A study of the properties of compacts from directly compressible fructose
Studium vlastností výlisků z přímo lisovatelné fruktosy
Práce se zabývá studiem pevnosti a doby rozpadu výlisků z přímo lisovatelné fruktosy Advantosy FS 95 a její směsi s mikrokrystalickou celulosou Vivapurem 102 v poměru 1 : 1 v závislosti na lisovací síle. Sleduje se vliv přídavku dvou mazadel stearanu hořečnatého a stearylfumarátu sodného v 1% koncentraci a přídavku léčivých látek kyseliny askorbové a kyseliny acetylsalicylové v 50% koncentraci. V případě Advantosy FS 95 nebyl zaznamenán statisticky významný rozdíl v hodnotách pevnosti výlisků v rámci typu použitého mazadla, s výjimkou lisovací síly 10 kN, kdy byla pevnost tablet nižší s Pruvem. Výlisky ze směsi Advantosy FS 95 s Vivapurem 102 měly pevnost vyšší. Její hodnoty se výrazně snížily vlivem mazadel, více v případě stearanu hořečnatého. Dobu rozpadu tablet v případě Advantosy FS 95 prodloužil více stearan hořečnatý, v případě směsi suchých pojiv stearylfumarát sodný. Tablety s kyselinou acetylsalicylovou měly významně vyšší pevnost a také delší dobu rozpadu než s kyselinou askorbovou.
Klíčová slova:
Advantosa FS 95 – Vivapur 102 – mazadla – pevnost tablet v tahu – doba rozpadu tablet
Authors:
J. Mužíková; S. Lišková
Authors‘ workplace:
Charles University in Prague, Faculty of Pharmacy in Hradec Králové, Department of Pharmaceutical Technology
Published in:
Čes. slov. Farm., 2009; 58, 14-20
Category:
Original Articles
Overview
The paper deals with a study of tensile strength and disintegration time of compacts from directly compressible fructose Advantose FS 95 and its mixture with microcrystalline cellulose Vivapur 102 in the ratio of 1 : 1 in dependence on compression force. It examines the effect of an addition of two lubricants, magnesium stearate and sodium stearyl fumarate, in the concentration of 1%, and an addition of active ingredients, ascorbic acid and acetylsalicylic acid in the concentration of 50%. In the case of Advantose FS 95, no statistically significant difference was observed in the values of strength of compacts within the type of the lubricant employed with an exception of the compression force of 10 kN, where the strength of tablets was lower with sodium stearyl fumarate. Compacts from the mixture of Advantose FS 95 with Vivapur 102 had a higher strength. Its values were markedly decreased by the action of lubricants, more in the case of magnesium stearate. Disintegration time of tablets in the case of Advantose FS 95 was prolonged more by magnesium stearate and in the case of the mixture of dry binders, sodium stearyl fumarate. Tablets with acetylsalicylic acid had a significantly higher strength and also a longer disintegration time than those with ascorbic acid.
Key words:
Advantose FS 95 – Vivapur 102 – lubricants – tensile strength of tablets – disintegration time of tablets
Introduction
Directly compressible sugars and sugar alcohols are employed as fillers for the manufacture of both normal and chewable tablets by direct compression. These substances by themselves are difficult to compress and that is why in direct compression they are used in their physically modified form, or in the form of coprocessed dry binders, in which the compression and flow properties are improved by an addition of a small amount of other auxiliary substance 1). Most coprocessed products consist of a large amount of brittle material, such as sugars, and a smaller amount of a plastically deforming material, such as cellulose or starch, fixed between or upon the particles of the brittle material. Plasticity, present in ductile materials, is important for the creation of a large area of contact 2). However, ductile materials are sensitive to lubricants and have high compaction speed sensitivity due to their viscoelasticity. During postcompaction relaxation of tablets compressed from ductile materials, stored elastic energy will break bonds and increase tablet porosity, which results in a lower tablet strength 3). In coprocessed filler-binders, the plastic material will be responsible for the good bonding properties because it creates a continuous matrix with a large surface area for bonding. The presence of a large amount of brittle material prevents the storage of too much elastic energy during compression, which results in a smaller stress relaxation. Moreover, the lubricant sensitivity will be low. Breakage of the coprocessed particle and the presence of a large amount of brittle material will prevent the formation of a coherent lubricant network 1).
