Some Possibilities in the Diagnosis of Early Acute Ischaemic Changes in the Heart Muscle in Sudden Death
Některé možnosti diagnostiky časných akutních ischemických změn srdečního svalu u náhlých úmrtí
Úvod:
Ischemická choroba srdeční patří mezi nejčastější onemocnění a značnou měrou se podílí na mortalitě naší populace (v letech 1995–2003 52–56,2 %). Náhlá úmrtí tvoří téměř 2/3 pitev, se kterými se soudní lékaři setkávají, a z nich více než 50 % je způsobeno ICHS. Proto je její diagnostika, a to zejména akutního infarktu myokardu, tak důležitá. Základním diagnostickým kritériem akutního infarktu myokardu je dle společné definice Evropské kardiologické společnosti a American College of Cardiology z roku 2000 pozitivita biochemických ukazatelů nekrózy srdečního svalu. Obecně platí závislost mezi aktivitou enzymů a množstvím poškozených buněk. Již asi 10 let se v odborné literatuře objevují práce, které doporučují využívat stanovení aktivity či koncentrace těchto biochemických ukazatelů i při diagnóze časných fází infarktu myokardu post mortem.
Metody:
Stanovovali jsme koncentrace myoglobinu, kreatinkinázy a troponinu I v krvi a v perikardiální tekutině zemřelých náhlou a násilnou smrtí. Soubor, který tvořilo 71 zemřelých, byl rozdělen do dvou skupin, první skupina (38 případů) byla tvořena zemřelými na akutní infarkt srdečního svalu (byl makroskopicky patrný nebo byla zjištěna ložiskově snížená aktivita SDH při histoenzymatické makroreakci v Šiklových řezech při výrazném zúžení či uzávěru věnčitých tepen a vyloučení jiné příčiny smrti). Zbylých 33 případů (tzv. non-IM smrti) tvořily 4 případy srdečního selhání, 6 případů intracerebrálního krvácení, 5 případů plicní embolie, 5 případů udušení (čtyřikrát oběšení, jednou traumatická asfyxie), tři případy zhmoždění mozku, dva případy otravy CO, dva případy utonutí, po jednom případu otravy kyanidy, otravy alkoholem, smrti vykrvácením, epilepsie, bronchopneumonie a hyperglykemického komatu.
Výsledky:
Kreatinkináza a myoglobin v krvi a v perikardiální tekutině a troponin I v perikardiální tekutině byly zvýšeny ve všech případech jak u IM, tak u non - IM smrtí, statisticky se nelišily ani jejich hladiny. Troponin I byl v krvi zvýšen v 87 % u IM a v 91 % u non-IM smrtí, rozdíl není statisticky významný, rovněž koncentrace se statisticky nelišily.
Závěr:
Nepodařilo se nám prokázat možné využití stanovení koncentrací myoglobinu, kreatinkinázy a troponinu I v krvi a perikardiální tekutině při diagnostice infarktu myokardu.
Klíčová slova:
ICHS – Infarkt myokardu – Troponin I – myoglobin – kreatinkináza
:
E. Tomášková; F. Vorel
:
Department of Forensic Medicine, České Budějovice Hospital
:
Soud Lék., 55, 2010, No. 3, p. 32-35
Introduction:
Ischaemic heart disease is the leading cause of death in industrialised countries. Sudden death accounts for approximately 2/3 of autopsies in forensic medicine, which is why the accurate diagnosis of IHD and first of all acute myocardial ischaemia is so important. On certain occasions in forensic medical practice it is difficult to diagnose AMI from morphological observation alone. In such cases, complementary diagnostic techniques, such as the determination of biochemical markers of the necrosis of the heart muscle (myoglobin, troponin I and creatine kinase) in cadaver fluids, take on special importance, so we decided to test the diagnostic evaluation of postmortem cTnI, myoglobin and creatine kinase determination in serum and pericardial fluid. Recently, cardiac troponins have gained attention as very specific markers of myocardial cell injury, and the European Society of Cardiology and the American College of Cardiology have suggested that these proteins should be preferred markers for the diagnosis of the necrosis of heart muscle.
