Hepatitis C treatment uptake and adherence among injecting drug users in the Czech Republic
Vstup do léčby a adherence k léčbě VHC u injekčních uživatelů drog v ČR
Východiska:
Injekční uživatelé drog (IUD) tvoří hlavní skupinu osob infikovaných virovou hepatitidou typu C (VHC) v rozvinutých zemích. Jejich vstup do léčby je však obecně nízký, navzdory dobře zdokumentované efektivitě léčby u bývalých nebo aktivních uživatelů drog. Prezentovaná studie je prvním pokusem o zmapování dostupnosti léčby VHC mezi IUD v ČR a o popis faktorů, které vstup IUD do léčby VHC ovlivňují.
Metody:
V období od ledna do března 2011 byla provedena dotazníková průřezová studie mezi centry pro léčbu virových hepatitid v ČR.
Výsledky:
Celkem bylo identifikováno 76 center pro léčbu virových hepatitid, 39 z nich poskytovalo léčbu VHC (především bývalým nebo abstinujícím) IUD. Většina kliniků uvedla obezřetnost před zahájením léčby VHC u IUD. Za nezbytné předpoklady zahájení léčby byly často považovány abstinence klienta od užívání návykových látek, zkušební doba před zahájením léčby, zařazení do opiátové substituční léčby a vyšetření externím specialistou. Centra pro léčbu virových hepatitid zřídka poskytují péči v oblasti návykových poruch. Uváděné limity pro úhradu péče mohou také omezovat zařazování IUD do léčby, neboť neuživatelé bývají preferování před uživateli drog. Z odpovědí kliniků nevyplynula existence rozdílů mezi uživateli drog a neuživateli drog, a ani mezi uživateli opioidů a metamfetaminu (pervitinu) ve vstupu do léčby a adherenci k léčbě.
Závěr:
I přes existenci národního standardu umožňujícího léčbu VHC u IUD se v praxi uplatňuje řada faktorů, které tvoří bariéru léčby VHC u IUD na straně poskytovatelů péče a léčebného systému v ČR. Opatření směrem k odstranění těchto faktorů jsou pro snížení výskytu VHC klíčová.
Klíčová slova:
virová hepatitida typu C – injekční užívání drog – léčba VHC – pervitin – opioidy
Authors:
V. Mravčík 1,2
; L. Strada 3; J. Reimer 3; B. Schulte 3
Authors‘ workplace:
National Monitoring Centre for Drugs and Drug Addiction, the Czech Republic
1; Department of Addictology, First Faculty of Medicine, Charles University in Prague and General University Hospital in Prague
2; Centre for Interdisciplinary Addiction Research, University of Hamburg
3
Published in:
Epidemiol. Mikrobiol. Imunol. 63, 2014, č. 4, s. 265-269
Category:
Review articles, original papers, case report
Overview
Background:
Injecting drug users (IDUs) represent a major subpopulation of hepatitis C virus (HCV)-infected people in developed countries. Yet their uptake to treatment is generally low despite well-documented effectiveness of HCV treatment among former and active IDUs. The present study represents the first attempt to describe the HCV treatment coverage among IDUs and identify factors that affect treatment uptake in the Czech Republic.
Methods:
From January to March 2011, a questionnaire survey was conducted among viral hepatitis treatment centres in the Czech Republic.
Results:
From a total of 76 identified hepatitis treatment centres existing in the country, 39 provided HCV treatment to (mainly former or abstaining) IDUs in 2010. Most clinicians reported being cautious in initiating HCV treatment in IDUs. Abstinence, a screening phase before treatment initiation, opioid substitution treatment and an external evaluation by a specialist were often prerequisites for skrting treatment. However, HCV treatment centres rarely provided drug-use specific services. Financial constraints were also reported, further limiting the inclusion of IDUs into treatment, as non-users are widely preferred to active drug users. Clinicians reported no difference in treatment uptake and adherence between drug users and non-users, nor between opioid and methamphetamine users.
Conclusion:
A number of system - and provider-related factors limit HCV treatment in IDUs in the Czech Republic, despite permissive national clinical guidelines. Targeting these factors is crucial to reduce HCV prevalence at population level.
Keywords:
viral hepatitis C – injecting drug use – HCV treatment uptake – methamphetamine – opioids
Introduction
Hepatitis C virus (HCV) infection represents a global public health concern. More than 185 million people are HCV--infected [1] and more than 350,000 individuals die each year due to end-stage liver diseases, such as cirrhosis and liver cancer [2]. Injecting drug use is a major route of transmission in developed countries [3]; 6 to 15 million injecting drug users (IDUs) are HCV-infected worldwide [4].
Despite high disease prevalence, HCV treatment uptake among IDUs has been found to be rather low [5, 6]. There are a number of system-, patient - and provider-related barriers to IDUs’ uptake and adherence to treatment, such as concerns regarding comorbidities, side effects of treatment including depression, fear of relapse to drug use, difficult social functioning, lack of information and counselling, referral-associated delays, gaps in funding schemes, and social factors such as stigmatization [7-9]. Treatment guidelines for HCV therapy in active IDUs are mostly restrictive or inconclusive [10, 11], although tolerance of active drug use has recently been recommended on the basis of extensive research evidence [12].
