Screening v 11.–13.+6 týdnu těhotenství
Screening v 11.–13.+6 týdnu těhotenství
Ultrazvukové vyšetření plodu a krevního zásobení dělohy mezi 11. a 13. týdnem těhotenství přináší důležité informace o stavu gravidity včetně výhledu do budoucnosti. Nuchální projasnění ve spojení s mateřskými sérovými markery (volná podjednotka β-hCG a pregnancy-associated plasma protein-A) se ukazují jako vysoce efektivní markery aneuploidií. To je dále posíleno dalšími ultrazvukovými markery I. trimestru, což vede k záchytu přesahujícímu 90 % s falešnou pozitivitou 2,5 %. Většina závažných strukturálních vad může být odhalena již v tomto období. Popsané vyšetření zadního mozku (intracranial translucency (IT)) v I. trimestru je efektivní screeningovou metodou pro vady neurální trubice. Dopplerovské vyšetření pulzatilního indexu uterinních arterií ve spojení s mateřskou anamnézou a biochemickým vyšetřením mateřského séra pomůže upřesnit riziko vývoje preeklampsie.
Klíčová slova:
screening v I. trimestru těhotenství, trisomie 21, aneuploidie, nuchální projasnění, dodatečné markery PAPP-A, free β-hCG, vady neurální trubice, preeklampsie.
Authors:
J. D. Sonek 1; K. H. Nicolaides 2; Petr Janků 3
Authors‘ workplace:
Department of Maternal Fetal Medicine, Wright State University, Dayton, Ohio, USA
1; Department of Fetal Medicine, King’s College Hospital and University College Hospital, London, UK
2; Department of Obstetrics and Gynecology, University Hospital Brno, Masaryk University, Czech Republic
3
Published in:
Ceska Gynekol 2012; 77(2): 92-104
Category:
Original Article
Overview
Ultrasound examination of the conceptus and the uterine blood supply between 11 and 13 weeks’ gestation provides important information about the state of the pregnancy at that point in time and about its future progress. Nuchal translucency measurement in conjunction with maternal serum markers (free-β -human chorionic gonadotropin and pregnancy-associated plasma protein-A), has been shown to be a highly effective method for screening for aneuploidy. This is further improved by the addition of other more recently discovered first trimester ultrasound markers resulting in detection rates that exceed 90% with a false positive rate of 2.5%. Many fetal structural anomalies can be detected at this gestational age. Recently described first trimester evaluation of the posterior brain (intracranial translucency (IT)) provides an effective screening tool for the presence of open neural tube defects. Doppler measurement of the pulsatility index in the uterine arteries in conjunction with maternal history and examination as well as maternal serum biochemistries helps to accurately establish the risk of developing preeclampsia.
Key words:
first-trimester screening, trisomy 21, aneuploidies, nuchal translucency, additional markers, serum PAPP-A, serum free β-hCG, open neural tube defects, preeclampsia.
INTRODUCTION
Over the past several decades prenatal screening, diagnosis, and treatment have progressively shifted to earlier stages in pregnancy. This process was significantly accelerated by the recognition that the combination of nuchal translucency measurement and maternal serum biochemistries in the first trimester is the most efficient method of screening for fetal aneuploidy. This efficiency of screening for chromosomal defects is improved by using several additional ultrasound markers that have been developed over the past ten years [1–4].
Improvement of the resolution of ultrasound equipment has been such that many fetal structural defects are now routinely diagnosed in the first trimester. The association of thickened nuchal translucency with a number of structural defects and a variety of fetal syndromes is now well accepted. It is recognized that some of the secondary markers used in screening for aneuploidy are also associated with an increased risk of certain structural defects. Conversely, a number of fetal structural defects are associated with an increased risk of aneuploidy. The existence of these associations creates a reciprocal interplay between screening and diagnosis.
Recently developed intracranial markers for open neural defects have overcome one of the last major limitations of first trimester ultrasound screening for fetal defects [5–7]. First trimester Doppler evaluation of the uterine arteries in conjunction with maternal biochemistries, and maternal examination may predict the development of diseases that do not become apparent until later in gestation: preeclampsia and growth restriction [8]. At the present time, the main benefit of this knowledge is to appropriately adjust the intensity of prenatal care. However, there is evidence that treatment with low dose aspiring may decrease the incidence of preeclampsia if instituted early in pregnancy [9]. The first trimester risk evaluation can result in an early identification of the at risk population and potentially optimize the effect of this treatment.
It is evident that much about the pregnancy can be predicted by examining the pregnancy in the first trimester. Therefore, the traditional approach to management of pregnancy where the initial stages are considered a time when very little can be done needs to be reassessed. First trimester may in fact be the time when the most intense scrutiny should be applied and a plan for monitoring the rest of the pregnancy developed.
SCREENING FOR ANEUPLOIDY IN THE FIRST TRIMESTER: OVERVIEW
Aneuploidy remains a major cause of fetal morbidity and mortality. It is, however, a condition that can be effectively screened for using ultrasound and maternal serum biochemical analysis and can be diagnosed using invasive prenatal procedures. Both screening and diagnosis are associated with a number of risks. Screening itself carries two major risks: producing unnecessary anxiety in those patients that are incorrectly assessed to be at an increased risk and incorrectly classifying patients carrying an affected fetus as low risk. All procedures used to diagnose aneuploidy are invasive and carry risks that include fetal loss. Therefore, it is clear that screening algorithms should maximize the identification of affected pregnancies as being at risk (as high a detection rate as possible) and minimize identifying unaffected pregnancies as being at risk (as low a false positive rate as possible).
The thickness of the nuchal translucency between 11 and 13+6 weeks gestation (crown-rump length [CRL]: 45-84 mm) has a high correlation with the risk for fetal aneuploidy: the risk increases as the measurement increases [10]. Certain maternal serum biochemical marker levels are also known to have different distributions in pregnancies with aneuploid fetuses as compared to those with euploid fetuses. The use of combination of nuchal translucency measurements and levels of maternal serum free-beta subunit of the human chorionic gonadotropin (free βhCG) and pregnancy-associated plasma protein A (PAPP-A) has been shown to have a superior screening efficiency in comparison to screening in the second trimester. Furthermore, over the past ten years, several additional ultrasound markers have been described that can be used to adjust the risk of aneuploidy. These improve the efficacy of screening in the first trimester even further. Even though maternal age alone is no longer recognized as a good screening method, maternal age must be an integral part of screening. It, along with maternal history establishes the a priori risk for chromosomal defects. The a priori risk must also be adjusted for gestational age. This is due to the fact that fetuses with a chromosomal abnormality have an increased risk of dying in utero; therefore, the overall prevalence of aneuploidy decreases with advancing gestational age.
MATERNAL AGE AND RISK OF ANEUPLOIDY
Overall, the risk of fetal aneuploidy increases with maternal age, an increase that accelerates after the age of 35. This fact has been recognized for many decades and was used to institute as the first screening method for aneuploidy in early 1970s. At that time, only approximately 5% of children were born to women who were 35 years of age or older. Therefore, using this method at that time had a false positive rate of 5%. The proportion of fetuses with trisomy 21 that were born to women in the advanced maternal age group was only 30%. The number of women that bear children at or beyond the age of 35 has steadily increased since then. In the developed countries, this proportion is now approximately 20%, making the false positive rate unacceptably high. Since the overall number of pregnancies in this age group has increased, the proportion of fetuses with trisomy 21 in it also increased and is now approximately 50%. Of note is that the risk of sex chromosome abnormalities (47XXX, 47XXY, and 47XYY) does not change with maternal age. The risk of monosomy X also does not increase with maternal age and there is evidence that its prevalence may actually decrease with maternal age.
GESTATIONAL AGE AND RISK OF ANEUPLOIDY
Fetuses affected by chromosomal abnormalities are more likely to die in utero than chromosomally normal fetuses. The rate of attrition differs according to specific types of aneuploidy. For example, a fetus that is identified as having trisomy 21 at 12 weeks’ gestation has a 30% chance of dying prior to term. The chance of a fetus with either trisomy 18 or 13 dying between 12 weeks’ gestation and term is 80%. Most fetuses with triploidy die prenatally and are rarely encountered at term. The lethality of a fetus with monosomy X is approximately 60% between 12 weeks’ gestation and term. However, the other sex chromosome abnormalities do not have an increased in utero lethality. They, like the euploid fetuses, have only an approximately 1–2% chance of dying between 12 weeks’ gestation and term [11–16].
As an example, the risk of a 20 year old woman having a fetus with trisomy 21 at 12 weeks’ gestation is 1 : 1,000 but her risk of delivering an affected child is only 1 : 1,500. For the same maternal age, the risk for trisomy 18 at 12 weeks’ gestation is 1 : 2,500 and 1 : 8,000 for trisomy 13 but only 1 : 18,000 and 1 : 42,000 respectively at term. This illustrates need to adjust for gestational age whilst screening for aneuploidy.
CRL AND SCREENING FOR ANEUPLOIDY
Since all markers have to be interpreted in the context of gestational age, an accurate and reproducible measurement of the crown-rump length (CRL) is absolutely critical [17, 18]. Briefly, the fetus needs to be in a neutral position and at rest. The fetus should be insonated along its longitudinal axis in the sagittal section. The magnification should be such that the fetus fills 30–50% of the image. The calipers are then placed on the top of the fetal crown and the bottom of the fetal rump (end-to-end) (Figure 1).

MATERNAL HISTORY AND FETAL ANEUPLOIDY
A history of a previous offspring or fetus with non-disjunction increases the risk of non-disjunction in subsequent pregnancies. The additional risk numerically is approximately 1 : 135 (0.75%) [19]. The a priori risk is calculated by adding this risk to the risk based on maternal age. The increase in risk is specific for the type of aneuploidy of the proband. For example, a 35 year old woman who had a previous child with trisomy 21 of the risk increases from 1 : 250 to 1 : 88. However, her risk for other trisomies is not increased beyond the age adjusted risk.