Methods:
We studied 71 cadavers, which were divided into 2 groups. The first group (38 cases) consisted of people where the acute myocardial infarction was the only cause of death (the myocardial lesion could be easily detected by macroscopic examination or by formazan test, other causes of death were excluded and the constriction or obturation of the coronary arteries was detected). The second group (non-AMI deaths) was formed by 4 cases of cardiac failure, 6 cases of intracerebral haemorrhage, 5 cases of pulmonary embolism, 7 cases of asphyxia (4 hangings, 1 traumatic asphyxia, 2 drownings), 3 cases of brain contusion, 2 cases of carbon monoxide poisoning, 1 case of cyanide poisoning, 1 case of alcohol poisoning, 1 case of epilepsy, 1 case of bronchopneumonia, 1 case of hyperglycaemic coma and 1 case of exsanguination.
Results:
The values of creatine kinase and myoglobin were increased in all cases (100%) of AMI-deaths and non-AMI deaths in blood and pericardial fluid and there was no statistically significant difference between these groups. Troponin I was increased in all cases of both groups in pericardial fluid, and again there was no significant difference in concentration between both groups. In blood, troponin I was increased by 87% in the group of AMI deaths and in 91% of non-AMI deaths. There was no significant statistical difference between the groups and there was no difference in concentration either.
Conclusion:
We did not find a statistically significant difference in pericardial fluid or in serum for cTnI, CK-MB and myoglobin between the group of AMI deaths and the group of non-AMI deaths and we cannot confirm the diagnostic efficacy of these biochemical markers in the postmortem diagnosis of acute myocardial infarction.
Key words:
IHD – acute myocardial infarction – troponin I – myoglobin – creatine kinase
Introduction
Ischaemic heart disease (IHD) is a general term for illnesses which have in common ischaemia of the myocardium based on a pathological process in the coronary arteries. The term “ischaemia of the myocardium” has a broad range; it includes additional aspects when the inadequate oxygen supply has a noncoronary origin (for example reduction of the blood transport capacity for oxygen, depression of the blood pressure, pathologically increased metabolic consumption).
The coronary bed ensures a supply to the myocardium of metabolic substrates (the crucial one is the supply of oxygen) and the recruitment of metabolic waste. The contractility and relaxation of the myocardium depends on adequate coronary flow, reduction of contractility appears with the restriction of perfusion about 10–20%. (4) There is already a high arteriovenous difference (75%) in the coronary circulation under normal resting conditions (2) and so it is not possible to increase the release of oxygen markedly under load. The greater metabolic requirements must be covered by increased coronary flow.
The imbalance between metabolic necessity and perfusion possibilities could be caused by:
- increased requirements of the myocardium;
- ireduced perfusion of the coronary bed;
- a combination of increased requirements and reduced perfusion (the most common). (4)
IHD is a clinical term for situations with different aetiopathogenesis but with the same consequence – the critical reduction of the blood flow through the coronary system. The basis of the defect of perfusion can be organic (atherosclerotic lesion, thrombosis, embolism, arteritis, coronary fistula or dissection) or functional (coronary spasm). Most often we see a combination of various pathogenetic factors, usually atherosclerotic plaque complicated by thrombosis or spasm).
Clinical classification of IHD: (4)
- Acute
(unstable) forms
- unstable angina pectoris
- acute myocardial infarction
- sudden coronary death
- Chronic
(stable) forms
- angina pectoris
- unmanifested ischaemia of the myocardium
- X syndrome
- IHD manifested by insufficiency of the myocardium
- IHD manifested by cardiac arrhythmia
Acute infarction of the myocardium (MI) is the most important form of IHD. It is a locus of acute ischaemic necrosis of the myocardium caused in most cases by the thrombotic closure of an atherosclerotic coronary artery. This is the most common cause of death in industrialised countries). (5)
The primary reason for the onset of MI is:
- an unstable atherosclerotic plaque (that is a plaque with exposed subendothelium);
- an occluding thrombus;
- critical stenosis closing the lumen partially or completely;
- spasm of the coronary artery (ordinarily imposed on atherosclerotic plaque).
