Can gram-negative-like biomarker values in Streptococcus pyogenes sepsis negatively influence right choice of initial antibiotic therapy?
Mohou hodnoty biomarkerů, imitující gramnegativní zánětlivou odpověď, negativně ovlivnit iniciální volbu antibiotika u pacientů se sepsí vyvolanou Streptococcus pyogenes?
Úvod: Biomarkery jsou u septických pacientů využívány jak k diagnostice sepse, tak k antibiotickému stewardshipu. Sepse vyvolaná gramnegativními bakteriemi mívá odlišné charakteristiky, především vysoký prokalcitonin vs C-reaktivní protein v porovnání se sepsí vyvolanou grampozitivními bakteriemi. Avšak jednotlivá infekční agens, především Streptococcus pyogenes, nemusí do tohoto schématu zapadat, což může vest k nesprávné iniciální volbě antibiotika.
Metody: Retrospektivní analýza biomarkerů, iniciální volby antibiotické léčby a výsledků léčby u pacientů se sepsí vyvolanou S. pyogenes, Escherichia coli a Staphylococcus aureus. Hodnoty biomarkerů byly porovnány pomocí Kruskal-Wallis testu s následným Dunn post-Hoc testem s prahem p < 0,05.
Výsledky: Hodnoty prokalcitoninu byly nejvyšší u sepse vyvolané S. pyogenes (12,51 ng/ml, IQR: 6,26–48,38 ng/ml) oproti sepsi vyvolaná E. coli (4,30 ng/ml, IQR: 1,50–10,00 ng/ml, p < 0,001) a S. aureus (0,75 ng/ml, QR: 0,34–1,62 ng/ml, p < 0,001). Poměr neutrofilů a lymfocytů vykazoval stejné charakteristiky jako prokalcitonin. Správná iniciální antibiotická léčba byla v souboru S. pyogenes 11,29 % v porovnání s 99,3 % a 100 % u S. aureus a E. coli skupin.
Závěr: Oproti předchozím studiím byly v našem souboru pozorovány nejvyšší hodnoty prokalcitoninu u pacientů se sepsí vyvolanou S. pyogenes spíše než gramnegativními bakteriemi. Vysoké hodnoty prokalcitoninu imitující gramnegativní zánětlivou odpověď přispěli k ovlivnění výběru iniciální antibiotické léčby (bez znalosti původce), což mohlo vést k vyšší mortalitě u této skupiny pacientů. Proto doporučujeme přehodnocení významu prokalcitoninu v diagnostice sepse pro zlepšení přežití i kvality života pacientů.
Klíčová slova:
GAS sepsis – procalcitonin – neutrophil/lymphocyte ratio – initial ATB therapy – Biomarkers – Clindamycin
Authors:
V. Adámková 1,2
; H. Lahoda Brodská 3; V. Adámková 4
; T. Zima 5
Authors‘ workplace:
Department of Clinical Microbiology and ATB centre, Institute of Medical Biochemistry and Laboratory Diagnostic, First Faculty of Medicine, Charles University and General University Hospital, Prague, Czech Republic
1; Department of Medical Microbiology, Faculty of Medicine, Palacky University, Olomouc, Czech Republic
2; Department of Clinical Biochemistry, Institute of Medical Biochemistry and Laboratory Diagnostic First Faculty of Medicine, Charles University and General University Hospital, Prague, Czech Republic
3; School of Biosciences, Cardiff University, Great Britain
4; Institute of Medical Biochemistry and Laboratory Diagnostic First Faculty of Medicine, Charles University and General University Hospital, Prague, Czech Republic
5
Published in:
Epidemiol. Mikrobiol. Imunol. 69, 2020, č. 3, s. 128-133
Category:
Original Papers
Overview
Úvod: Biomarkery jsou u septických pacientů využívány jak k diagnostice sepse, tak k antibiotickému stewardshipu. Sepse vyvolaná gramnegativními bakteriemi mívá odlišné charakteristiky, především vysoký prokalcitonin vs C-reaktivní protein v porovnání se sepsí vyvolanou grampozitivními bakteriemi. Avšak jednotlivá infekční agens, především Streptococcus pyogenes, nemusí do tohoto schématu zapadat, což může vest k nesprávné iniciální volbě antibiotika.
