Pediatric oral solutions with propranolol hydrochloride for extemporaneous compounding: the formulation and stability study
Pediatrické perorální roztoky s propranolol-hydrochloridem pro magistraliter přípravu: formulace a hodnocení stability
Cílem práce je formulace pediatrického perorálního přípravku s propranolol-hydrochloridem (PRO) 2 mg/ml pro magistraliter přípravu, určeného k terapii infantilního hemangiomu nebo hypertenze u cílové skupiny dětí od 1 měsíce do školního věku, a hodnocení jeho stability. K dosažení pH okolo 3, optimálnímu pro PRO, byl jako vehikulum využit roztok kyseliny citronové nebo citráto-fosfátový pufr. K maskování hořké chuti PRO byl použit prostý sirup. Všechny roztoky byly uchovávány v dobře uzavřené hnědé lékovce při 5 ± 3 °C a/nebo 25 ± 3 °C. V časových intervalech 0–180 dní byla hodnocena koncentrace PRO a protimikrobní látky, benzoanu sodného, validovanou HPLC metodou. Všechny přípravky byly stabilní při obou teplotách s hodnotou pH v rozmezí 2,8–3,2. V souladu s požadavky lékopisu byla prokázána účinnost protimikrobní látky, benzoanu sodného (Ph. Eur., 5.1.3). Podle našich zkušeností má přípravek s citráto-fosfátovým pufrem lepší chuť než přípravek s kyselinou citronovou.
Klíčová slova:
propranolol-hydrochlorid • pediatrický přípravek • magistraliter přípravek • roztok • testování stability • HPLC
Authors:
Sylva Klovrzová; Lukáš Zahálka; Ludmila Matysová; Petr Horák; Zdenka Šklubalová
Published in:
Čes. slov. Farm., 2013; 62, 35-39
Category:
Original Articles
Overview
The aim of this study is to formulate an extemporaneous pediatric oral solution of propranolol hydrochloride (PRO) 2 mg/ml for the therapy of infantile haemangioma or hypertension in a target age group of 1 month to school children and to evaluate its stability. A citric acid solution and/or a citrate-phosphate buffer solution, respectively, were used as the vehicles to achieve pH value of about 3, optimal for the stability of PRO. In order to mask the bitter taste of PRO, simple syrup was used as the sweetener. All solutions were stored in tightly closed brown glass bottles at 5 ± 3 °C and/or 25 ± 3 °C, respectively. The validated HPLC method was used to evaluate the concentration of PRO and a preservative, sodium benzoate, at time intervals of 0–180 days. All preparations were stable at both storage temperatures with pH values in the range of 2.8–3.2. According to pharmacopoeial requirements, the efficacy of sodium benzoate 0.05 % w/v was proved (Ph.Eur., 5.1.3). The preparation formulated with the citrate-phosphate buffer, in our experience, had better palatability than that formulated with the citric acid solution.
Keywords:
propranolol hydrochloride • pediatric preparation • extemporaneous preparation • solution • stability testing • HPLC
Introduction
Propranolol hydrochloride (PRO) is a non-cardio selective beta blocker. It is usually administered in the form of tablets or capsules in therapy of cardiovascular diseases, to control symptoms of hyperthyroidism, the prophylaxis of migraine, and many other indications1). A successful treatment of infantile hemangioma has been observed recently; PRO is orally administered from newborns to school children at an initial dose of 2 to 3 mg/kg daily in two or three divided doses1–3).
A liquid preparation is the best dosage form for paediatric patients as young children are simply unable to swallow conventionally sized tablets or capsules. Unfortunately, no pediatric oral liquid dosage form is on the market until now. Under these circumstances, the pharmacist needs to compound such a preparation extemporaneously. When formulating a pediatric preparation in a hospital pharmacy, the pharmacist should attend to the stability of the active pharmaceutical substance for a labelled time period, the suitability and safety of excipients for children in the indicated target age groups, and expected duration of treatment4, 5). A simple way of preparing an oral liquid preparation is to crush commercial tablets to make a fine powder and mix it with a suitable vehicle.
Many empirical formulations prepared that way have been published for PRO6–8). Unfortunately, some authors of the earlier publications have used excipients which are not suitable for paediatric patients; a commercial suspending vehicle consisting of ethanol 1%, saccharin 0.05%, and cherry-flavoured 33% polyethylene glycol 8000 base, is an example7). The lack of valid stability data is the second common disadvantage of earlier publications.