Secondarily there are factors which can contribute to the expansion of the focus of MI:
- deteriorating coronary circulation (hypotension, tachycardia, physical load, stress);
- reduced oxygen supply (hypoxia, anaemia);
- increasing metabolic requirements of the myocardium (hypertension, tachycardia).
However 6% of all patients and almost 25% of patients with MI younger than 35 years do not have any detectable signs of coronary atherosclerosis by coronarography or autopsy and they do not have any risk factors of atherosclerosis except for smoking (9). The reason for MI in these patients may be protracted spasm, exceptionally embolism of a coronary artery, sometimes inflammatory changes, trauma, post-thrombotic conditions, congenital anomaly of the coronary arteries, the acute onset of a disproportion between oxygen supply and consumption in the myocardium (CO poisoning, aortic stenosis, cocaine abuse) etc. (9).
The infarct of the myocardium can affect any part of the heart, an infarct in the left ventricle is the most often and clinically most consequential; infarct in the right ventricle appears together with lesions of the posterior wall; infarct in the atria is usually silent but it can manifest as faults of formation and conduction of impulses.
The cells of the myocardium can survive about 20–30 minutes with the ability to recover completely by restoring the oxygen supply after the closure of a coronary artery. The progression of necrosis from the endocardium to the epicardium and from the centre to the periphery of the coronary artery basin lasts 4–12 hours (usually about 6 hours, exceptionally 24 hours). The ability to contract of the ischaemic myocardial part disappears progressively for several seconds after total closure of a coronary artery. Hypokinesis (depression of contractions) is the least severe grade of lesion, akinesis is the more severe one (extinction of contractions) and dyskinesis is the most severe grade (extinction of contractions and passive paradoxical systolic bulge of the affected region).
According to the European Society of Cardiology and The American College of Cardiology, the basic clinical diagnostic criterion of acute MI (AMI) is the positivity of the biochemical markers of myocardial necrosis (9). Generally the relation between the activity of the enzymes and the amount of cellular damage is valid.
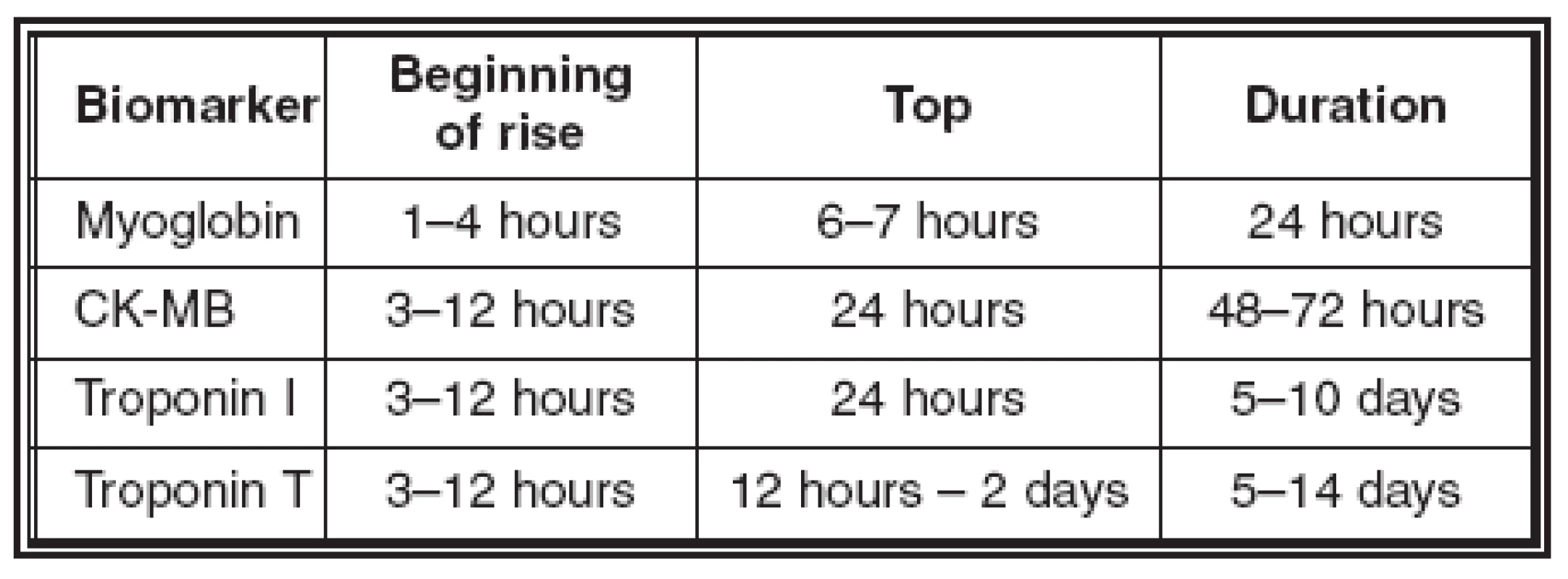
The determination of creatine-kinase (CK) activity and its specific myocardial fraction (CK-MB) is one of the most specific tests. The normal values of CK are 0.2–3.2 μkat/l, CK-MB up to 0.4 μkat/l. At least a double increase is required for the positive diagnosis of AMI. Problems with diagnosis can occur from simultaneous contusion of skeletal muscles (for example after cardiopulmonary resuscitation – CPR, cardioversion) or after prolonged exercise, from myopathy or hypothyroidism.