Clinicians are generally hesitant to treat active IDUs. They fear IDUs’ low adherence and thus lower efficacy of antiviral HCV treatment (measured as sustained virologic response, SVR). They are also afraid of reinfections after relapsing to risky drug injecting behaviour. However, recent systematic reviews demonstrate that IDUs can achieve successful treatment outcomes. Hellard et al. [13] found SVR in chronically HCV-infected IDUs (mean rate 54.3%; range: 18.1-94.1%) comparable to that of non/ex-users (54-63%). Similarly, Dimova et al. [14] found high SVR rates of 55.5% among drug users (95% CI: 50.6–60.3 %), together with a high completion rate of 83.4% (95% CI: 77.1-88.9%), which was especially high in patients receiving addiction treatment and other support services. Also the most recent meta-analysis found pooled SVR 56% (95% CI: 50-61%) for all genotypes, 37% (95% CI: 26-48%) for genotypes 1 and 4, 67% (95% CI: 56-78%) for ge-notypes 2 and 3 and a pooled adherence rate was 82% (95% CI: 74-89%).[15] SVR persists and reinfection is rare (2-5%), even in active drug users and those who relapse to injecting drug use [15.17].
Vaccine against HCV is not available due to high genetic variability of the virus. Evidence-based prevention strategies in IDUs therefore primarily include opioid substitution treatment (OST) and needle and syringe programs (NSP), if possible provided simultaneously, as this increases their individual effects [18-20]. However, recent model projections show that these interventions do not necessarily lead to substantial reductions in HCV within a decade (for 40% baseline chronic prevalence) unless the intervention coverage is scaled-up to 60% for both OST and NSP [21, 22]. Modelling analyses also indicate a strong preventive potential of HCV treatment in IDUs [6, 23]. This prevention effect is larger when baseline HCV prevalence is lower [24, 25] and even for low treatment rates, a large reduction in HCV prevalence can be achieved [26].
Combination of pegylated interferon α (PEG-IFN) and ribavirin (RBV) is the standard treatment of chronic HCV infection with different durations of treatment: 24 weeks for HCV genotypes 2 and 3, and 48 weeks for genotypes 1 and 4 [27]. Recently introduced directly acting antivirals (DAAs) in combination with PEG-IFN and RBV present higher SVR. Moreover, new DAAs administered without IFN for a shorter duration will lead to higher efficacy, better tolerance, and fewer side-effects [28].
In 2011, the Czech Republic had an estimated 40,200 problem drug users, including 38,600 IDUs. Methamphetamine (locally called “pervitin”) was used by an estimated 30,900 individuals and opioids by 9,300 individuals [29]. This large proportion of methamphetamine injectors in the Czech Republic is unique in Europe [30]. As pharmacological substitution is not available for this drug, methamphetamine users may represent a more problematic group of patients in HCV treatment. Anti-HCV prevalence rates in Czech IDUs was found to be 15% and 70% depending on sample characteristics and inclusion criteria; prevalence rates of 15–40% were usually found in samples from non-clinical recruitment setting [29, 31]. An annual HCV incidence of 11–15% was found among IDUs in the previous decade [32, 33].
Antiviral hepatitis treatment is guaranteed jointly by the hepatological and infectological societies of the Czech Medical Association. Czech clinical guidelines are permissive, encouraging HCV treatment of active drug users after considering patient’s individual risks and benefits. Furthermore, the guidelines recommend measures to increase adherence and treatment response, such as a multidisciplinary approach to treatment, substitution therapy, supervised medication and regular visits to treatment centres. However, it is unclear to what extent clinicians follow these recommendations and how they decide on HCV treatment initiation in their patients in clinical practice.
The current study represents the first attempt to map the availability of HCV treatment for IDUs in the Czech Republic. Furthermore, the study aims to describe the rules and practices that clinicians consider for HCV treatment initiation of IDUs and to identify possible differences in providers’ experiences with the treatment of opioid and methamphetamine users. Preliminary results were previously published in Czech [43]
Methods
From January to March 2011, the National Monitoring Centre for Drugs and Drug Addiction conducted a cross-sectional survey among viral hepatitis treatment centres in the Czech Republic. The Czech Society for Hepatology and the Medical Society for Infectiology provided lists of specialised centres for treatment of viral hepatitis. This list was expanded with HCV treatment centres that are promoted on a public website http://www.virova-hepatitida.cz/. Seventy-six centres were identified, all of them were individually invited to participate by e-mail. Of them 40 (53%) completed the online questionnaire designed for this study; 5 centres did not submit the questionnaire, although they provided basic information about the HCV treatment provision in the centre. The questionnaire consisted of 80 items divided into 5 sections: identification of the centre, number of treated patients, rules and practices for initiating HCV treatment in IDUs, financial issues, and experience in the treatment of opioid versus methamphetamine users. Recall period for recent quantitative data was the year 2010. Selection of the respondent representing the centre was up to each centre, though in most cases head physicians completed the questionnaire. Clinicians were asked to provide exact figures or their best estimate. No incentives were provided to respon-dents. HCV treatment was defined as treatment with PEG-IFN/RBV for 24 or 48 weeks, depending on the virus genotype.
Results
Data was analysed from 40 centres, of which 17 were gastroenterological and 23 infectological. All 14 Czech admi-nistrative regions were represented to various extents except for one.
