The use of authorized medicinal products for human use in veterinary practice in the Czech Republic
Používání humánních hromadně vyráběných léčivých přípravků ve veterinární praxi v České republice
Cílem studie bylo vyhodnotit preskripční zvyklosti spojené s použitím humánních hromadně vyráběných léčivých přípravků (HVLP) při léčbě zvířat. Byla provedena analýza humánních HVLP pro perorální podání předepsaných jak ve veterinárních ordinacích na Veterinární a farmaceutické univerzitě Brno (VFU), tak i mimo ně (v terénu). Byly použity dotazníky (11 terénních veterinárních lékařů) a rozbor dat o výdejích humánních přípravků v lékárně VFU (2006–2007; veterinární recepty vystavené na univerzitních pracovištích). Hodnoceny byly přítomnost údajů o dávkování v Českém lékopisu, farmakoterapeutická skupina a existence ekvivalentů v sortimentu veterinárních HVLP. V předpisech z ordinací na VFU se vyskytovalo: 243 různých humánních HVLP (202 druhů) obsahujících 168 různých účinných látek zařazených do 47 farmakoterapeutických skupin. Doporučené terapeutické dávky byly nalezeny v Českém lékopisu pro 127 účinných látek. Třicet tři druhů humánních HVLP (16 %) by mohlo být nahrazeno analogickými veterinárními HVLP. Terénní vetrinární lékaři používali pouze 152 humánních HVLP, tj. 112 druhů (55 % druhů HVLP používaných veterinárními lékaři na VFU), což představuje 106 účinných látek (63 % ze spektra používaného veterinárními lékaři na VFU), zařazených ve 34 farmakoterapeutických skupinách (72 %). Pro 56 % ze 106 předepsaných účinných látek neudává Český lékopis doporučené dávkování. Z nalezených 24 druhů humánních HVLP by mohlo být 21 % nahrazeno nahrazeno analogickými veterinárními HVLP. Preskripce na žádankách (VFU) splňuje kritéria racionální farmakoterapie (většinou pro jednu léčivou látku – jeden HVLP druh) a dodržování právních předpisů (“terapeutické kaskády”). Studie ukázala, že existují rozdílné přístupy k používání humánních HVLP ve veterinární praxi mezi veterinárními lékaři na univerzitních pracovištích a v terénu.
Klíčová slova:
veterinární lékař – lékárník – humánní léčivý přípravek – veterinární léčivý přípravek – dávkování
Authors:
B. Macešková; L. Smejkalová; P. Brauner; J. Frenzelová
Authors‘ workplace:
Veterinary and Pharmaceutical University Brno
Published in:
Čes. slov. Farm., 2010; 59, 256-262
Category:
Original Articles
Overview
The aim of this study was to evaluate the patterns in using authorized medicinal products for human use (HMPs) in treating animals. An analysis of the HMPs for oral administration prescribed both at the veterinary surgeries at the University of Veterinary and Pharmaceutical Sciences Brno (VFU) as well as outside the university (rural) was accomplished. Questionnaires (11 small animal practice surgeons) were processed and an analysis of the data on the human medicines dispensed at the pharmacy at VFU (2006–2007; veterinary prescriptions written in the university settings) was performed. The presence of dosage instructions in the Czech Pharmacopoeia, the pharmacotherapeutic group and the presence of equivalents among the VMPs (authorized veterinary medicinal product) were taken into consideration. The prescriptions at the surgeries at VFU comprised: 243 human HMPs (202 kinds) containing 168 different active substances involved in 47 pharmacotherapeutic groups. The recommended therapeutic dosage is stated in the Czech Pharmacopoeia for 127 substances. Thirty three of the HMP kinds (16%) could be replaced by the analogical VMPs. The small animal practice surgeons use only 152 HMPs, i.e. 112 kinds (55% of the HMP kinds used in VFU surgeries), which represents 106 active substances (63% of the scope prescribed in VFU surgeries) and 34 (72%) pharmacotherapeutic groups. For 56% of the 106 prescribed substances, no dosage is stated in the Czech Pharmacopoeia. Twenty four of the HMP kinds (21%) could be replaced by analogical VMPs. The prescription originated from order forms (VFU) shows the criteria of rational pharmacotherapy (mostly for one active substance – one HMP kind) and of the adherence to the law (“therapeutic cascades”). This study indicates that there exist different patterns in using HMP in veterinary practice among veterinary surgeons working at the University and in the small animal practice settings.
