell complex parameters and visual field parameters after tumor resection in chiasmal compression. Jpn J Ophthalmol, 56(1); 2012 : 68-75.
30. Tieger, MG., Hedges, TR. 3rd., Ho, J. et al.: Ganglion cell complex loss in chiasmal compression by brain tumors. J Neuroophthalmol, 37(1); 2017 : 7-12.
31. Unsöld, R., Hoyt, WF.: Band atrophy of the optic nerve. The histology of temporal hemianopsia. Arch Ophthalmol, 98(9); 1980 : 1637-1638.
32. Wybar, K.: Chiasmal compression. Presenting ocular features. Proc R Soc Med, 70(5); 1977 : 307-317.
33. Yoneoka, Y., Hatase, T., Watanabe, N. et al.: Early morphological recovery of the optic chiasm is associated with excellent visual outcome in patients with compressive chiasmal syndrome caused by pituitary tumors. Neurol Res, 37(1); 2015 : 1-8.
34. Yum, HR., Park, SH., Park, HY. et al.: Macular Ganglion Cell Analysis Determined by Cirrus HD Optical Coherence Tomography for Early Detecting Chiasmal Compression. PLoS One,11(4); 2016: e0153064. doi: 10.1371/journal.pone.0153064. eCollection 2016.
Labels
OphthalmologyArticle was published in
Czech and Slovak Ophthalmology

2019 Issue 3
-
All articles in this issue
- The use of optical coherence tomography in chiasmal compression
- Selective angiography with the possibility of thrombolysis in patients with central retinal artery occlusion
- Influence of cornea on intraocular pressure measurement by ICARE PRO and ORA
- Methods of improving the quality of life in patients with stable maculopathy-pilot results of a new study
- Ranibizumab for the treatment of choroidal neovascularization due to cause other than age related macular degeneration
- Pupillary abnormalities in childhood – 2 case presentations
- Czech and Slovak Ophthalmology
- Journal archive
- Current issue
- About the journal
Most read in this issue
- Pupillary abnormalities in childhood – 2 case presentations
- Influence of cornea on intraocular pressure measurement by ICARE PRO and ORA
- Ranibizumab for the treatment of choroidal neovascularization due to cause other than age related macular degeneration
- Methods of improving the quality of life in patients with stable maculopathy-pilot results of a new study
