Diclofenac sodium entrapment and release from halloysite nanotubules
Enkapsulace a uvolňování diklofenaku sodné soli z halloysitových nanotubulů
Halloysit je vzhledem ke svému zajímavému tvaru prázdných nanotubulů vhodným materiálem pro poutání různých účinných látek. Tento experiment studoval možnost zachycení aniontového modelového léčiva diklofenaku sodné soli v halloysitových tubulech, a tím zpomalení rychlosti jeho uvolňování. Halloysit může vázat léčiva třemi různými mechanismy: adsorpcí, interkalací a uzavřením v tubulech. Výsledky adsorpčních studií prokázaly určitou adsorpci sodné soli diklofenaku na povrch minerálu i přes jeho trvalý záporný náboj. Výsledky rentgenové práškové difrakce (XRPD) vyloučily interkalační reakci. Nejdůležitějším mechanismem bylo uzavření léčiva do prázdných tubulů s enkapsulační účinností 48,1 %. Uvolňování léčiva z halloysitových tubulů bylo ve srovnání s disolucí čistého léčiva prodloužené. Halloysit samotný i s včleněným léčivem se ukázal být vhodným materiálem pro formování pelet metodou extruze/sferonizace.
Klíčová slova:
halloysit • diklofenak sodná sůl • vázání léčiva • pelety • prodloužené uvolňování léčiva
Authors:
Kateřina Krejčová; Patrick B. Deasy; Miloslava Rabišková
Published in:
Čes. slov. Farm., 2013; 62, 28-34
Category:
Original Articles
Overview
Halloysite was found to have interesting nanotubular geometry viable for the entrapment of various active agents. In this experiment, the ability of hollow halloysite cylinders to entrap the anionic model drug diclofenac sodium and to retard drug dissolution rate was investigated. Drugs could be incorporated into layered tubules via three different mechanisms: adsorption, intercalation and tubular entrapment. Based on the adsorption studies, some diclofenac sodium was shown to be adsorbed to the polyionic mineral surface despite its permanent negative charge. The X-ray powder diffraction analysis (XRPD) results did not prove any intercalation reaction to occur. The most important drug-loading mechanism involved the tubular entrapment with encapsulation efficiency 48.1%. The drug release from halloysite was prolonged in comparison with the dissolution of pure drug. Halloysite itself as well as halloysite loaded with the drug proved to be appropriate material to form pellets by extrusion /spheronization method.
Keywords:
halloysite • diclofenac sodium • drug entrapment • pellets • prolonged drug release
Introduction
Halloysite is a naturally occurring mineral possessing special particle shape of multilayered hollow cylinders with the diameter of 10–50 nm and the length of 2–40 μm1–3). This aluminosilicate clay is mined from natural deposits of several countries in America and Asia. Samples from different regions might slightly differ in dimensions. Halloysite is chemically similar to kaolin, which however has different plate-like structure. Halloysite has two forms, i.e. hydrated (10 Å) form binding a monolayer of water molecules in between the layers (intercalation) and dehydrated (7 Å) form. Molecules of water in hydrated form can be replaced by other small molecules, e.g. glycerin4). Dehydrated form of halloysite cannot be changed to hydrated form by simple exposure of water5), but special procedure involving intermediary intercalation complex with potassium acetate can be used6).
Outer surface area of halloysite is bearing negative charge as determined by zeta potential measurements. Silica is mainly positioned on the outer surfaces of the tubules and its oxide when exposed to water behaves as an acidic oxide giving the negative charge to the surface of tubules over a wide range of pH. This negative surface charge is small at very low pH increasing significantly when pH value of the solution is becoming higher. Aluminium present mainly on the inner surface and edges of the tubules shows more amphoteric behavior after the exposure to water. Based on the outer tubules area, halloysite tends to have a polyanionic surface and should readily bind cationic drugs7).
