Effect of six antiretroviral drugs (delavirdine, stavudine, lamivudine, nelfinavir, amprenavir and lopinavir/ritonavir in association) on albino pregnant rats (Rattus norvegicus Albinus, Rodentia, Mammalia): biological assay
Vliv šesti antiretrovirotik (delavirdinu, stavudinu, lamivudinu, nelfinaviru, amprenaviru a lopinaviru/ritonaviru v kombinaci) na březí bílé krysy (Rattus norvegicus Albinus, Rodentia, Mammalia): biologický test
Cíl:
Srovnat chronické účinky antiretrovirotické léčby (lamivudin, stavudin, delavirdin, nelfinavir, amprenaviru a kombinace lopinaviru/ritonaviru) u březích potkanů albínů.
Typ studie:
Review.
Název a sídlo pracoviště:
Gynekologicko-porodnická klinika, Federal University of São Paulo (UNIFESP), Sao Paulo, SP, Brazílie.
Metodika:
Retrospektivní srovnávací studie se zabývala 18 skupinami po 10 březích samičkách potkanů, které byly téměř tři měsíce staré a vážily 200 gramů. Každé z nich byla denně aplikována léčba pomocí žaludeční sondy, zatímco kontrolní skupině byl aplikován 1 ml destilované vody. Studijní skupiny obdržely lamivudin (5, 15 a 45 mg/kg/den); stavudin (na 1, 3 a 9 mg/kg/den); nelfinavir (40, 120 a 360 mg/kg/den); amprenavir (na 46, 138 a 414 mg/kg/den); lopinavir/ritonavir (12.8/3.2, a 38.4/9.6 115/28.8 mg/kg/den) a delavirdin (20 a 60 mg/kg/den). To představuje jedno-, troj - a devítinásobek terapeutické dávky pro člověka, s výjimkou posledního léku, kde nebyla nejvyšší dávka podána. Byla hodnocena hmotnost samice, plodu a placenty, počet implantací a reabsorpcí, hlavní zevní malformace plodu a odumření samice nebo plodu. Pro statistické zpracování byly použity test Kruskalův-Wallisův a χ2 test.
Výsledky:
U všech tří dávek stavudin zvyšoval hmotnost samice (p = 0,001), zatímco lamivudin při trojnásobné a devítinásobné dávce hmotnost snižoval (p < 0,001). Amprenavir u všech dávek, a lopinavir//ritonavir u troj - a devítinásobné dávky zvyšoval úmrtnost samic (p < 0,001). Z hlediska plodů nebylo žádné z antiretrovirotik škodlivé, pokud jde o implantaci, reabsorpci, teratogenitu a úmrtnost (p > 0,05). Stavudin při všech dávkách snižoval hmotnost mláďat(p < 0,001); Nicméně lamivudin u trojnásobné, delavirdin u trojnásobné a amprenavir u trojnásobné dávky hmotnost mláďat zvyšoval (p < 0,001).
Závěr:
U březích samic jsme pozorovali letální toxicitu u krys, kterým byl aplikován amprenavir a ritonavir/lopinavir; hmotnost krys se měnila při užití lamivudinu a stavudinu . U plodů byly pozorovány nežádoucí účinky v souvislosti s hmotností mláďat u stavudinu, lamivudinu, amprenaviru a delavirdinu..
Klíčová slova:
březí krysy, antiretrovirotika, teratologie, biologický test
Authors:
M. U. Nakamura *; Edward Araujo Júnior *
; Simões M. J.+; Oliveira Filho R. M.§; L. Kulay Júnior *
Authors‘ workplace:
Department of Obstetrics, Federal University of São Paulo (UNIFESP), São Paulo, SP, Brazil
+Department of Morphology, Federal University of São Paulo (UNIFESP), São Paulo, SP, Brazil
§Department of Pharmacology, São Paulo University (USP), São Paulo, SP,
*
Published in:
Ceska Gynekol 2014; 79(4): 295-304
Overview
Objective:
To compare the chronic effects of antiretrovirals (lamivudine, stavudine, delavirdine, nelfinavir, amprenavir and an association of lopinavir/ritonavir) on albino pregnant rats.
Design:
Review.
Setting:
Department of Obstetrics, Federal University of São Paulo (UNIFESP), São Paulo, SP, Brazil.
Methods:
This was a comparative retrospective study formed by 18 groups of 10 pregnant rats each, which were nearly three months of age and weighed 200 g. All of them were medicated every day using a stomach probe, while the control group was given 1 mL of distilled water. The study groups received lamivudine (at 5, 15 and 45 mg/kg/day); stavudine (at 1, 3 and 9 mg/kg/day); nelfinavir (at 40, 120 and 360 mg/kg/day); amprenavir (at 46, 138 and 414 mg/kg/day); lopinavir/ritonavir (at 12.8/3.2, 38.4/9.6 and 115/28.8 mg/kg/day) and delavirdine (at 20 and 60 mg/kg/day). These represented 1, 3 and 9 times the human therapeutic dose, except for the last drug, for which the 9-times dose was not used. Maternal, litter and placental weights, implantation and reabsorption numbers, major external fetal malformations and fetal and maternal deaths were evaluated. The Kruskal-Wallis test was used to compare quantitative variables and the chi-square test was used to compare qualitative variables.
Results:
At all three doses, stavudine increased the maternal weight (p=0.001), while lamivudine at 3 - and 9-times doses reduced it (p<0.001). Amprenavir at all of the doses, and lopinavir/ritonavir at 3 - and 9-times doses, caused higher rates of maternal death (p<0.001). Regarding the fetuses, none of the antiretroviral drugs studied were harmful with regard to implantation, reabsorption, teratogenity and mortality (p>0.05). Stavudine at all doses reduced the litter weights (p<0.001); however, lamivudine at the usual and 3-times doses, delavirdine at 3-times dose, and amprenavir at 3-times dose increased the litter weight (p<0.001).
Conclusion:
In the maternal compartment, we observed lethal toxicity in the pregnant rats that received amprenavir and ritonavir/lopinavir; and maternal weight change with lamivudine and stavudine. In the fetal compartment, adverse effects were observed in relation to litter weight from stavudine, lamivudine, delavirdine and amprenavir.
Keywords:
pregnant rats, antiretroviral drugs, teratology, biological assay
INTRODUCTION
More than 30 years after the acquired immunodeficiency syndrome was first recognized, the human immunodeficiency virus (HIV) continues to be at the focus of worldwide investment [10]. With regard to its reproductive issues, even though the percentage of women living with HIV has remained stable at 50% for several years, it has been estimated that 370,000 children under the age of 15 years become infected with HIV every year, thus showing that worldwide, the prevalence within this age group increased from 1.6 million in 2001 to 2.0 million in 2007 [42]. These figures would have been even higher if reductions had not occurred since 2002 through prevention of mother-child vertical transmission.
The preventive strategies include combating sexual transmission, blood transmission and vertical transmission from mother to child. Strategies centered on antiretroviral drugs, for preventing vertical transmission and providing post-exposure prophylaxis and probably indirect prevention secondary to reduction of the viral load in individuals with the virus, have expanded because of their effectiveness. In the United States, the incidence of AIDS is decreasing, with substantially increased survival after the disease has been diagnosed. This has come about especially since highly active antiretroviral therapy (HAART) was introduced, which became the standard treatment in 1996 [7].