Quantitative data on the extent to which HCV treatment is provided
HCV treatment was provided by 39 centres; 1 had provided treatment in the past. The majority of centres (22 out of 39) started offering HCV treatment between the years 2000 and 2005, while the first started as early as in 1990. Thirty-two centres provided treatment to IDUs, 3 had treated IDUs in the past, and 5 centres had never treated IDUs. The following data was provided by 33–36 centres, depending on the level of detail. From 1990 until the end of 2010, the number of treated patients ranged between 10 and 1500 in individual centres (Mean = 162, Median = 60). In total, 5842 patients were treated in 36 centres. Thirty-five of these centres reported 2202 former or active IDUs (38% of all patients), of which 1568 were male (71%). In 2010 alone, 35 centres reported that 664 patients were referred to treatment, of which 397 were active or former IDUs (60%). Treatment was initiated in 448 patients (68% of all referred), of which 263 were former or abstaining IDUs (84 opioid users and 187 methamphetamine users), 25 were in substitution treatment, and 3 were active IDUs.
Estimated total number of centres and patients
Data from the survey was extrapolated to all 76 treatment centres in the Czech Republic. Information on past HCV treatment coverage in the 36 non-responding centres was estimated using information about the 5 centres that responded without completing a questionnaire. One of them never treated HCV, 4 centres treated HCV in the present and past (of which 2 in IDUs) and 3 treated HCV in 2010 (of which one in IDUs). Assuming the same distribution in the 36 non-responding centres as in the 5 centres (i.e. 80% have ever treated HCV, 60% treated HCV in 2010, 40% ever treated IDUs, 20% treated IDUs in 2010), it can be estimated that 61 centres treated HCV in the Czech Republic in 2010, of which 39 treated IDUs (Table 1).
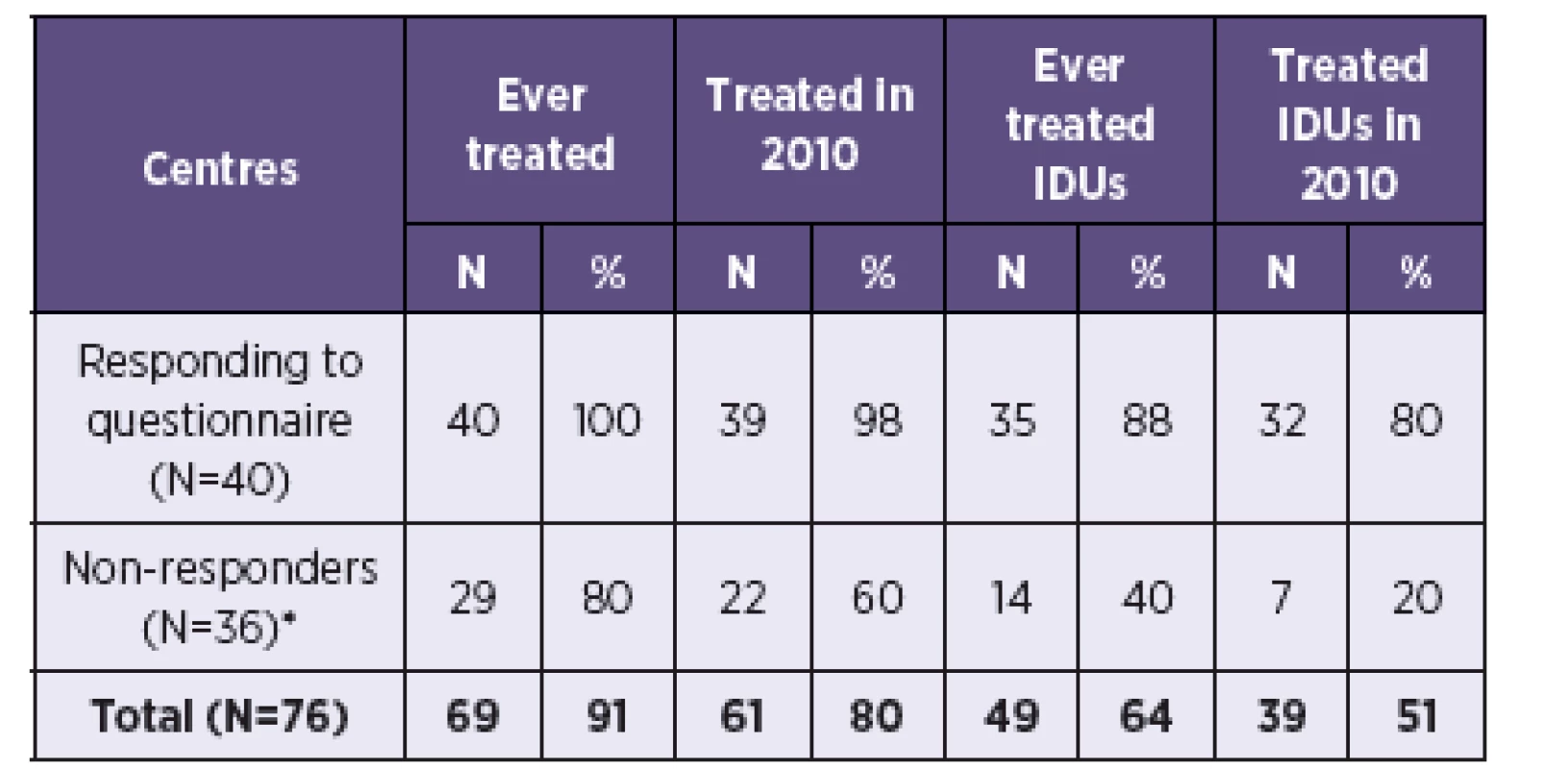
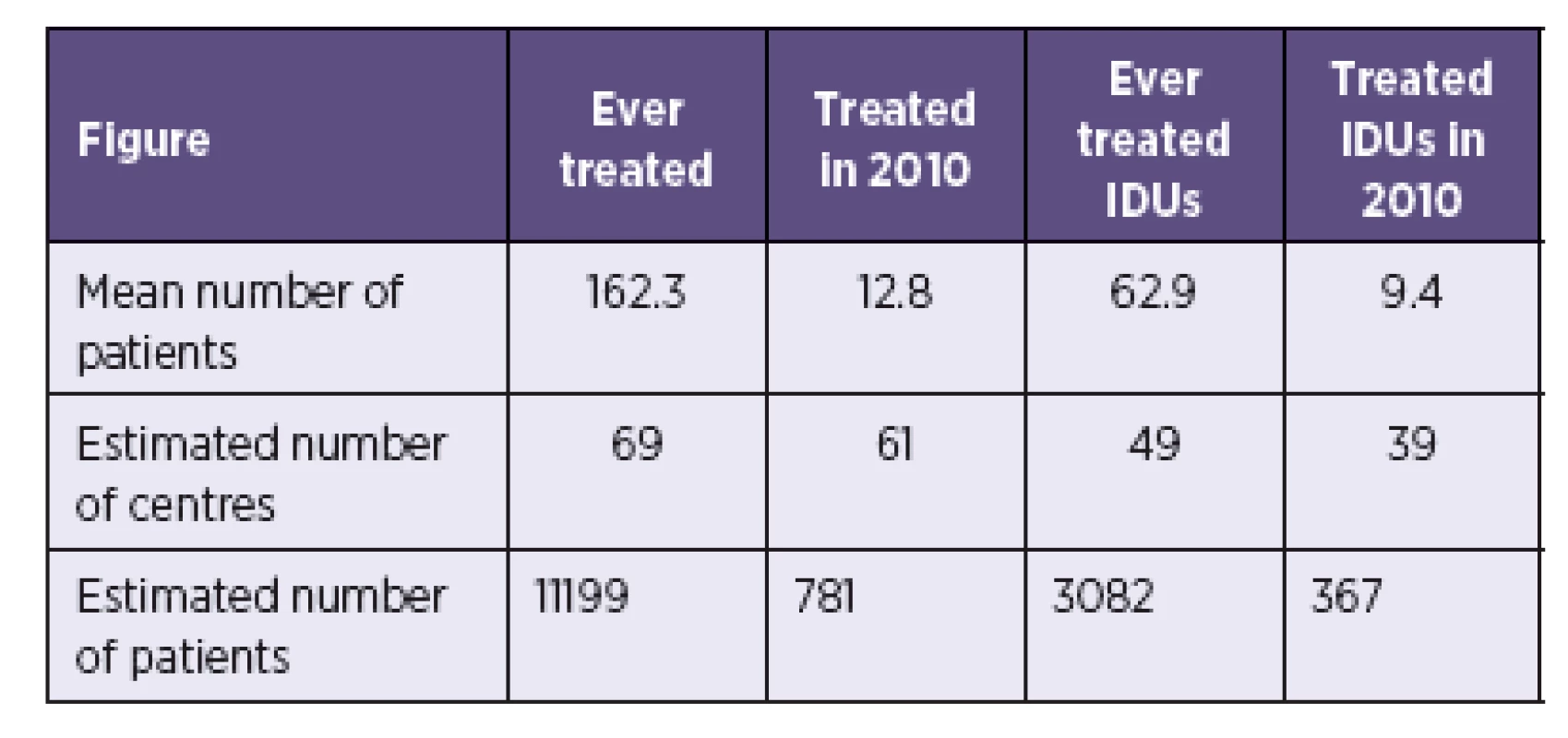
Mean number of treated patients from 40 responding centres was applied to estimated number of centres in order to calculate the estimated total number of HCV treated patients in the Czech Republic (Table 2). An estimated 781 patients were in HCV treatment in 2010, of which 367 (47%) were estimated to be IDUs.
Rules and practices for initiating HCV treatment
Thirty-four centres (85%) reported rules for initiating treatment in IDUs; clinicians mostly referred to national HCV treatment guidelines. Both strict and lenient clinicians reported that they apply an individual approach to each patient. Those treating drug users emphasized the risk of low adherence of active IDUs.