Metody: Retrospektivní analýza biomarkerů, iniciální volby antibiotické léčby a výsledků léčby u pacientů se sepsí vyvolanou S. pyogenes, Escherichia coli a Staphylococcus aureus. Hodnoty biomarkerů byly porovnány pomocí Kruskal-Wallis testu s následným Dunn post-Hoc testem s prahem p < 0,05.
Výsledky: Hodnoty prokalcitoninu byly nejvyšší u sepse vyvolané S. pyogenes (12,51 ng/ml, IQR: 6,26–48,38 ng/ml) oproti sepsi vyvolaná E. coli (4,30 ng/ml, IQR: 1,50–10,00 ng/ml, p < 0,001) a S. aureus (0,75 ng/ml, QR: 0,34–1,62 ng/ml, p < 0,001). Poměr neutrofilů a lymfocytů vykazoval stejné charakteristiky jako prokalcitonin. Správná iniciální antibiotická léčba byla v souboru S. pyogenes 11,29 % v porovnání s 99,3 % a 100 % u S. aureus a E. coli skupin.
Závěr: Oproti předchozím studiím byly v našem souboru pozorovány nejvyšší hodnoty prokalcitoninu u pacientů se sepsí vyvolanou S. pyogenes spíše než gramnegativními bakteriemi. Vysoké hodnoty prokalcitoninu imitující gramnegativní zánětlivou odpověď přispěli k ovlivnění výběru iniciální antibiotické léčby (bez znalosti původce), což mohlo vést k vyšší mortalitě u této skupiny pacientů. Proto doporučujeme přehodnocení významu prokalcitoninu v diagnostice sepse pro zlepšení přežití i kvality života pacientů.
Keywords:
sepse – prokalcitonin – poměr neutrofilů/lymfocytů – iniciální ATB terapie – biomarkery – klindamycin
INTRODUCTION
Sepsis, defined as life-threatening organ dysfunction caused by a dysregulated host response to infection, is the major cause of mortality from any infectious disease worldwide [1]. In 2005, the WHO reported a global estimate of 18.1 million cases of severe Streptococcus pyogenes (GPOS) disease, with 1.78 million new cases of severe disease and 517,000 deaths per year [2]. Group A streptococci (GAS) not only causes superficial diseases, but it has also the capacity to breach epithelial barriers and cause a variety of invasive diseases which lead to a death of 8 to 23% patients with GAS invasive disease within 7 days of infection [3].
Being the first 3–6 hours after the clinical suspicion critical for establishment of therapeutic measures that improve prognosis, the keystone of sepsis management is timely administration of active microbials, Surviving Sepsis Campaign guidelines [4]. Under routine clinical praxis, patients with suspected sepsis are immediately screened for biomarkers and blood culture is drawn. In the best-case scenario, preliminary results of microbial diagnosis of sepsis from the blood culture are available after 24 h. Therefore, the choice of empiric initial ATB therapy is commonly based on the level of available biomarkers and clinical symptoms [5] and once the microbiological results are available, the initial therapy is evaluated and changed if inappropriate.
In general, empiric initial treatment of a suspected GPOS sepsis consists of a use of glycopeptides. Suspected GNEG sepsis is covered by the administration of carbapenems, aminoglycosides or colistin based on a local epidemiological situation [6]. However, the gold standard for GAS sepsis is a combination of cell wall synthesis inhibitors (i. e. beta-lactams or glycopeptides) with protein synthesis inhibitors (MLS antibiotics) [7]. Although S. pyogenes is susceptible to penicillin in vitro [8], penicillin monotherapy treatment of GAS infections with toxin production has been associated with high morbidity and mortality [7] due to “Eagle effect” [9]. Addition of clindamycin decreases morbidity and mortality [10] via inhibition of GAS virulence factors production. Worldwide increasing resistance of S. pyogenes to clindamycin might decrease its effect, however, data from the Czech Republic do not show the trend in increasing resistance [11, 12]. Furthermore, clindamycin is associated with longer post-antibiotic effect than penicillin and its effect is not influenced by inoculum size [13]. On the other hand, the use of clindamycin is associated with increased risk of post-antibiotic colitis caused by Clostridium difficile [14].