Antiretroviral therapy has changed the course of HIV infection, from an infection that was almost invariably fatal to a chronic disease. Recognition of different drug classes acting on different stages of the life cycle of the virus may inhibit its replication more effectively. This is the most important and furthest advanced point within this field, both for treatment and for prevention. The challenge for healthcare worldwide is to able to provide the right antiretroviral drugs for the right people, given that after the short-lived success of the first such agent (azidothymidine, AZT), it became clear that HIV has the ability to generate drug-resistant mutations. To combat this, a combination of agents, generally three, would be required and this ultimately became known as HAART. The best choice of antiretroviral agent depends not only on its toxicity but also on its cost, which varies widely between regions according to the financial and technological situation of each region [10]. Usually, the treatment combines two nucleoside reverse transcriptase inhibitors (NRTIs) plus one non-nucleoside reverse transcriptase inhibitor (NNRTI), or combinations of two NRTIs plus one protease inhibitor (PI), which is equally effective.
HIV transmission from a pregnant woman to her child has been studied since 1989. In the 1990s, the transmission rate was 20% without treatment, and this reduced to 10.4% among those who received monotherapy consisting of zidovudine, 3.8% for therapy with two antiretroviral drugs and 1.2% with HAART. The transmission rate also varied according to the viral load: 1.0% for <400 copies/mL; 5.3% for 400 to 3499; 9.3% for 3500 to 9999; 14.7% para 10,000 to 29,999; and 23.4% for > 30,000 [11]. Because of the good results from HAART, this regimen was recommended for pregnant women in order to prevent vertical transmission, and many women became pregnant under this treatment. According to estimates obtained through data pooled between UNAIDS, WHO, UNICEF and countries that report their rates, it seems that the coverage of antiretrovirals for HIV-pregnant women, for preventing vertical transmission in developing countries, increased from 9% in 2004 to 33% in 2007 [42].
However, therapy during pregnancy may lead to selection of drug resistance in viral replication and, consequently, to treatment failure in the future. The main reason for this is poor adherence among pregnant women. Pharmacokinetic variability may also have an important role, since it involves inter-actions between two or more drugs, or between drugs and food, sex, age, hepatic/renal function and pregnancy-related physiological modifications. More recently, it was reported that several genetic polymorphisms influenced drug exposure, toxicity and treatment response [12].
New drugs for combating AIDS continue to be produced because of the rapid development of viral resistance to these drugs. On the other hand, molecular studies on pharmacokinetics have enabled greater understanding of the mechanisms involves in drug transportation through the placenta, thereby exposing or protecting the fetus to differing degrees.
The sources of data on the potential toxicity of antiretrovirals during pregnancy include studies on animals, cohort studies and pregnancy and antiretroviral records in case reports. However, the data available on the safety of using these new drugs among pregnant women are limited. This shortage of comparative studies on the adverse effects of antiretrovirals (lamivudine, delavirdine, stavudine, amprenavir, nelfinavir and ritonavir/lopinavir in association) during pregnancy motivated us to analyze them during pregnancy among albino rats. The present study shows the results from six previous investigations conducted at our service, which were analyzed together here.
Thus, the objective of the present study was to compare the action of six antiretrovirals (lamivudine, stavudine, delavirdine, nelfinavir, amprenavir and an association of lopinavir/ritonavir) during pregnancy among albino rats in relation to maternal mortality, maternal and fetal weight, numbers of implantations and reabsorptions, malformations and fetal deaths.
MATERIALS AND METHODS
The guidelines for care and use of animals in research approved by the local institution were followed. The Research Ethics Committee of the Federal University of São Paulo (UNIFESP) approved the experimental protocol. Virgin female adult EPM-1 Wistar rats were selected after three regular consecutive estrous cycles and were kept under specific pathogen-free conditions with a constant day/night cycle (lights on from 07 : 00 to 19 : 00). The animals were provided with Purina One® rat chow and tap water ad libidum and were mated in the proportions of one male to two females overnight. The immediate 24-hour period after mating was taken to be day zero of pregnancy since spermatozoids were detected in vaginal smears [19].
One hundred and eighty pregnant rats were randomly distributed into six animal groups as follows: the control group (C) (n=10) consisted of rats that were treated daily with 0.5 mL of propylene glycol orally (drug vehicle controls); the other five groups (n=170) were subdivided and treated with antiretroviral drugs at three different doses: the habitual dose (similar to that recommended for humans) and 3 and 9 times the habitual dose. Group D was treated with delavirdine at only two different doses: the habitual dose and 3 times this dose. Group L (n=30) was administered lamivudine at doses of 5 mg/kg, 15 mg/kg and 45 mg/kg, respectively [34]; group S (n=30) was administered stavudine at doses of 1 mg/kg, 3 mg/kg and 9 mg/kg,respectively [3]; group A (n=30) was administered amprenavir at doses of 46 mg/kg, 138 mg/kg and 414 mg/kg, respectively [32]; group D (n=20) was administered delavirdine at doses of 20 mg/kg and 60 mg/kg, respectively [13]; group N (n=30) was administered nelfinavir at doses of 40 mg/kg,120 mg/kg and 360 mg/kg, respectively [30]; and group R/L (n=30) was administered ritonavir in association with lopinavir at doses of 12.8 mg/kg with 3.2 mg/kg, 38.4 mg/kg with 9.6 mg/kg and 115.2 mg/kg with 28.8 mg/kg, respectively [24].
The vehicle and drugs were administered by means of gavage once a day, with a final volume of 1.0 mL, starting on the day zero and continuing until the term of pregnancy (20th day), when the rats were anesthetized.
Body weights were recorded for all animals on day zero and on the 7th, 14th and 20th days of pregnancy, and were expressed as percentages of body weight gain (OHAUS – Triple Bean Balance). At term (20th day), the animals were weighed and anesthetized with a mixture of xylazine (20 mg/kg) and ketamine (100 mg/kg), intraperitoneally. From wide open laparotomy and hysterotomy, the following parameters were recorded: fetal and placental weights, number of implantations, number of reabsorptions and number of live and dead fetuses. The fetuses were also evaluated under a stereomicroscope for gross external abnormalities, such as congenital thoracic abnormalities, spina bifida, cleft lip/palate, micrognathia, microglossia, hydrocephalus, axial skeletal deviations, polydactyly, rib malformations or limb abnormalities.
The data were transferred to a spreadsheet in the Excel 2007 software (Microsoft Corp., Redmond, WA, USA) and were analyzed using the SPSS software (version 18.0, SPSS Inc., Chicago, IL, USA). The data were expressed as means ± standard deviations and medians, and also as absolute and relative frequencies (%). The results were subjected to analysis of variance (ANOVA) and were further analyzed by means of the Kruskal-Wallis multiple comparison test. Contingency tables and chi-square tests were used to analyze the death rates. The significance level was set at p<0.05.
RESULTS
The stavudine group presented increases in maternal weight with the habitual dose that were greater than in all the other groups (p=0.001), except for delavirdine with the habitual dose, which showed a similar increase. The amprenavir group showed greater maternal mortality than in any other group, with the habitual dose (p<0.001) (Table 1).