MATERNAL SERUM BIOCHEMISTRIES AND FETAL ANEUPLOIDY
Abnormalities in the chromosomal complement are translated into abnormalities in the biochemical products of the fetal-placental unit [20–25]. These result in abnormal levels of biochemical markers rather than alteration in their structure. The two analytes that have been tested most extensively in the first trimester and that were found to be useful are free β-hCG and PAPP-A. Maternal serum ADAM12 (A disintegrin and metalloprotease domain 12) is lower in trisomic pregnancies than in euploid pregnancies [26]. However, there is a strong association between the levels of ADAM12 and both free β-hCG and PAPP-A reducing the utility of this analyte [27]. Recent evidence suggests that serum alpha-fetoprotein (AFP) and placental growth factor (PlGF) may also be useful in first trimester screening [28, 29].
The process that converts a maternal serum biochemical marker level into a usable number involves the following general steps. The distributions of the serum levels in affected and unaffected pregnancies are initially developed. These are converted to multiples of the mean (MoM) and distribution of the log10 MoM plotted. Since the distributions are Gaussian, the difference in the heights at a given MoM reflects the difference in prevalence of a given aneuploidy for that MoM. The ratio of the heights produces a likelihood ratio that can be used to adjust the a priori risk of an aneuploidy.
Each of the common aneuploidies are associated with distributions of free β-hCG and PAPP-A that differ to a varying degree from those in unaffected pregnancies. The patterns of the differences are specific for individual aneuploidies. In trisomy 21, the mean free β-hCG levels are approximately twice as high as in euploid pregnancies and the PAPP-A levels are about half as high [30]. Conversely, in trisomy 18 and 13 both free β-hCG and PAPP-A are reduced [31–32]. Fetuses with abnormalities of the number of sex chromosomes have a decreased level of PAPP-A but the free β-hCG levels do not differ appreciably from those in unaffected pregnancies [33]. Levels in triploidy depend on the type: diandric triploidy (incomplete molar gestation) is associated with very high free β -hCG levels and PAPP-A levels are mildly decreased whereas both analytes are significantly decreased in the case of digynic triploidy [34].
It is now apparent that there are certain maternal characteristics historical factors that affect the levels of free β-hCG and PAPP-A: maternal weight, racial origin, smoking, and method of conception. Additionally, different reagents and method for measuring the levels of the maternal serum analytes yield different results. Therefore, all of these factors need to be accounted for in order for a risk assessment to be accurate [35]. It also appears that there is a correlation between free β-hCG and PAPP-A between successive pregnancies. Therefore, in multiparous patients with a previous unaffected offspring the likelihood ratio associated with free β-hCG and PAPP-A levels in subsequent pregnancies should be adjusted according to the results in the previous pregnancy [36]. For example, if the maternal serum biochemistries are abnormal in the first pregnancy but the fetus itself is normal, the risk associated with such an abnormality will be less in the second pregnancy as compared to the first one.
Using only the combination of free β-hCG, PAPP-A, and maternal age in screening for trisomy 21 the overall detection rate is 65% for a screen-positive rate of 5%. However, the screening performance of these analytes improves if they are collected earlier in pregnancy (9-10 weeks) [37–40]. This is due to the fact that the difference in PAPP-A levels between unaffected pregnancies and those with trisomic fetuses is greater at an earlier gestational age. Even though the difference in free β hCG increases with increasing gestational age, it is not sufficiently great to compensate for the decreasing difference in PAPP-A [30, 40–42].
NUCHAL TRANSLUCENCY AND FETAL ANEUPLOIDY
Between 11 and 13+6 weeks’ gestation (CRL of 45-84 mm), the amount of fluid contained in the subcutaneous space behind the fetal neck is variable and tends to increase in the presence of a variety of fetal pathological conditions. The estimation of the fluid amount has been standardized by measuring the nuchal translucency (NT) [10].
The technique for NT measurement includes the following: a true sagittal section of the fetal head and neck that is magnified so only the head and upper portion of the fetal thorax occupy the image. The face of the transducer should be as parallel to the longitudinal axis of the fetus as possible as this results in the sharpest delineation of the nuchal membrane. There are several anatomic landmarks that are looked for to establish that the fetal section is sagittal. If the fetus is facing the transducer, the contour of the fetal profile should be visible and a short echogenic line representing the fetal nasal tip should be present. The upper palate in this view is a roughly rectangular in shape. The presence in the image of an echogenic projection extending toward the nose from the front of the upper palate, which represents the frontal process of the maxilla, indicates that the section is not truly mid sagittal. Regardless of whether the fetus is facing toward or away from the transducer, a hypoechoic round structure located approximately in the center of the fetal head representing the developing thalamus should be seen if the section is sagittal [43].
The calipers best used for NT measurement are those that have a shape of a cross. The calipers need to be placed on the nuchal skin and the soft tissue overlying the fetal spine so that the horizontal cross hatch lies completely within the echogenic borders and is flush with the inner border of the hypoechoic space between the two. The place to measure is selected by identifying the thickest point of the NT. The ultrasound settings should be adjusted so the lines that border the nuchal translucency are not too thick. The amniotic membrane needs to be identified as a separate structure from the NT. If a nuchal cord is present, the NT thickness is estimated by measuring it above and below the nuchal cord and averaging the two measurements. (For a detailed description of how to perform NT measurement correctly, please click on http://www.fetalmedicine.com/ fmf/training-certification/certificates-of-competence/ the-11-136-week-scan/ and follow the prompts).
It must be kept in mind that regardless of the degree of increase, a thickened NT is always a marker, not a pathological condition. Indeed, NT thickening can result from a number of fetal conditions most of which are benign and in most individual cases their specific nature is unclear. The proposed mechanisms for nuchal thickening in the presence of pathological conditions include altered composition of the extracellular matrix, abnormal or delayed lymphatic system formation, impaired lymphatic drainage due to poor fetal movement, cardiac malfunction due to either a structural defect or myocarditis, redistribution of blood flow due to a cardiovascular abnormality, venous congestion of the superior mediastinum, and fetal anemia, hypoproteinemia, or infection. Since NT thickening can result from a number of different and unrelated etiologies, a number of different and often unrelated fetal conditions can lead to NT thickening. It is also likely that in the presence of a pathological condition, more than one fetal problem contributes to the nuchal thickening.
It must be stressed that it is the thickness, not the appearance of the NT that matters when it comes to calculating the risk of aneuploidy [44, 45]. The artificial differentiation between “nuchal edema” and “cystic hygroma” in the first trimester does not improve risk calculation and has lead to confusion both in the literature and in actual risk calculation.
In screening for aneuploidy, the optimal way to compare the distributions of normal and chromosomally abnormal fetuses has been a subject of ongoing discussion. Traditionally, this comparison has been one in one of two ways. Either by using the MoM method, which is akin to the one used in the case of maternal biochemistries [46] (please, see above) or by using a method whereby the NT measurement in the screened subject is subtracted from the median NT measurement of the normal population at the same gestational age. The likelihood ratio that is used to adjust the a priori risk is then based on this difference (delta NT method) [47, 48].
Most recently, a statistical analysis (mixture model) first described by Pearson in 1894 [49] was applied to the distributions of NT measurements in both the normal and aneuploid populations[50]. This analysis is designed to detect the presence of differently performing subpopulations within a population in which a single Gaussian or other standard distribution fails to provide an adequate fit. The mixture model approach suggests that both the populations of euploid fetuses and aneuploid fetuses contain two subpopulations: one where the median nuchal measurements are gestational age-dependent and a second one where the medians remain the same across the 11-13+6 week gestational ages. In the euploid population, the medians of the NT measurements in 95% of the fetuses change with gestational age but 5% remain constant. This proportion is reversed in fetuses with trisomy 21 where 95% are unchanged and only 5% change with gestational age. The proportion of fetuses where the NT median measurements are gestational age dependent in trisomy 18, trisomy 13, and monosomy X is 30%, 15% and 10% respectively.
Nuchal translucency measurement is the most extensively studied marker used in prenatal screening for fetal aneuploidy [51–54]. Multiple prospective studies that contain a combined number of hundreds of pregnancies where NT measurements were performed have demonstrated that using maternal age and nuchal translucency measurement alone has a trisomy 21 detection rate of 75-80% for a screen-positive rate of 5%. The numbers are similar for other major chromosomal defects.
Additional ultrasound markers and aneuploidy
Additional markers that have been shown to be useful in the first trimester include those that are evaluated using gray scale ultrasound (nasal bone [NB] and frontomaxillary [FMF] angle) and those where Doppler ultrasound is required (ductus venosus [DV], tricuspid valve [TCV]) (for a detailed description of how to evaluate each of these markers correctly, please click on http://www.fetalmedicine.com/fmf/training-certifi cation/certificates-of-competence/the-11-136-week-scan/ and follow the prompts).
The NB is absent in approximately 60% of trisomy 21 fetuses but only in 2.5% of euploid fetuses. The FMF angle is above the 95th percentile in approximately 50% of fetuses with trisomy 21. The drawback of this marker is that a precise midline view of the face is even more critical than in the case of NT measurement and may be difficult to consistently obtain using 2D ultrasound. Tricuspid regurgitation is present in approximately 55% of fetuses with trisomy 21 and 1% of euploid fetuses. Reversal of flow through the ductus venosus during an atrial contraction is seen in 66% of fetuses with trisomy 21 and in 3% of euploid fetuses. The likelihood ratios associated with all of these markers need to be adjusted for gestational age and NB is affected by ethnicity [55, 65].