The third and most important drug loading mechanism involves tubular entrapment. This can be achieved by the “vacuum method” as reported in several papers2, 8–10). Briefly, during this procedure the inner gas is replaced by saturated drug solution following solvent evaporation.
Up to now, several original papers dealing with drug entrapment into halloysite nanotubules were published. These included the encapsulation of oxytetracycline hydrochloride, khellin and nicotinamide adenine dinucleotide2, 8), diltiazem hydrochloride and propranolol hydrochloride9), tetracycline hydrochloride or tetracycline base10). These experiments proved the possibility of entrapment of hydrophilic as well as lipophilic drugs into halloysite nanotubules. Oxytetracycline hydrochloride, dilthiazem hydrochloride and propranolol hydrochloride as representatives of highly soluble hydrophilic drugs were successfully encapsulated into halloysite nanotubules and the drug release was prolonged depending on the drug solubility. The entrapment of khellin, a hydrophobic vasodilator, needed the pretreatment of halloysite with ethylene glycol to permit the encapsulation of hydrophobic drug. Khellin was entrapped in a melted state and then allowed to solidify in the clay. Its release was very slow, as expected, due to drugęs low solubility in water. For encapsulation of some drugs, e.g. nicotinamide adenine dinucleoside, it was found necessary to add a water based polymer, in this case povidone, in order to increase the viscosity of the solution to aid in drug retention.
The aim of this work was to investigate the ability of halloysite tubules to entrap the anionic model drug – diclofenac sodium (DS) and to retard its dissolution rate. Diclofenac sodium, anti-inflammatory drug used in the treatment of chronic inflammatory diseases and in neurology, is administered several times a day due to its rapid systemic clearance. This warrants the use of controlled release formulations to improve the patient compliance11). Following the characterization of the raw aluminosilicate carrier (halloysite, grade G) which is necessary due to the batch-to-batch variability, prolonged release product formation for oral administration via tubular entrapment into the halloysite tubules was examined.
Experimental part
Materials and methods
As starting materials for all experiments, diclofenac sodium (donated by Zentiva, a.s., Czech Republic) and halloysite “G” clay mineral (NZ China Clays Ltd., New Zealand) were used. Methanol (VWR International Ltd., Poole, UK) and purified water were the solvents. For the preparation of dissolution media and buffers, citric acid monohydrate and disodium hydrogenphosphate dodecahydrate (Merck KGaA, Darmstadt, Germany), acetic acid glacial and sodium acetate trihydrate (Sigma-Aldrich Chemie, GmbH, Germany) were used.
Determination of binding curves
50 ml of aqueous drug solutions, containing various amounts of diclofenac sodium were added to 100 mg samples of sieved halloysite “G” powder and stirred for 20 min at 500 rpm. After sample filtration (the first filtrate part was discarded), UV analysis (Hewlett Packard 8452A Diode Array Spectrophotometer, Hewlett Packard Co., USA) was performed at the wavelength of 276 nm. The concentrations of diclofenac sodium in the solution were calculated for each sample using calibration curve. The difference in concentration of the drug solution before and after interaction corresponded to the amount bound to the halloysite. All measurements were performed in triplicate.
Drug loading into halloysite
For drug loading, the method described by Levis and Deasy9), and Kelly et al.10) was adapted. The sieved halloysite “G” clay mineral (< 185 μm ) was loaded (in the ratio 3 : 5) with a 12% w/v drug solution. The wetted halloysite powder was placed in a sealed desiccator vessel, and a vacuum applied (vacuum pump Edwards Model RV5, Edwards High Vacuum International, UK), until all gas bubbles were removed. The halloysite suspensions were subsequently filtrated to remove the residual drug solution. The drug-loaded samples were then dried for 24 hours at the temperature of 60 °C. The dried powders were ground down using a porcelain mortar and pestle and sieved again through a 185 μm sieve. This procedure was repeated once more to ensure that the drug solution had fully filled the inner space of mineral tubules.