Nevertheless, currently there is no single commercially available biomarker that would be 100% specific and sensitive to discriminate between GNEG and GPOS sepsis [15] This hinders clinical sepsis pathway implementation, potentially leading to an inappropriate choice of ATB therapy, which in the worst-case results in patient’s death [5].
Frequently used biomarkers, such as the total white blood cells, neutrophil count, and CRP, lack the specificity to discriminate between SIRS and sepsis [15]. In this sense, PCT – a prohormone of calcitonin – was shown to have the best accuracy to identify patients with invasive bacterial infections because inflammatory stimuli including severe infection leads to its upregulated production in different tissues [16]. Despite the fact that elevated PCT serum concentrations are not exclusive to infections (they can also be elevated during paraneoplastic processes, in patients with solid tumours or with major trauma [17], at this moment, PCT is considered among the best clinically available biomarkers to diagnose sepsis [18] and can be used as a guide to fulfil the principles of antimicrobial stewardship (AS). Brodská et al. and other researchers [19, 20] have published that PCT values are significantly higher in GNEG sepsis compared to GPOS and yeast sepsis and currently, if PCT level is > 3 [20], patients are treated for GNEG sepsis until microbiological results are available. Nonetheless, Ruddel et al., 2018 [21] questioned the validity of those findings.