An example of a directly compressible filler of this type is Advantose FS 95, which is a coprocessed dry binder, directly compressible fructose with a 5% addition of starch. Fructose as such is sweeter than saccharose, dextrose, and the sugar alcohols mannitol and sorbitol, which are employed also as tablet fillers 4). Fructose in tablets efficaciously masks unpleasant tastes, but tablets of pure fructose of an appropriate strength and wear can be directly compressed at a relatively slow speed. In this case, in order to achieve suitable parameters of direct compression, it is suitable to combine crystalline fructose and sorbitol in the ratio of 3 : 1, or regranulate it with 3.5% povidon 5). Fructose is well soluble in water, it is also hygroscopic, and it absorbs a significant amount of moisture at a relative humidity higher than 60% 4).
A combination of fructose with a 5% addition of starch yielded a coprocessed dry binder with excellent properties for the use in direct compression, the above-mentioned Advantose FS 95. An improved physical form of the particles and a more advantageous distribution of particle size significantly improved the flowability of the material. The product also possesses lower hygroscopicity than the standard fructose. Compressibility of the material exceeds that of directly compressible dextrose and saccharose. The material is more than two times more soluble than saccharose and by 20% more sweetening, so it masks the unfavourable taste of active ingredients in an excellent way 6).
The paper aimed to study the properties of tablets made from directly compressible fructose Advantose FS 95, specifically the tensile strength and disintegration time of compacts in dependence on compression force, addition of two types of lubricants in one concentration and two types of model active ingredients. The mixtures of Advantose FS 95 and microcrystalline cellulose Vivapur 102 in the ratio of 1 : 1 underwent the same evaluation.
EXPERIMENTAL PART
Materials
Advantose™ FS 95 fructose – co-dried system of fructose (95%) and of starch (5%) (SPI Pharma Group, France);
Vivapur®102 – microcrystalline cellulose (J. Rettenmaier & Söhne GmbH + Co, Rosenberg, Germany);
magnesium stearate (Acros Organics, New Jersey, USA);
Pruv® – sodium stearyl fumarate (J. Rettenmaier & Söhne GmbH+Co, Rosenberg, Germany);
acetylsalicylic acid (Schűtz and Co. GmbH, Hamburg, Germany);
ascorbic acid (Northeast General Pharmaceutical Factory, Shenyang, China).
Preparation of tableting materials and tablets
A list of tableting materials evaluated in the study:
- Advantose FS 95 with 1% magnesium stearate
- Advantose FS 95 with 1% Pruv
- Advantose FS 95 and Vivapur 102 1 : 1
- Advantose FS 95 and Vivapur 102 1 : 1 with 1% magnesium stearate
- Advantose FS 95 and Vivapur 102 1 : 1 with 1% Pruv
- Advantose FS 95 with 50% acetylsalicylic acid and with 1% magnesium stearate
- Advantose FS 95 with 50% acetylsalicylic acid and with 1% Pruv
- Advantose FS 95 with 50% ascorbic acid and with 1% magnesium stearate
- Advantose FS 95 with 50% ascorbic acid and with 1% Pruv
- Advantose FS 95 and Vivapur 102 1 : 1 with 50% acetylsalicylic acid and with 1% magnesium stearate
- Advantose FS 95 and Vivapur 102 1 : 1 with 50% acetylsalicylic acid and with 1% Pruv
- Advantose FS 95 and Vivapur 102 1 : 1 with 50% ascorbic acid and with 1% magnesium stearate
- Advantose FS 95 and Vivapur 102 1 : 1 with 50% ascorbic acid and with 1% Pruv
The substances were mixed gradually always 5 minutes in a stainless steel cube KB 15S (Erweka GmbH, Hausenstamm, Germany). In multicomponent mixtures, the dry binders were mixed first (5 min), then the active ingredient (5 min), and finally the lubricants sodium stearyl fumarate or magnesium stearate were added (5 min). The mixing rate was 17 revolutions per min, the total amount always being 30 g.
All tableting materials were used to produce 16 tablets compressed with the use of a special die with an upper and a lower punch on a material testing equipment T1 – FRO 50 TH.A1K Zwick/Roell (Zwick GmbH & Co, Ulm, Germany). Proper compaction took place by applying the pressure on the upper punch. The tablets were of a cylindrical shape without facets with a diameter of 13 mm and weight 0.5 ± 0.0010 g. Compression velocity was 40 mm/min and compression forces of 10, 12.5 and 15 kN. Mixtures including active ingredients were compressed using only a compression force of 12.5 kN.