The next specific sign of necrosis is the rapid increase of the concentration of myoglobin and troponin in the serum. The increased value of myoglobin lasts for 1–2 days only, the value of troponin about 1 week. The normal value of myoglobin is 5–70 μg/l, but an increased value is not just specific for myocardial lesions, we can find it in lesions of the skeletal muscles and in renal insufficiency.
Troponins are regular proteins supervising calcium mediated interaction between actin and myosin. Cardiac forms of these proteins (cTnI a cTnT) are products of specific genes, which occur only in the heart. Their increase is mostly proof of “minimal cardiac necrosis”. Normal values of troponin T are up to 0.05 μg/l, of troponin I up to 0.5 μg/l. False positive results are found in dialysed patients with advanced renal failure. Almost 29% of these patients show a positive troponin T value without a simultaneous lesion of the myocardium. We do not see an increase of troponin I so often, but its value increases in patients with serious heart congestion, probably in connection with myofibrillar degeneration and in patients with hepatic cirrhosis with an alcoholic aetiology. Acute lesions of skeletal muscles do not have any influence on increasing values of troponins (Table 1, Graph 1).

The earliest macroscopically apparent pathologic-anatomical changes arise after 6 hours of ischaemia, usually even later. Non-specific changes (paleness, red and bluish cyanosis, “boiled look”, thinning of the left ventricular wall in the area of the infarct) are apparent usually after 12–18 hours. The completely developed yellow-coloured coagulative necrosis with haemorrhagic border can be seen after 3–5 days.
The focus of ischaemia becomes greyer at the end of the first week; this is caused by gradual substitution of necrotic muscle by granular tissue. Complete cicatricose transformation forms over 6–8 weeks, it depends on the size of the infarct.
The early diagnosis of myocardial infarct after 3–6 hours is possible by the use of histoenzymatic reaction (macroreaction) with nitro-blue-tetrazolium on cross-section of the ventricles of the heart; necrotic myocardium loses its enzymes (mainly dehydrogenases) and so the resulting colour of the necrotic tissue after the reaction is different in comparison with the healthy muscle.
It is possible to demonstrate the irreversible cellular changes in the myocardium 20 minutes after the onset of experimental ischaemia by using the electron-microscope. The electron-microscope is not usable in autopsy diagnosis because of post mortem autolysis of tissues.