Determination of the drug content
The amount of diclofenac sodium within samples (20 mg) was determined by extracting the drug into methanol in a 100 ml Erlenmeyer flask. The samples were kept at 37 °C in a water bath and occasionally shaken. After 72 hours, the total mixture was filtered through a 0.45 μm membrane filter (Supor®-450 membrane filter, Pall Corporation, USA) and the concentration of the drug within the filtrate was determined spectrophotometrically (λ = 276 nm) after reference to a pre-constructed calibration curve. Each determination was carried out in triplicate.
Scanning Electron Microscopy Analysis
The samples were mounted on an aluminium stub using a double-sided sticky tape. The samples were coated with a gold layer in a Polaron SC-500 sputter coater (VG Microtech, Sussex, UK). The samples were then examined using a Hitachi S-4300 Scanning Electron Microscope (Hitachi Scientific Instruments, Japan) at 5.0 kV.
X-ray Powder Diffraction Analysis (XRPD)
X-ray patterns were obtained using a Siemens D500 X-ray powder diffractometer (Siemens, Cambridge, UK). Powdered samples were studied by placing a thin layer of the powder in conventional cavity mounts. The samples were scanned from 5 to 40 ° 2θ. The CuKα1 anode (wavelength λ = 1.54056 Å) was operated at 40 kV and 20 mA. From X-ray scans, the d-spacings were calculated according to the Bragg’s Law [Eq. 1]:
nλ = 2 d sinθ [Eq. 1]
where the integer n is the order of the diffracted beam, λ is the wavelength of the incident X-ray beam, d is the distance between adjacent planes of atoms (d-spacings), and θ is the angle of incidence of the X-ray beam.
Thermogravimetric Analysis (TGA)
The mass change of the substance as a function of temperature was examined using thermogravimetric analysis. TGA was performed on powdered samples using the thermogravimetric analyser Mettler TG 50 coupled with a Mettler MT 5 thermobalance (Mettler Toledo, GmbH., Switzerland). The temperature range 25–300 °C with a heating rate 10 °C per minute was selected.
Preparation of drug-free and drug-loaded halloysite pellets
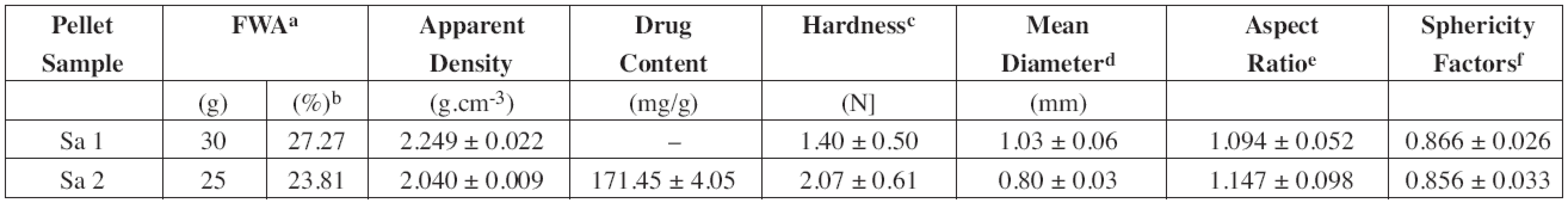
The halloysite pellets were produced by the extrusion/ spheronization method using a rotary gravity-fed cylinder type extruder (Alexanderwerk Type GA65, Germany) and a 120 mm diameter spheronizer (Caleva Ltd. Model 120, UK) fitted with a cross-hatch friction plate. The dry powder (80 g) and the appropriate amount of wetting liquid (purified water) were mixed and kept in an air tight container for approx. 24 h to ensure homogenous liquid distribution. Subsequently, the wetted mass was extruded through a 1 mm diameter screen at the extruder rotational speed of 30 rpm. The extrudate was spheronized at 1500 rpm for 20 min. The final product was dried at room temperature for at least 48 h before its characterization. The size and size distribution (Retsch® Sieve Shaker, Retsch GmbH & Co, Germany), shape analysis (optical microscope DN 45, Lambda Praha, Czech Republic, connected to the CCD camera Alphaphot, Nicon, Japan and operated by Ia32software, LECO Corporation), apparent density (helium gas pycnometer AccuPyc 1330, Micrometrics Instruments Corp., USA), hardness (C50 Tablet Hardness & Compression Tester, Engineering Systems, UK, fitted with a C 5 load cell used for pellet evaluation), and dissolution behavior of selected pellet fraction (850–1180 μm) were investigated.