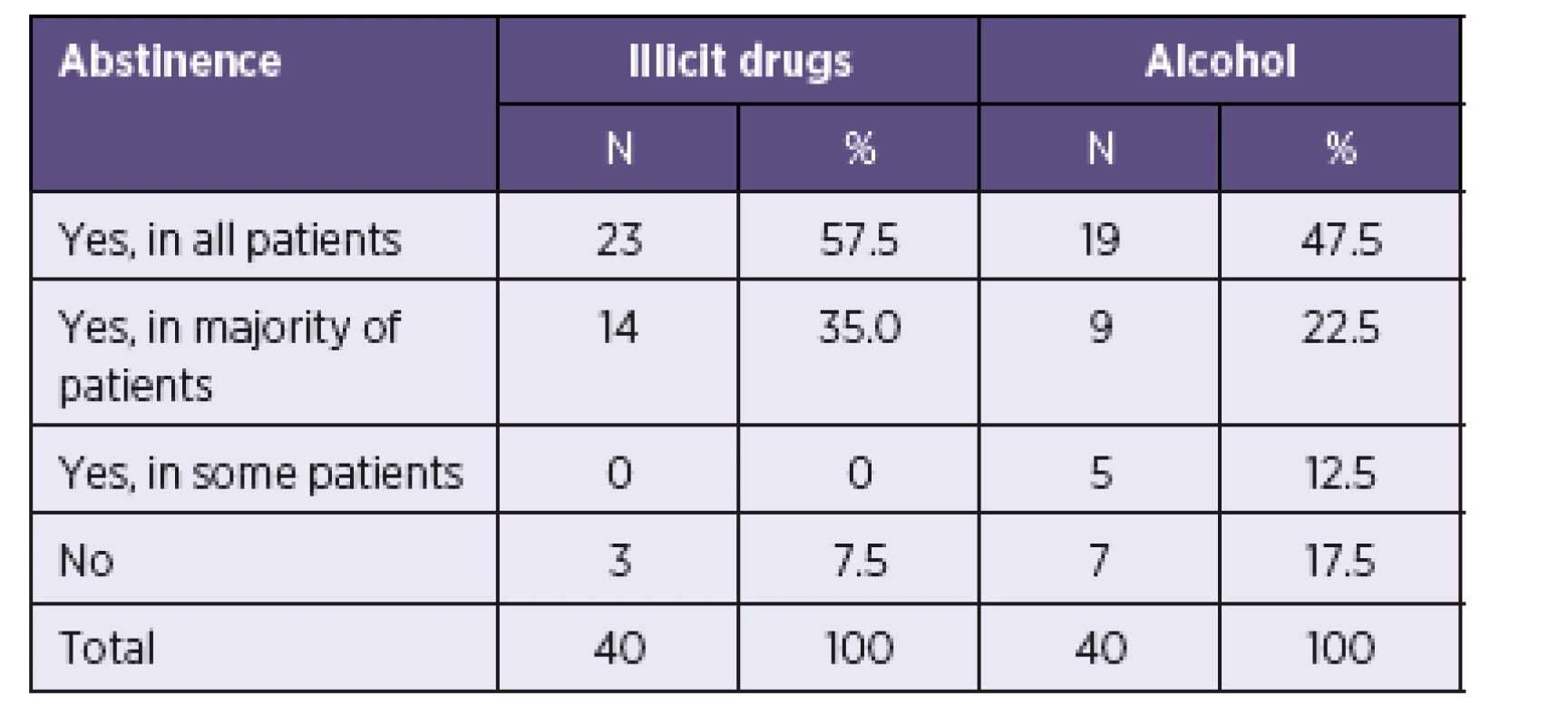
All centres, except for 3, considered abstinence from illicit drugs an absolute or relative precondition for HCV treatment (Table 3). Clinicians were less strict regarding the consumption of alcohol. Rare or moderate alcohol consumption was widely tolerated, although a zero tolerance policy was applied to alcohol abuse. The required period of abstinence ranged between 3-24 months (Mean = 6.7) for drugs and 2-12 months (Mean = 5.6) for alcohol (Median = 6 and Modus = 6 in both drugs and alcohol).
Before initiating treatment, 90% of clinicians applied a “probationary” phase (Mean = 4.5; Modus = 6; range: 1-12 months) to test patient adherence and 50% of clinicians requested active opioid users to be in substitution treatment. Many centres required specialist assessment (62.5% by a psychiatrist; 32.5% by an addiction specialist) before beginning HCV therapy. Special addiction treatment and counselling services were, however, mostly lacking in hepatitis treatment centres. Addiction specialists were permanent members of the hepatitis treatment team in only 5 centres. OST was provided by merely 3 centres, although 21 centres (53%) cooperated with other health care providers in this regard. Other addiction treatment or counselling was provided by 8 hepatitis treatment centres and 22 (55%) cooperated with other addiction care providers (17 with medical facilities, 16 with non-medical facilities and 11 with both).
Funding and affordability of HCV treatment
Twenty-four centres (60%) stated that patients’ uptake into treatment was limited due to centre’s low budget. Ten centres reported that treatment was not started in a total of 62 patients in 2010 due to financial constraints. Four centres had a waiting list with a total of 43 patients by the end of 2010, of which 18 were active or former IDUs. Thirty centres (75%) reported a preference for abstaining (former) drug users and non-users to active drug users due to fear of low adherence.
Experience with the treatment of opioid users versus methamphetamine users
The following proportions were weighted by the total number of treated patients as of 31 December 2010. Treatment uptake in IDUs, as reported by 30 centres, was 60% on average (range 0-90%). Clinicians claimed that there was a difference in treatment uptake between IDUs and non-users, as well as between opioid users and methamphetamine users (58% and 33% of clinicians respectively). However, opinions differed on which groups presented better uptake. Treatment completion was reported as 93% on average (range 0–100%). The majority of clinicians did not observe a difference in completion rate between IDUs and non-users, nor between opioid and methamphetamine users (71% and 81% of clinicians respectively). Twelve to fourteen centres responded to questions regarding differences in clinical status, treatment uptake, and completion between opioid and methamphetamine users. The majority (68-93%) observed no difference between groups in terms of severity of hepatological clinical status, motivation to start treatment, ability to comply with the treatment regime and risk of relapse to drug use.