This study was focused on the formulation of an extemporaneous solution containing PRO 2 mg/ml, suitable for therapy of infantile hemangioma in a target group of children from 1 month to approximately 6 years for hospital and/or home care. The stability of PRO was evaluated under two different conditions of storage within a shelf life of 180 days using high performance liquid chromatography (HPLC).
Experimental part
Materials
Citric acid monohydrate, sodium phosphate dibasic dodecahydrate, sodium benzoate (SB), and propranolol hydrochloride (PRO) of pharmaceutical quality were used. Simple sucrose syrup (64% w/w) was obtained from Fagron (Czech Republic). Water for injection (WFI) was used throughout the study as the solvent in the preparation of the vehicles and solutions.
Analytical reagents
The following reagents of analytical grade were used: acetonitrile, sulphuric acid (≥ 95–97%), and sodium dodecyl sulphate (≥ 98.5%) (all obtained from Sigma-Aldrich, Germany), butylparaben and tetrabutylammonium dihydrogenphosphate (≥ 97.0%) (both from Fluka, Germany), and sodium hydroxide (Penta, Czech Republic).
Methods
Compounding of buffer solution
To prepare a citrate-phosphate buffer solution of pH 3 (CPB), 1.67 g of citric acid and 1.47 g of dibasic sodium phosphate were dissolved in WFI and made up to 100.0 ml of a solution with WFI. The stock solution was stored in a tightly closed brown glass bottle, protected from light, and refrigerated (5 ± 3 °C).
Compounding of solutions of PRO
The composition of all prepared solutions F1–F3 is shown in Table 1.
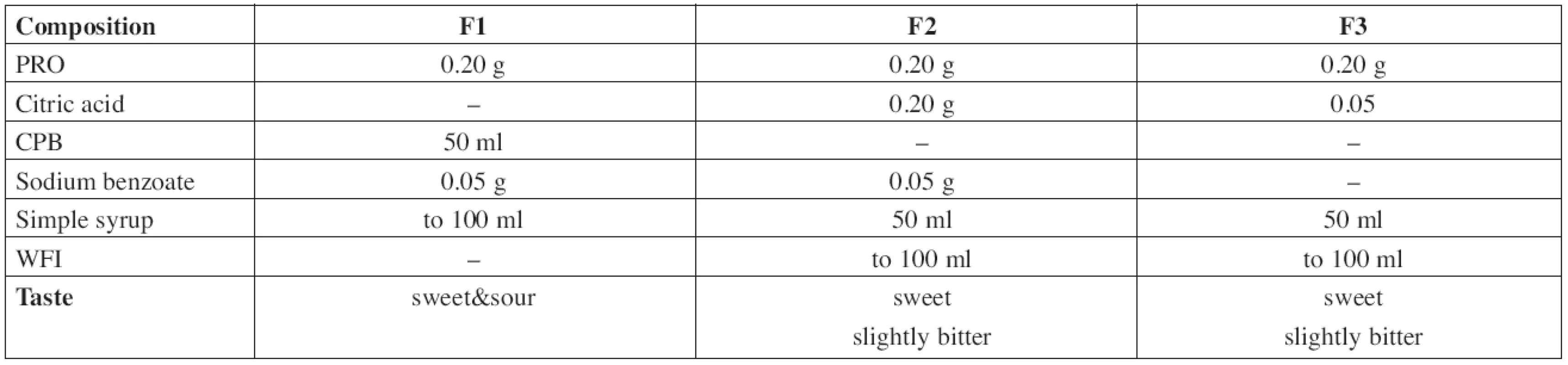
The F1 solution of PRO 2 mg/ml was prepared by dissolution of 0.20 g of the substance and 0.05 g of sodium benzoate in an appropriate volume of CPB, then filled with buffer solution up to 50 ml and made up to the total volume of 100.0 ml with Simple Sucrose Syrup.
In the formulation F2, 0.2 g of propranolol hydrochloride, 0.05 g of sodium benzoate, and 0.2 g of citric acid were dissolved in an appropriate volume of WFI, made up to 50 ml with WFI and then filled up to a total volume of 100.0 ml with Simple Sucrose Syrup.
The solution F3 was prepared by dissolution of 0.20 g of propranolol hydrochloride and 0.05 g of citric acid that way as the previous one. This solution was preservative-free.