The earliest, but not specific, changes of ischaemia are thinning, waving and stretching of the muscle fibres observed with a light microscope, which are caused by the traction of surrounding live musculature. After 12-24 hours, coagulative necrosis of myocardial cells can be seen – the plasma is becoming more eosinophilic and the nuclei are becoming pale (karyolysis) or shrunken and darker (pycnosis); in the end, the myocardial cells are changed into shrunken, eosinophilic anuclear formations without cross-striation. After 24 hours, an infiltrate of neutrophils occurs in the interstitium; the infiltrate is the most extensive after 2–3 days.
For 3–7 days there is disintegration of necrotic cells and macrophages can be seen in the infiltrate. Substitution of the necrotic myocardium by granular tissue (capillaries with endothelium, fibroblasts and sparse lymphocytes) begins on the 5th–7th day. The majority of the infarcted focus is substituted by about the 10th day. The granular tissue turns completely into stable collagenous cicatrix over the next 6 weeks. (5)
In most cases of interest to the forensic pathologist, the death is sudden and the morphological changes of infarction do not have time to develop. In these occasions the positive diagnosis of AMI is difficult. In such cases, complementary diagnostic techniques, such as the determination of biochemical markers (myoglobin, troponin I and CK-MB) in cadaver fluids, were suggested. (7, 3, 1, 6) We decided to test the diagnostic value of postmortem cTnI, myoglobin and creatine kinase determinations in serum and pericardial fluid.
Materials and methods
We studied 71 cadavers, which were divided into 2 groups. The first group (38 cases) was formed by people who died with acute myocardial infarction (the myocardial lesion could be easily detected by macroscopic examination or by formazan test and the constriction or obturation of the coronary arteries was detected and other causes of death were excluded) – AMI deaths. The second group (non-AMI deaths) was formed by 4 cases of cardiac failure, 6 cases of intracerebral haemorrhage, 5 cases of pulmonary embolism, 7 cases of asphyxia (4 hangings, 1 traumatic asphyxia, 2 drownings), 3 cases of brain contusion, 2 cases of carbon monoxide poisoning, 1 case of cyanide poisoning, 1 case of alcohol poisoning, 1 case of epilepsy, 1 case of bronchopneumonia, 1 case of hyperglycaemic coma and 1 case of exsanguination. The blood was collected from the femoral vein.
Results
- The average age of all the deceased was 58.4 years, the average age of the AMI group was 60.3 years.
- Women formed 26.8 % of all cases (but only one was in the AMI group).
- Cardiopulmonary resuscitation was pursued in 44.7% in the AMI group and in 12% in the non-AMI group. Other than post-resuscitation traumatic changes were found in 2.6% of the AMI group (i.e. haematomas, fracture of femur) and in 27% of the non-AMI group (i.e. brain contusion, chest contusion, many excoriations, extensive haematomas).
- The heart weighed more than 350 grams in 94.7% of the group of AMI deaths (average weight 452 grams), and in 82% of the non-AMI group (average weight 387 grams).
- Pulmonary oedema was found in 97.4 % of the AMI group and in 60.6 % in the group of non-AMI deaths.
- Alcohol was positive in 21% of the AMI group and in 30% of the non-AMI group.
- The formazan macroreaction test was positive in 3 cases of non-AMI deaths (alcohol intoxication, drowning, pulmonary embolism).
- There were macroscopically visible acute ischaemic changes in the heart in 68.4% and total atherothrombotic closure in 13% of the deceased in the AMI deaths group.
- Chronic ischaemic changes (i.e. myofibrosis) were macroscopically visible in 63% of cases in the AMI deaths group and in almost 58% of cases in the non-AMI deaths group.