The aim of this retrospective study was to evaluate the administration of an appropriate initial ATB therapy based on available biomarkers in patients with microbiologically confirmed cases of GAS sepsis.
METHODS
Patient population
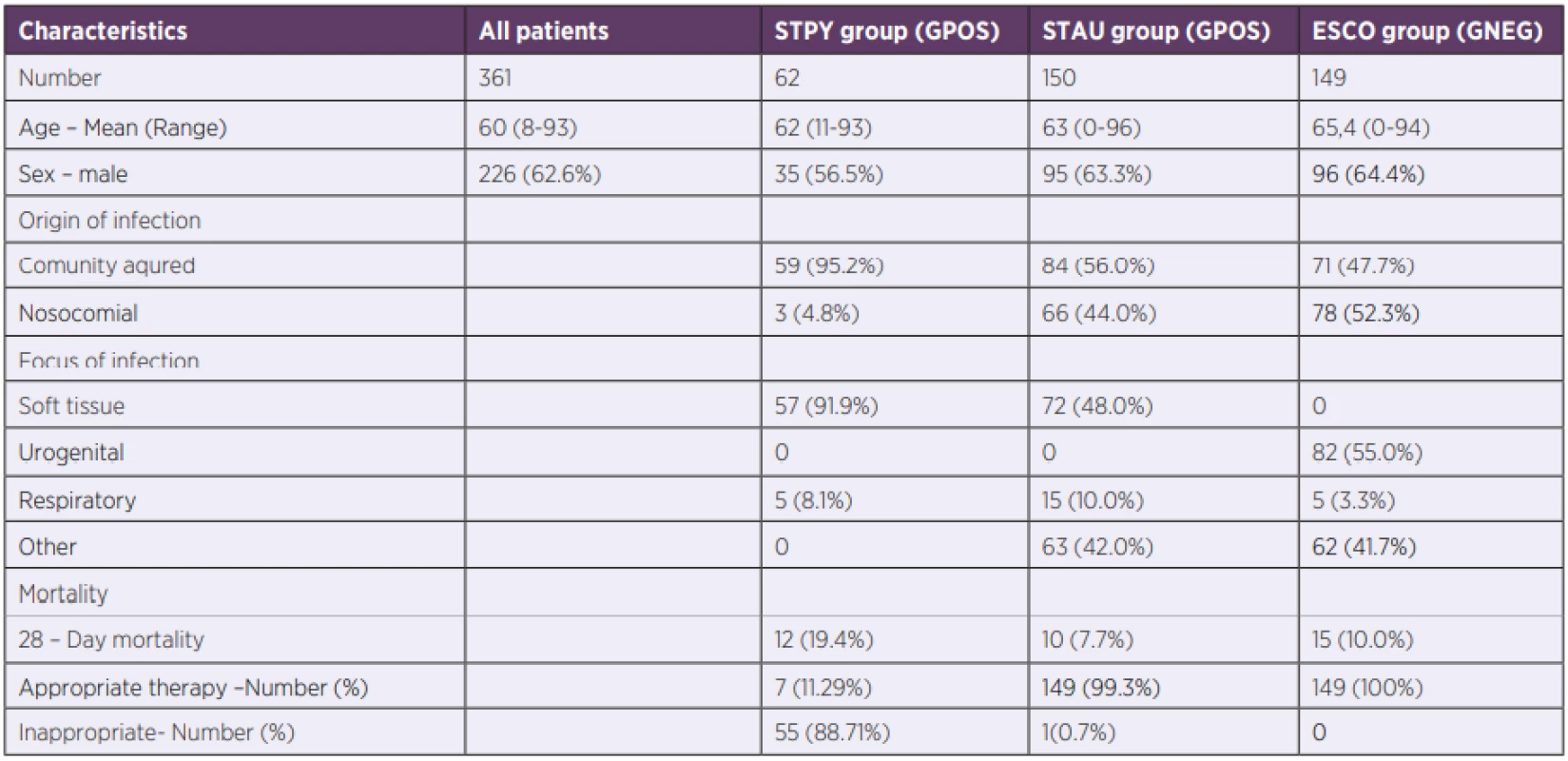
Retrospectively, we identified and reviewed all cases of GAS sepsis (n = 62) between 2006 and 2019 in a single tertiary-care centre. Control groups were patients with sepsis between years 2018 and 2019 caused by the most frequent causative GPOS agent (n = 150), Staphylococcus aureus, and the most frequent GNEG agent (n = 149) Escherichia coli. Only confirmed cases with complete data (PCT, CRP, NLR, WBC, blood cultures, recommended ATB treatment) were analysed.
Our outcome was the incidence of an appropriate initial ATB therapy, which was based on the levels of PCT and NLR.
Biomarkers determination
PCT, CRP, NLR, WBC measurements were performed in hospital laboratory using commercially available assays as part of routine care. All PCT values and other laboratory parameters were recorded within the first 24h after the onset of sepsis as baseline data. Blood cultures were drawn at sepsis onset before the start of antimicrobial therapy and processed and analysed according to local standards.
The investigators considering all available clinical and microbiological data identified the focus of infection retrospectively. For analysis, foci of infection were grouped into four categories (bones/soft tissue, respiratory, urogenital and other).