Measurement of pH
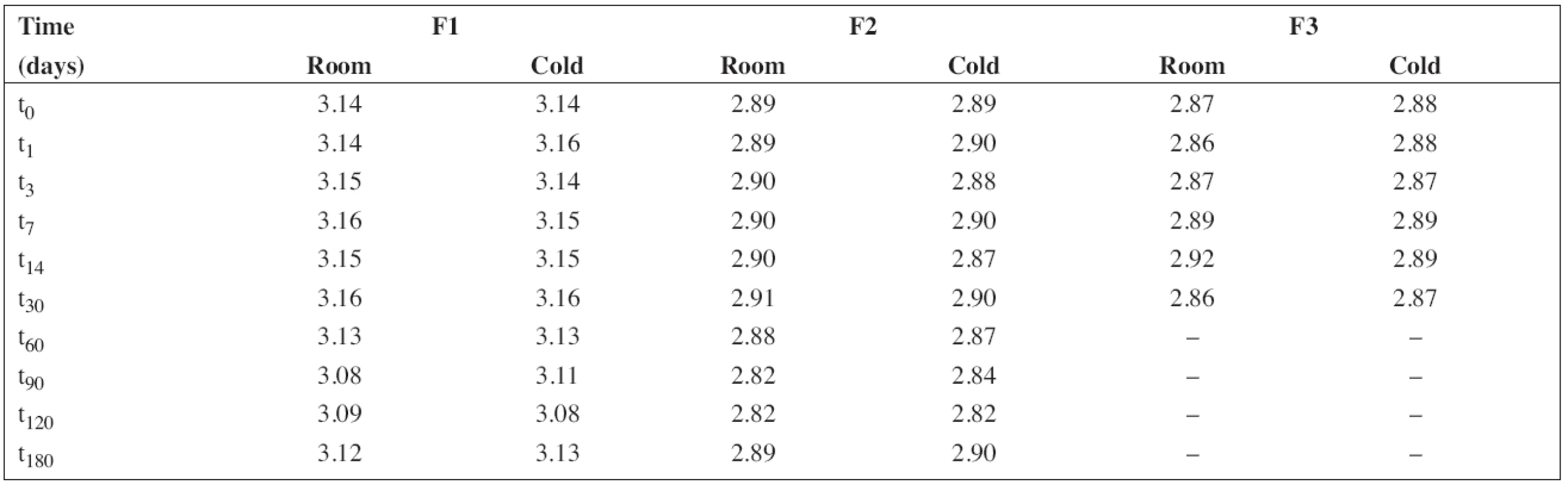
The pH value was measured under stabilized conditions using a pH meter (pH 212 Microprocessor pH Meter, Hanna instruments, Germany) with a combined pH electrode. The pH meter was calibrated at pH 4.01 and 7.00 at 20 °C using standard buffer solutions (WTW, Germany). The results obtained at the time intervals chosen in the stability study are presented in Table 2.
Instrumentation and analytical conditions
A stability indicating HPLC assay was developed for PRO and sodium benzoate, using butylparaben as an internal standard. The HPLC system consisted of a Shimadzu LC-2010C (CLASS-VP Software, Shimadzu, Japan) with a Dual – Absorbance UV Detector. Separation was achieved using a Supelco Discovery® C18 column (25 cm x 4.6 mm x 5 μm) (Supelco, USA). The isocratic flow rate was 1.8 ml/min and the UV detector was set at a wavelength of 230 nm.
The mobile phase consisted of 1.6 g of sodium dodecyl sulphate, 0.31 g tetrabutylammonium dihydrogenphosphate, 1 ml of sulphuric acid, 450 ml of HPLC grade water, and 550 ml of acetonitrile, and was adjusted to the pH value of 3.3 using sodium hydroxide solution. The mobile phase solution was filtrated through a 0.45 μm filter (Glass Microfiber Filters, Whatman, UK) and then was sonicated for a few minutes (Sonorex Digitec, Bandelin, Germany) before HPLC analysis.