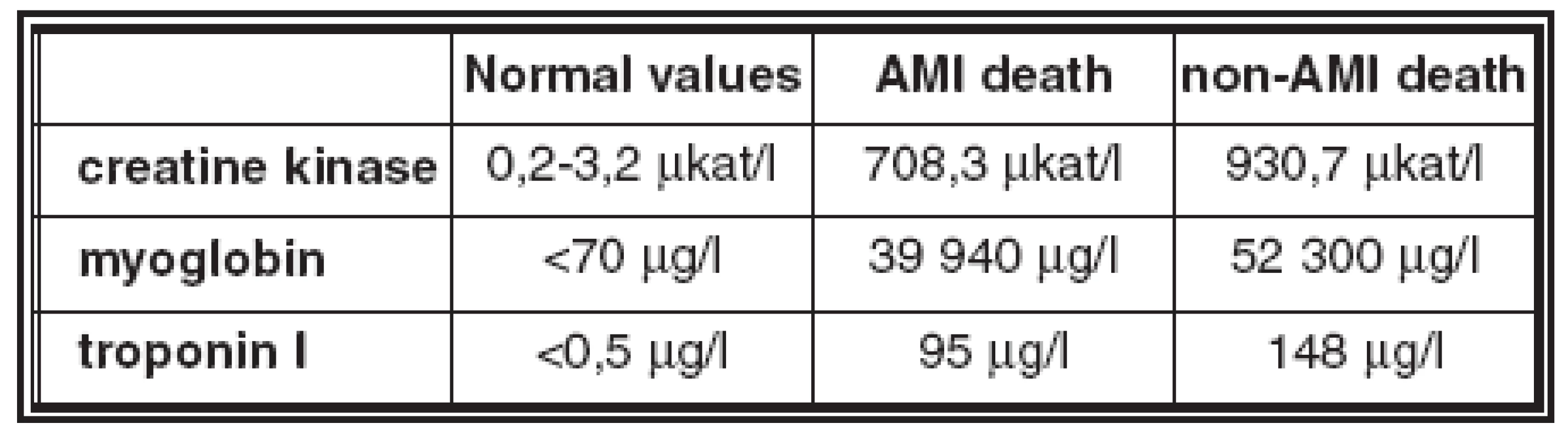
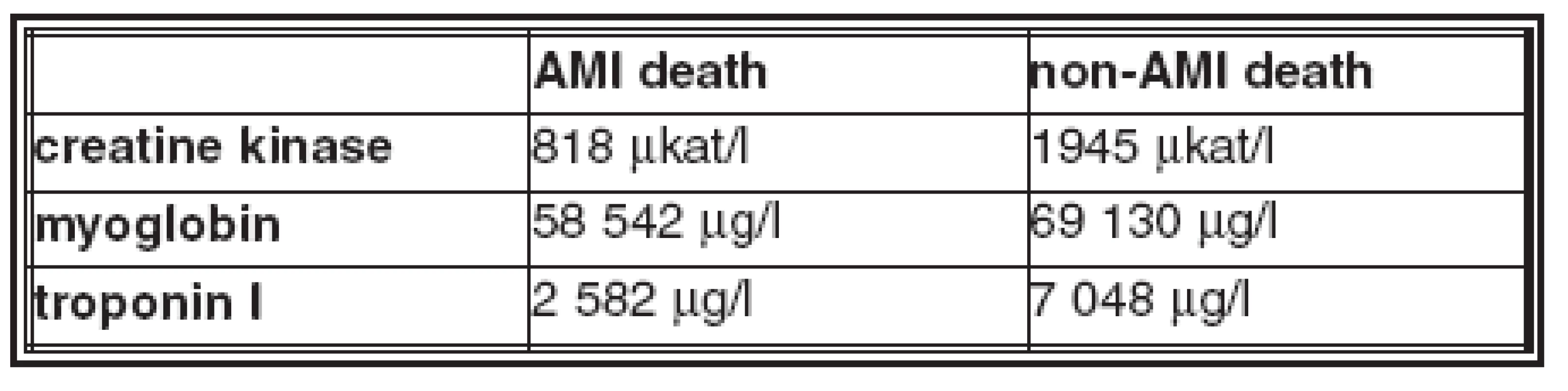
- The average values of biomarkers in blood and in pericardial fluid in the AMI deaths and non - AMI deaths groups are in Table 2 and Table 3.
- Creatine kinase and myoglobin were increased in blood and in pericardial fluid in all AMI and non-AMI deaths; there was no statistical difference in their concentrations between these groups.
- Troponin I was increased in pericardial fluid in all cases of both groups, there was no statistical difference in concentrations in these groups.