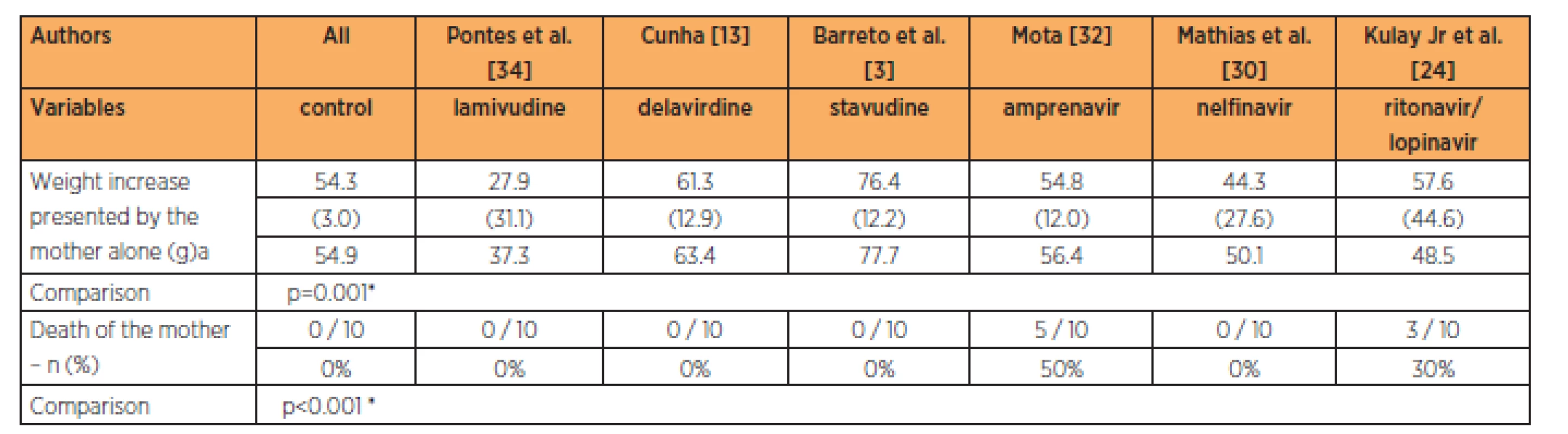
The stavudine group showed maternal weight gains with the 3-times habitual dose that were greater than in all the other groups studied (p<0.001). The groups with delavirdine, amprenavir, nelfinavir and the association between lopinavir and ritonavir presented maternal weight gains that were similar to that of the control group, at the 3-times habitual dose. The groups treated with amprenavir and with the association of lopinavir/ritonavir presented higher mortality than in the other groups at the 3-times habitual dose (Table 2).
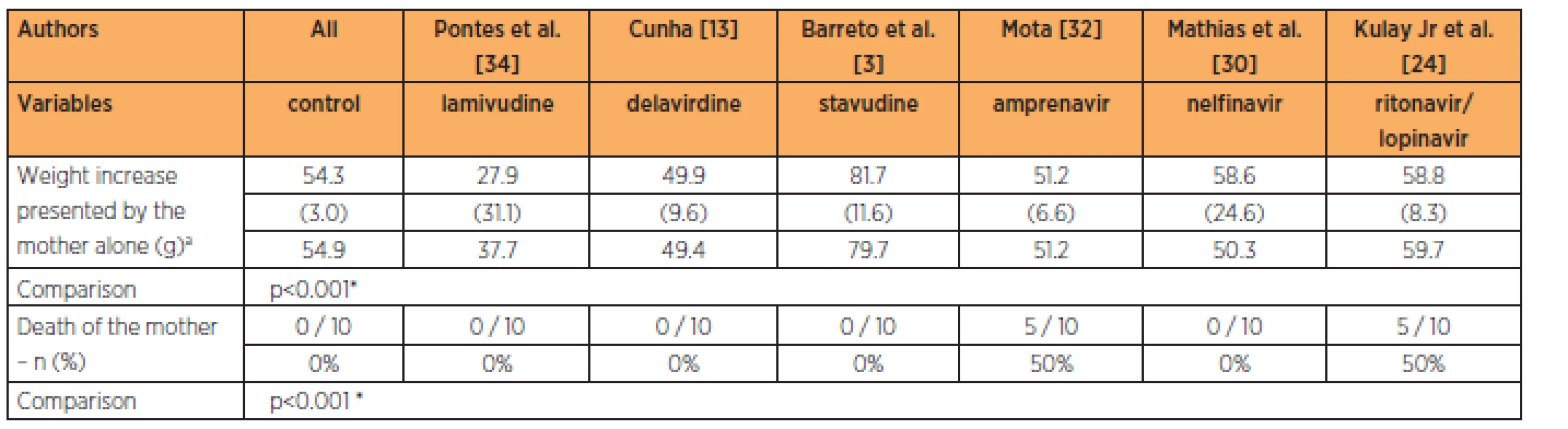
The lamivudine group presented maternal weightgains that were smaller than those of the control group. The groups with amprenavir, lopinavir/ritonavir in association and nelfinavir had maternal weight gains that were similar to that of the control group at the 9-times habitual dose (p<0.001). The groups treated with amprenavir and the association of lopinavir/ritonavir had higher maternal mortality rates than in the other groups (p<0.001) (Table 3).
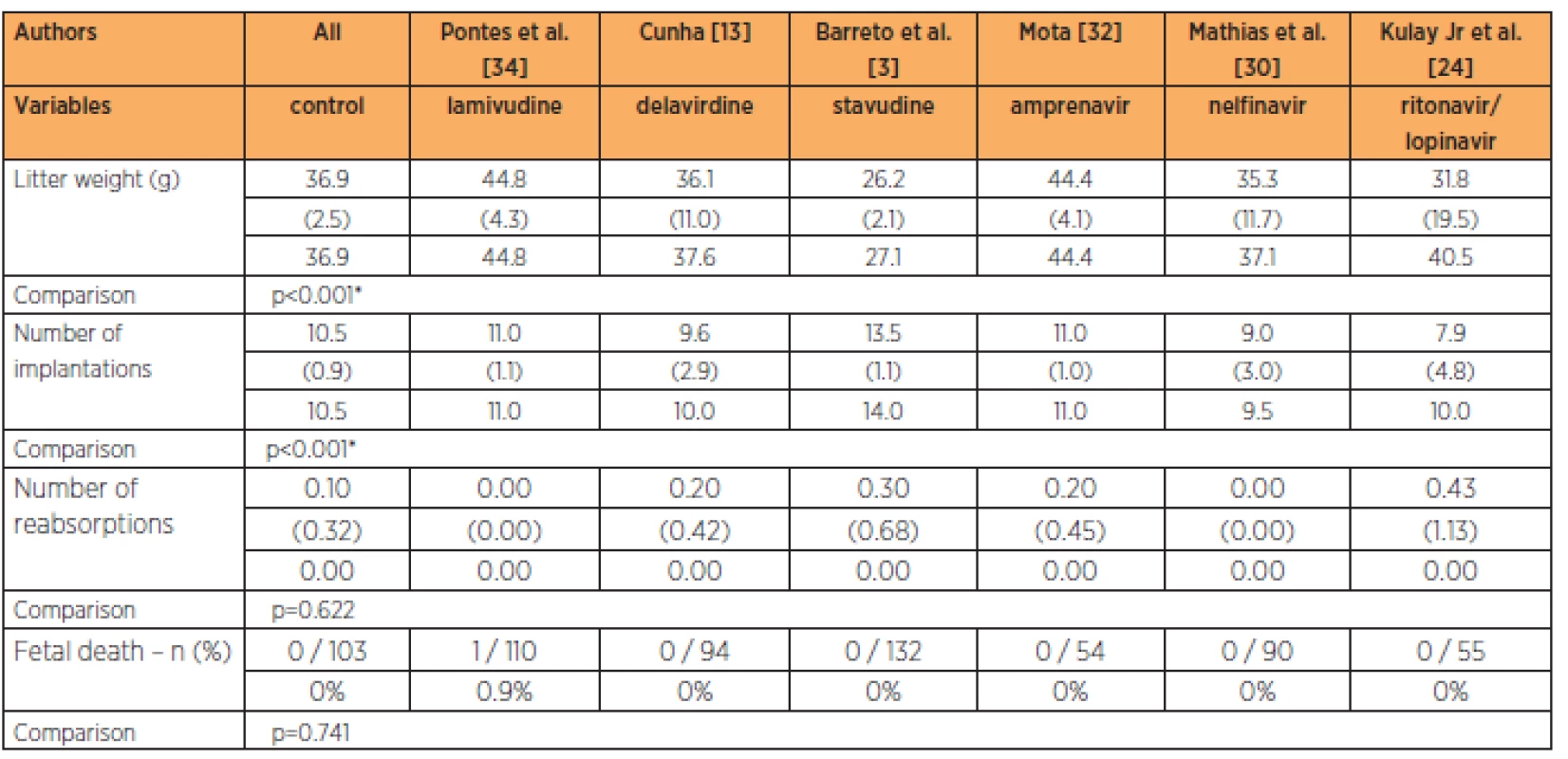
Only the lamivudine group presented fetalweights greater than in the control group (p<0.001), while the lamivudine group also had a greater number of implantations than in all the other groups at the habitual dose (p<0.001). No significant differences in the numbers of reabsorptions (p=0.622) or fetal deaths (p=0.741) were observed (Table 4).