Discussion
Cross-sectional design of the study, subjective character of information collected via questionnaires and providers’ responses as the only source of data represent major limitations of our findings. Low response rate in some questions further impedes an extrapolation and generalisation of our results (for example as regards differences between methamphetamine and opioid users).
Nevertheless, the study for the first time outlines the availability and provision of HCV treatment to IDUs in the Czech Republic. According to the extrapolated data, 64% of centres treating HCV in the Czech Republic in 2010 provided HCV treatment to IDUs and 47% of patients treated that year were abstaining, former or (rarely) active IDUs. A number of provider - and system-related factors were identified, which hamper higher treatment uptake.
Financial limits of treatment centres represent a major barrier to higher treatment rates. In the financial scheme of public health insurance, reimbursement of HCV treatment is not based on the cost of individual patients, but is provided in a fixed overall flat rate for the centre as a whole, thus not reflecting the increasing number of treated patients. Moreover non-users are widely preferred to active drug users, which further negatively influences treatment uptake among IDUs. This is counterproductive from a public health perspective since recent findings show that HCV treatment in active IDUs has a strong preventive potential, reducing further transmissions and decreasing prevalence of HCV infection at population level, even with low coverage of treatment [e.g. 24, 25, 26]. As HCV prevalence in Czech IDUs is quite low, this preventive effect may already be taking place in the Czech Republic, even with low HCV treatment rate.
Many clinicians require IDUs to be in OST or to be assessed by a psychiatrist or addiction specialist before initiating treatment. However, drug-use specific services and specialists are usually not part of HCV treatment centres. This lack of integrated care can be seen as a considerable weak point of the existing HCV treatment system in the Czech Republic. A multidisciplinary approach can effectively address these barriers to HCV therapy, as well as manage the needs of patients; for instance, relating to the adverse side-effects of treatment [34–36]. The most effective strategy for increasing treatment uptake and adherence is a one-stop-shop model, in which HCV and addiction treatment, as well as other related counselling services, are offered in one centre [37–39].
Clinicians’ conservative attitudes towards HCV-treatment of active IDUs represent a further limitation. While the Czech Republic has permissive guidelines for the treatment of IDUs, many clinicians require abstinence from drugs. Active drug use is associated with a chaotic lifestyle and social destabilisation, which in turn is related to decreased adherence and treatment efficacy [e.g. 40, 41]. However, research shows that active IDUs can achieve treatment adherence and virologic response comparable to non-users, especially when risk factors and barriers associated with drug use are managed appropriately [e.g. 13, 14, 42]. When Czech clinicians do consider IDUs for HCV treatment, they broadly apply pre-treatment assessment to manage factors that can affect adherence.
No difference was reported between IDUs and non-IDUs, nor between opioid and methamphetamine users, in terms of treatment uptake and completion. This is promising information (even if based on clinicians’ reported experience) considering methamphetamine users represent the vast majority of IDUs in the Czech Republic and an effective method for increasing treatment adherence (a substitution treatment) is not available to them.
In conclusion, low financial resources, insufficient integra-tion of HCV and addiction treatment, and clinicians’ conservative attitudes towards treatment of active IDUs represent the main limits in treating HCV in IDUs in the Czech Republic. Addressing these barriers is the key to increasing HCV treatment rates in (active) IDUs and reducing HCV prevalence in the Czech Republic in the long-term.
Acknowledgements
This work was supported by the Internal Grant Agency of the Ministry of Health [grant number IGA 10034-4: Social costs of tobacco, alcohol and illicit drugs in the Czech Republic]. The founder had no role in the review design, collection, analysis, and interpretation of data.
Authors acknowledge Dr. Jiřina Hobstová, Dr. Jan Galský, and Dr. Petr Urbánek for their comments and piloting of the online questionnaire, and Dr. Vlastimil Nečas for the programming of the online questionnaire and data collection.
Conflicts of interest
None declared.
Authors’ contributions
VM designed and performed the data collection. VM, LS, JR and BS participated in the analysis of the data and drafting the manuscript. All the authors read and approved the final manuscript.
Key points
Hepatitis C virus (HCV) infection in injecting drug users (IDUs) represents a considerable global public health concern.
Antiviral treatment in IDUs can reduce HCV prevalence at population level and leads to adherence and effectiveness comparable to that in non-users. However, HCV treatment rate in IDUs is generally low.
The present study found a number of system - and provider-related factors limiting uptake and adherence of IDUs to HCV treatment in the Czech Republic. These factors represent important targets for public health policy interventions.
Do redakce došlo dne 30. 4. 2014.
Corresponding author:
Viktor Mravčík, MD, PhD
National Monitoring Centre for Drugs and Drug Addiction, Office of the Government of the Czech Republic,
Nábřeží E. Beneše 4
118 01 Prague 1
the Czech Republic
E-mail: mravcik.viktor@vlada.cz, tel.: (+420) 950 192 751
Sources
1. Hanafiah KM, Groeger J, Flaxman AD, Wiersma ST. Global epidemiology of hepatitis C virus infection: New estimates of age-specific antibody to hepatitis C virus seroprevalence. Hepatology, 2013;57(4):1333–1342.