Key words:
veterinary surgeon – pharmacist – medicinal product for human use – veterinary medicinal product – dosage
Introduction
Using drugs in treating animals is an integral part of veterinary medicine. The production of veterinary drugs is controlled worldwide 1). The main European directive is the Directive 2001/82 EC (as amended by the Directive 2004/28 EC and in the wording of the latest amendments) 2). It defines the requirements for correct veterinary drugs production. The dispensation of the required drugs is performed by veterinary surgeons in compliance with Act No 378/2007 Code of Law (CR) 3) and Act No 166/1999 Code of Law (CR) 4), if they have the necessary product. If not, they prescribe it and pharmacists dispense the medication (Reg. No 54/2008 Code of Law 5)). The dispensation of less common human and veterinary authorized medicinal products and all “magistral formula” drugs lies within the pharmacists’ authority. Communication between veterinary surgeons and pharmacists is very sporadic as can be seen in the Czech surveys, as well as in those from abroad 6). The number of prescribed veterinary “magistral formula” drugs is very small. The use of authorized medicinal products for human use (HMPs) is subject to the law both abroad 7) and in the Czech Republic, where there exists therapeutic “extra label” usage, which means that the drug is administered with no reference to either the authorized documentation or to the instructions established by the producer 8). The veterinary surgeon is not prohibited from using a necessary medicinal product, if the preconditions are accomplished (Reg. No 344/2008 Code of Law CR 9)). The correct dosage for a particular animal species appears, then, to be the most important question. The information source has always been and still is the formulary literature (the former editions of the Czech Pharmacopoeia and the one valid in the time of this research, Pharmacopoea Bohemica 2005 10), Ph.B. MMV including the Addenda11–13)), where the recommended therapeutic dosages for particular animal species and different ways of administration of common active substances are introduced. The European Pharmacopoeia (Ph. Eur. 6.0) 14) does not state the dosage for animals. The most comprehensive and detailed information can be found in sources from abroad, such as US Pharmacopeia (2008) 15) and its Veterinary Pharmaceutical Information Monographs, VetBase 4 (2007) 16) and its instructions for 800 active substances applying to 130 animal species, Sounders Handbook of Veterinary Drugs 17) or VPR V2.6 18) where the dosage calculator and interaction descriptions are included. Some information is also conveyed by magazines, e.g. in the USA 19). The dosage of each authorized veterinary medicinal product is mentioned in detail in the Summary Product Characteristics (SPC). Abroad, some obligatory rules for the “magistral formulas” of veterinary drugs as well as the algorithms for the “off label” use of human drugs in veterinary practice can be found at pharmacies 9).
Aims of the study
- formulary literature analysis with regard to the recommended dosages for animals,
- survey of the range of authorized medicinal products for human use (HMPs) prescribed by veterinary surgeons,
- analysis of the active substances from the aspect of the dosage,
- evaluation of the found drugs collection from the therapeutic cascade standpoint.
EXPERIMENTAL PART
Methodology
All the publications of the Pharmacopoeia (Formulary) valid in the Czech (Czechoslovak) Republic since 1947 (including the Addenda) 20–33) were included in the analysis. The results are summarized in Tables.
The authorized medicinal products for human use (HMPs) range used in veterinary medicine was investigated in detail:
- a questionnaire survey of 11 veterinary surgeons working at rural veterinary care centers (small animal practice) in North Moravia, 2002;
- analysis of the electronic records of the dispensation of the authorized medicinal products for human use (HMPs) prescribed on veterinary prescriptions at the pharmacy on the premises of VFU, 2006–2007.