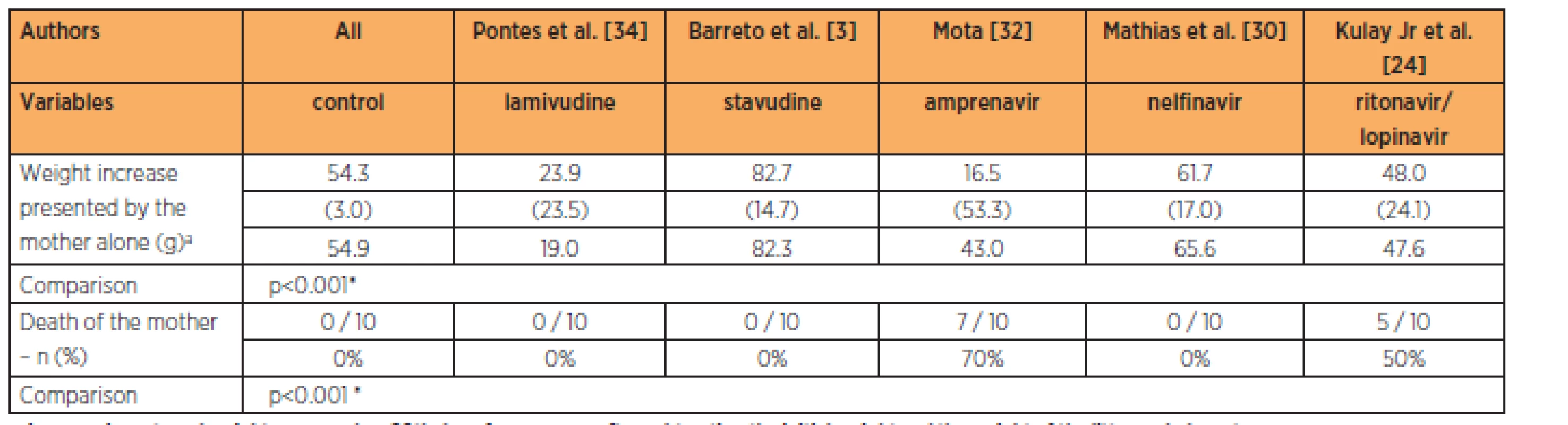
The group treated with stavudine had lower fetal weight while the groups treated with delavirdine, lamivudine and amprenavir had higher fetal weights than in the control group, at the 3-times habitual dose (p<0.001). The groups treated with lamivudine and stavudine had greater numbers of implantations than in the control group, while the groups treated with the lopinavir/ritonavir association, nelfinavir, delavirdine and amprenavir had numbers of implantations that were similar to those of the control group, at the 3-times habitual dose (p=0.002). No significant differences in the numbers of reabsorptions (p=0.069) and fetal deaths were observed (Table 5).
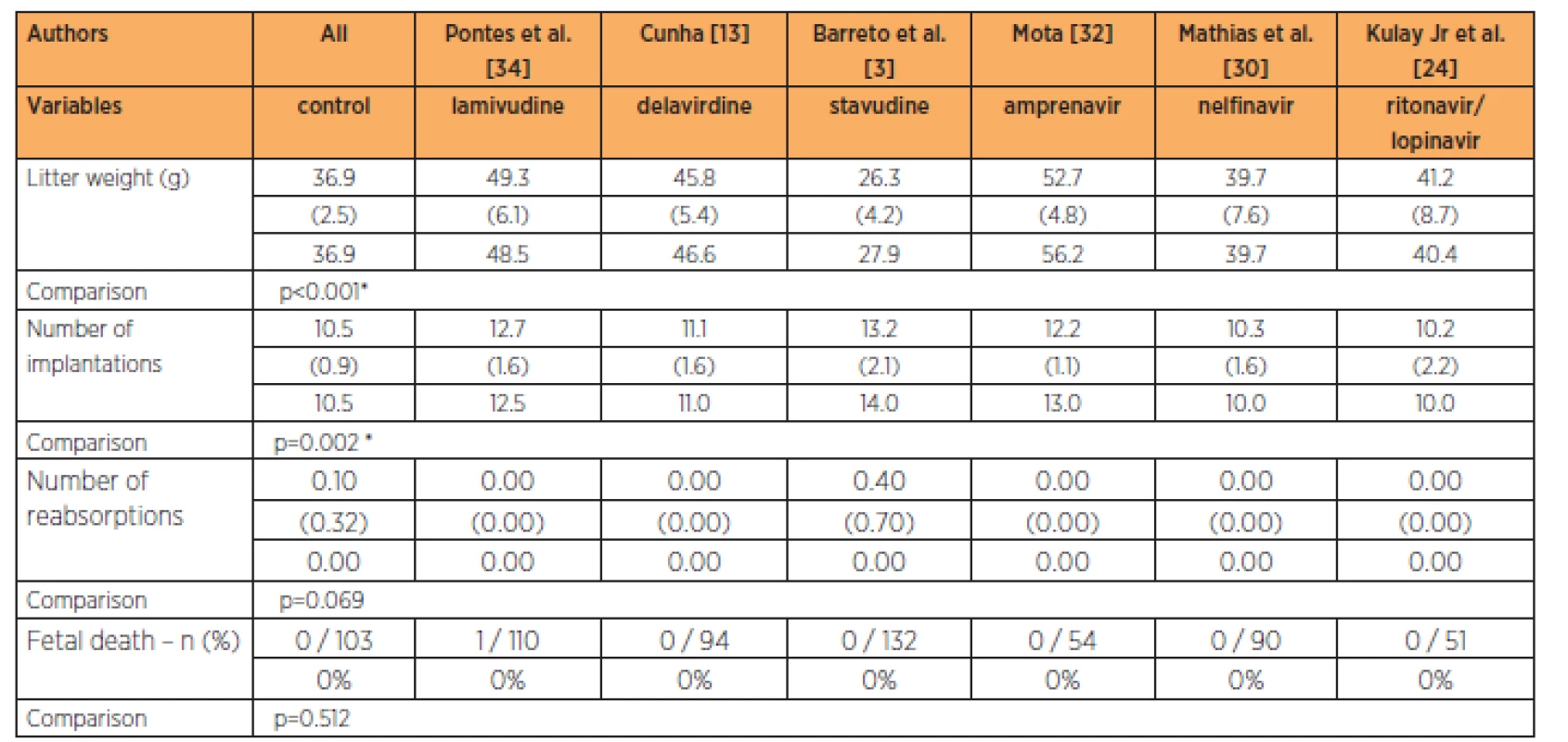
The group treated with stavudine had lower fetal weight than all the other groups studied, at the 9-times habitual dose (p=0.002). The group treated with stavudine also had a greater number of implantations than in all the other groups studied at the 9-times habitual dose (p=0.002). No significant differences in the numbers of reabsorptions (p=0.369) and fetal deaths were observed (Table 6).