Dissolution studies
The drug release from powder or pellet samples was measured using the basket dissolution apparatus Erweka DT-D6 (Erweka, GmbH., Germany). The dissolution profiles of 200 mg samples or 35 mg of pure drug were determined in 1,000 ml of dissolution medium (purified water or McIlvaineęs buffer pH 3.2 or McIlvaineęs buffer pH 6.8, respectively) at a rotation speed 100 rpm and temperature 37 °C. The powder samples were initially loaded into empty tea-bags and sealed at the opened end, before being placed into the basket. At predetermined time intervals, the samples (10 ml) were withdrawn, replaced with fresh medium, filtered and assayed spectrophotometrically (Lambda 25, Perkin Elmer, USA) at 276 nm. The dissolution test for each sample was carried out at least in triplicate. The data obtained were treated according to zero order [Eq. 2], first order [Eq. 3] and Higuchięs [Eq. 4] models:
Mt = M0 + k0t [Eq. 2]
ln Mt = M0 + k1t [Eq. 3]
Mt = M0 + kHt1/2 [Eq. 4]
In these equations, Mt is the cumulative amount of drug released at any specified time point, M0 is the dose of the drug incorporated in the delivery system, k0, k1 and kH are rate constants and t is time.
Results and Discussion
Halloysite characterization
Raw halloysite G clay is a tinted yellowish or brownish powder with the apparent density of 2.283 ± 0.006 g.cm-3. Under the scanning electron microscope, it is possible to distinguish individual halloysite microtubules (Fig. 1). To minimize the content of occasional agglomerates, sieving through the 185 μm sieve was employed and the < 185 μm sieve fraction (the apparent density 2.291 ± 0.012 g.cm-3) only was used for all subsequent studies.

Figure 2 shows the XRPD pattern of the halloysite G sample used in subsequent experiments. The sharp peak around 12.2° 2θ (corresponding to the basal spacing of approximately 7.25 Å) can be clearly distinguished. This phase represents the dehydrated 7 Å form of halloysite (so called metahalloysite). The typical 10 Å peak indicating the presence of a water molecules sheet between two adjacent aluminosilicate layers12) is missing and thus it can be concluded that the halloysite G sample was almost fully dehydrated to metahalloysite. This observation is supported by the minimal weight loss (~ 3.79% ± 0.32%) observed during drying to the constant weight and TG analysis (~ 5.07% ± 0.70%) carried out on raw clay material. Unlike kaolinite which occurs as well-formed, large plate-like crystals (1–10 μm) of clear hexagonal outline, halloysite has a tubular morphology. This structural order manifests itself as the next important diagnostic feature of the X-ray pattern13) – as the intense reflection peak at approx. 20 °2θ (~ 4.4 Å).

Drug-loading mechanisms
Layered clay mineral halloysite can interact with both organic and inorganic chemicals through a number of mechanisms such as adsorption, intercalation and cation exchange14) or can enclose the drug by its mechanical entrapment within hollow cylinders.