The information on the dosage of the active substances included in particular authorized medicinal products for human use (HMPs) and on their possible analogs in the range of the authorized veterinary medicinal products (VMPs) distributed in the Czech Republic was found with the help of the following resources:
- Automated System of Medicinal Products (ASMP), publication 2008.4 34),
- Czech Pharmacopoeia 2005 10) and its Addenda 2006, 2007 11, 12),
- Authorized Veterinary Medicinal Products 2007 35).
RESULTS
The analysis of editions of the Czech (Czechoslovak) Pharmacopoeia
The analysis of eight Czech (Czechoslovak) Pharmacopoeia publications and their Addenda revealed that all the editions except Czechoslovak Pharmacopoeia 1st ed. (PhBs I) and Czechoslovak Pharmacopoeia 4th ed. (PhBs IV) introduced instructions for veterinary medicinal products dosages (Table 1). PhBs I states that there are 151 active substances that cannot be dispensed without a medical (veterinary) prescription. The Czechoslovak Pharmacopoeias use the term “the highest dosages”. This dosage cannot be exceeded by the pharmacist unless a veterinary surgeon asks for it on the prescription. The Pharmacopoeias based on particular editions of the European Pharmacopoeia (Ph. B. MCMXCVII 28), Ph. B. MMII 30), Ph. B. MMV 10), Ph. B. MMIX 33)) supplemented with “national specificities” use the term “recommended therapeutic dosage used in animals” defined as “the average dosage”, with the postscript that “the veterinary surgeon often prescribes different dosages depending on the circumstances”.
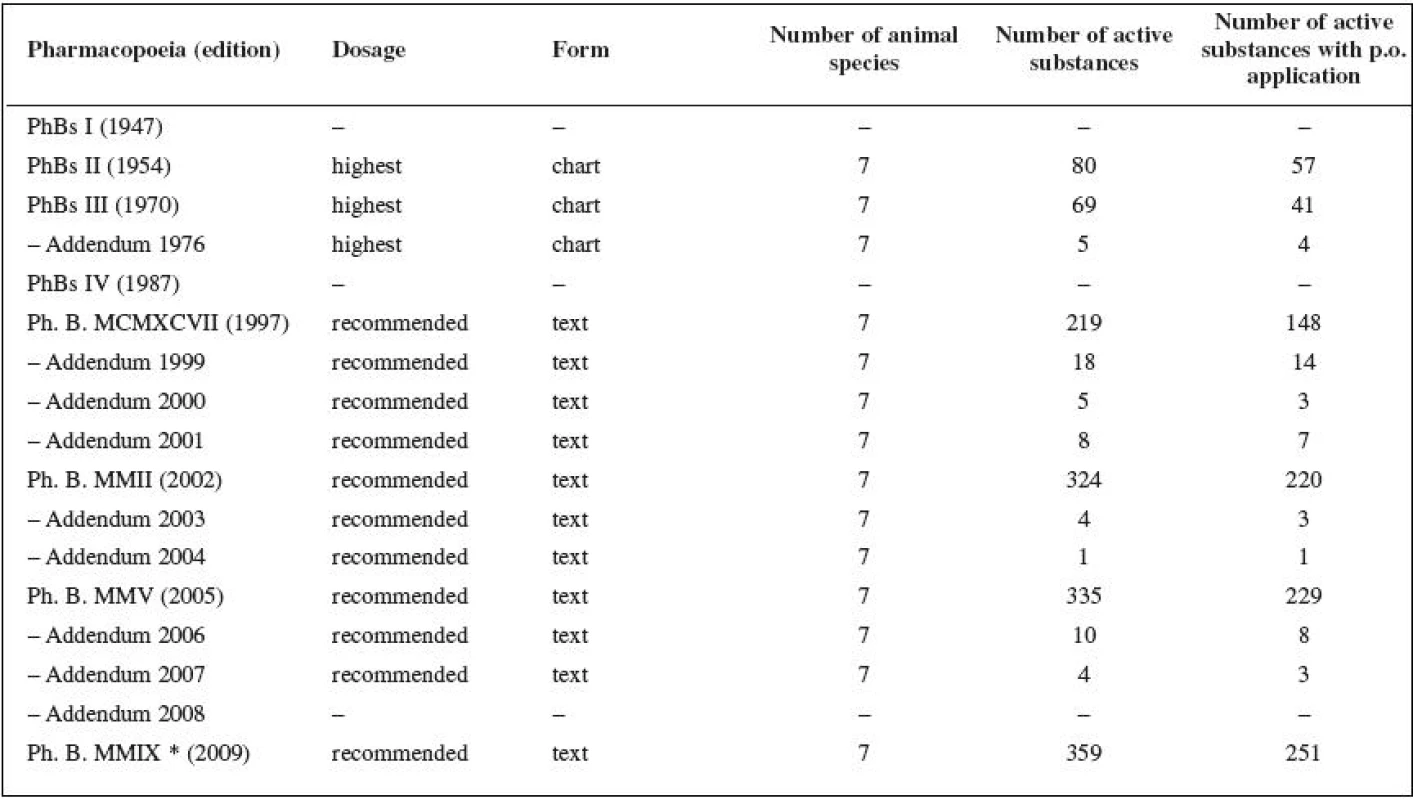
Prescribers’ behaviour evaluation
For the purpose of this study, the number of prescribed HMPs was transformed to the number of HMP kinds, which means that the different packages sizes and strengths of the same HMP were considered to be one HMP kind.
The questionnaire survey (outside VFU premises prescriptions)