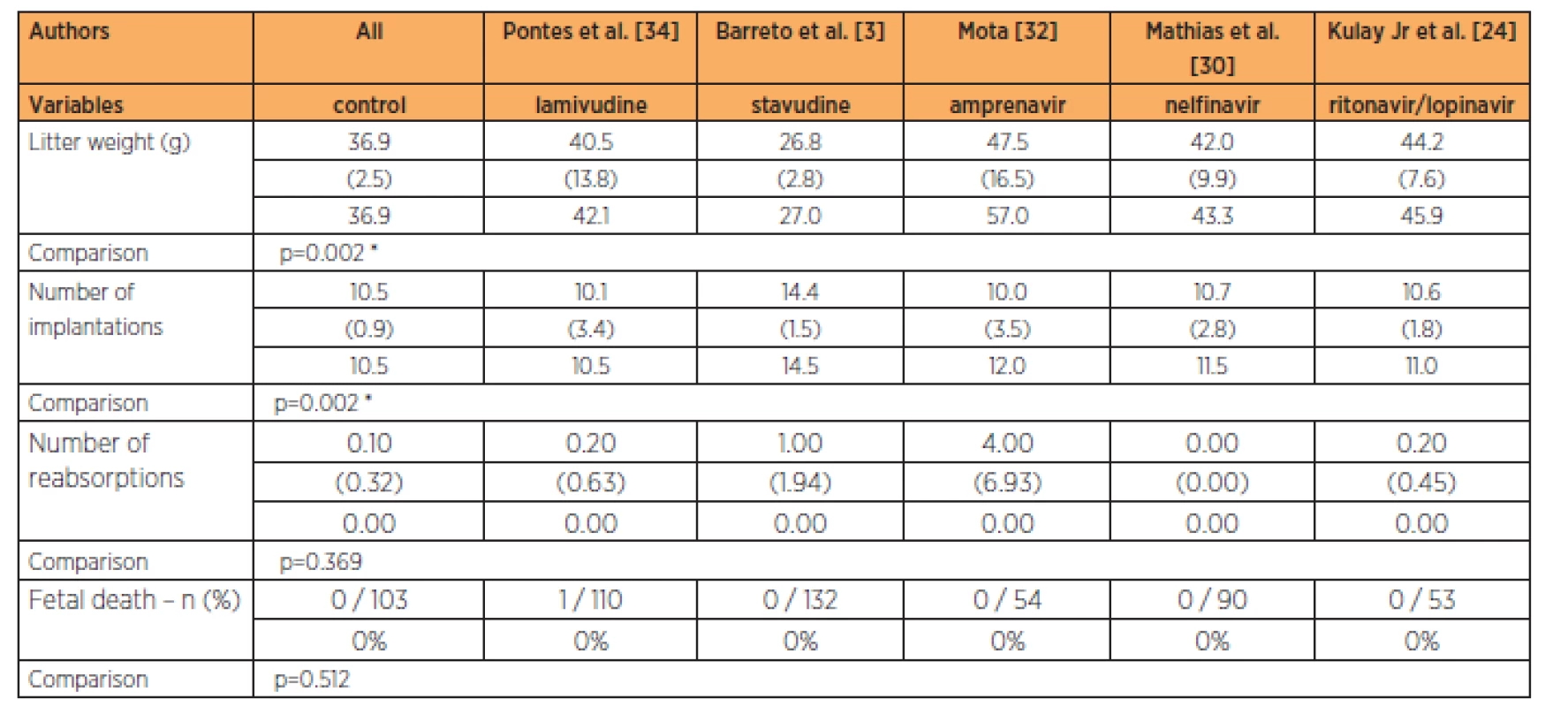
DISCUSSION
The six studies included in this paper were chosen because of their homogeneity of methodology [3, 13, 24, 30, 32, 34], i.e. the doses were standardized at the same as and three and nine times greater than the doses recommended for administration to humans, except for delavirdine, for which the 9-times dose was missing [13]. The studies evaluated toxicity in biological assays on monotherapy, except in the case of the association between lopinavir and ritonavir, given that lopinavir alone is not available for therapeutic use. The laboratory concerned was already aware of the pharmacokinetics of these two protease inhibitors and was producing the drugs as a manufactured association.
Viral resistance, which is one of the most feared complications from AIDS treatment, may come from administration of suboptimal doses. During pregnancy, transplacental transportation of drugs, their concentrations in the embryo/fetus and placenta, biotransformation by the fetus and placenta and fetal elimination may affect the pharmacokinetics of the drug in the maternal compartment. These physiological alterations during pregnancy may reduce the plasma concentration of nelfinavir (with administration of 750 mg three times a day), but do not change the pharmacokinetics of lamivudine, stavudine and nelfinavir (with administration of 1,250 mg twice a day). Furthermore, there are no data on the pharmacokinetics of delavirdine and amprenavir [31]. These particular features may be important in choosing the best pharmacological therapy during pregnancy, from the perspective of avoiding development of subsequent viral resistance, and with regard to lesser adverse reaction both for the mother and for the fetus, thereby maximizing the therapeutic success. Thus, studies on animals become necessary in order to gain knowledge of the biosafety of these medications and to make it possible to compare the medications studied.
In analyzing our results from administering one, three and nine times the usual drug dose to the female rats, it could be inferred that the change to the maternal weight (probably lipodystrophy, with accumulation of body fat in the mother) may have occurred more evidently with stavudine than with the other antiretrovirals. This lipodystrophy brings back the stigma of AIDS, with breakage of secrecy and consequent difficulties in socialization.