2. Perz JF, Armstrong GL, Farrington LA, Hutin YJ, Bell BP. The contributions of hepatitis B virus and hepatitis C virus infections to cirrhosis and primary liver cancer worldwide. Journal of Hepatology, 2006;45(4):529–538.
3. Alter MJ. HCV routes of transmission: what goes around comes around. Semin Liver Dis, 2011;31(4):340–346.
4. Nelson PK, Mathers BM, Cowie B, et al. Global epidemiology of hepatitis B and hepatitis C in people who inject drugs: results of systematic reviews. Lancet, 2011;378(9791):571–583.
5. Mehta SH, Genberg BL, Astemborski J, et al. Limited Uptake of Hepatitis C Treatment Among Injection Drug Users. Journal of Community Health, 2008;33(3):126–133.
6. Grebely J, Matthews GV, Lloyd AR, Dore GJ. Elimination of Hepatitis C Virus Infection Among People Who Inject Drugs Through Treatment as Prevention: Feasibility and Future Requirements. Clin Infect Dis, 2013;57(7):1014–1020.
7. Grebely J, Raffa JD, Lai C, et al. Low uptake of treatment for hepatitis C virus infection in a large community-based study of inner city residents. J Viral Hepat, 2009;16(5):352–358.
8. Harris M, Rhodes T. Hepatitis C treatment access and uptake for people who inject drugs: a review mapping the role of social factors. Harm Reduct J, 2013;10 : 7.
9. Mravčík V, Strada L, Štolfa J, et al. Factors associated with the uptake, adherence and efficacy of hepatitis C treatment among people who inject drugs: a literature review. Patient Prefer Adherence, 2013;7 : 1067–1075.
10. Reimer J, Schulte B, Castells X, et al. Guidelines for the Treatment of Hepatitis C Virus Infection in Injection Drug Users: Status Quo in the European Union Countries. Clinical Infectious Diseases, 2005;40(Suppl 5):373–378.
11. European Association of the Study of the Liver. 2011 European Association of the Study of the Liver hepatitis C virus clinical practice guidelines. Liver Int, 2012;32 (Suppl 1):2–8.
12. Robaeys G, Grebely J, Mauss S, et al. Recommendations for the Management of Hepatitis C Virus Infection Among People Who Inject Drugs. Clinical Infectious Diseases, 2013;57 (Suppl. 2):S129–137.
13. Hellard M, Sacks-Davis R, Gold J. Hepatitis C treatment for injection drug users: a review of the available evidence. Clin Infect Dis, 2009;49(4):561–573.
14. Dimova RB, Zeremski M, Jacobson IM, Hagan H, Des Jarlais DC, Talal AH. Determinants of hepatitis C virus treatment completion and efficacy in drug users assessed by meta-analysis. Clin Infect Dis, 2013;56(6):806–816.
15. Aspinall EJ, Corson S, Doyle JS, et al. Treatment of hepatitis C virus infection among people who are actively injecting drugs: a systematic review and meta-analysis. Clin Infect Dis, 2013;57 Suppl 2:S80–89.
16. Dalgard O, Bjoro K, Hellum K, et al. Treatment of chronic hepatitis C in injecting drug users: 5 years’ follow-up. Eur Addict Res, 2002;8(1):45–49.
17. Grebely J, Pham ST, Matthews GV, et al. Hepatitis C virus reinfec-tion and superinfection among treated and untreated participants with recent infection. Hepatology, 2011;55(4):1058–1069.
18. Hagan H, Pouget ER, Des Jarlais DC. A systematic review and meta-analysis of interventions to prevent hepatitis C virus infection in people who inject drugs. J Infect Dis, 2011;204(1):74–83.
19. van den Berg CH, Smit C, Van Brussel G, Coutinho RA, Prins M. Full participation in harm reduction programmes is associated with decreased risk for human immunodeficiency virus and hepatitis C virus: evidence from the Amsterdam cohort studies among drug users. Addiction, 2007;102 : 1454–1462.
20. Turner KM, Hutchinson S, Vickerman P, et al. The impact of needle and syringe provision and opiate substitution therapy on the incidence of hepatitis C virus in injecting drug users: pooling of UK evidence. Addiction, 2011;106(11):1978–1988.
21. Vickerman P, Martin N, Turner K, Hickman M. Can needle and syrin-ge programmes and opiate substitution therapy achieve substantial reductions in hepatitis C virus prevalence? Model projections for different epidemic settings. Addiction, 2012;107(11):1984–1995.
22. Vickerman P, Martin NK, Hickman M. Understanding the trends in HIV and hepatitis C prevalence amongst injecting drug users in dif-ferent settings – implications for intervention impact. Drug Alcohol Depend, 2012;123(1–3):122-131.
23. Martin NK, Vickerman P, Foster GR, Hutchinson SJ, Goldberg DJ, Hickman M. Can antiviral therapy for hepatitis C reduce the prevalence of HCV among injecting drug user populations? A modeling analysis of its prevention utility. J Hepatol, 2011;54(6):1137–1144.
24. Martin NK, Vickerman P, Hickman M. Mathematical modelling of hepatitis C treatment for injecting drug users. Journal of Theoretical Biology, 2011;274(1):582–566.
25. Vickerman P, Martin N, Hickman M. Can Hepatitis C virus treatment be used as a prevention strategy? Additional model projections for Australia and elsewhere. Drug and Alcohol Dependence, 2010;113(2-3):83-85.