152 different HMPs (i.e. 112 kinds) representing 106 different active substances out of 34 pharmacotherapeutic groups were identified, assuming that HMPs with different concentrations and sets are considered as one medicinal products kind. The recommended therapeutic dosage in animals is included in the Pharmacopoeia valid at that time for 47 of the aforementioned substances. Twenty four of the HMP kinds identified have analogs in the range of the authorized veterinary medicinal products (VMPs) in the same or a similar form (e.g. pills = pellets, capsules).
Analysis of archival data (ambulatory centres and clinics of VFU)
The analysis is focused on the electronic records from February 2006 to April 2007 collected in the pharmacy located in the campus (VFU).
File “VFU – Order Forms”
This file contains 114 different HMPs (i. e. 107 kinds) with 100 different active substances out of 42 pharmacotherapeutic groups. The recommended therapeutic dosages for 65 of the used active substances are introduced in the Pharmacopoeia valid at that time. There are analogs of the same or a similar form of medicine for 14 of the HMP kinds found in the range of the authorized veterinary medicinal products (VMPs).
The “magistral formula” drugs for oral application were assessed as well. Seven different “magistral formula” drugs with five different active substances were found. The recommended therapeutic dosage involved in Ph. B. MMV 10) (including the Addenda 11–13)) was found for two active substances.
File “VFU – Prescriptions”
243 HMPs (202 kinds) out of 47 pharmacotherapeutic groups (with 168 different active substances) were found in this file. The recommended therapeutic dosage for 127 of the used active substances is stated in the Pharmacopoeia valid at that time (Ph. B. MMV 10), including the Addenda 11–13)). There are analogs of the same or a similar drug form for 33 of the found HMP kinds in the range of the authorized veterinary medicinal products (VMPs).
The comparison of the found results is shown in Figures 1 and 2.