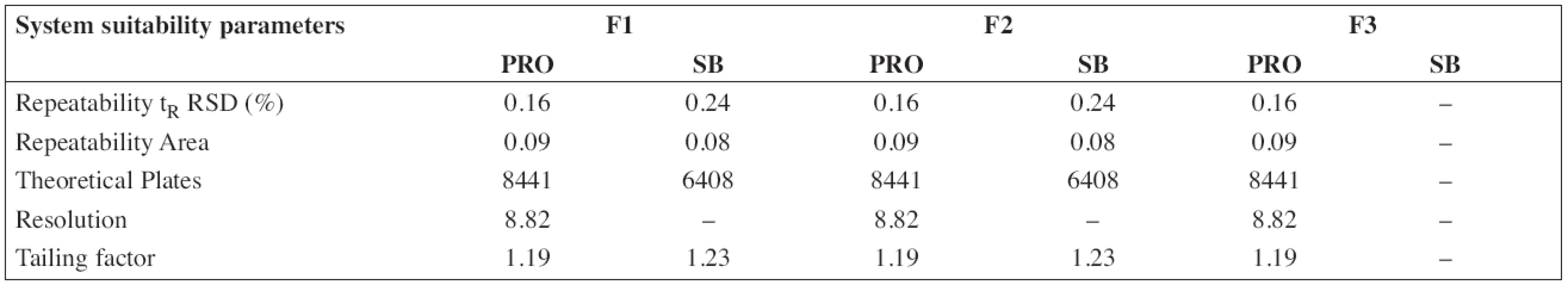
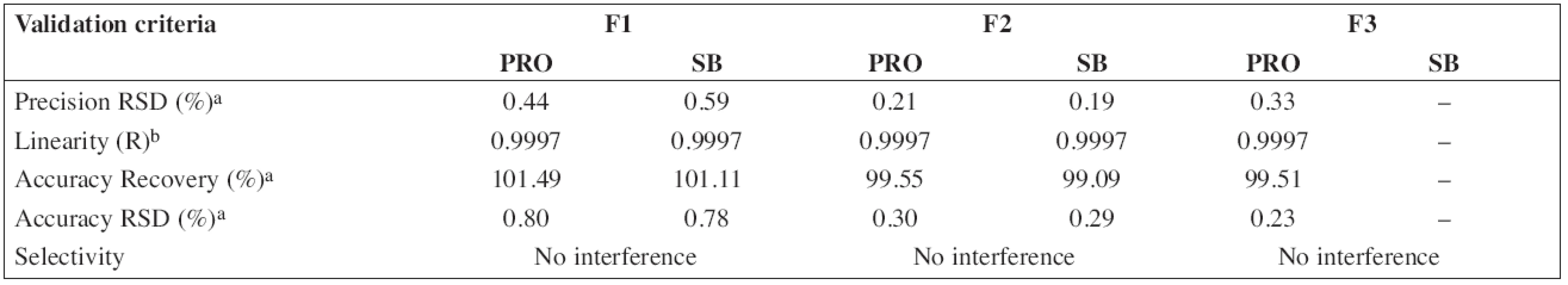
The HPLC method for the analysis of the proposed oral solution was successfully and completely validated by following the Q2(R1) ICH guideline (1997). System suitability parameters (n = 6) and validation data are summarized in Tables 3 and/or 4, respectively.
Stability study
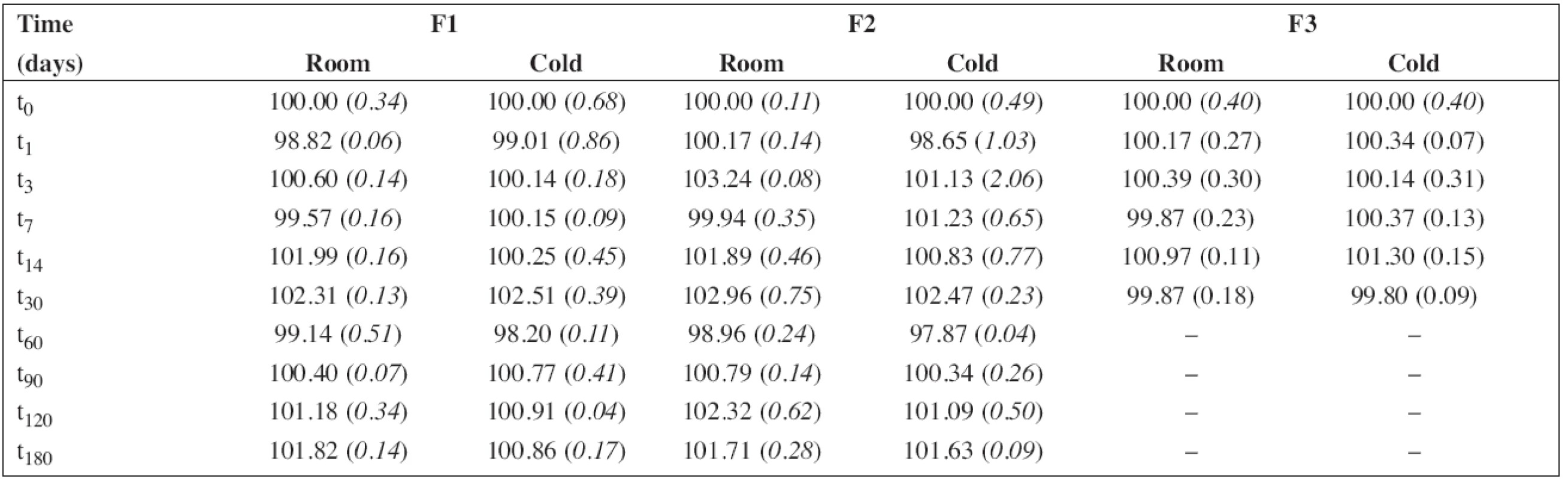
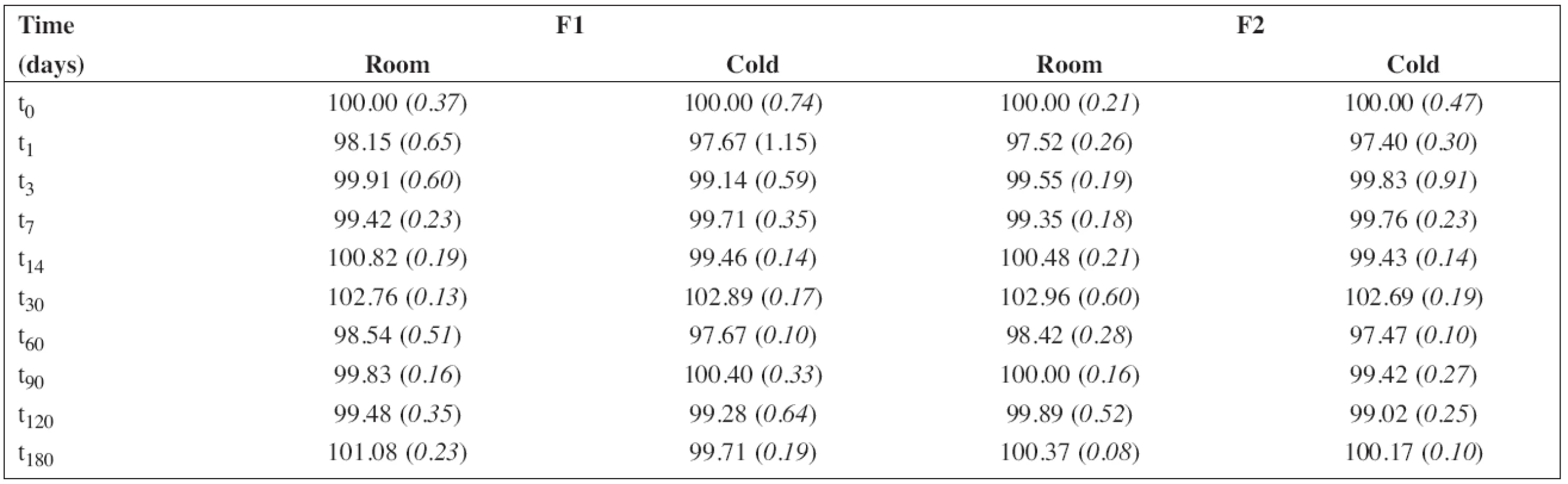
The batch of the preparation was divided into two separate samples and stored in a tightly closed brown glass bottle at room temperature (25 ± 3 °C) and in a refrigerator (5 ± 3 °C). The concentration of propranolol hydrochloride and the preservative, sodium benzoate, in the preparations F1 and F2 were evaluated at the beginning of the stability assay (t0, a content of 100 %) and thereafter at time intervals of 1 – 3 – 7 – 14 – 30 – 60 – 90 – 120 – 180 days. The concentration of propranolol hydrochloride in solution F3 was evaluated the same way but only at the time interval up to 30 days. Stability limit of maximum 5% degradation of the drug and the preservative contents were the basic criteria.