Adsorption of the drug onto mineral surface: Kaolinite minerals can be regarded as a hydrated mix of silica (SiO2) and alumina (Al2O3) where each component plays a role in determining the surface charge properties of the whole structure. Both oxides exhibit a strongly pH-dependent surface charge, being positive and negative at pH values lower and higher than the pH corresponding to their point of zero charge (pHPZC). Silica is an acidic oxide (pHPZC ~ 2) and is negatively charged over a substantial range of pH values, while alumina shows a more amphoteric behaviour15). In the range of pH from 4 to 9, the zeta potential of halloysite has been shown to change from about –12 mV to –50 mV7, 8, 15). The permanent negative surface charge of clay minerals tends to electrostatically attract cations in order to ensure the electroneutrality of the system. However, alumina has a well-defined pHPZC at about pH 7.6 15) and at the pH of the adsorption studies (the pH values of all halloysite bulk suspensions varied from 6.98 to 7.21), the presence of positively charged inner core surfaces and edges of the tubules cannot be excluded7, 8). Thus, the anionic drug could be adsorbed to some, albeit little, extent (~ 72 mmol/kg for diclofenac sodium).
The equilibrium adsorption process is usually described by an isotherm. Lee and Kim13) demonstrated that the adsorption of a cationic surfactant from solution onto solid surfaces of clay minerals (kaolinite and halloysite) follows the Langmuir isotherm. In the case of the adsorption of diclofenac sodium by halloysite, the assumptions of Langmuir sorption were not always completely satisfied (Fig. 3), especially when low concentrations of the drug solution were added. The diclofenac sodium-halloysite binding curve exhibits an unusual shape suggesting that probably two different concentration-dependent adsorption mechanisms or multilayer adsorption take place16).

Intercalation reaction: Some layered inorganic materials can accommodate polar organic compounds between the layered lamellae and form intercalation complex. Reactive guest molecules enter the interlayer spaces and cause expanding of the layers. In the case of halloysite, intercalation process of small molecules containing two functional groups, preferably –OH and/or –NH2 (e.g. glycerol) through hydrogen bonding was described4, 17). When the halloysite is intercalated with some guest structure, the XRD analysis can clearly show that the expansion of the halloysite layers occurs in one direction only, i.e. along the C-axis, and only the parameters for the 001 spacing are altered14). By comparison of the drug-free and drug-loaded halloysite XRD patterns (Fig. 2), no shift of a 001 spacing of 7.25 Å was observed and the pattern remained unchanged. This does not indicate any intercalation reaction of the dehydrated halloysite and the drug.
Tubular entrapment: The third and most important drug loading mechanism involves the tubular entrapment. The “vacuum method“ applied has been previously reported2, 8-10). During this procedure, the entrapped air within tubules is replaced with the saturated liquid and the drug should enter the lumen of halloysite tubules and remain there after solvent removal by filtration and evaporation. The encapsulation efficiency (EE) of the process was calculated according to Kelly et al.10). The maximal amount of diclofenac sodium which could be theoretically available for drug loading was 0.4 g per one gram of halloysite (~ 100% EE). The actual content of drug encapsulated within the halloysite tubules was determined to be approx. 192.4 ± 6.7 mg per gram of drug-loaded halloysite thus giving the encapsulation efficiency of 48.1%.
Extrusion-spheronization studies
Almost all clay minerals can be made plastic when mixed with a small quantity of water. This ability to be moulded into various shapes that harden when dried18) could be used for the production of spheric pellets by the extrusion-spheronization technique. The process resulted in a production of round, smooth and relatively dense pellets (Fig. 4). Using drug-loaded halloysite as a starting material, the amount of purified water used as a wetting agent decreased from 30 g to 25 g owing to the presence of water soluble compound (DS). Due to the reduced moisture content of the “plastic” mass, the resulted pellets were smaller (Table 1) and less spherical (sphericity factor 0.866 vs. 0.856). Diclofenac sodium being slightly soluble in water could recrystallize during pellet drying and form solid bridges and thus harder pellets (1.40 N vs. 2.07 N) were provided.