26. Zeiler I, Langlands T, Murray JM, Ritter A. Optimal targeting of Hepatitis C virus treatment among injecting drug users to those not enrolled in methadone maintenance programs. Drug Alcohol Depend, 2010;110(3):228–233.
27. Rosen HR. Clinical practice. Chronic hepatitis C infection. N Engl J Med, 2011;364(25):2429–2438.
28. Assis DN, Lim JK. New pharmacotherapy for hepatitis C. Clin Pharmacol Ther, 2012;92(3):294–305.
29. Mravčík V, Grohmannová K, Chomynová P, et al. Annual Report. The Czech Republic 2011 Drug Situation. Prague: Office of the Government of the Czech Republic; 2012.
30. Griffiths P, Mravčík V, Lopez D, Klempova D. Quite a lot of smoke but very limited fire-the use of methamphetamine in Europe. Drug and Alcohol Review, 2008;27(5):236–242.
31. Zábranský T, Mravčík V, Korčišová B, Řehák V. Hepatitis C Virus Infection among Injecting Drug Users in the Czech Republic – Prevalence and Associated Factors. European Addiction Research, 2006;12 (3):151–160.
32. Mravčík V, Šebáková H. Výskyt virových hepatitid typu B a C u injekčních uživatelů drog v okrese Karviná. Adiktologie, 2002;2(2):19–27.
33. Mravčík V, Petrošová B, Zábranský T, Coufalová M, Řehák V. Výskyt VHC u injekčních uživatelů drog. Výsledky studie prováděné mezi klienty nízkoprahových zařízení v letech 2002–2005. Praha: Úřad vlády ČR; 2009.
34. Grebely J, Genoway K, Khara M, et al. Treatment uptake and outcomes among current and former injection drug users receiving directly observed therapy within a multidisciplinary group model for the treatment of hepatitis C virus infection. Int J Drug Policy, 2007;18(5):437–443.
35. Backmund M, Meyer K, Von Zielonka M, Eichenlaub D. Treatment of hepatitis C infection in injection drug users. Hepatology, 2001;34(1):188–193.
36. Reimer J, Haasen C. Need-adapted HCV-treatment setting for injection drug users. Lancet, 2009;373(9681):2090–2091.
37. Treloar C, Newland J, Rance J, Hopwood M. Uptake and delivery of hepatitis C treatment in opiate substitution treatment: perceptions of clients and health professionals. J Viral Hepat, 2010;17(12):839–844.
38. Litwin AH, Soloway I, Gourevitch MN. Integrating Services for Injection Drug Users Infected with Hepatitis C Virus with Methadone Maintenance Treatment: Challenges and Opportunities. Clinical Infectious Diseases, 2005;40 (Suppl. 5):S339–345.
39. Moussalli J, Delaquaize H, Boubilley D, et al. Factors to Improve the Management of Hepatitis C in Drug Users: An Observational Study in an Addiction Centre. Gastroenterology Research and Practice, 2010;2010:pii: 261472.
40. Dore GJ, Hellard M, Matthews GV, et al. Effective Treatment of Injecting Drug Users With Recently Acquired Hepatitis C Virus Infection. Gastroenterology, 2010;138(1):123–135.
41. Alvarez-Uria G, Day JN, Nasir AJ, Russell SK, Vilar FJ. Factors associated with treatment failure of patients with psychiatric diseases and injecting drug users in the treatment of genotype 2 or 3 hepatitis C chronic infection. Liver International, 2009;29(7):1051–1055.
42. Schulte B, Schutt S, Brack J, et al. Successful treatment of chronic hepatitis C virus infection in severely opioid-dependent patients under heroin maintenance. Drug Alcohol Depend, 2010;109(1-3):248–251.
43. Mravčík, V. Léčba VHC u injekčních uživatelů drog v ČR – průzkum mezi centry pro léčbu virových hepatitid. Adiktologie, 2012; 12 (1):10–22.
Labels
Hygiene and epidemiology Medical virology Clinical microbiologyArticle was published in
Epidemiology, Microbiology, Immunology

2014 Issue 4
-
All articles in this issue
- Prevalence study of nosocomial infections in university hospitals in the Czech Republic
- Outbreak of viral hepatitis B and C in hospitalized cancer patients
- Pertussis trend in children under one year of age in the Czech Republic in 1997–2013
- Current situation in clinical trials with vaccines in the Czech Republic
- Epidemiology and risk factors in legionellosis
-
Infections caused by non-Typhi serovars of Salmonella at the
Infectious Diseases Clinic of the University Hospital Brno in 2011–2013 - Seroprevalence of Anaplasma phagocytophilum in patients with suspected Lyme borreliosis
- An uncommon detection of Mycobacterium tuberculosis in pericardial effusion
- Surveillance of West Nile fever in horses in the Czech Republic from 2011 to 2013
- Hepatitis C treatment uptake and adherence among injecting drug users in the Czech Republic
- Epidemiology, Microbiology, Immunology
- Journal archive
- Current issue
- About the journal
Most read in this issue
- Prevalence study of nosocomial infections in university hospitals in the Czech Republic
- Seroprevalence of Anaplasma phagocytophilum in patients with suspected Lyme borreliosis
- Current situation in clinical trials with vaccines in the Czech Republic
-
Infections caused by non-Typhi serovars of Salmonella at the
Infectious Diseases Clinic of the University Hospital Brno in 2011–2013