Each sample was measured in triplicate. The average values of the percentage content (n = 6) of propranolol hydrochloride with relative standard deviations (RSD, %) in brackets are summarized in Table 5. Similarly, the results for sodium benzoate are shown in Table 6.
Results and Discussion
In an aqueous vehicle, PRO has good solubility (50 mg/mL). Solutions are stable at about pH 2.8 – 4 with the best at pH 39). A disadvantage of PRO is a bitter taste leading to the necessity of the addition of a sweetener.
In this study, three formulations of PRO solution were compounded (Table 1). The citric acid and/or the citrate-phosphate buffer solution, respectively, were used as the vehicles to achieve pH value of about 3. Generally, a multi-dose preparation needs an addition of a preservative. Since there are some references indicating possible incompatibility between PRO and parabens resulting in the degradation of the parabens6), sodium benzoate was used as an alternative8, 10) assuming the use in a children target group of 1 month and older (the formulations F1 and F2). Simple Sucrose Syrup is added to improve palatability of the solutions. The preparation F3 was formulated preservative-free assuming the use for neonates below 1 month.
According to the analytical procedures validation ICH guidelines (Q2(R1)), the HPLC method was completely validated. In Tables 3 and 4, system suitability parameters (n = 6) and validation data are presented.
All solutions were stored in tightly closed brown glass bottles at 5 ± 3 °C and/or 25 ± 3 °C, respectively. At time intervals mentioned in the experimental section, samples were withdrawn and used to estimate pH value and the content of PRO and SB (preserved preparations F1 and F2). The results in Table 2 show good consistency in pH value during the stability study. This is important particularly in the case of the preserved solutions F1 and F2 as sodium benzoate has an alkaline effect on pH value, which might lead to degradation of PRO9).
The percentage content of PRO and SB content estimated using HPLC during the stability study at room temperature and/or refrigerator are summarized in Table 5 and/or Table 6, respectively. As F3 did not contain sodium benzoate, only the results for F1 and F2 are shown in Table 6. In all cases, the concentration of drug and/or preservative, respectively, was within recommended limits of ± 5% of the initial concentration at the beginning of the stability assay (t0)11). Based on the results, the estimated shelf-life12) of 180 days was proved at both temperatures of storage for F1 and F2 formulations when stored in a tightly closed brown glass bottle.
Conclusions
The aim of the study was to find an optimal vehicle for paediatric oral solution of PRO and to verify its stability at two temperatures of storage. The proposed oral aqueous solutions F1 and F2 for extemporaneous compounding were stable at room temperature and/or refrigerator for 180 days. In accordance with the European Pharmacopoeia (Ph.Eur. 7.0, 5.1.3 Efficacy of antimicrobial preservation), the efficacy of the antimicrobial preservative, sodium benzoate 0.05 % w/v, was demonstrated by an accredited laboratory. A labelled shelf-life of 3 months, storage in a refrigerator at 5 ± 3 °C, and protection from light can be recommended. The formulation F1 consisting of citrate-phosphate buffer mixed with sugar syrup we considered better than F2 for a sweet and sour taste, particularly in the therapy of older children. Formulation F3 represents the composition formulated with a minimal content of excipients and is preservative-free. It must, therefore, be prepared under aseptic conditions. It can be expected for use in the therapy of neonates under supervision of a caregiver. A labelled shelf-life of 7 days can be recommended for extemporaneous compounding in real-life situations if stored in a refrigerator at 5 ± 3 °C. To protect from microbial contamination and to allow easy administration, preparations should be packaged in a glass container with a screw cap suitable for administration using a syringe for oral use.
Acknowledgements
Supported by the project of the Ministry of Health of the Czech Republic for conceptual development of research organization 00064203 (University Hospital Motol, Prague, Czech Republic) and Student Grants SVV-2012265 002 and SVV-2012265 001. The publication is co-financed by the European Social Fund and the state budget of the Czech Republic. TEAB, project no. CZ.1.07/2.3.00/20.0235.
Conflicts of interest: none.