Dissolution studies
The solubility of the pure drug is strongly influenced by pH, ionic strength and composition of the aqueous medium19) (Fig. 5). In dissolution media with pH values below its pKa (~ 4.2), the concentration of DS after 3 hr of dissolution did not exceed 28.7 μg.ml-1. Owing to suppressed dissociation of carboxyl groups, the drug is present mostly in its free acid form which is less soluble (water solubility 17.8 mg.l-1 20)) than the salt. As the pH of the medium increased, the solubility of diclofenac sodium increased due to the contribution from the ionized form. In the alkaline media, the powdered drug displayed a very fast and almost complete dissolution. The highest solubility was reached in purified water due to the minimum ionic strength and absence of Na+ ions coming from the buffer constituents.

DS-loaded halloysite: Similar dissolution behaviour of diclofenac sodium was observed also in the case of its release from halloysite tubules. Figure 6 shows the release profiles in three different dissolution media - purified water, McIlvaineęs buffers pH 3.2 and 6.8 simulating gastric and small intestine pH conditions, respectively. When compared with the dissolution of the pure drug powder, the drug release from mineral tubules was retarded and the burst release reduced. In buffered medium at pH 6.8, only 65% of encapsulated drug was dissolved within 6 h of dissolution testing and even after 24 h, approximately 18% of drug still remained unreleased. The release profiles of DS from the extruded-spheronized product were similar but slightly slower to those observed for the non-pelletized drug-loaded halloysite. This effect was expected due to smaller surface area exposed to the dissolution medium in the case of pellets.

Conclusions
The use of halloysite clay material for the encapsulation of diclofenac sodium was examined. It was shown that the halloysite tubules could act as modified release drug delivery systems due to the entrapment of anionic model drug within the hollow tubular cores and binding the drug to the polyionic surfaces of the mineral. The drug release from loaded halloysite was retarded in comparison with the dissolution of the pure drug powder. In order to reduce the burst effect and optimize drug dissolution profile, cationic polymers, e.g. Eudragit® RS or chitosane, and viscosity increasing agents will be used in further experiments.
Acknowledgement
Authors are grateful to Zentiva, a.s. Praha (Czech Republic) for the generous donation of diclofenac sodium.
Conflicts of interest: none.
Received 12 December 2012 / Accepted 16 Januar 2013
K. Krejčová • P. B. Deasy
School of Pharmacy, Trinity College Dublin, Dublin, Ireland
prof. PharmDr. Miloslava Rabišková, CSc. (✉ )
Department of Pharmaceutical Technology, Faculty of Pharmacy, Charles University
Heyrovského 1203, 500 05 Hradec Králové, Czech Republic
e-mail: rabiskom@faf.cuni.cz
Sources
1. Christie T., Fletcher W. K. Contamination from forestry activities: implications for stream sediment exploration programmes. J. Geo. Exp. 1999; 67, 201–210.
2. Price R. R., Gaber B. P., Lvov Y. M. In-vitro release characteristics of tetracycline HCl, khellin and nicotinamide adenine dineculeotide from halloysite. J. Microencapsulation 2001; 18, 713–722.
3. Rabišková M. Halloysite – interesting nanotubular carrier for drugs – review. Čes. a slov. Farm. 2012; 61, 255–260.
4. Carr R. M. Chih H. Complexes of halloysite with organic compounds. Clay Miner 1971; 9, 153–166.
5. Harrison J. L., Greenburg S. S. Dehydration of fully hydrated halloysite from Lawrence county Indiana. Clay Miner. 1962; 9, 374–377.
6. Wada K. Lattice expansion of kaolinite minerals by treatment with potassium acetate. Am. Miner. 1961; 46, 78–91.
7. Levis S. R., Deasy P. B. Characterization of halloysite for use as a microtubular drug delivery system. Int. J. Pharm. 2002; 243, 125–134.
8. Lvov Y. M., Price R., Gaber B., Ichinose I. Thin film nanofabrication via layer-by-layer adsorption of tubule halloysite, spherical silica, proteins and polycations. Colloids and Surfaces A: Physicochem. Eng. Aspects 2002; 198–200, 375–382.
9. Levis S. R., Deasy P. B. Use of coated microtubular halloysite for the sustained release of diltiazem hydrochloride and propranolol hydrochloride. Int. J. Pharm. 2003; 253, 145–157.