Measurement of tensile strength of tablets and evaluation of the lubricant sensitivity of tableting materials
Tensile strength was always evaluated in 10 tablets, first no sooner than 24 hours after compaction. Measurements were performed on a Schleuniger apparatus (Dr. Schleuniger Pharmatron AG, Solothurn, Switzerland), which measured tablet sizes accurate to 0.01 mm and destruction force in N. Tensile strength of tablets was calculated according to Eq. [1]:
where P is tensile strength of tablets (MPa), F is destruction force (N), d is tablet diameter (mm), and h is thickness of the tablet (mm) 7).
LSR (lubricant sensitivity ratio) values, which make it possible to quantify and mutually compare the lubricant sensitivity of tableting materials, were calculated according to Eq. [2]:
where Csu is the crushing strength of tablets without an added lubricant and Csl is the crushing strength with a lubricant. The more this value approaches 1, the more the dry binder is sensitive to an added lubricant from the viewpoint of decreased strength of tablets 8). In the present paper, the values of tensile strength, not those of crushing strength, are used in the equation.
Measurement of disintegration time of tablets
Disintegration times of tablets were evaluated earliest 24 hours after compaction always in 6 tablets. Measurements were performed on an apparatus for the determination of disintegration time of tablets Erweka ZT 301 (Erweka GmbH, Hausenstamm, Germany) following the method described in the chapter Pharmaceutical Technical Procedures in the Ph. Eur. 2005. The test was carried out without discs in the medium of purified water tempered to 37 °C ± 1 °C. The tablet was considered disintegrated at the moment when there was no remainder on the net.
The results of strengths and disintegration times were statistically processed by means of the computer programmes Excel and Qcexpert. Elementary data analysis yielded the mean values with standard deviations, which were plotted into dependences on compression force. In the cases of unclear significance of differences in the values, unpaired t-test at a level of significance of 0.05 was employed.
RESULTS AND DISCUSSION
Advantose FS 95 is a directly compressible fructose with a 5% addition of starch, which improves its tableting characteristics. The paper aimed to study the properties of compacts made from this substance, specifically the tensile strength and disintegration time. Tablets made from Advantose FS 95 with microcrystalline cellulose Vivapur 102 in the ratio of 1 : 1 were also evaluated. The influential factors included compression force, addition of two types of lubricants, magnesium stearate and sodium stearyl fumarate in the concentration of 1%, and an addition of two types of model active ingredients, ascorbic acid and acetylsalicylic acid in the concentration of 50%. Compression forces were selected with regard to assumed decreases in strength in tablets with active ingredients, i.e. in such a way that the strength of tablets from Advantose FS 95 with lubricants and its mixture with Vivapur 102 ranged around or above the upper limit of the optimal tensile strength of tablets (1.11 MPa) 9). Specifically it was 10, 12.5 and 15 kN, the mixtures with active ingredients being compressed only with the use of the compression force of 12.5 kN. Active ingredients were selected on the basis of different mechanisms of compression and different solubility in water.
Figure 1 shows the dependence of tensile strength of tablets on compression force for Advantose FS 95 with lubricants. With an exception of the compression force of 10 kN, where the strength of compacts is lower with Pruv, within the framework of the type of the lubricant employed there is no statistically significant difference between the values. The same dependence is shown in Figure 2, this time for the mixture of Advantose FS 95 and Vivapur 102 in the ratio of 1 : 1. In this case also a mixture of dry binders without a lubricant was compressed, because it was possible thanks to the lubricating effect of microcrystalline cellulose. Compacts made from pure Advantose FS 95 could not be compressed without a lubricant due to high friction of the substance during compression and its sticking to the punches. In the mixture of Advantose FS 95 and Vivapur 102, plastic deformation of microcrystalline cellulose was observed, because in the substances with this mechanism of compression there occurs a decrease in the strength of compacts due to the added lubricant 10). This decrease was more marked in the case of added magnesium stearate. The values of LSR (lubricant sensitivity ratio) were thus higher for magnesium stearate and in the case of both lubricants they decreased with compression force (see Table 1). Figure 3 compares the strength of compacts from Advantose FS 95 and its mixture with Vivapur 102. Unambiguously the highest strength was observed in the tablets from a pure mixture of dry binders without lubricants. A mixture of dry binders yields stronger tablets even with lubricants and with a significantly lower strength due to magnesium stearate. Here the role is played most probably by a larger specific surface of magnesium stearate, which is important for covering of the intersurfaces of plastically deformed microcrystalline cellulose, the presence of which causes a decrease in strength.