The summary of the data from the veterinary prescriptions brought the following facts to light:
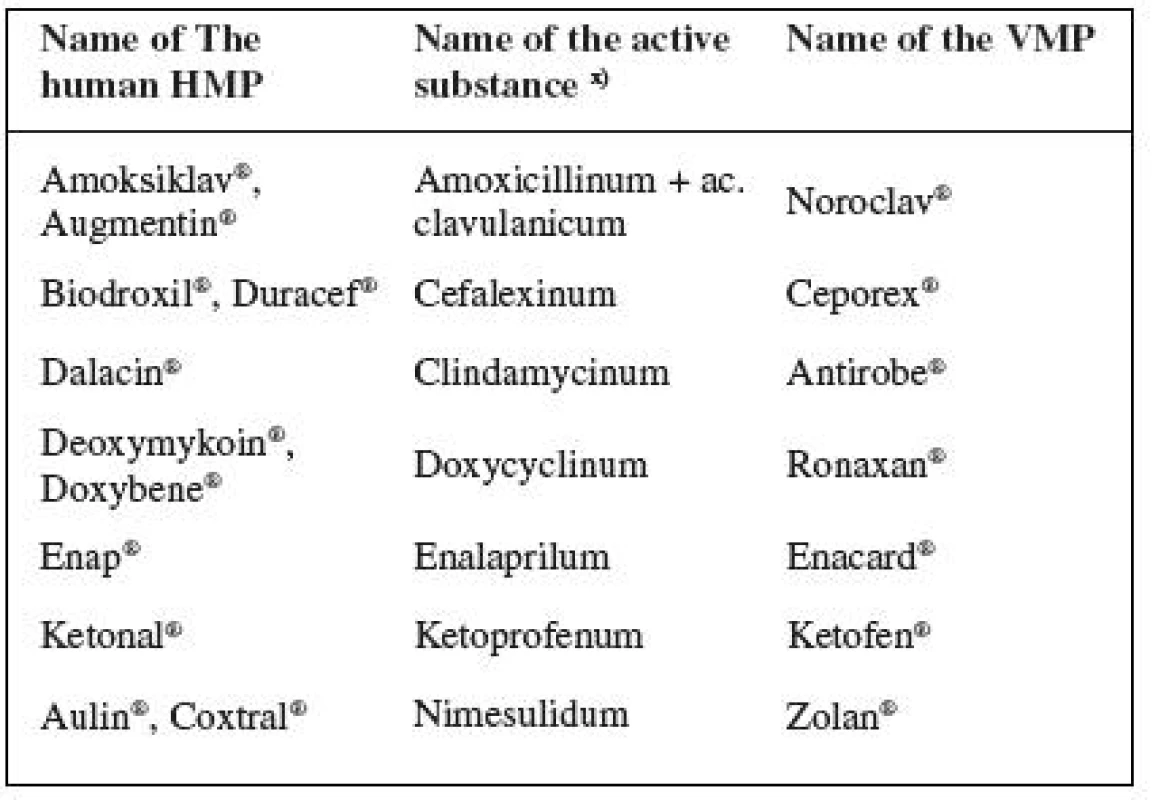
1. Prescribed authorized medicinal products for human use (oral administration) that do have analogs in the range of authorized veterinary medicinal products are listed in Table 2.
2. Active substances often prescribed in the form of authorized medicinal products for human use (HMPs) on the grounds that there are no authorized veterinary medicinal products (VMPs) available (oral administration): acetylcysteinum, aciclovirum, allopurinolum, ambroxololum, cetirizinum, ciprofloxacinum, diazepamum, diltiazemum, famotidinum, furosemidum, ibuprofenum, metoprololum, pancreatinum, phenobarbitalum, piroxicamum, zolpidemum.
Ph. B. MMV 10) (including Addenda11–13)) states the dosages for animals for 11 of the above listed substances.