Doppler evaluation of the hepatic artery [HA] has recently been shown as a potentially useful marker in screening for trisomy 21 [66, 67]. It has been found that in 70% of fetuses with trisomy 21 the peak systolic velocity is above the 95th percentile of the normal distribution and the pulsatility index is below 2.
COMBINING MARKERS IN SCREENING FOR ANEUPLOIDY
In order for various markers to be included in a single screening protocol, ideally they should be independent of each other. If there is an association between markers, it has to be relatively minor and needs to be accounted for in the screening algorithm. There does not appear to be any association between NT, maternal serum biochemistries, NB, and FMF angle [57, 59, 68–71]. There are minor associations between NT measurements and the DV and TCV findings.
The a priori risk is must always be adjusted for gestational age, maternal age, and any appropriate maternal history. All biochemical markers and ultrasound markers need to take into account the gestational age. The biochemical markers and the additional ultrasound markers need to be adjusted based on a number of additional maternal factors, most of which are listed in the above text. Based on several prospective interventional studies, the combination of NT measurement and free β-hCG and PAPP-A (combined screening) levels yields a 90% detection rate for trisomy 21 at a screen-positive rate of 5% [72–80].
By adding the more recently developed markers, the detection rate increases to 95% and the false positive rate decreases to 2.5% [58, 61, 65]. Similar results can be achieved if the examination of the additional markers is done in all patients or on contingent basis. The contingent protocols usually begin with a combined screen. Patients are assigned to one of three categories based on the risk for trisomy 21: high risk (risk >1 : 50), intermediate risk (risk 1 : 51–1 : 1,000), and low risk (risk <1 : 1,000). The high risk group (1.3% of the population, contains about 85% of trisomy 21) is offered invasive diagnostic testing and the low risk group (80% of the population, contains 1% of trisomy 21) is reassured but a second trimester targeted scan is still recommended. Examination of the additional markers is only performed in the intermediate group (18-20% of the population, contains 14% of trisomy 21). Another type of a contingent approach begins with ultrasound examination only (NT measurement and tricuspid valve or ductus venosus evaluation). Biochemistries are measured only if the risk falls into the intermediate risk group (20% of the total population). These results are similar to using a combined screen in all subjects along with either the DV or TV evaluation [81].
Fetal heart rate is only marginally helpful in screening for trisomy 21. However, it improves screening for trisomy 18 where it is decreased and for trisomy 13 where it is increased [82–84].
The use of first trimester combined screening and additional markers results in the diagnosis of 95% of trisomies 18 and 13 for a false positive rate of .1% [58, 61, 85].
SCREENING FOR ANEUPLOIDY IN MULTIPLE GESTATIONS
The use of nuchal translucency and additional ultrasound markers is an effective method for screening in twins and higher order multiple gestations [86–89].
The use of first trimester ultrasound in screening in multiple gestations offers several advantages. Firstly, the chorionicity can be determined with essentially complete certainty based on the appearance of the dividing membrane at a point where it reaches the placenta: thickening (so-called lambda sign) indicates a dichorionic (D/D) gestation and thin membrane without thickening (so-called T sign) is indicative of a monochorionic/diamniotic (M/D) gestation [90]. This information is then included in the risk calculation. The NT measurement-based risk in M/D gestation is calculated by measuring NT in each fetus and taking the average risk based on the two measurements and maternal serum biochemistries [91].
The false positive rate in a M/D gestation is higher than expected. This is due to the fact that NT thickening can also result from an early form of twin-to-twin transfusion syndrome [92, 93]. After the appropriate adjustment for chorionicity, the use of maternal serum biochemistries improves screening in twins [94].
In D/D gestations, a separate risk is assigned to each fetus based on the combination of ultrasound findings and maternal serum biochemistries. It is now evident that even in euploid dichorionic gestations there is a correlation between the NT measurements in the two fetuses [95–97]. This fact is now accounted for in the FMF screening algorithm for dichorionic twins.
There is a difference in the level of maternal serum biochemistries between euploid D/D gestations and M/D gestations. In D/D gestations, the levels are roughly double those of singletons. They are also increased in M/D gestations but not as much as in D/D ones [97–100].
The efficiency of first trimester combined screening in twins is similar to that in singletons [101]. The use of maternal serum biochemistries less well defined in high order multiple gestations.
VARIATIONS IN FETAL ANATOMY AND ANOMALIES AS MARKERS FOR ANEUPLOIDY
Occasionally, ultrasound findings are identified that in themselves are not detrimental to fetal well-being but are associated with an increased risk of chromosomal abnormalities (“soft” markers). In addition to all of the markers mentioned above, these include choroid plexus cysts, echogenic intracardiac focus, hyperechogenic bowel, and pyelectasis (renal pelvis >1.5 mm) [102]. As isolated findings, none of these markers significantly increase the risk of aneuploidy. However, if they are found in conjunction with other abnormal findings, they do elevate the level to which the risk of aneuploidy is increased.
Some fetal anomalies are associated with an increase in the risk of certain specific chromosomal abnormalities. These include congenital defects that are fairly reliably diagnosable in the first trimester: holoprosencephaly (risk of 1 : 2 for trisomy 13), diaphragmatic hernia (risk of 1 : 4 for trisomy 18), atrioventricular septal defect (risk of 1 : 2 for trisomy 21), omphalocele (risk of 1 : 4 for trisomy 18 and risk of 1 : 10 for trisomy 13), megacystis defined as bladder length of ≥7mm (risk of 1 : 10 for either trisomy 18 or 13 [103–106].
SCREENING IN THE FIRST TRIMESTER FOLLOWED BY A SECOND TRIMESTER ULTRASOUND EVALUATION
The second trimester ultrasound examination (usually at 18-22 weeks’ gestation) may reveal abnormal findings that were either not appreciated or not present at the first trimester examination and that may play a role in adjusting the risk of aneuploidy. As in the first trimester, these can be divided into “soft” markers for aneuploidy and anomalies that are associated with an increased risk of aneuploidy. The presence of any of above described major anomalies should trigger a discussion regarding invasive diagnosis even if the first trimester results were normal. However, second trimester soft markers also need to be interpreted in the context of whether or not they are isolated. As in the first trimester, each marker has a different likelihood ratio associated with it. Some markers such as significant thickening of the nuchal fold, absence of the nasal bone, thickened prenasal thickness, hyperechogenic bowel, and a short humerus increase the risk of aneuploidy even when they are isolated. Others, such as mild hydronephrosis, intracardiac echogenic focus, choroid plexus cysts, and short femur do not increase risk if they are isolated. Even though likelihood ratios associated with second trimester markers have been estimated, they are less firmly established than likelihood ratios used in the first trimester [53, 103, 107–114].
In a pregnancy where a first trimester risk assessment had been performed, the risk of aneuploidy at the second trimester ultrasound must take into account the results of the first trimester screen, i.e. the a priori risk is the result of the first trimester screen. For example, in a patient that is of advanced maternal age whose risk had been reduced by the first trimester risk assessment, the significance of a marker found in the second trimester will have less of an impact than if the first trimester screening had never been done. It has been estimated that the second trimester genetic scan after first trimester screening can add 6% detection of trisomy 21 for an additional false positive rate of 1% [115].
SCREENING IN THE FIRST TRIMESTER FOLLOWED BY SECOND TRIMESTER MATERNAL SERUM BIOCHEMISTRIES
The use of maternal serum biochemistries in screening for aneuploidy was initially described in the second trimester. The four biochemical markers that are at the present most commonly used are alpha fetoprotein (AFP), free β-hCG, unconjugated estriol (uE3), and inhibin [52, 116–118].
There are essentially three models that have been proposed that combine screening in the first trimester (ultrasound markers and first trimester biochemistries) and second trimester biochemistries. One is an integrated non-disclosure screening algorithm where a first trimester screen using NT measurement and PAPP-A is performed followed by a second trimester maternal serum screening consisting of AFP, uE3, free β-hCG, and inhibin levels. The risk assessment is based on the combination of the first and second trimester screening. The results are not disclosed until the second trimester at which time an amniocentesis is offered to the patients who are at an increased risk [119]. This approach is probably ethically untenable as even information regarding grossly abnormal NT measurements is withheld from the patient. The second approach is a step-wise sequential method. Here all patients undergo first trimester screening and those patients that are at an increased risk are offered a CVS. The remaining patients undergo second trimester maternal serum screening using AFP, uE3, free β-hCG, and inhibin levels. In this group the results of the first trimester and second trimester screening are combined and the ones that are at an increased risk are offered an amniocentesis. The false positive rate used in the first trimester is usually set at a low level in order to avoid an unacceptably high overall false positive rate. The third approach is similar to the step-wise contingent screening in the first trimester described earlier. The difference is that the patients assigned to the intermediate risk group go through second trimester maternal serum screening rather than utilizing the additional first trimester ultrasound markers [120]. The estimated detection for these approaches is 90-94% for a 5% false positive rate [120–122].