Statistical analysis
Data analysis was performed in RStudio. Categorical data were expressed as percentage and continuous variables as the mean and medians with interquartile range (IQR) between 25th and 75th percentile. For the comparison of nonparametric data, Kruskal-Wallis test followed by Dunn Post-Hoc tests were used. The threshold for significance was set at p < 0.05.
RESULTS AND DISCUSSION
Patient’s population
During the 13-years long study period, 62 patients with GAS sepsis were hospitalized in the General University Hospital, Prague. Demographic and outcome clinical data are summarized in Table 1. GAS sepsis with positive blood culture is rare [22, 23] and therefore, our cohort was unique due to its large number of GAS patients.
Biomarker levels associated with bacterial sepsis
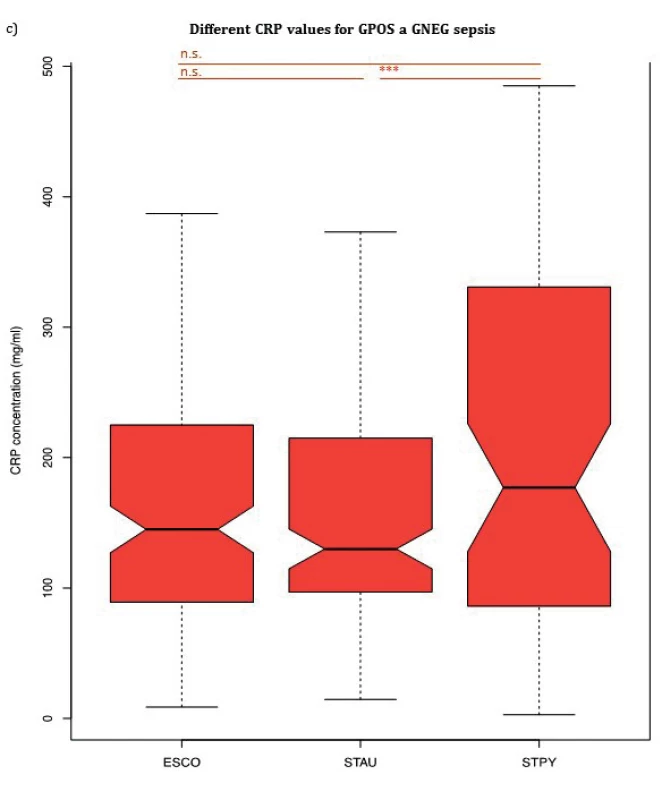
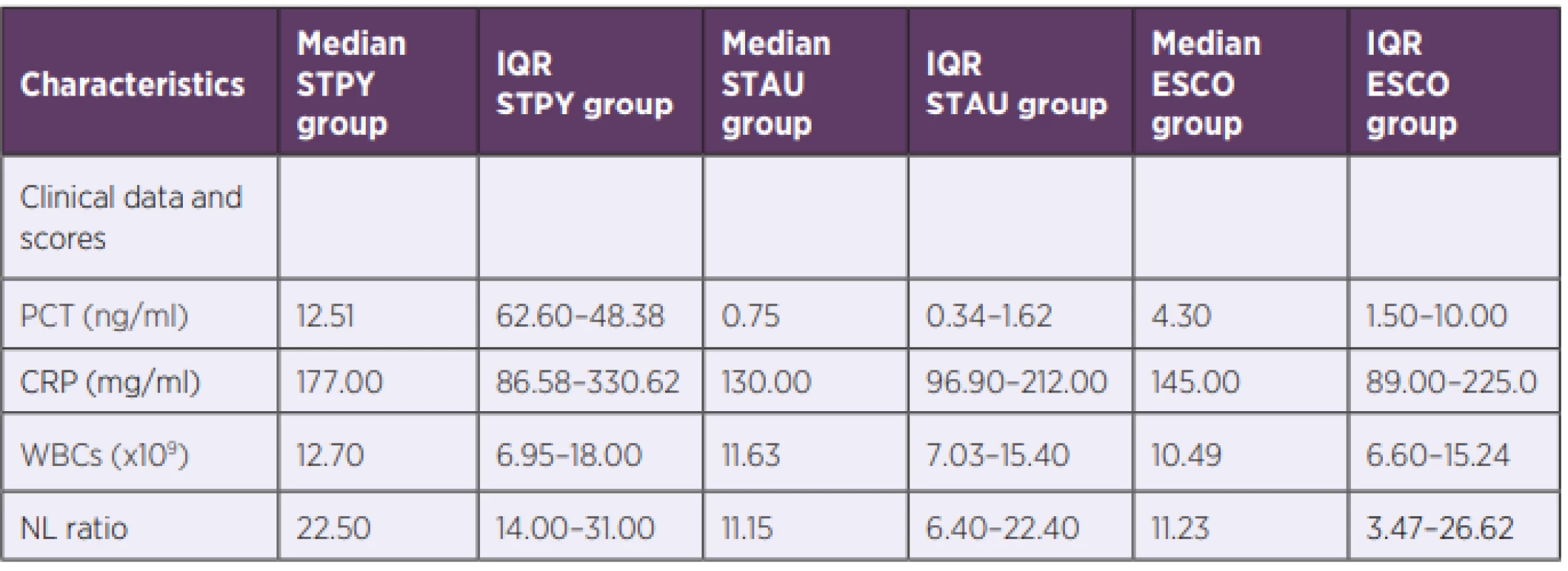
PCT, NLR and CRP median values for three bacterial species (Escherichia coli, Staphylococcus aureus, Streptococcus pyogenes) are summarized in Table 2 and shown in Figure 1a, 1b and Figure 1c, respectively. PCT median value of GAS sepsis 12.51 ng/ml (IQR: 6.26–48.38 ng/ml) was significantly higher than the values obtained for both E. coli and S. aureus sepsis 4.30 ng/ml (IQR: 1.50–10.00 ng/ml, p < 0,001) and 0.75 ng/ml (IQR: 0.34–1.62 ng/ml, p < 0,001), respectively. Up to our knowledge, we are the first to report that S. pyogenes can produce an inflammatory response similar to or higher than GNEG bacteria.