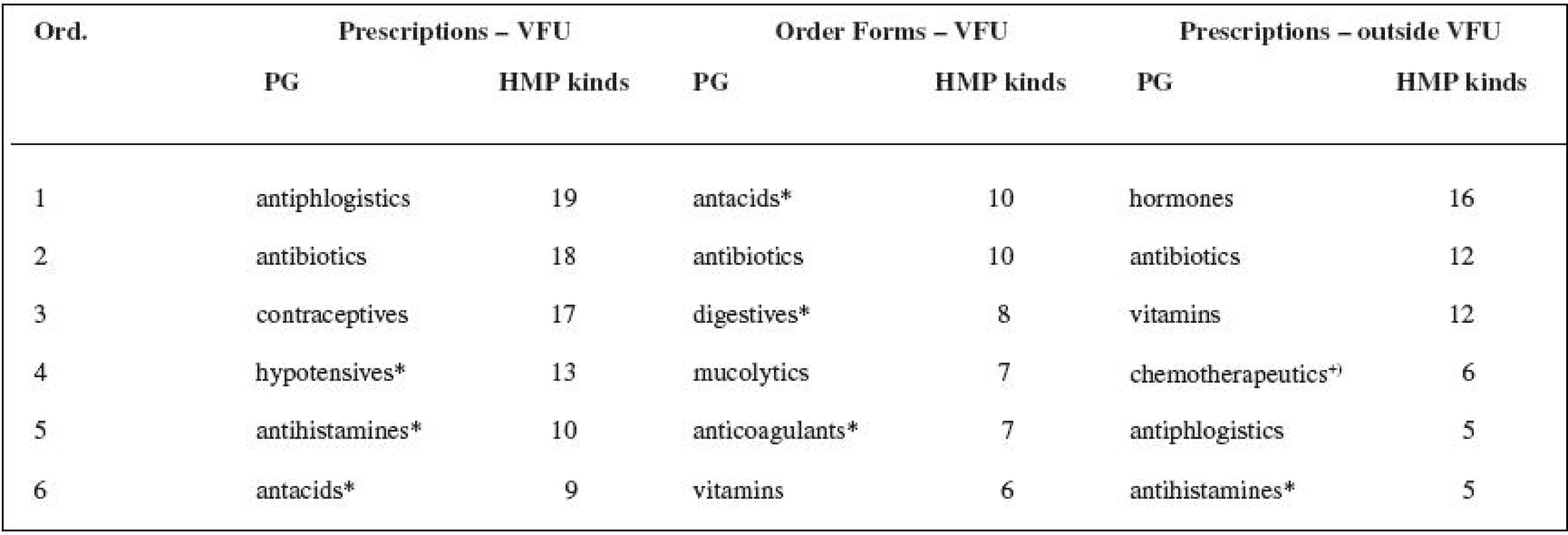
3. Assessment of pharmacotherapeutic groups (defined in Automated System of Medicinal Products 34) prescribed by veterinary surgeons, see Table 3.
DISCUSSION
The majority of the Pharmacopoeias issued in the Czech (Czechoslovak) Republic contains information on the drug dosage for animals. The spectrum of animal species has not changed for more than 60 years, but the range of the drugs used is continually increasing (by 64% in the last 20 years). Compared both with the European Pharmacopoeia valid nowadays and with its previous editions, there is no similar detailed information. Nevertheless, veterinary surgeons can use other sources which provide instructions on the dosage for a large number of drugs in a wider range of animal species than those covered by the valid Pharmacopoeia edition. The Formulary Board – Department of Veterinary Medicinal Products – has repeatedly dealt with the problem of the further need in the medicinal table VI. Recommended therapeutic dosages in animals. The newest edition of the Czech Pharmacopoeia (Ph. B. MMIX33)) continues to keep it. From the practical viewpoint, the more important fact is that the dosage of each authorized veterinary medicinal product is mentioned in detail in Summary Product Characteristics (SPC).