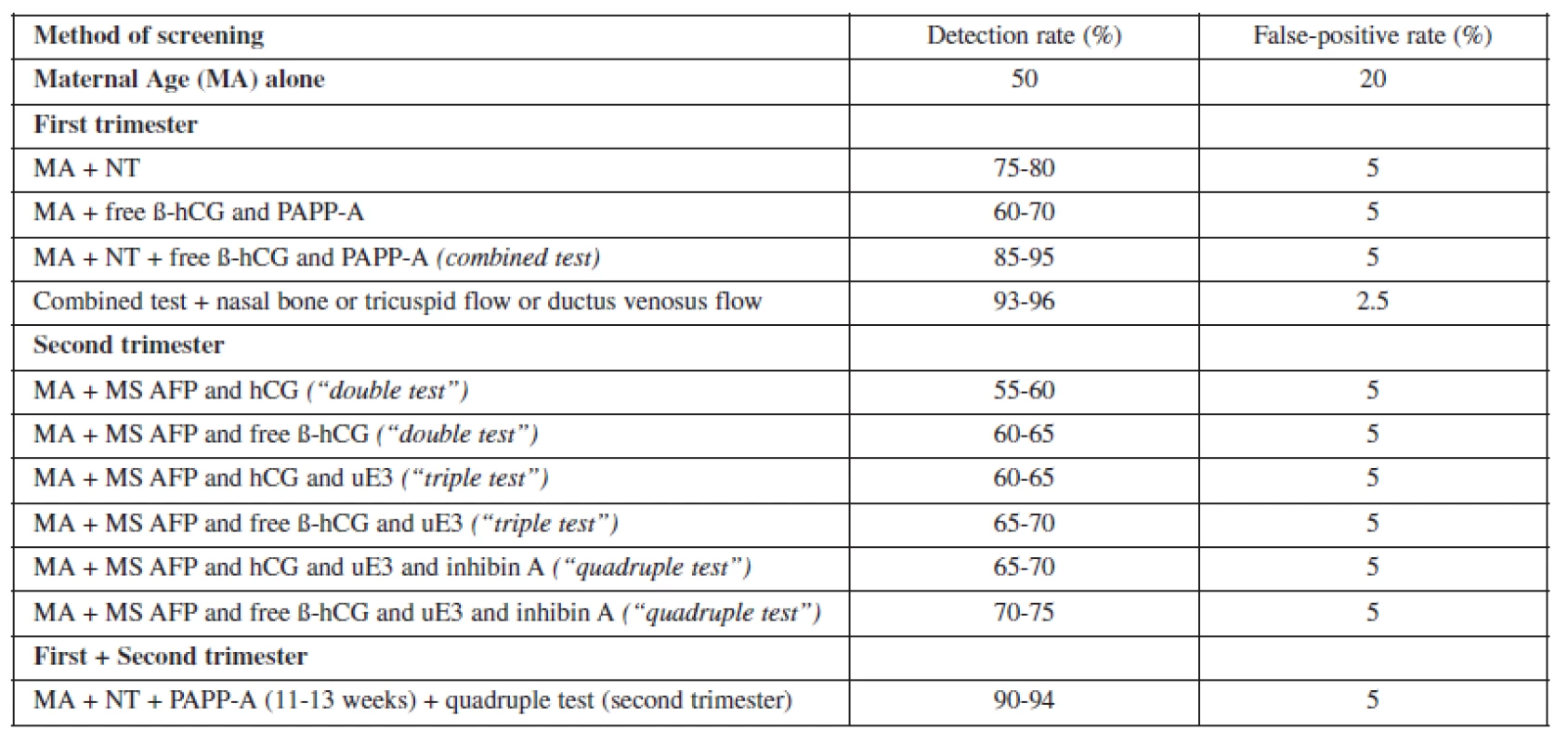
None of these approaches are an improvement over first trimester screening and may be inferior if the additional first trimester ultrasound markers are used (Table 1). The complexity of screening across trimesters potentially increases the cost of screening and increases the risk of some patients never completing the screening process. Therefore, it is expected that the actual real-world performance of such a screening algorithm would actually be inferior to the one where the screening process is completed in the first trimester.
FIRST TRIMESTER SCREENING FOR FETAL PROBLEMS OTHER THAN CHROMOSOMAL DEFECTS
Fetuses that have a significantly thickened NT but are chromosomally normal are still at an increased risk for poor pregnancy outcome [123–133]. This increase does not become statistically significant until the NT measurement reaches or exceeds 3.5 mm, which represents the 99th percentile across the 11-13+6 week gestational period [127].
The prevalence of major fetal abnormalities increases exponentially as the nuchal translucency measurement increases >3.5mm: it is approximately 2.5% for an NT of 3.5mm and reaches 45% for an NT of 6.5 mm or more [124, 132].
The utility of NT measurement in screening for cardiac defects, anomalies that are especially difficult to diagnose prenatally has been shown in a number of studies. A statistically significant increase in the prevalence of congenital heart defects is noted with thickened NT measurements: 3% (3.5-4.5mm), 7% (4.5-5.4mm), 20% (5.4-6.4mm), 30% (>6.5 mm) [134–138]. Furthermore, NT thickening is not associated with only certain type of heart defects. Left and right heart lesions, septal defects, outflow tract defects, complex cardiac defects have all been found to be associated with a thickened NT [139, 140]. Of note is that many of the major cardiac defects may now be diagnosed at the time of the 11-13+6 week scan [141–143].
A short list of additional anomalies that have been found in association with thickened NT includes diaphragmatic hernia [144], omphalocele [145], body stalk anomaly [146], skeletal defects [147], and certain genetic syndromes such as congenital adrenal hyperplasia [148], fetal akinesia deformation sequence [149], Noonan syndrome [150], Smith-Lemli-Opitz syndrome [151], and spinal muscular atrophy [152]. The incidence of fetal demise is increased in fetuses in which the NT measurement significantly thickened even if a specific fetal defect is not detected [124, 132]. The majority of fetal losses and anomalies are diagnosed prior to 20-22 weeks’ gestation. In chromosomally normal fetuses that survive to the mid second trimester and in which a targeted ultrasound fails to reveal any anomalies or increased nuchal fold thickness, the risk for perinatal or long-term morbidity and mortality does not appear to be increased [129, 130, 153–156].
FIRST TRIMESTER SCREENING FOR OPEN NEURAL TUBE DEFECTS
Even though the optimal time to perform a fetal anatomic survey is the mid second trimester, it is evident that many major fetal anomalies can already be diagnosed in the first trimester [157, 158]. However, until recently the diagnosis of open neural defects was a glaring exception.
In the second trimester, the existence of specific abnormalities of the posterior fossa (Chiari type II malformation) and the fetal bony forehead (bifrontal scalloping) in association with the presence of open neural tube defects (ONTD) has been recognized for decades [159]. However, in the first trimester useful secondary ultrasound findings were not available until recently. This rendered first trimester diagnosis of ONTD extremely difficult.
The first trimester posterior fossa anatomy is easily evaluated in the sagittal view of the fetal head, a view that is similar to that needed for NT, NB and FMF angle evaluation. In this view, two of echogenic lines are normally seen between the occipital bone posteriorly and the sphenoid bone anteriorly. They run roughly along the longitudinal axis of the fetus and are parallel to each other. The more anterior line represents the interface between the brain stem and the floor of the fourth ventricle and the second line represents roof of the fourth ventricle (developing cerebellum and the choroid plexus). The anechoic space between the two lines has been termed the intracranial translucency (IT). This space is obliterated in many fetuses with ONTD, making this finding a useful screening method for this condition [5].
A technique that improves the standardization of this method has been proposed recently. It is based on a ratio of the measurement of the distance between the sphenoid bone and the floor of the fourth ventricle (the brain stem diameter [BS]) and the distance between the floor of the fourth ventricle and the occipital bone [BSOB]) (Figure 2). In normal fetuses, the BS/BSOB ratio is below 1 whereas in fetuses with ONTD this ratio is above 1. This posterior fossa abnormality may represent an early form of Chiari Type II malformation [7].

It has also been shown that that 90% of first trimester fetuses with ONTD have a FMF angle that is below the 5th percentile.(6) This finding may represent a first trimester precursor of the frontal bone abnormalities seen in the second trimester found in association with ONTD.
An abnormal ratio of the distances between the echogenic lines in the posterior fossa and/or an unusually acute FMF angle should lead to an extremely careful ultrasound evaluation of the spine at the time of the first trimester ultrasound. If the appearance of the spine is normal on the initial scan, a scan should be repeated at approximately 16 weeks. If this scan is normal, the fetus should be reexamined at 20 weeks’ gestation.
FIRST TRIMESTER SCREENING FOR PREECLAMPSIA
Even though the clinical signs and symptoms of preeclampsia do not become evident until the second half of the pregnancy, it is recognized that changes that lead to its development occur much earlier. The fundamental defect is thought to be abnormal development of the vascular supply to the placenta [160]. One method of evaluating the vascular supply on the maternal side is to examine the resistance to blood flow in the uterine artery by the measuring its pulsatility index (PI) using pulsed Doppler. (For a detailed description of how to measure the uterine artery pulsatility index the correctly, please click on http://www.fetalmedicine.com/fmf/training-certifica tion/certificates-of-competence/the-11-136-week-scan/ and follow the prompts.)
It appears that increased impedance in uterine arteries in the first trimester is associated with an increased risk of developing preeclampsia [8, 161–163]. This is especially true for early onset preeclampsia which is commonly associated with intrauterine growth restriction (IUGR). In addition to uterine artery PI, other parameters that contribute to preeclampsia risk assessment include maternal history (age, ethnicity, smoking, parity), maternal examination (BMI, mean arterial blood pressure), maternal serum levels of PAPP-A and placental growth factor [PlGF] (decreased levels of one or both increase the risk of preeclampsia) [8].
For a 5% false positive rate, the combination of the above mentioned risk factors was shown to predict 90% of early preeclampsia, 35% of late preeclampsia, and 20% of gestational hypertension. This compares favorably with screening based on maternal history alone where only 30% of early and 20% of late preeclampsia are predicted for a 5% false positive rate [8].
CONCLUSION
The first trimester is a critical time in pregnancy. In many ways, it is the time when processes that determine the progress of the rest of the pregnancy are set in motion.