S. pyogenes GNEG-like inflammatory response might be explained via recognition of GAS pore-forming toxin streptolysin O by TLR4 leading to high expression of pro-inflammatory cytokines [24].
TLR4 is a receptor for lipopolysaccharide of GNEG bacteria, while peptidoglycan of GPOS bacteria activates TLR2 [25] and it has been shown that administration of lipopolysaccharide leads to increased PCT mRNA expression in peripheral blood mononuclear cells [26]
Furthermore, there was also a significant difference in PCT median values between E. coli and S. aureus sepsis (p < 0.001). Data from control groups are in agreement with other studies showing that generally, patients with GNEG sepsis have significantly higher PCT values compared to GPOS septic patients [27]. Due to a very low incidence of S. pyogenes positive blood cultures [3], even their inclusion in GPOS group does not significantly increase PCT level (computational modelling, data not shown).
Similarly, there was a significant difference in NLR median value between GAS sepsis 22.5 (IQR: 14.00–31.00) and both E. coli and S. aureus sepsis 11.23 (IQR: 3.47–26.62, p < 0.001) and 11.15 (IQR: 6.40–22.40, p < 0.001), respectively. In contrast to PCT, there was no significant difference in NLR median values between E. coli and S. aureus (p > 0.05).
Although there was no significant difference in median CRP values between septic patients with E. coli and S. aureus nor between patients with S. pyogenes and E. coli, CRP levels of patients with S. pyogenes 177.00 mg/ml (IQR: 86.58–330.62 mg/ml) were significantly increased compared to patients with S. aureus 130.00 mg/ml (IQR: 96.90–212.00 mg/ml), p = 0.016. WBC median values showed no significant difference between any of the groups confirming the findings by Brodská et al [19].
ATB therapy
According to patient’s records, all patients with E. coli sepsis and 149 out of 150 patients with S. aureus received an appropriate initial therapy. Patients with E. coli received either a monotherapy (meropenem, 34.3%) or a combination of: beta-lactams with aminoglycosides (38.8%); carbapenems with aminoglycosides (15.2%) or ciprofloxacin with aminoglycosides (11.7%). S. aureus infected patients were treated by vancomycin (35.2%) or oxacillin (25.6%) in monotherapy or by a combination of vankomycin and one of these: meropenem (14.3%), amikacin (18.4), ciprofloxacin (6.5%). The one patient (0.7%) with an inappropriate initial therapy received amikacin in monotherapy. Nevertheless, appropriate initial therapy in GAS sepsis was given to only 7 out of 62 patients (11.29%). These patients received a combination of clindamycin (protein synthesis inhibitor) with one of these: piperacillin/tazobactam (4.84%); penicillin (3.23%); oxacillin (1.61%); ertapenem (1.61%). The decision was made either based on patient’s history in last three months (presence of confirmed GAS infection) or on a presence of skin and soft tissue infection. The rest of GAS patients (88.71%) was treated only by bactericidal antibiotics without the agent inhibiting protein synthesis as their PCT levels were high, currently acknowledged as an indicator of GNEG sepsis [20], and the source of infection was unknown. 28-Day mortality in GAS patients was 19.4% but all of them died in the first 7 days. On the other hand, mortality of patients in the control groups in the first 7 days was < 5% which increased to 7.7% in S. aureus cohort and to 10.0% in E. coli cohort after 28 days.
CONCLUSION
Contrasting previous reports, the highest PCT and NLR were observed in S. pyogenes cohort. This contributed to a high percentage of initial inappropriate choice ATB therapy. Therefore, if the etiology of sepsis is unknown, the PCT and/or NLR values are high and microbiological results are not yet available, addition of protein synthesis inhibiting antibiotic is required. This therapy would prevent a worsening of symptoms in case the sepsis was not caused by GNEG bacterium but S. pyogenes mimicking GNEG immune response.
Furthermore, our data highlight an urgent need for a development of fast diagnostic tests based on next-generation sequencing and implementing them into a routine clinical praxis in order to prevent deaths related to a wrong initial ATB therapy due to bacterial sepsis misclassification.