The active metabolite stavudine triphosphate not only inhibits synthesis of viral DNA but also inhibits the DNA polymerase from undergoing cell growth and further reduces synthesis of mitochondrial DNA (by more than 50%). This is related to lipoatrophy [43] and the ability to postpone the toxicity of the drug [9]. Ananworanich et al. [1] found in a Thai population that when the stavudine/didanosine regimen was replaced by tenofovir/lamivudine while receiving saquinavir/ritonavir, the lipodystrophy and mitochondrial toxicity were reversed.
Another two antiretrovirals that also suggest occurrences of increased body fat are delavirdine and the ritonavir/lopinavir association. In clinical cases studies that were retrospectively analyzed, no greater incidence of lipodystrophy was observed among groups treated with delavirdine [40]. With regard to the ritonavir/lopinavir association, the manifestation of this adverse effect in humans occurred after 48 weeks, while in rats it was after eight weeks. This period was greater than the length of pregnancy in these animals [5, 35]. In the present study, in the nelfinavir group at the 9-times dose, there was no difference with the 9-times dose of stavudine, which also suggests that this dose of nelfinavir has potential for lipodystrophy. In a clinical study, it was observed that after the initial increase of the first 16 weeks, there was a reduction in fat at the extremities, which would be a sign of lipodystrophy. This change was greater in the didanosine/stavudine group (-16.8%) and in the nelfinavir group (-13.1%) [14].
Regarding amprenavir, nelfinavir and lamivudine, in comparison with controls, no accumulation of excess body fat was observed in the present study. In the study by Lagathu et al. [25], amprenavir acted weakly on adipocytes, in comparison with other protease inhibitors. The association between abacavir and lamivudine has also been taken to have little lipodystrophic effect [2], and likewise, the association between zidovudine and lamivudine [14].
In the rats that were treated with lamivudine at higher doses, the gain in fat was less than in the control, and this low level was also shown by Figueiró Filho et al. [17]. This finding was interpreted as a symptoms coming from lipoatrophy. Lamivudine is considered to be one of the NRTIs with least risk of lipodystrophy [39]. However, an in vitro study showed that lamivudine gave rise to significantly decreased intracellular lipid accumulation [23]. It was also observed that in the groups treated with lamivudine, the way in which lipodystrophy occurred differed from the occurrences in groups treated with protease inhibitors. In the groups treated with lamivudine, the symptoms started early on and the weight loss was more significant [18]. The prevalence of lipodystrophy among protease inhibitor users has been found to be around 64%, when used concomitantly with lamivudine [18]. Studies correlating weight gain and use of protease inhibitors have reported that the weight may remain unchanged [44] or may increase by more than 5 to 8 kg [33, 41].
Another aspect of maternal toxicity that was observed was the lethal potential of the drugs. Amprenavir and the ritonavir/lopinavir association were the most toxic: amprenavir at the habitual dose for humans, and the ritonavir/lopinavir association at the doses of 3 and 9 times.
Cytochrome P450 (CYP)3A4 is the most abundant intestinal and hepatic enzyme and it metabolizes approximately 50% of drugs [45]. Drugs can be classified as substrates, inducers and/or inhibitors of CYP3A4, and this makes it possible to predict whether the availability, efficacy or toxicity of the drug might be altered through coadministration of compounds that are known to be substrates of CYP3A. The substrates of CYP3A4 overlap considerably with those of glycoprotein P. Inhibition or induction of CYP3A4 through antiretroviral drugs such as ritonavir and delavirdine frequently causes unfavorable and residual biochemical interactions and probably fatal toxicity, but depends on many other associated factors such as enzymes, steroids, drugs and patients [45].
It is now known that nuclear receptors are involved in genetic regulation of P450. Among these, the pregnane X receptor (PXR) and the constitutive androstane receptor (CAR) are the major determinants of gene expression associated with various aspects of drug metabolism, including oxidative metabolism, conjugation and transportation. PXR is expressed mainly in the liver and intestine, where it has an important role in detoxification and elimination of xenobiotics from the body [31]. PXR also seems to be involved in regulating and diminishing the expression of multidrug resistance protein 1 (MDR1/ABCB1) and protein 2 (MRP2/ABCC2) [22].
During the perinatal period, it seems that PXR performs a role in protecting the maternal-fetal system from the toxic effects of endogenous steroids and xenobiotics [29]. Steroidal hormones such as progesterone, glucocorticoid and pregnenolone stimulate expression of PXR in the liver and ovaries by around 50-fold in full-term pregnancy, compared with the non-gestational state, and this expression decreases after delivery. PXR has a different response according to the animal used, and it is problematic in rodents. In transgenic mice, rifampicin (which is a PXR binder that is specific for humans) was found to strongly induce hepatic CYP3A4, thereby reducing metabolic stability of amprenavir, nelfinavir and saquinavir in the liver microsomes by 52, 53 and 99%, respectively, with a reduction in the area under the amprenavir time/concentration curve of approximately 80% [26]. However, this response was not observed with 16-alpha-carbonitrile pregnenolone, which is a PXR binder that is specific for rodents. On the other hand, in human lymphocytes, amprenavir and other protease inhibitors like atazanavir, lopinavir, nelfinavir and ritonavir are known for their capacity to induce drug-metabolizing enzymes through a PXR activation mechanism [20], and are therefore less toxic to humans than to rodents. It is possible that the greater toxicity of amprenavir in rats that was shown in our study, with occurences of maternal death even at the dose recommended for humans, may be related to PXR. This would corroborate other studies [20, 26] that have shown that the response to the drug varies according to the animal specimen. The necropsies on these animals showed multiple organ failure. With regard to ritonavir, even though this is a PXR agonist and therefore induces CYP3A4 expression, it has been recognized as a potent inhibitor of CYP3A4. However, the inhibition outweighs the induction and the resultant from this is that the activity of this enzyme becomes reduced [20].
Despite the above points showing that the ritonavir/lopinavir association has greater toxicity for rodents than for humans, this association was found to present a high percentage (44%) of abandonment of treatment, of which 28% was due to toxicity [6]. In another study, 18.5% of the patients abandoned the treatment because of toxicity, gastrointestinal symptoms, hyperlipidemia or elevated hepatic transaminase levels, which increased with length of use and coinfection with hepatitis C [4].
Other antiretrovirals have not been found to cause maternal death, although nelfinavir and amprenavir were found to lead to hepatotoxicity at doses 9 times greater than the usual dose [30]. Furthermore, amprenavir was also found to affect the maternal kidneys, with vascular dilatation [34]. Stavudine was also found to cause hepatic and pancreatic lesions [3].