The first trimester used to be considered a time when very little could be done from the standpoint of prenatal care. This is due to the fact that until recently the early pregnancy had been relatively inaccessible. However, advances in ultrasound equipment and our understanding of early fetal and placental development allow us now to perform a detailed and informative examination of the fetus, placenta, and the blood supply to the uterus. This, along with measuring certain fetal-placental products in the maternal circulation, allows us to not only to establish whether or not the development of the fetus and/or the placenta is normal at that point in time but to also may predict the risks for certain adverse pregnancy outcomes later in pregnancy. This knowledge may be used to divide pregnant women into various risks groups and to design individualized, targeted prenatal care for them. Additionally, by being able to segregate a group that is truly at risk for diseases such as preeclampsia, possible treatments may be tested more effectively. It is likely that treatments for the prevention of diseases that develop later in pregnancy will be more effective if started as early in pregnancy as possible.
As the utility of ultrasound examination expands, our responsibility to perform the best possible ultrasound examination increases as well. This can be achieved only with proper training and expertise followed by an ongoing and rigorous external quality assurance program. A factor that is difficult to quantify but is none-the-less crucial in performing a thorough ultrasound examination is a high level of commitment on the part of each individual operator.
J. Sonek, MD RDMS
Center for Maternal Fetal Medicine, Ultrasound, and Genetics
Miami Valley Hospital
1 Wyoming Street
Dayton, Ohio 45409
e-mail: jdsonek@mvh.org
Sources
1. Nicolaides KH. 2004. Nuchal translucency and other first-trimester sonographic markers of chromosomal abnormalities. Am J Obstet Gynecol 191 : 45-67.
2. Sonek J. 2007. First Trimester Ultrasonography in Screening and Detection of Fetal Anomalies. Am J Med Genet C Semin Med Genet 145 (1):45-61.
3. Sonek J, Nicolaides KH. 2010. Additional first trimester ultrasound markers. Clin Lab Med 30 : 573-592.
4. Nicolaides KH. 2011. Screening for fetal aneuploidy at 11 to 13 weeks. Prenat Diagn 31 : 7-15.
5. Chaoui R, Benoit B, Mitkowska-Wozniak H et al. 2009. Assessment of intracranial translucency (IT) in the first trimester in the detection of spina bifida at the 11-13 week scan. Ultrasound Obstet Gynecol 34 : 249-252.
6. Lanchmann R, Picciarelli G, Moratalla J, et al. 2010. Frontomaxillary facial angle in fetuses with open spina bifida at 11-13 weeks’ gestation. Ultrasound Obstet Gynecol, 36 : 268-271.
7. Lanchmann R, Chaoui R, Moratalla J, et al. 2011. Posterior brain in fetuses with open spina bifida at 11-13 weeks. Prenat Diagn 31 : 103-106.
8. Poon LCY, Kametas NA, Chelemen T, et al. 2010. Maternal risk factors for hypertensive disorders in pregnancy: a multivariate approach. J Hum Hypertens. 24 : 104-110.
9. Boujold E, Roberge S, Lacasse Y, et al. 2010. Prevention of preeclampsia and intrauterine growth restriction with aspirin started in early pregnancy: a meta-analysis. Obstet Gynecol 116(2 Pt 1):402-414.
10. Nicolaides KH, Azar G, Byrne D, et al. 1992. Fetal nuchal translucency: ultrasound screening for chromosomal defects in first trimester of pregnancy. Br Med J 304 : 867–869.
11. Hecht CA, Hook EB. 1994. The imprecision in rates of Down syndrome by 1-year maternal age intervals: a critical analysis of rates used in biochemical screening. Prenat Diagn 14 : 729–738.
12. Halliday JL, Watson LF, Lumley J, et al. 1995. New estimates of Down syndrome risks at chorionic villus sampling, amniocentesis, and livebirth in women of advanced maternal age from a uniquely defined population. Prenat Diagn 15 : 455–465.
13. Snijders RJM, Holzgreve W, Cuckle H, Nicolaides KH. 1994. Maternal age-specific risks for trisomies at 9–14 weeks’ gestation. Prenat Diagn 14 : 543–552.
14. Snijders RJM, Sebire NJ, Cuckle H, Nicolaides KH. 1995. Maternal age and gestational age-specific risks for chromosomal defects. Fetal Diag Ther 10 : 356–367.
15. Snijders RJM, Sundberg K, Holzgreve W, et al. 1999. Maternal age and gestation - specific risk for trisomy 21. Ultrasound Obstet Gynecol 13 : 167–170.
16. Morris JK, Wald NJ, Watt HC. 1999. Fetal loss in Down syndrome pregnancies. Prenat Diagn 19 : 142–145.
17. Chalouhi GE, Bernard JP, Ville Y, Salomon LJ. 2011. A comparison of first trimester measurements for prediction of delivery date. J Matern Fetal Neonatal Med 24 : 51-57.
18. Salomon LJ, Bernard M, Amarsy R, et al. 2009 The impact of crown-rump length measurement error on combined Down syndrome screening: a simulation study. Ultrasound Obstet Gynecol 33 : 506-511.
19. Nicolaides KH (ed). 1999. The 11-14 week scan: the diagnosis of fetal abnormalities. Parthenon Publishing: Carnforth, UK.
20. Merkatz IR, Nitowsky HM, Macri JN, Johnson WE. 1984. An association between low maternal serum alpha-fetoprotein and fetal chromosomal abnormalities. Am J Obstet Gynecol 148 : 886-894.
21. Canick J, Knight GJ, Palomaki GE, et al. 1988. Low second trimester maternal serum unconjugated oestriol in pregnancies with Down’s syndrome. BJOG 95 : 330-333.
22. Macri JN, Kasturi RV, Krantz DA, et al. 1990. Maternal serum Down syndrome screening: free beta protein is a more effective marker than human chorionic gonadotrophin. Am J Obstet Gynecol 163 : 1248-1253.
23. Van Lith JM, Pratt JJ, Beekhuis JR, Mantingh A. 1993. Second trimester maternal serum immuno-reactive inhibin as a marker for fetal Down’s syndrome. Prenat Diagn 12 : 801-806.
24. Brambati B, Macintosh MCM, Teisner B, et al. 1993. Low maternal serum level of pregnancy associated plasma protein (PAPP-A) in the first trimester in association with abnormal fetal karyotype. BJOG 100 : 324-326.
25. Aitken DA, Wallace EM, Crossley JA, et al. 1996. Dimeric inhibin A as a marker for Down’s syndrome in early pregnancy. N Engl J Med 334 : 1231-1236.
26. Christiansen M, Pihl K, Hedley PL, et al. 2010. ADAM 12may be used to reduce false positive rate of first trimester combined screening for Down syndrome. Prenat Diagn 30 : 110-114.
27. Poon LC, Chelemen T, Minekawa R, et al. 2009. Maternal serum ADAM12 (A disintegrin and metalloprotease) in chromosomally abnormal pregnancy at 11-13 weeks. Am J Obstet Gynecol 508.e1-6.
28. Bredaki FE, Wright D, Matos P, Syngelaki A, Nicolaides KH. 2011. Fetal Diagn Ther 30 : 215-218.
29. Pandya P, Wright D, Syngelaki A, et al. Maternal serum placental growth factor in prospective screening for aneuploidies at 8-13 weeks’ gestation. Fetal Diagn Ther, in press.
30. Kagan KO, Wright D, Spencer K, et al. 2008. First-trimester screening for trisomy 21 by free beta-human chorionic gonadotropin and pregnancy-associated plasma protein-A: impact of maternal and pregnancy characteristics. Ultrasound Obstet Gynecol 31 : 493–502.
31. Tul N, Spencer K, Noble,P et al. 1999. Screening for trisomy 18 by fetal nuchal translucency and maternal serum free beta hCG and PAPP-A at 10-14 weeks of gestation. Prenat Diagn 19 : 1035-1042.
32. Spencer K, Ong C, Skentou H, et al. 2000. Screening for trisomy 13 by fetal nuchal translucency and maternal serum free beta hCG and PAPP-A at 10-14 weeks of gestation. Prenat Diagn 20 : 411-416.
33. Spencer K, Tul N, Nicolaides KH. 2000. Maternal serum free beta hCG and PAPP-A in fetal sex chromosome defects in the first trimester. Prenat Diagn 20 : 390-394.
34. Spencer K, Liao A, Skentou H, et al. 2000. Screening for Triploidy by fetal nuchal translucency and maternal serum free ß-hCG and PAPP-A at 10-14 weeks of gestation. Prenat Diagn 20 : 495-499.
35. Kagan KO, Wright D, Valencia C, et al. 2008. Screening for trisomies 21, 18 and 13 by maternal age, fetal nuchal translucency, fetal heart rate, free {beta}-hCG and pregnancy-associated plasma protein-A. Hum Reprod 23 : 1968-1975.
36 Wright D, Syngelaki A, Birdir C, et al. 2011. First-trimester screening for trisomy 21 with adjustment for biochemical results of previous pregnancies. Fetal Diagn Ther 30 : 194-202.
37. Borrell A, Casals E, Fortuny A, et al. 2004. First-trimester screening for trisomy 21 combining biochemistry and ultrasound at individually optimal gestational ages. An interventional study. Prenat Diagn 24 : 541–545.
38. Kagan KO, Wright D, Baker A, et al. 2008. Screening for trisomy 21 by maternal age, fetal nuchal translucency thickness, free beta-human chorionic gonadotropin and pregnancy-associated plasma protein-A. Ultrasound Obstet Gynecol 31 : 618-624.
39. Kirkegaard I, Petersen OB, Uldbjerg N, TŅrring N. 2008. Improved performance of first-trimester combined screening for trisomy 21 with the double test taken before a gestational age of 10 weeks. Prenat Diagn 28 : 839-844.
40. Wright D, Spencer K, Kagan KO, et al. 2010. First-trimester combined screening for trisomy 21 at 7-14 weeks. Ultrasound Obstet Gynecol 36 : 404-411.