Abbreviations
STPY – Streptococcus pyogenes
STAU – Staphylococcus aureus
ESCO – Escherichia coli
GPOS – Gram-positive bacteria
GNEG – Gram-negative bacteria
CRP – C-reactive protein
PCT – prokalcitonin
GAS – Group A Streptococcus
WBC – white blood count
NLR – Neutrophil lymphocyte ratio
AS – antimicrobial stewardship
MLS – macrolide-lincosamide-streptogramin B
Conflicts of Interest
The authors declare no conflict of interest.
Do redakce došlo dne 5. 3. 2020.
Adresa pro korespondenci:
prim. MUDr. Václava Adámková
Ústav lékařské biochemie a laboratorní diagnostiky Všeobecná fakultní nemocnice a 1. LFUK
Ke Karlovu 455
120 00 Praha-Nové Město
e-mail: vaclava.adamkova@vfn.cz
Sources
1. Rello J, van Engelen TSR, Alp E, et al. Towards precision medicine in sepsis: a position paper from the European Society of Clinical Microbiology and Infectious Dis-eases. Clin Microbiol Infect, 2018;24(12):1264–1272.
2. Carapetis JR, Steer AC, Mulholland EK, et al. The global burden of group A streptococcal diseases. Lancet Infect Dis, 2005;5 : 685–694.
3. Lamagni TL, Darenberg J, Luca-Harari B, et al. Epidemiology of severe Streptococcus pyogenes disease in Europe. J Clin Microbiol, 2008;46 : 2359–2367.
4. Puskarich MA, Trzeciak S, Shapiro NI, et al. Outcomes of patients undergoing early sepsis resuscitation for cryptic shock compared with overt shock. Resuscitation, 2011;82 : 1289–1293.
5. Candel FJ, Borges Sá M, Belda S, et al. Current aspects in sepsis approach. Turning things around. Rev Esp Quimioter, 2018;31(4):298–315.
6. Adamkova V. Charakteristika jednotlivých antibiotik. In Adamkova V. Antibiotika v chrurgických oborech. Praha: Mladá fronta; 2016 : 17–48.
7. Stevens DL, Tanner MH, Winship J, et al. Severe group A strepto-coccal infections associated with a toxic shock-like syndrome and scarlet fever toxin A. N Engl J Med, 1989;321(1):1–7.
8. Allen U, Moore D. Invasive group A streptococcal disease: management and chemoprophylaxis. Can J Infect Dis Med Microbiol, 2010;21 : 115–118.
9. Stevens DL, Gibbons AE, Bergstrom R, et al. The Eagle effect revisited: efficacy of clindamycin, erythromycin, and penicillin in the treat-ment of streptococcal myositis. J Infect Dis, 1988;158 : 23–28.
10. Carapetis JR, Jacoby P, Carville K, et al. Effectiveness of clindamycin and intravenous immunoglobulin, and risk of disease in contacts, in invasive group a streptococcal infection. Clin Infect Dis, 2014;59(3):358–365.
11. Pesola AK, Sihvonen R, Lindholm L, et al. Clindamycin resistant emm33 Streptococcus pyogenes emerged among invasive infec-tions in Helsinki metropolitan area, Finland, 2012 to 2013. Euro Surveill, 2015;20(18):pii=21117.
12. Databáze výsledků studie „RESPIRAČNÍ PATOGENY“ [online]. Dostupné na www: https://apps.szu.cz/rp/rezistence.php.
13. Mascini EM, Jansze M, Schouls LM, et al. Penicillin and clindamycin differentially inhibit the production of pyrogenic exotoxins A and B by group A streptococci. Int J Antimicrob Agents, 2001;18(4):395–398.
14. Teng Ch, Reveles KR, Obodozie-Ofoegbu OO, et al. Clostridium difficile Infection Risk with Important Antibiotic Classes: An Analysis of the FDA Adverse Event Reporting Systém. Int J Med Sci, 2019;16(5):630–635.
15. Raveendran AV, Kumar A, Gangadharan S. Biomarkers and newer laboratory investigations in the diagnosis of sepsis. J R Coll Physicians Edinb, 2019;49 : 207–216.
16. Meisner M. Pathobiochemistry and clinical use of procalcitonin. Clin Chim Acta, 2002;323(1–2):17–29.
17. Aziz SA, Nelwan EJ, Sukrisman L, et al. Higher cut-off serum procalcitonin level for sepsis diagnosis in metastatic solid tumor patients. BMC Res Notes, 2018;11(1):84.