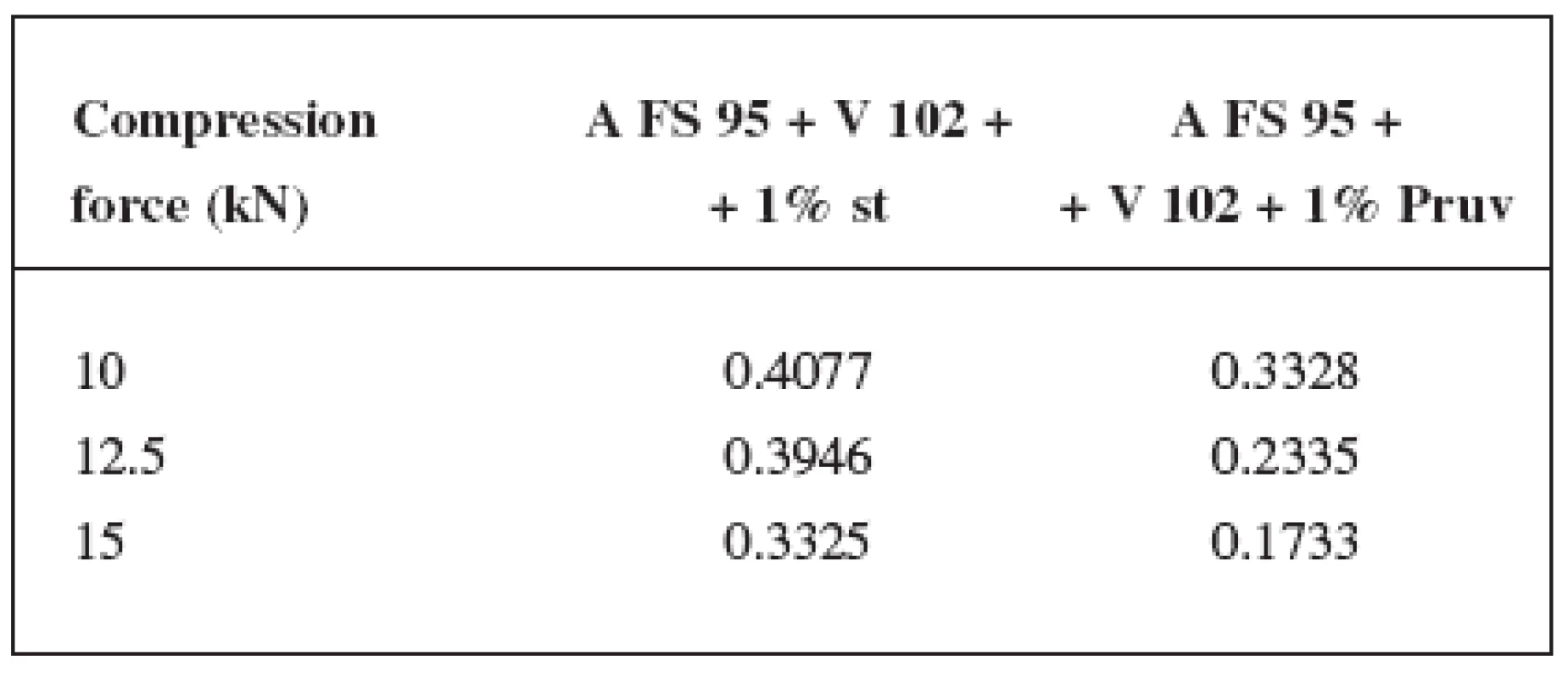
Figure 4 presents the dependence of the disintegration time on compression force for Advantose FS 95 with lubricants. A shorter disintegration time occurs in tablets with Pruv, which would correspond to smaller hydrophobicity of this lubricant. A growing dependence was recorded only between the compression forces of 12.5 and 15 kN. An identical dependence, but for the compacts from the mixture of Advantose FS 95 and Vivapur 102, is shown in Figure 5. An addition of the lubricant to the mixture of dry binders prolonged disintegration time, but in this case the intervention of Pruv was more marked. Due to greater hydrophobicity of magnesium stearate, its greater intervention into disintegration could be expected. In addition, at the compression force of 15 kN no statistically significant difference between the values for the pure mixture and the mixture with magnesium stearate was observed. Figure 6 compares the dependences of disintegration time of tablets on compression force for Advantose FS 95 and its mixture with Vivapur 102. It follows from the above-mentioned observations that the longest disintegration time was recorded in tablets made from the mixture of dry binders with Pruv. The shortest disintegration time of compacts was not identical for all compression forces, at the compression force of 10 kN the shortest disintegration time was recorded for the mixtures of dry binders without lubricants, at the compression forces of 12.5 and 15 kN it was observed in Advantose FS 95 with Pruv.