41. Cuckle HS, van Lith JMM. 1999. Appropriate biochemical parameters in first-trimester screening for Down syndrome. Prenat Diagn 19 : 505-512.
42. Spencer K, Crossley JA, Aitken DA, et al. 2003. The effect of temporal variation in biochemical markers of trisomy 21 across the first and second trimesters of pregnancy on the estimation of individual patient specific risks and detection rates for Down’s Syndrome. Ann Clin Biochem 40 : 219-231.
43. Plasencia W, Dagklis T, Sotiriadis A, et al. 2007. Frontomaxillary facial angle at 11 + 0 to 13 + 6 weeks’ gestation-reproducibility of measurements.
44. Molina F, Avgidou K, Kagan K, et al. 2006. Cystic hygromas, nuchal edema, and nuchal translucency at 11-14 weeks of gestation. Obstet Gynecol, 107 : 678-683. 130.
45. Sonek J, Croom C, McKenna D, et al. 2006. Letter to the Editor, Obstet Gynecol 107 : 424.
46. Nicolaides KH, Snijders RJ, Cuckle HS. 1998. Correct estimation of parameters for ultrasound nuchal translucency screening. Prenatal Diagnosis 18 : 519-523.
47. Pandya PP, Snijders RJM, Johnson S, et al. 1995. Screening for fetal trisomies by maternal age and fetal nuchal translucency thickness at 10 to 14 weeks of gestation. BJOG 102 : 957–962.
48. Spencer K, Bindra R, Nix ABJ, et al. 2003. Delta - NT or NT MoM: which is the most appropriate method for calculating accurate patient-specific risks for trisomy 21 in the first trimester? Ultrasound Obstet GynecoI 22 : 142-148.
49. Pearson K. 1894. Contributions to the mathematical theory of evolution. Phil Trans Roy Soc London 185 : 71-110.
50. Wright D, Kagan KO, Molina FS, et al. 2008. A mixture model of nuchal translucency thickness in screening for chromosomal defects. Ultrasound Obstet Gynecol 31 : 376-383.
51. Snijders RJ, Noble P, Sebire N et al. Fetal Medicine Foundation First Trimester Screening Group.1998. UK multicentre project on assessment of risk of trisomy 21 by maternal age and fetal nuchal-translucency thickness at 10-14 weeks of gestation. Lancet 352 : 343-346.
52. Wald NJ, Rodeck C, Hackshaw AK, et al. (SURUSS Research Group. 2003). First and second trimester antenatal screening for Down’s syndrome: the results of the Serum, Urine and Ultrasound Screening Study (SURUSS). Health Technol Assess 7 : 1-88.
53. Nicolaides KH. 2003. Screening for chromosomal defects. Ultrasound Obstet Gynecol 21 : 313-321.
54. Malone FD, Canick JA, Ball RH, et al. (First - and Second-Trimester Evaluation of Risk (FASTER) Research Consortium). 2005. First-trimester or second-trimester screening, or both, for Down‘s syndrome. N Engl J Med 353 : 2001-2011.
55. Sonek J, Nicolaides K. 2002 Prenatal ultrasonographic diagnosis of nasal bone abnormalities in three fetuses with Down syndrome. Am J Obstet Gynecol 186 : 139-141.
56. Cicero S, Curcio P, Papageorghiou A, Sonek J, Nicolaides KH. 2001. Absence of nasal bone in fetuses with Trisomy 21 at 11-14 weeks of gestation: an observational study. Lancet 358 : 1665-1667.
57. Cicero S, Avgidou K, Rembouskos G, et al. 2006. Nasal bone in first-trimester screening for trisomy 21. Am J Obstet Gynecol 195 : 109–114.
58. Kagan KO, Cicero S, Staboulidou I, et al. 2009. Fetal nasal bone in screening for trisomies 21, 18 and 13 and Turner syndrome at 11-13 weeks of gestation. Ultrasound Obstet Gynecol 33 : 259-264.
59. Sonek J, Borenstein M, Dagklis T, et al. 2007. Fronto-maxillary Facial Angle in Fetuses with Trisomy 21 at 11-13 (+6) Weeks’. Am J Obstet Gynecol 196(3):271.
60. Matias A, Gomes C, Flack N, et al. 1998. Screening for chromosomal abnormalities at 10-14 weeks: the role of ductus venosus blood flow. Ultrasound Obstet Gynecol 12 : 380-384.
61. Maiz N, Valencia C, Kagan KO, et al. 2009. Ductus venosus Doppler in screening for trisomies 21, 18 and 13 and Turner syndrome at 11-13 weeks of gestation. Ultrasound Obstet Gynecol 33 : 512-517.
62. Huggon IC, DeFigueiredo DB, Allan LD. 2003. Tricuspid regurgitation in the diagnosis of chromosomal anomalies in the fetus at 11–14 weeks of gestation. Heart 89 : 1071–1073.
63. Faiola S, Tsoi E, Huggon IC, et al. 2005. Likelihood ratio for trisomy 21 in fetuses with tricuspid regurgitation at the 11 to 13 + 6-week scan. Ultrasound Obstet Gynecol 26 : 22–27.
64. Falcon O, Faiola S, Huggon I, et al. 2006. Fetal tricuspid regurgitation at the 11 + 0 to 13 + 6-week scan: association with chromosomal defects and reproducibility of the method. Ultrasound Obstet Gynecol 27 : 609-612.
65. Kagan KO, Valencia C, Livanos P, et al. 2009. Tricuspid regurgitation in screening for trisomies 21, 18 and 13 and Turner syndrome at 11+0-13+6 weeks of gestation. Ultrasound Obstet Gynecol 33 : 18-22.
66. Bilardo CM, Timmerman E, Robles de Medina PG, Clur SA. 2011. Increased hepatic artery flow in first trimester fetuses: an ominous sign. Ultrasound Obstet Gynecol 37 : 438-443.
67. Zvanca M, Gielchinsky Y, Abdeljawad F, et al. 2010. Hepatic artery Doppler in trisomy 21 and euploid fetuses at 11-13 weeks. Prenat Diagn 31 : 22-27.
68. Brizot ML, Snijders RJM, Bersinger NA, et al. 1994. Maternal serum pregnancy associated placental protein A and fetal nuchal translucency thickness for the prediction of fetal trisomies in early pregnancy. Obstet Gynecol 84 : 918–922.
69. Brizot ML, Snijders RJM, Butler J, et al. Maternal serum hCG and fetal nuchal translucency thickness for the prediction of fetal trisomies in the first trimester of pregnancy. 1995. Br J Obstet Gynaecol 102 : 1227-1232.
70. Noble PL, Abraha HD, Snijders RJ, et al. 1995. Screening for fetal trisomy 21 in the first trimester of pregnancy: maternal serum free beta-hCG and fetal nuchal translucency thickness. Ultrasound Obstet Gynecol 6 : 390-395.
71. Spencer K, Souter V, Tul N, et al. 1999. A screening program for trisomy 21 at 10–14 weeks using fetal nuchal translucency, maternal serum free ß-human chorionic gonadotropin and pregnancy-associated plasma protein-A. Ultrasound Obstet Gynecol 13 : 231–237.
72. Krantz DA, Hallahan TW, Orlandi F, et al. 2000. First-trimester Down syndrome screening using dried blood biochemistry and nuchal translucency. Obstet Gynecol 96 : 207-213.
73. Bindra R, Heath V, Liao A, et al. 2002. One Stop Clinic for Assessment of Risk for Trisomy 21 at 11-14 weeks: A Prospective Study of 15,030 Pregnancies. Ultrasound Obstet Gynecol 20 : 219-225.
74. Schuchter K, Hafner E, Stangl G, et al. 2002. The first trimester ‘combined test’ for the detection of Down syndrome pregnancies in 4939 unselected pregnancies. Prenat Diagn 22 : 211-215.
75. Spencer K, Spencer CE, Power M, et al. 2003. Screening for chromosomal abnormalities in the first trimester using ultrasound and maternal serum biochemistry in a one stop clinic: A review of three years prospective experience. Br J Obstet Gynaecol 110 : 281-286.
76. Wapner R, Thom E, Simpson J, et al. 2003. First trimester maternal serum biochemistry and fetal nuchal translucency screening (BUN) study group. First trimester screening for trisomies 21 and 18. N Engl J Med 349 : 1405-1413.
77. Nicolaides KH, Spencer K, Avgidou K, et al. 2005. Multicenter study of first-trimester screening for trisomy 21 in 75 821 pregnancies: results and estimation of potential impact of individual risk-oriented two-stage first-trimester screening. Ultrasound Obstet Gynecol 25 : 221-226.
78. Ekelund CK, JŅrgensen FS, Petersen OB, et al. (Danish Fetal Medicine Research Group). 2008. Impact of a new national screening policy for Down’s syndrome in Denmark: population based cohort study. BMJ 337: a2547. doi: 10.1136/bmj.a2547.
79. Kagan KO, Etchegaray A, Zhou Y, et al. 2009. Prospective validation of first-trimester combined screening for trisomy 21. Ultrasound Obstet Gynecol 34 : 14-18.
80. Leung TY, han LW, aw LW, et al. 2009. First trimester combined screening for Trisomy 21 in Hong Kong: outcome of the first 10,000 cases. 2 : 300-304.
81. Kagan KO, Staboulidou I, Cruz J, et al. 2010. Two-stage first-trimester screening for trisomy 21 by ultrasound assessment and biochemical testing. Ultrasound Obstet Gynecol 36 : 542-547.
82. Hyett JA, Noble PL, Snijders RJ, et al. 1996. Fetal heart rate in trisomy 21 and other chromosomal abnormalities at 10–14 weeks of gestation. Ultrasound Obstet Gynecol 7 : 239-244.