Received 3 December 2012 / Accepted 10 Januar 2013
S. Klovrzová • P. Horák
University Hospital Motol, Hospital Pharmacy, Prague, Czech Republic
doc. PharmDr. Zdenka Šklubalová, Ph.D. (✉ )
Charles University in Prague, Faculty of Pharmacy, Department of Pharmaceutical Technology, Hradec Králové
Heyrovského 1203, 500 05 Hradec Králové, Czech Republic
e-mail: zdenka.sklubalova@faf.cuni.cz
L. Zahálka • L. Matysová
Charles University in Prague, Faculty of Pharmacy, Department of Analytical Chemistry, Hradec Králové, Czech Republic
Sources
1. Propranolol hydrochloride. In Sweetman S. C. ed. Martindale: The Complete Drug Reference, 37th Ed. London: Pharmaceutical Press 2011; 989–990.
2. Léauté-LabrŹze C., Dumas de la Roque E., Hubiche T., Boralevi F., Thambo J. B., Taēeb A. Propranolol for severe hamangiomas of infanty. N Engl J Med 2008; 358, 2649–2651.
3. Bagazgoitia L., Torrelo A., Gutiérrez J. C. L., Hernández-Martín A., Luna P., Gutiérrez M., Baňo A., Tamariz A., Larralde M., Alvarez R., Pardo N., Baselga E. Propranolol for infantile hemangiomas. Ped Dermatol 2011; 28, 108–114.
4. European Pharmacopoeia Commision. Quality Guideline On The Pharmaceutical Development Of Medicines For Paediatric Use. Strassbourg, 2010. EMEA/CHMP/PEG/194810/2005: Reflection Paper: Formulations of choise for the paediatric population, 45 s., date accessed 25.11.2012 (http://www.ema. europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003782.pdf)
5. Resolution on quality and safety assurance requirements for medicinal products prepared in pharmacies for the special needs of patients. 2011. date accessed 25.11.2012 https://wcd.coe.int/View Doc.jsp?id=1734101
6. Brown G. C., Kayes J. B. The stability of suspensions prepared extemporaneusly from solid oral dosage forms. J Clin Pharm 1976; 1, 29–37.
7. Henry D. W., Repta A. J., Smith F. M., White S. J. Stability of propranolol hydrochloride suspension compounded from tablets. Am J Hosp Pharm 1986; 43, 1492–1495.
8. Ahmed G. H., Steward P. J., Tucker I. G. The stability of extemporaneus paediatric formulations of propranolol hydrochloride. Aust J Hosp Pharm 1988; 18, 312–318.
9. Propranolol hydrochloride. In: Trissel L. A. ed Stability of compounded Formulations, 4th Ed, American Pharmacists Association: Washington 2009; 478–481.
10. Gupta V., Stewart K. R. Stability of propranolol hydrochloride suspension and solution compounded from injection or tablet. Am J Hosp Pharm 1987; 44, 360–361.
11. Bardin C., Astier A., Vulto A., Sewell G., Vigneron J., Trittler R., Daouphars M., Paul M., Trojniak M., Pinguet F. Guidelines for the practical stability studies of anticancer drugs: a European consensus conference. Eur J Hosp Pharm 2012; 19, 278–285.
12. Capen R., Christopher D., Forenzo P., Ireland C., Liu O., Lyapustina S., O’Neill J., Patterson N., Quinlan M., Sandell D., Schwenke J., Stroup W., Tougas T. On the shelf life of pharmaceutical products. AAPS Pharm Sci Tech 2012; 23 June; DOI: 10.1208/ s12249-012-9815-2.
Labels
Pharmacy Clinical pharmacologyArticle was published in
Czech and Slovak Pharmacy

2013 Issue 1
-
All articles in this issue
- Testing of the potentially probiotic lactobacilli for use in food supplements
- Organization and management of nationalized pharmaceutical industry in Slovakia from 1945 to 1948
- Oral mucosa and therapy of recurrent aphthous stomatitis
- Design and development of diltiazem hydrochloride transmucosal drug delivery system
- Diclofenac sodium entrapment and release from halloysite nanotubules
- Pediatric oral solutions with propranolol hydrochloride for extemporaneous compounding: the formulation and stability study
- Mucoadhesive films as perspective oral dosage form
- Czech and Slovak Pharmacy
- Journal archive
- Current issue
- About the journal
Most read in this issue
- Pediatric oral solutions with propranolol hydrochloride for extemporaneous compounding: the formulation and stability study
- Oral mucosa and therapy of recurrent aphthous stomatitis
- Organization and management of nationalized pharmaceutical industry in Slovakia from 1945 to 1948
- Mucoadhesive films as perspective oral dosage form