The strength of tablets with active ingredients is shown in Figure 7. The compacts with acetylsalicylic acid are significantly stronger, both in the cases of Advantose FS 95 alone and the mixture of dry binders. The strengths of these compacts are at the upper limit of the optimal strength of tablets (0.56–1.11 MPa) 9), identically as the only value of tablets with ascorbic acid, which is in the case of the mixture of the active ingredient and dry binders without lubricants. Other compacts containing ascorbic acid possess very low strength at the given compression force, below the limit of the optimal strength. Plastic deformability of acetylsalicylic acid enabled good compressibility of the mixtures of this active ingredient with Advantose FS 95 and its mixture with Vivapur 102. The compression force of 12.5 kN thus yielded compacts with sufficient strength. Ascorbic acid is compressed mainly with the use of the mechanism of the fragmentation of particles11).

Disintegration time of compacts is shown in Figure 8. Compacts containing acetylsalicylic acid had a markedly longer disintegration time not only due to their greater strength, but also due to bad solubility of the active ingredient in water, in contrast to ascorbic acid, which is well soluble in water. Extremely long disintegration times were observed in compacts with pure Advantose FS 95 and with lubricants (with Mg-stearate, 59 min; with Pruv, 91 min). These values are not shown in the Graph as the significantly lower values would not be visible there. The disintegration times of compacts from the mixtures of Advantose FS 95 and Vivapur 102 clearly show the disintegrating effect of microcrystalline cellulose.

The study was supported by the grant MSM 0021620822 and by the firm SPI Pharma group and J. Rettenmaier & Söhne GmbH + Co, which supplied the samples of the dry binders tested.
Received 5 November 2008 / Accepted 20 December 2008
Address for correspondence:
PharmDr. Jitka Mužíková, PhD.
Department of Pharmaceutical Technology, Charles University in Prague, Faculty of Pharmacy
Heyrovského 1203, 500 05 Hradec Králové
e-mail: muzikova@faf.cuni.cz
Sources
1. Bolhuis, G.K., Armstrong, N. A.: Pharm. Dev. Technol., 2006; 11, 111–124.
2. Van der Voort Maarschalk, K., Bolhuis, G.K.: Pharm. Technol., 1999; 23, 34–42.
3. Van der Voort Maarschalk, K., Vromans, H., Bolhuis, G.K., Lerk, C. F.: Eur. J. Pharm. Biopharm., 1996; 42, 49–55.
4. Kibbe, A. H.: Handbook of pharmaceutical excipients. 3rd Ed. London: APhA Washington and PhP 2000; 210–212.
5. Osberger, T. F.: Pharmaceut. Technol., 1979; 3, 81–86.
6. SPI Pharma: Advantose ™FS 95 Fructose, Firm. lit. – http://www.spipharma.com/ ProductsFolder/103Advantose /103/Advantose95.html (2007).
7. Fell, J. T., Newton, J. M.: J. Pharm. Sci., 1970; 59, 688–691.
8. Bos, C. E., H. Bolhuis, H., Van Doorne, Lerk, C. F.: Pharm. Weekbl., 1987; Sci. Ed. 9, 274–282.
9. Belousov, V. A.: Khim. Farm. Zh., 1976; 10, 105–111.
10. Jarosz, P. J., Parrott, E. L.: Drug Dev. Ind. Pharm., 1984; 10, 259–273.
11. Bolhuis, G. K., Z. T. Chowhan, Z. T.: Materials for direct compaction. In: Alderborn, G, Nystrőm, Ch. (eds.) Pharmaceutical Powder Compaction Technology, Inc.: New York: Marcel Dekker 1996; 419–500.
Labels
Pharmacy Clinical pharmacologyArticle was published in
Czech and Slovak Pharmacy

2009 Issue 1
-
All articles in this issue
- The standard prescriptions for the preparation of pharmaceuticals in pharmacies II. Proposals for innovation and standardization of prescriptions for dermal treatment
- Chitosan pellets produced by extrusion-spheronisation
- Pharmacotherapy of cardiovascular diseases in the population of the Czech Republic
- A study of the properties of compacts from directly compressible fructose
- Evaluation of pellet friability
- Czech and Slovak Pharmacy
- Journal archive
- Current issue
- About the journal
Most read in this issue
- The standard prescriptions for the preparation of pharmaceuticals in pharmacies II. Proposals for innovation and standardization of prescriptions for dermal treatment
- Pharmacotherapy of cardiovascular diseases in the population of the Czech Republic
- Chitosan pellets produced by extrusion-spheronisation
- Evaluation of pellet friability