83. Liao AW, Snijders R, Geerts L, et al. 2000. Fetal heart rate in chromosomally abnormal fetuses. Ultrasound Obstet Gynecol 16 : 610–613.
84. Papageorghiou AT, Avgidou K, Spencer K, et al. 2006. Sonographic screening for trisomy 13 at 11 to 13(6) weeks of gestation. Am J Obstet Gynecol 194 : 397–401.
85. Kagan KO, Anderson JM, Anwandter G, et al. 2008. Screening for triploidy by the risk algorithms for trisomies 21, 18 and 13 at 11 weeks to 13 weeks and 6 days of gestation. Prenat Diagn 28 : 1209-1213.
86. Pandya PP, Hilbert F, Snijders RJ, Nicolaides KH. 1995. Nuchal translucency thickness and crown-rump length in twin pregnancies with chromosomally abnormal fetuses. J Ultrasound Med 14 : 565-568.
87. Sebire NJ, Snijders RJM, Hughes K, et al. 1996. Screening for trisomy 21 in twin pregnancies by maternal age and fetal nuchal translucency thickness at 10–14 weeks of gestation. BJOG 103 : 999-1003.
88. Sebire NJ, Noble PL, Psarra A, et al. 1996. Fetal karyotyping in twin pregnancies: selection of technique by measurement of fetal nuchal translucency. BJOG 103 : 887-890.
89. Maymon R, Jauniaux E, Holmes A, et al. 2001. Nuchal translucency measurement and pregnancy outcome after assisted conception versus spontaneously conceived twins. Hum Reprod 16 : 1999-2004.
90. Sepulveda W, Sebire NJ, Hughes K, et al. 1996. The lambda sign at 10-14 weeks of gestation as a predictor of chorionicity in twin pregnancies. Ultrasound Obstet Gynecol 7 : 421-423.
91. Vandecruys H, Faiola S, Auer M, et al. 2005. Screening for trisomy 21 in monochorionic twins by measurement of fetal nuchal translucency thickness. Ultrasound Obstet Gynecol 25 : 551-553.
92. Sebire NJ, Hughes K, D’Ercole C, et al. 1997. Increased fetal nuchal translucency at 10–14 weeks as a predictor of severe twin-to-twin transfusion syndrome. Ultrasound Obstet Gynecol. 10 : 86-89.
93. Kagan KO, Gazzoni A, Sepulveda-Gonzalez G, et al. 2007. Discordance in nuchal translucency thickness in the prediction of severe twin-to-twin transfusion syndrome. Ultrasound Obstet Gynecol 29 : 527-532.
94. WŅjdemann KR, Larsen SO, Shalmi AC, et al. 2006. Nuchal translucency measurements are highly correlated in both mono - and dichorionic twin pairs. Prenat Diagn 26 : 218-220.
95. Cuckle H, Maymon R. 2010. Down syndrome risk calculation for a twin fetus taking account of the nuchal translucency in the co-twin. Prenat Diagn 30 : 827-833.
96. Wright D, Syngelaki A, Staboulidou I, et al. 2010. Screening for trisomies in dichorionic twins by measurement of fetal nuchal translucency thickness according to the mixture model. Prenat Diagn 31 : 16-21.
97. Spencer K, Nicolaides KH. 2000. First trimester prenatal diagnosis of trisomy 21 in discordant twins using fetal nuchal translucency thickness and maternal serum free beta-hCG and PAPP-A. Prenat Diagn 20 : 683-684.
98. Spencer K, Nicolaides KH. 2003. Screening for trisomy 21 in twins using first trimester ultrasound and maternal serum biochemistry in a one-stop clinic: a review of three years experience. BJOG 110 : 276-280.
99. Spencer K, Kagan KO, Nicolaides KH. 2008. Screening for trisomy 21 in twin pregnancies in the first trimester: an update of the impact of chorionicity on maternal serum markers. Prenat Diagn 28 : 49-52.
100. Linskens IH, Spreeuwenberg MD, Blankenstein MA, van Vugt JM. 2009. Early first-trimester free beta-hCG and PAPP-A serum distributions in monochorionic and dichorionic twins. Prenat Diagn 29 : 74-78.
101. Spencer K, Nicolaides KH. 2003. Screening for trisomy 21 in twins using first trimester ultrasound and maternal serum biochemistry in a one-stop clinic: a review of three years experience. BJOG 110 : 276-280.
102. Dagklis T, Plasencia W, Maiz N, et al. 2008. Choroid plexus cyst, intracranial echogenic focus, hyperechoic bowel and hydronephrosis in screening for trisomy 21 at 11+0 to 13+6 weeks. Ultrasound Obstet Gynecol. 31 : 132-135.
103. Nicolaides KH, Snijders RJM, Gosden CM, et al. 1992. Ultrasonographically detectable markers of fetal chromosomal abnormalities. Lancet 340 : 704-707.
104. Liao A, Sebire N, Geerts L, et al. 2003. Megacystis at 10-14 weeks of gestation: chromosomal defects and outcome according to bladder length. Ultrasound Obstet Gynecol 21 : 338-341.
105. Sebire NJ, Von Kaisenberg C, Rubio C, et al. 1996. Fetal megacystis at 10-14 weeks of gestation. Ultrasound Obstet Gynecol 8 : 387-390.
106. Kagan KO, Staboulidou I, Syngelaki A, et al. 2010. The 11-13 weeks scan: Diagnosis and outcome of holoprosencephaly, exomphalos and megacystis. Ultrasound Obstet Gynecol 36 : 10-14.
107. Benacerraf BR, Neuberg D, Bromley B, Frigoletto FD Jr. 1992. Sonographic scoring index for prenatal detection of chromosomal abnormalities. J Ultrasound Med 11 : 449-458.
108. Vintzileos AM, Egan JF. 1995. Adjusting the risk for trisomy 21 on the basis of second-trimester ultrasonography. Am J Obstet Gynecol 172 : 837–844.
109. Vintzileos AM, Campbell WA, Rodis JF, et al. 1996. The use of second-trimester genetic sonogram in guiding clinical management of patients at increased risk for fetal trisomy 21. Obstet Gynecol 87 : 948–952.
110. Nicolaides KH (ed.). 1996. Ultrasound Markers for Fetal Chromosomal Defects. Parthenon Publishing: Carnforth, UK.
111. Bahado-Singh R, Deren O, Oz U, et al. 1998. An alternative for women initially declining genetic amniocentesis: individual Down syndrome odds on the basis of maternal age and multiple ultrasonographic markers. Am J Obstet Gynecol 179 : 514–519.
112. Nyberg DA, Souter VL, El-Bastawissi A, et al. 2001. Isolated sonographic markers for detection of fetal Down syndrome in the second trimester of pregnancy. J Ultrasound Med 20 : 1053-1063.
113. Smith-Bindman R, Hosmer W, Feldstein V, et al. 2001. Second-trimester ultrasound to detect fetuses with Down syndrome a meta-analysis. JAMA 285 : 1044–1055.
114. Bromley B, Lieberman E, Shipp TD, Benacerraf BR. 2002. The genetic sonogram. A method of risk assessment for Down syndrome in the second trimester. J Ultrasound Med 21 : 1087-1096.
115. Krantz DA, Hallahan TW, Macri VJ, Macri JN 2007. Genetic sonography after first-trimester Down syndrome screening. Ultrasound Obstet Gynecol 29 : 666-670.
116. Cuckle H, Benn P, Wright D. 2005. Down syndrome screening in the first and/or second trimester: model predicted performance using meta-analysis parameters. Seminars Perinatology 29 : 252-257.
117. Cuckle H, Benn P. 2009. Multianalyte maternal serum screening for chromosomal defects. In Genetic Disorders and the Fetus: Diagnosis, Prevention and Treatment, 6th edition, Milunsky A (ed.). Johns Hopkins University: Baltimore.
118. Wald NJ, Huttly WJ, Hackshaw AK. 2003. Antenatal screening for Down’s syndrome with the quadruple test. Lancet 361 : 835-836.
119. Wald NJ, Watt HC, Hackshaw AK. 1999. Integrated screening for Down’s syndrome on the basis of tests performed during the first and second trimesters. N Engl J Med 341 : 461-467.
120. Wright D, Bradbury I, Benn P, et al. 2004. Contingent screening for Down syndrome is an efficient alternative to non-disclosure sequential screening. Prenat Diagn 24 : 762-766.
121. Benn P, Wright D, Cuckle H. 2005. Practical strategies in contingent sequential screening for Down syndrome. Prenat Diagn 25 : 645-652.
122. Cuckle HS, Malone FD, Wright D, et al. 2008. Contingent screening for Down syndrome - results from the FaSTER trial. Prenat Diagn 28 : 89-94.
123. Brady AF, Pandya PP, Yuksel B, et al. 1998. Outcome of chromosomally normal live births with increased fetal nuchal translucency at 10-14 weeks’ gestation. J Med Genet 35 : 222-224.
124. Souka AP, Krampl E, Bakalis S, et al. 2001. Outcome of pregnancy in chromosomally normal fetuses with increased nuchal translucency in the first trimester. Ultrasound Obstet Gynecol 18 : 9-17.
125. Mangione R, Guyon F, Taine L, et al. 2001. Pregnancy outcome and prognosis in fetuses with increased first-trimester nuchal translucency. Fetal Diagn Ther 16 : 360-363.
126. Bilardo CM, Pajkrt E, de Graaf IM, et al. 1998. Outcome of fetuses with enlarged nuchal translucency and normal karyotype. Ultrasound Obstet Gynecol 11 : 401-406.
127. Michailidis GD, Economides DL. 2001. Nuchal translucency measurement and pregnancy outcome in karyotypically normal fetuses. Ultrasound Obstet Gynecol 17 : 102-105.