18. Wacker C, Prkno A, Brunkhorst FM, et al. Procalcitonin as diagnostic marker for sepsis: a systematic review and meta-analysis. Lancet Infect Dis, 2013;13(5):426–435.
19. Brodska H, Malickova K, Adamkova V, et al. Significantly higher procalcitonin levels could differentiate Gram-negative sepsis from Gram-positive and fungal sepsis. Clin Exp Med, 2012;13(3):165–170.
20. Li S, RongH, GuoQ, et al. Serum prokalcitonin levels distinguish Gram-negative bacterial sepsis from Gram-positive bacterial and fungal sepsis. J Res Med Sci, 2016;21 : 39.
21. Thomas-Ruddel DO, Poidinger B, Kott M, et al. Influence of pathogen and focus of infection on procalcitonin values in sepsis patients with bacteremia or candidemia. Crit Care, 2018;22(1):128.
22. Ullberg M, Özenci V. Identification and antimicrobial susceptibility testing of Gram-positive and Gram-negative bacteria from positive blood cultures using the Accelerate Pheno™ system. Eur J Clin Microbiol Infect Dis, 2020;39(1):139–149.
23. Vincent J-L, Rello J, Marshall J, et al. International study of the prevalence and outcomes of infection in intensive care units. JAMA, 2009;302(21):2323–2329.
24. Valderrama JA, Nizet V. Group A Streptococcus encounters with host macrophages. Future Microbiol, 2018;13(1):119–134.
25. Elson G, Dunn-Siegrist I, Daubeuf B, et al. Contribution of Toll-like receptors to the innate immune response to Gram-negative and Gram-positive bacteria. Blood, 2007;109(4):1574–1583.
26. Oberhoffer M, Stonans I, Russwurm S, et al. Procalcitonin expres-sion in human peripheral blood mononuclear cells and its modulation by lipopolysaccharides and sepsis-related cytokines in vitro. J Lab Clin Med, 1999;134(1):49–55.
27. Leli C, Ferranti M, Moretti A, et al. Procalcitonin levels in gram-positive, gram-negative, and fungal bloodstream infections. Dis markers, 2015;2015 : 701480.
This work was supported under Grant RVO VFN 64165 of the General University Hospital in Prague, Czech Republic and under Grant HORIZON 2020 (no. 687697) (www.SmartDiagnos.eu).
Labels
Hygiene and epidemiology Medical virology Clinical microbiologyArticle was published in
Epidemiology, Microbiology, Immunology

2020 Issue 3
-
All articles in this issue
- Epidemiology of vancomycin-resistant enterococci in the Hradec Králové University Hospital in 2017
- Seroprevalence of IgG antibodies against measles in health care workers of the Strakonice Hospital
- Implementation and use of whole genome sequencing (WGS) in the surveillance of invasive pneumococcal disease, Czech Republic, 2017–2019
- Drain fly – Clogmia albipunctata (Diptera: Psychodidae) – a fly with epidemiological potential and posing risk of myiasis
- Jubileum profesora Vladimíra Vonky
- Životní jubileum RNDr. Vratislava Němečka, CSc.
- Vzpomínky na MUDr. Evu Jílkovou
- MUDr. Jarmila Kaustová (* 8. 3. 1945 – † 1. 5. 2020)
- Smuteční oznámení: zemřel doc. MUDr. Vlastimil Obdržálek, CSc.
- Increase in RNASEL gene expression by miR-29-3p inhibitors in HEK293T cells
- Can gram-negative-like biomarker values in Streptococcus pyogenes sepsis negatively influence right choice of initial antibiotic therapy?
- The duration of SARS-CoV-2 shedding in patients recovering from COVID-19
- Epidemiology, Microbiology, Immunology
- Journal archive
- Current issue
- About the journal
Most read in this issue
- Epidemiology of vancomycin-resistant enterococci in the Hradec Králové University Hospital in 2017
- Drain fly – Clogmia albipunctata (Diptera: Psychodidae) – a fly with epidemiological potential and posing risk of myiasis
- The duration of SARS-CoV-2 shedding in patients recovering from COVID-19
- Seroprevalence of IgG antibodies against measles in health care workers of the Strakonice Hospital