The number of the active substances found in the research shows that there is and has been a lack of the authorized veterinary medicinal products (VMPs) needed for therapeutic practice both in the past and nowadays. The small animal practice surgeons used authorized medicinal products for human use (HMP kinds) in 21% of the cases when analogous authorized veterinary medicinal products were available, the veterinary surgeons at VFU in 16% for prescriptions and in 13% for order forms. The total number of authorized veterinary medicinal products (VMPs) that are available did not change significantly during the research: 2002 – 1307 VMPs, 2006 – 1279 VMPs (Authorized Veterinary Medicinal Products 2007)35). The small animal practice surgeons more often prescribed drugs containing active substances which have no recommended dosages in the valid Pharmacopoeia – 56% of all prescribed authorized medicinal products for human use (HMP kinds). The same cases appeared at VFU in 24% (prescriptions), or 35% (order forms). The small animal practice surgeons use, for their prescriptions, 55% of the range of the authorized medicinal products for human use (HMP kinds) prescribed by the veterinary surgeons at VFU (which represents 63% of the active substances and 72% of the pharmacotherapeutic groups found in veterinary prescription at VFU). The veterinary practice outside VFU is probably based on a smaller range of drugs according to the previous experience of the veterinary surgeon, and apparently it was not influenced by the marketing efforts of the pharmaceutical companies producing generic medicinal products in the period of the study. The prescription originated from the order forms (VFU) reveals the criteria of the rational pharmacotherapy (mostly for one active substance – one HMP kind) and of the adherence to the law (“therapeutic cascades”). The most frequent pharmacotherapeutic groups from the whole range of prescribed human medicinal products are: antibiotics, hormones, vitamins, antacids including antiulcer drugs, antiphlogistics, and hypotensive drugs. Four out of six of the most frequent pharmacotherapeutic groups of authorized medicinal products for human use (HMPs) are common for both groups of veterinary surgeons. Some pharmacotherapeutic groups are not available in the range of the authorized veterinary medicinal products (VMPs) at all. If they do appear there, there are no medicinal products with the indicated substances involved in these groups. The reason for the use of the authorized medicinal products for human use (HMPs) in the other cases, which means when there is an analogous VMP (the most frequent groups are antibiotics, hypotensive drugs, antiphlogistics), can be both a professional decision and also a decision guided by the different price of the same product in human and veterinary forms.
In conclusion, the range of authorized medicinal products for human use (HMPs) used in veterinary medicine is rather extensive. There may be various reasons for this, such as that it would be uneconomical to produce an analogous VMP, or that there is at that moment a shortage of the required VMP. Authorized medicinal products for human use (HMPs) when used for animals always follow the “off label” use rules. Although such prescriptions are always issued in accordance with the contract between the veterinary surgeon and the animal owner, the pharmacist should be able to give some professional advice as well as some necessary information when the HMP is actually dispensed. A large number of drugs are commonly used, yet still the pharmacist does not have enough reliable sources of information and has to look up the appropriate references abroad. The use of authorized medicinal products for human use (HMPs) in veterinary practice, especially in small animal practice, often becomes the only reasonable therapeutic solution. Veterinary surgeons at VFU prescribe a wide range of authorized medicinal products for human use (HMPs). The dosage of the contained substances is most often included in commonly available literary sources, and they use less often those authorized medicinal products for human use (HMPs) whose analogs can be found in the range of the authorized veterinary medicinal products (VMPs), as compared with the small animal practice surgeons. This study indicates that there exist different patterns in using authorized medicinal products for human use (HMPs) in veterinary practice among veterinary surgeons working at the University and in the small animal practice.
Received 18. November 2010 / Accepted 15. October 2010
Address for correspondence:
PharmDr. Lenka Smejkalová, Ph.D.
Department of Applied Pharmacy
Faculty of Pharmacy VFU
Brno
Palackého 1–3,
612 42 Brno
e-mail:
smejkaloval@vfu.cz
Sources
1. Snirc, J., Sokol, J., Seginko, J., Hera, A.: Clinical veterinary pharmacology. Martin: Neografia 2007.
2. Directive 2001/82 EC (as amended by Directive 2004/28 EC and in the wording of the latest amendments. http://www.uskvbl.cz/id_menu = 144 (18. 3. 2008).
3. Act No 378/2007 Code of Law (CR).
4. Act No 166//1999 Code of Law (CR).
5. Reg. No 54/2008 Code of Law (CR).
6. Kernan, F.: Veterinarian and Pharmacist – yesterday, today and tomorrow. Can. Vet. J., 1997; 18, 265 – 273.
7. Medicines, Ethics and Practice: A Guide for Pharmacists and Pharmacy Technicians. Section 1.8: Medicines for veterinary use. Section 1.9: Alphabetical list of medicines for veterinary use. http://www.rspgb.org.uk (18. 3. 2008).