128. Shulman LP, Emerson DS, Grevengood C, et al. 1994. Clinical course and outcome of fetuses with isolated cystic nuchal lesions and normal karyotypes detected in the first trimester. Am J Obstet Gynecol 171 : 1278-1281.
129. Cheng C, Bahado-Singh RO, Chen S, et al. 2004. Pregnancy outcomes with increased nuchal translucency after routine Down syndrome screening. Int J Gynaecol Obstet 84 : 5-9.
130. Senat MV, De Keersmaecker B, Audibert F, et al. 2002. Pregnancy outcome in fetuses with increased nuchal translucency and normal karyotype. Prenat Diagn 22 : 345-349.
131. Cha’Ban FK, van Splunder P, Los FJ, et al. 1996. Fetal outcome in nuchal translucency with emphasis on normal fetal karyotype. Prenat Diagn 16 : 537-541.
132. Souka AP, Snidjers RJM, Novakov A, et al. 1998. Defects and syndromes in chromosomally normal fetuses with increased nuchal translucency thickness at 10–14 weeks of gestation. Ultrasound Obstet Gynecol 11 : 391-400.
133. Souka AP, Von Kaisenberg CS, Hyett JA, et al. 2005. Increased nuchal translucency with normal karyotype. Am J Obstet Gynecol 192 : 1005-1021.
134. Hyett J, Moscoso G, Papapanagiotou G, et al. 1996. Abnormatlities of the heart and great arteries in chromosomally normal fetuses with increased nuchal translucency thickness at 11-13 weeks of gestation. Ultrasound Obstet Gynaecol 7 : 245-250.
135. Hyett JA, Perdu M, Sharland GK, et al. 1997. Increased nuchal translucency at 10-14 weeks of gestation as a marker for major cardiac defects. Ultrasound Obstet Gynecol 10 : 242-246.
136. Lopes LM, Brizot ML, Lopes MA, et al. 2003. Structural and functional cardiac abnormalities identified prior to 16 weeks’ gestation in fetuses with increased nuchal translucency. Ultrasound Obstet Gynecol 22 : 470-478.
137. McAuliffe F, Winsor S, Hornberger L, et al. 2003. Fetal cardiac defects and increased nuchal translucency thickness. Am J Obstet Gynecol 189, Abstract 571.
138. Hyett J, Perdu M, Sharland G, et al. 1999. Using fetal nuchal translucency to screen for major congenital cardiac defects at 10-14 weeks of gestation: population based cohort study. Brit Med J 318 : 81-85.
139. Makrydimas G, Sotiriadis A, Ioannidis JP. 2003. Screening performance of first-trimester nuchal translucency for major cardiac defects: a meta-analysis. Am J Obstet Gynecol 189 : 1330-1335.
140. Makrymidas G, Sotiradis A, Huggon IC, et al. 2005. Nuchal translucency and fetal cardiac defects: A pooled analysis of major fetal echocardiography centers. Am J Obstet Gynecol 192 : 85-89.
141. Carvalho JS, Moscoso G, Ville Y. 1998 First trimester transabdominal fetal echocardiography. Lancet 351 : 1023-1027.
142. Zosmer N, Souter VL, Chan CSY, et al. 1999. Early diagnosis of major cardiac defects in chromosomally normal fetuses with increased nuchal translucency. Br J Obstet Gynaecol 106 : 829-833.
143. Simpson JM, Jones A, Callaghan N, et al. 2000. Accuracy and limitations of transabdominal fetal echocardiography at 12-15 weeks of gestation in a population at high risk for congenital heart disease. Br J Obstet Gynaecol 16 : 30-36.
144. Sebire NJ, Snijders RJM, Davenport M, et al. 1997. Fetal nuchal translucency thickness at 10–14 weeks of gestation and congenital diaphragmatic hernia. Obstet Gynecol 90 : 943-947.
145. Snijders RJM, Sebire NJ, Souka A, Santiago C, Nicolaides KH. 1995. Fetal exomphalos and chromosomal defects: relationship to maternal age and gestation. Ultrasound Obstet Gynecol 6 : 250-255.
146. Smrcek JM, Germer U, Krokowski M, et al. 2003. Prenatal ultrasound diagnosis and management of body stalk anomaly: analysis of nine singleton and two multiple pregnancies. Ultrasound Obstet Gynecol 21 : 322-328.
147. Makrydimas G, Souka A, Skentou H, et al. 2001. Osteogenesis imperfecta and other skeletal dysplasias presenting with increased nuchal translucency in the first trimester. Am J Med Genet 98 : 117-120.
148. Fincham J, Pandya PP, Yuksel B, et al. 2002. Increased first-trimester nuchal translucency as a prenatal manifestation of salt-wasting congenital adrenal hyperplasia. Ultrasound Obstet Gynecol 20 : 392-394.
149. Hyett J, Noble P, Sebire NJ, et al. 1997. Lethal congenital arthrogryposis presents with increased nuchal translucency at 10–14 weeks of gestation. Ultrasound Obstet Gynecol 9 : 310-313.
150. Achiron R, Heggesh J, Grisaru D, et al. 2000. Noonan syndrome: a cryptic condition in early gestation. Am J Med Genet 92 : 159-165.
151. Hyett JA, Clayton, PT, Moscoso G, et al. 1995. Increased first trimester nuchal translucency as a prenatal manifestation of Smith-Lemli-Opitz syndrome. Am J Med Genet. 58 : 374-376.
152. de Jong-Pleij EA, Stoutenbecek P, van der Mark-Batseva NN, et al. 2002. The association of spinal muscular atrophy type II and increased nuchal translucency. Ultrasound Obstet Gynecol 19 : 312-313.
153. Brady AF, Pandya PP, Yuksel B, et al. 1998. Outcome of chromosomally normal livebirths with increased fetal nuchal translucency at 10-14 weeks’ gestation. J Med Genet 35 : 222-224.
154. Adekunle O, Gopee A, El-Sayed M, et al. 1999. Increased first-trimester nuchal translucency: Pregnancy and infant outcomes after routine screening for Down’s syndrome in an unselected antenatal population. Br J Radiol 72 : 457-460.
155. Maymon R, Jauniaux E, Cohen O, et al. 2000. Pregnancy outcome and infant follow-up of fetuses with abnormally increased first trimester nuchal translucency. Hum Reprod 15 : 2023-2027.
156. Hiippala A, Eronen M, Taipale P, et al. 2001. Fetal nuchal translucency and normal chromosomes: A long-term follow-up study. Ultrasound Obstet Gynecol. 18 : 18-22.
157. Souka AP, Pilalis A, Kavalakis Y et al. 2004. Assessment of fetal anatomy at the 11–13-week ultrasound examination. Ultrasound Obstet Gynecol 24 : 730–734.
158. Becker R, Wegner RD. 2006. Detailed screening for fetal anomalies and cardiac defects at the 11-13 week scan. Ultrasound Obstet Gynecol 27 : 613-618.
159. Nicolaides KH, Campbell S, Gabbe SG. 1986. Ultrasound screening for spina bifida: cranial and cerebellar signs. Lancet 2 : 72-74.
160. Khong TY, De Wolf F, Robertson F, Brosens I. 1986. Inadequate maternal vascular response to placentation in pregnancies complicated by preeclampsia and by small for gestational age infants. Brit J Obstet Gynaecol. 93 : 1049-59.
161. Poon LCY, Staboulidou I, Maiz N, et al. 2009. Hypertensive disorders in pregnancy: screening by uterine artery Doppler at 11-13 weeks. Ultrasound Obstet Gynecol 34 : 142-148.
162. Poon, LCY, Karagiannis G, Leal A, et al. 2009. Hypertensive disorders in pregnancy: screening by uterine artery Doppler and blood pressure at 11-13 weeks. Ultrasound Obstet Gynecol 34 : 487-502.
163. Poon LC, Stratieva V, Piras S, et al. Hypertensive disorders in pregnancy: combined screening by uterine Doppler, blood pressure and serum PAPP-A at 11-13 weeks. Prenat Diagn Epub 2010 Jan 27.
Labels
Paediatric gynaecology Gynaecology and obstetrics Reproduction medicineArticle was published in
Czech Gynaecology

2012 Issue 2
-
All articles in this issue
- New methods increasing assisted reproduction results
- Adrenocorticotropin hormone – possible marker of pregnancy pathologies
- Immunotherapy in ovarian cancer
- Heterotopic pregnancy as an complication during pregnancy and labour – the case report
- Fetomaternal haemorrhage in delivery by cesarean section
- Medical, legal and etical aspects of fertility preservation in cancer survivor
- Urinary tract infections in pregnancy: when to treat, how to treat, and what to treat with
- Thrombophilic mutation by women with serious pregnancy complications
- Possibilities of IVF in native cycle
- History and present state of University Department of Obstetrics and Gynecology in Brno, Czech Republic
- Laparoscopically assisted neovagina formation – updated Vechieti surgery
- Effect of GnRH analogues pre-treatment on myomectomy outcomes in reproductive age women
- Ovarian tissue cryopreservation in cancer patients – six years of clinical experience
- Vaginal birth after previous caesarian section – outcomes analysis 2007–2010
- Triple negative breast cancer – prognostically highly unfavourable group cancer of breast
- Screening v 11.–13.+6 týdnu těhotenství
- Czech Gynaecology
- Journal archive
- Current issue
- About the journal
Most read in this issue
- Triple negative breast cancer – prognostically highly unfavourable group cancer of breast
- Possibilities of IVF in native cycle
- Urinary tract infections in pregnancy: when to treat, how to treat, and what to treat with
- Vaginal birth after previous caesarian section – outcomes analysis 2007–2010