10. Kelly H. M., Deasy P. B., Ziaka E., Claffey N. Formulation and preliminary in vivo dog studies of a novel drug delivery system for the treatment of periodontitis. Int. J. Pharm. 2004; 274, 167–183.
11. Zambito Y., Di Colo G. Preparation and in vitro evaluation of chitosan matrices for colonic controlled drug delivery. J. Pharm. Pharm. Sci. 2003; 6, 274–281.
12. Kloprogge J. T., Frost R. L. Raman microprobe spectroscopy of hydrated halloysite from a neogene cryptoclast from southern Belgium. J. Raman Spectroscopy 1999; 30, 1079–1085.
13. Lee S. Y., Kim S. J. Adsorption of naphthalene by HDTMA modified kaolinite and halloysite. Appl. Clay Sci. 2002; 22, 55–63.
14. Frost R. L., Tran T. H., Kristof J. FT-Raman spectroscopy of the lattice region of kaolinite and its intercalates. Vibrational Spectroscopy 1997; 13, 175–186.
15. Tari G., Bobos I., Gomes C. S. F., Ferreira J. M. F. Modification of surface charge properties during kaolinite to halloysite-7Å transformation. J. Colloid Interface Sci. 1999; 210, 360–366.
16. Lee G., Rupprecht H. Adsorption at solid surfaces. In: Swarbrick J., Boylan J.C., editors. Encyclopedia of Pharmaceutical Technology. 1st ed. New York – Basel: Marcel Dekker, Inc. 1998, 73–114.
17. Suh Y. J., Kil D. S., Chung K. S., Abdullayev E., Lvov Y. M., Montagyt D. Natural nanocontainer for the controlled delivery of glycerol as moisturizing agent. J. Nanoscience Nanotechnology 2001; 11, 661–665.
18. Christie T., Thompson B., Brathwaite B. Mineral Commodity Report 20 – Clays. New Zealand Mining 2000; 27, 26–43.
19. Kincl M., Vrečer F., Veber M. Characterization of factors affecting the release of low-solubility drug from prolonged release tablets. Anal. Chim. Acta 2004; 502, 107–113.
20. Fini A., Fazio G., Gonzales-Rodriguez M., Cavallari C., Passerini N., Rodriguez L. Formation of ion-pairs in aqueous solutions of diclofenac sodium. Int. J. Pharm. 1999; 187, 163–173.
21. Podczeck F., Rahman S. R., Newton J. M. Evaluation of a standardised procedure to assess the shape of pellets using image analysis. Int. J. Pharm. 1999; 192, 123–138.
22. Deasy P. B., Law M. F. L. Use of extrusion-spheronization to develop an improved oral dosage form of indomethacin. Int. J. Pharm. 1997; 148, 201–209.
Labels
Pharmacy Clinical pharmacologyArticle was published in
Czech and Slovak Pharmacy

2013 Issue 1
-
All articles in this issue
- Testing of the potentially probiotic lactobacilli for use in food supplements
- Organization and management of nationalized pharmaceutical industry in Slovakia from 1945 to 1948
- Oral mucosa and therapy of recurrent aphthous stomatitis
- Design and development of diltiazem hydrochloride transmucosal drug delivery system
- Diclofenac sodium entrapment and release from halloysite nanotubules
- Pediatric oral solutions with propranolol hydrochloride for extemporaneous compounding: the formulation and stability study
- Mucoadhesive films as perspective oral dosage form
- Czech and Slovak Pharmacy
- Journal archive
- Current issue
- About the journal
Most read in this issue
- Pediatric oral solutions with propranolol hydrochloride for extemporaneous compounding: the formulation and stability study
- Oral mucosa and therapy of recurrent aphthous stomatitis
- Organization and management of nationalized pharmaceutical industry in Slovakia from 1945 to 1948
- Mucoadhesive films as perspective oral dosage form