8. Lust, E.: Compounding for Animal Patients: Contemporary Issues. J. Am. Pharm. Assoc., 2004; 44, 375–386.
9. Reg No 344/ 2008 Code of Law (CR).
10. Czech Pharmacopoeia 2005. 1st ed. Praha: Grada Publishing 2005.
11. Czech Pharmacopoeia 2005, Addendum 2006, part II. 1st ed. Praha: Grada Publishing 2006.
12. Czech Pharmacopoeia 2005, Addendum 2007 1st ed. Praha: Grada Publishing 2007.
13. Czech Pharmacopoeia 2005, Addendum 2008 In: Vestnik SÚKL 2008; 7, 22.
14. European Pharmacopoeia (Ph. Eur. 6.0. http://www. edqm.eu (18. 3.2008).
15. US Pharmacopeia. www.usp.org (18. 3. 2008).
16. VETBASE 4 http://www.petalk.com (10. 9. 2007).
17. Papich, M.: Saunders Handbook of Veterinary Drugs. 2nd ed. Edinburgh: Elsevier Saunders 2007.
18. VPR V2.6. http://www.vproline.com (11. 9. 2007).
19. Lust, E.: Veterinary Information for Pharmacists. US Pharmacist., 2004; 29, 16–23 www.uspharmacist.com. (18. 3. 2008).
20. Czechoslovak Pharmacopoeia 1st ed. Praha: State Printing – office 1947.
21. Czechoslovak Pharmacopoeia 1st ed. Addendum. Praha: Health Care – Publishing 1952.
22. Czechoslovak Pharmacopoeia 2nd ed. Praha: Health care – Publishing 1954.
23. Czechoslovak Pharmacopoeia 2nd ed. Addendum. Praha: Health care – Publishing 1959.
24. Czechoslovak Pharmacopoeia 3rd ed. part I. Praha: AVICENUM 1970.
25. Czechoslovak Pharmacopoeia 3rd ed. Addendum. Praha: AVICENUM 1976.
26. Czechoslovak Pharmacopoeia 4th ed. part I. Praha: AVICENUM 1987.
27. Czechoslovak Pharmacopoeia 4th ed. Addendum. Praha: AVICENUM 1991.
28. Czech Pharmacopoeia 1997 part I. 1st ed. Praha: Grada Publishing 1997.
29. Czech Pharmacopoeia 1997, Addendum 1999 part I. 1st ed. Praha: Grada Publishing 1999.
30. Czech Pharmacopoeia 2002. 1st ed. Praha: Grada Publishing 2002 (electronic version).
31. Czech Pharmacopoeia 2002, Addendum 2003. 1st ed. Praha: Grada Publishing 2003 (electronic version).
32. Czech Pharmacopoeia 2002, Addendum 2004. 1st ed. Praha: Grada Publishing 2004 (electronic version).
33. Czech Pharmacopoeia 2009. 1st ed. Praha: Grada Publishing 2009.
34. Automated System of Medicinal Products (ASMP), publication 2008.4
35. Authorized Veterinary Medicinal Products 2007. Prion: Hradec Králove 2007.
Labels
Pharmacy Clinical pharmacologyArticle was published in
Czech and Slovak Pharmacy

2010 Issue 6
-
All articles in this issue
- Orodispersable dosage forms and technologies used in their production
- A study of the process of homogenization of powder mixtures using NIR spectroscopy
-
Study of local anesthetics. Part 193.
Study of temperature and salt-induced micellization of hepatacainium perchlorate in aqueous solution - On the history of the Pharmacy of the Brothers of Mercy in Skalica till the year 1919
- The use of authorized medicinal products for human use in veterinary practice in the Czech Republic
-
Study of local anaestetics – Part 192*
Formulation of lidocaine into gels with anti-inflammatory effect
- Czech and Slovak Pharmacy
- Journal archive
- Current issue
- About the journal
Most read in this issue
- Orodispersable dosage forms and technologies used in their production
- A study of the process of homogenization of powder mixtures using NIR spectroscopy
- On the history of the Pharmacy of the Brothers of Mercy in Skalica till the year 1919
- The use of authorized medicinal products for human use in veterinary practice in the Czech Republic