In relation to the permeability of the placental barrier, studies have shown that both lamivudine and stavudine are able to cross the placental barrier by means of passive diffusion [8]. The proportion of the nelfinavir concentration in the umbilical cord blood in relation to the maternal blood was found to be 22.5% (< 250/1110 ng/mL) and for the ritonavir/lopinavir association, this was 8.1% (< 250/3105 ng/mL) [28]. The present study showed that protease inhibitors generally cross the placenta in low quantities. This limitation seems to result from their high degree of binding with plasma proteins and from retrograde transportation through glycoprotein P, which is extensively expressed in the placenta, thus minimizing the exposure of the fetus to potentially toxic drugs. In our study, none of the groups presented any fetal damage, with regard to implantation, fetal weight and number of implantations, considering that all of these items were greater than or equal to the controls, except in the stavudine group. In this last group, the fetal weight at all doses was significantly lower, although with a greater number of implantations. This leads to a belief that the lower weight may have been due both to the drug and to the intrinsic nature of the animal. We observed that the fetal weight was higher in relation to the control group among the animals that were treated with lamivudine (doses of 1 and 3 times), delavirdine (3 times) and amprenavir (3 times). Figueiró-Filho et al. [17] administered lamivudine to pregnant rats at a dose 10 times greater than usual and found that their litter had lower weights. Lower fetal weight was also observed among pregnant rats exposed to amprenavir and ritonavir/lopinavir at doses 2, 0.7 and 1.8 times the therapeutic dose for humans, respectively [36]. Since lamivudine passes through the placenta through passive diffusion, it is possible that it acts on the fetal weight, as a consequence of lipoatrophy or lipohypertrophy. Delavirdine may increase cholesterol and HDL cholesterol levels [37], and this elevation may lead to birth of macrosomic fetuses from these mothers, who also have high insulin levels even before presenting diabetes [16]. Studies on amprenavir in relation to glucose and lipid metabolism have demonstrated that this drug may increase cholesterol and triglyceride levels, peripheral insulin resistance and weight [15, 38]. These alterations in the mother are substrates for obtaining macrosomic fetuses. Figueiró-Filho et al. [17] found smaller numbers of fetuses per animal, along with lower fetal viability rates, both for nelfinavir and for lamivudine.
In studies on transporters and flow from the apical membrane of the syncytiotrophoblast that have been conducted more recently, the importance of their moderating role on drug penetration through the placental barrier has been recognized. The first of these transporters to be recognized was glycoprotein P [27]. The two other transporters are the multidrug resistance protein 2 (MRP2) and the breast cancer resistance protein (BCRP) [21]. While BCRP and glycoprotein P expression decrease as full term is approached, MRP2 expression increases in the placenta with progression towards full term [27]. BCRP can transport a broad spectrum of substrates, including zidovudine and lamivudine. A variety of BCRP inhibitors have now been identified, including protease inhibitors such as nelfinavir and ritonavir, thus at least partly explaining the placental permeability to lamivudine and the placental barrier to nelfinavir and ritonavir.
CONCLUSION
In summary, in this biological assay, in the maternal compartment, we observed lethal toxicity in the pregnant rats that received amprenavir and the ritonavir/lopinavir association; and maternal weight change with use of lamivudine and stavudine. In the fetal compartment, adverse effects were observed only in relation to litter weight from stavudine, lamivudine, delavirdine and amprenavir.
Prof. Edward Araujo Júnior, PhD
Department of Obstetrics, Federal University of São Paulo (UNIFESP)
Rua Carlos Weber, 956 apt. 113 Visage
Vila Leopoldina
São Paulo – SP, Brazil
CEP 05303-000
e-mail: araujojred@terra.com.br
Sources
1. Ananworanich, J., Nuesch, R., Cote, HC., et al. Changes in metabolic toxicity after switching from stavudine/didanosine to tenofovir/lamivudine – a Staccato trial substudy.J Antimicrob Chemother, 2008, 61, p. 1340–1343.
2. Anderson, AM., Bartlett, JA. Fixed dose combination abacavir/lamivudine in the treatment of HIV-1 infection. Expert Rev Anti Infect Ther, 2005, 3, p. 871–883.
3. Barreto, RL., de Jesus Simões, M., Amed, AM., et al. Stavudine effects on rat pregnancy outcome. J Obstet Gynaecol Res, 2004, 30, p. 242–245.
4. Bongiovanni, M., Cicconi, P., Landonio, S., et al. Predictive factors of lopinavir/ritonavir discontinuation for drug-related toxicity: results from a cohort of 416 multi-experienced HIV-infected individuals. Int J Antimicrob Agents, 2005, 26, p. 88–91.
5. Carr, A., Ritzhaupt, A., Zhang, W., et al. Effects of boosted tipranavir and lopinavir on body composition, insulin sensitivity and adipocytokines in antiretroviral-naive adults. AIDS, 2008, 22, p. 2313–2321.
6. Casau, NC., Glesby, MJ., Paul, S., Gulick, RM. Brief report: efficacy and treatment-limiting toxicity with the concurrent use of lopinavir/ritonavir and a third protease inhibitor in treatment-experienced HIV-infected patients. J Acquir Immune Defic Syndr, 2003, 32, p. 494–498.
7. Centers for Disease Control and Prevention (CDC). Epidemiology of HIV/AIDS – United States, 1981–2005. MMWR Morb Mortal Wkly Rep, 2006, 55, p. 589–592.
8. Chappuy, H., Treluyer, JM., Jullien, V., et al. Maternal-fetal transfer and amniotic fluid accumulation of nucleoside analogue reverse transcriptase inhibitors in human immunodeficiency virus-infected pregnant women. Antimicrob Agents Chemother, 2004, 48, p. 4332–4336.
9. Chen, CH., Vazquez-Padua, M., Cheng, YC. Effect of anti-human immunodeficiency virus nucleoside analogs on mitochondrial DNA and its implication for delayed toxicity. Mol Pharmacol, 1991, 39, p. 625–628.
10. Cohen, MS., Hellmann, N., Levy, JA., et al. The spread, treatment, and prevention of HIV-1: evolution of a global pandemic. J Clin Invest, 2008, 118, p. 1244–1254.
11. Cooper, ER., Charurat, M., Mofenson, L., et al. Combination antiretroviral strategies for the treatment of pregnant HIV-1-infected women and prevention of perinatal HIV-1 transmission. J Acquir Immune Defic Syndr, 2002, 29, p. 484–494.
12. Cressey, TR., Lallemant, M. Pharmacogenetics of antiretroviral drugs for the treatment of HIV-infected patients: an update. Infect Genet Evol, 2007, 7, p. 333–342.
13. Cunha, AM. Action of delaverdine on albino pregnant rats. Biological assay. Dissertation. [MSD thesis]. São Paulo: Federal University of São Paulo, 2000.
14. Dube, MP., Parker, RA., Tebas, P., et al. Glucose metabolism, lipid, and body fat changes in antiretroviral-naive subjects randomized to nelfinavir or efavirenz plus dual nucleosides. AIDS, 2005, 19, p. 1807–1818.
15. Dube, MP., Qian, D., Edmondson-Melancon, H., et al. Prospective, intensive study of metabolic changes associated with 48 weeks of amprenavir-based antiretroviral therapy. Clin Infect Dis, 2002, 35, p. 475–481.
16. Evagelidou, EN., Kiortsis, DN., Bairaktari, ET., et al. Lipid profile, glucose homeostasis, blood pressure, and obesity – anthropometric markers in macrosomic offspring of nondiabetic mothers. Diabetes Care, 2006, 29, p. 1197–1201.
17. Figueiró-Filho, EA., Duarte, G., Silva, AA., et al. [Effects of antiretroviral drugs on fertility of Wistar rats]. Rev Bras Ginecol Obstet, 2002, 24, p. 647–652.
18. Gervasoni, C., Ridolfo, AL., Trifiro, G., et al. Redistribution of body fat in HIV-infected women undergoing combined antiretroviral therapy. AIDS, 1999, 13, p. 465–471.
19. Hamilton, JB., Wolfe, JM. The effect of male hormone substance upon birth and prenatal development in the rat. Anat Rec, 1938, 70, p. 433–440.
20. Healan-Greenberg, C., Waring, JF., Kempf, DJ., et al. A human immunodeficiency virus protease inhibitor is a novel functional inhibitor of human pregnane X receptor. Drug Metab Dispos, 2008, 36, p. 500–507.
21. Kobayashi, D., Ieiri, I., Hirota, T., et al. Functional assessment of ABCG2 (BCRP) gene polymorphisms to protein expression in human placenta. Drug Metab Dispos, 2005, 33, p. 94–101.
22. Kojima, K., Nagata, K., Matsubara, T., Yamazoe, Y. Broad but distinct role of pregnane x receptor on the expression of individual cytochrome p450s in human hepatocytes. Drug Metab Pharmacokinet, 2007, 22, p. 276–286.
23. Kosmiski, LA., Miller, HL., Klemm, DJ. In combination, nucleoside reverse transcriptase inhibitors have significant effects on 3T3-L1 adipocyte lipid accumulation and survival. Antivir Ther, 2006, 11, p. 187–195.
24. Kulay, L. Jr., Hagemann, CC., Nakamura, MU., et al. Administration of lopinavir/ritonavir association during rat pregnancy: maternal and fetal effects. Clin Exp Obstet Gynecol, 2013, 40, p. 151–154.
25. Lagathu, C., Eustace, B., Prot, M., et al. Some HIV antiretrovirals increase oxidative stress and alter chemokine, cytokine or adiponectin production in human adipocytes and macrophages. Antivir Ther, 2007, 12, p. 489–500.
26. Ma, X., Cheung, C., Krausz, KW., et al. A double transgenic mouse model expressing human pregnane X receptor and cytochrome P450 3A4. Drug Metab Dispos, 2008, 36, p. 2506–2512.
27. Mao, Q. BCRP/ABCG2 in the placenta: expression, function and regulation. Pharm Res, 2008, 25, p. 1244–1255.
28. Marzolini, C., Rudin, C., Decosterd, LA., et al. Transplacental passage of protease inhibitors at delivery. AIDS, 2002, 16, p. 889–893.
29. Masuyama, H., Hiramatsu, Y., Mizutani, Y., et al. The expression of pregnane X receptor and its target gene, cytochrome P450 3A1, in perinatal mouse. Mol Cell Endocrinol, 2001, 172, p. 47–56.
30. Mathias, CV., Mathias, CF., Simões, MJ., et al. Safety of nelfinavir use during pregnancy. An experimental approach in rats. Clin Exp Obst Gyn, 2005, 32, p. 163–165.
31. Mirochnick, M., Capparelli, E. Pharmacokinetics of antiretrovirals in pregnant women. Clin Pharmacokinet, 2004, 43, p. 1071–1087.
32. Mota, DR. Action chronic amprenavir on pregnant rats (Rattus norvegicus albinos, Rodentia, Mammalia). Biological assay. [MSD thesis]. São Paulo: Federal University of São Paulo, 2003.
33. Nguyen, A., Calmy, A., Schiffer, V., et al. Lipodystrophy and weight changes: data from the Swiss HIV Cohort Study, 2000–2006. HIV Med, 2008, 9, p. 142–150.
34. Pontes, RD., Amed, AM., Simões, RS., et al. A morphological and biochemical appraisal of the liver and renal effects of lamivudine on rat pregnancy. Clin Exp Obstet Gynecol, 2006, 33, p. 209–212.
35. Prot, M., Heripret, L., Cardot-Leccia, N., et al. Long-term treatment with lopinavir-ritonavir induces a reduction in peripheral adipose depots in mice. Antimicrob Agents Chemother, 2006, 50, p. 3998–4004.
36. Public Health Service Task Force. Recommendations for Use of Antiretroviral Drugs in Pregnant HIV-Infected Women for Maternal Health and Interventions to Reduce Perinatal HIV Transmission in the United States. Available at http://aidsinfo.nih.gov/contentfiles/PerinatalGL.pdf. Accessed 20 April 2009.
37. Roberts, AD., Liappis, AP., Chinn, C., et al. Effect of delavirdine on plasma lipids and lipoproteins in patients receiving antiretroviral therapy. AIDS, 2002, 16, p. 1829–1830.
38. Rosenkranz, SL., Yarasheski, KE., Para, MF., et al. Antiretroviral drug levels and interactions affect lipid, lipoprotein, and glucose metabolism in HIV-1 seronegative subjects: a pharmacokinetic-pharmacodynamic analysis. Metab Syndr Relat Disord, 2007, 5, p. 163–173.
39. Sattler, FR. Pathogenesis and treatment of lipodystrophy: what clinicians need to know. Top HIV Med, 2008, 16, p. 127–133.
40. Scott, LJ., Perry, CM. Delavirdine: a review of its use in HIV infection. Drugs, 2000, 60, p. 1411–1444.
41. Stricker, RB., Goldberg, B. Weight gain associated with protease inhibitor therapy in HIV-infected patients. Res Virol, 1998, 149, p. 123–126.
42. UNAIDS and WHO. Report on the global HIV/AIDS epidemic 2008: executive summary. Available at: 43. van der Valk, M., Casula, M., Weverlingz, GJ., et al. Prevalence of lipoatrophy and mitochondrial DNA content of blood and subcutaneous fat in HIV-1-infected patients randomly allocated to zidovudine - or stavudine-based therapy. Antivir Ther, 2004, 9, p. 385–393.
44. Young, J., Rickenbach, M., Weber, R., et al. Body fat changes among antiretroviral-naive patients on PI - and NNRTI-based HAART in the Swiss HIV cohort study. Antivir Ther, 2005, 10, p. 73–81.
45. Zhou, SF. Drugs behave as substrates, inhibitors and inducers of human cytochrome P450 3A4. Curr Drug Metab, 2008, 9, p. 310–322.
Labels
Paediatric gynaecology Gynaecology and obstetrics Reproduction medicineArticle was published in
Czech Gynaecology

2014 Issue 4
-
All articles in this issue
- Prebioptic methods in the cervical cancer screening
- Correlation between the incidence of PIK3CA mutations in breast cancer and histopathological characteristics of the tumor
- Characteristics of sexual maturation in girls in Bratislava region
- Pregnancy and delivery in patients with an implanted shunt for hydrocephalus
- Intraindividual variability of uroflowmetry in women
- Principles and limits of clinical methods in the diagnosis of fetal hypoxia
- Shute´s parallel forceps
- The most valuable predictorsof endometrial receptivity
- Effect of six antiretroviral drugs (delavirdine, stavudine, lamivudine, nelfinavir, amprenavir and lopinavir/ritonavir in association) on albino pregnant rats (Rattus norvegicus Albinus, Rodentia, Mammalia): biological assay
- Tuberculosis in pregnancy:a challenging differential diagnosisfor inflammatory bowel disease
- Czech Gynaecology
- Journal archive
- Current issue
- About the journal
Most read in this issue
- Prebioptic methods in the cervical cancer screening
- Pregnancy and delivery in patients with an implanted shunt for hydrocephalus
- Principles and limits of clinical methods in the diagnosis of fetal hypoxia
- Tuberculosis in pregnancy:a challenging differential diagnosisfor inflammatory bowel disease