- Troponin I was increased in blood in 87% of AMI deaths and in 91% of non-AMI deaths; this difference is not statistically significant; there is no statistical difference in concentration in these groups.
Discussion
Several studies discussing the efficacy of the postmortem determination of biochemical markers in IHD and the possibility of using them in the diagnosis of acute myocardial infarction have been undertaken recently (7, 3, 1). Statistically significant differences in pericardial fluid for cTnI, CK-MB and myoglobin between myocardial infarction and other deaths were obtained (7), but in serum only cTnI showed statistically significant differences. The authors of this study recommended the use of CK-MB and cTnI in pericardial fluid in postmortem diagnosis as a factor with a high negative predictive value for AMI.
From the study (8) it can be concluded that blood is not a suitable medium for determination of biochemical markers of cTnI and atrial natriuretic peptide (pro-ANP) for postmortem diagnosis of myocardial damage and for determining the diagnosis of sudden cardiac death in a manner similar to the diagnosis of myocardial damage in living patients.
Similarly, in our study we did not find statistically significant differences in pericardial fluid and in serum for cTnI, CK and myoglobin between the group of AMI deaths and the group of non-AMI deaths and we cannot confirm the diagnostic efficacy of these biochemical markers in postmortem diagnosis of acute myocardial infarction. We cannot attribute the increased values of all biochemical markers in both groups to heart damage as a consequence of resuscitation. The proportion of resuscitation was higher in the AMI group (44%) in comparison to the non-AMI group.
Conclusion
In the evaluation of postmortem diagnosis of early myocardial infarction we should still rely on up to the present time well known methods - macroscopic examination, formazan test or histological evidence of acute infarction.
MUDr. František Vorel, CSc.
Oddělení soudního lékařství
Nemocnice České Budějovice, a.s.
B. Němcové 585/54
370 01 České Budějovice
Sources
1. Cina S. J., Li D. J, Chan D. W, Boitnott J. K., Hruban R. H., Smialek J. E: Serum concentrations of cardiac troponin I in sudden death, The American Journal of Forensic Medicine and Pathology 19(4), 1998, p. 324–328
2. Ganong F. W.: Přehled lékařské fyziologie, H&H, 1999, p. 476.
3. Hansen S. H., Rossen K.: Evaluation of cardiac troponin I immunoreaction in autopsy heart: a possible marker of early myocardial infarction, Forensic Science International 99 1999, p. 189-196.
4. Klener P. et al.: Vnitřní lékařství, Galén, 2001, p. 151–153.
5. Kolektiv autorů: Speciální patologie, Galén, 1999, p. 28-29.
6. Osuna E., Pérez-Cárceles M. D., Noguera J., Alvarez M. V., Noguera J., Luna A.: Cardiac troponin I (cTn I) and the postmortem diagnosis of myocardial infarction. Int J Legal Med 111, 1998, p. 173–176.
7. Pérez-Cárceles M. D., Noguera J., Jimenéz J. L., Martínez P., Luna A., Osuna E.: Diagnostic efficacy of biochemical markers in diagnostic post-mortem of ischemic heart disease, Forensic Science International 142, 2004, p. 1–7.
8. Šidlo J, Parrák V., Kvasnička P., Majdan M., Šidlová H.: On the use of biochemical markers in diagnostics of sudden cardiac death, The Czech-Slovak Journal of Pathology and Forensic medicine 2, 2008, p. 31–34
9. Špaček R., Widimský P.: Infarkt myokardu, Galén, 2003.
10. Štejfa M. a spol.: Kardiologie, Grada Publishing, 2007, str. 21‑24, str. 45–49.
Labels
Anatomical pathology Forensic medical examiner ToxicologyArticle was published in
Forensic Medicine

2010 Issue 3
Most read in this issue
- 3,5-Dimethoxyfenol – Marker Intoxication with Taxus baccata
- Mass Disasters with Higher Quantity of Dead Bodies and DVIT Concept in the Czech Republic
- Sexual Assault in the woman
- Některé možnosti diagnostiky časných akutních ischemických změn srdečního svalu u náhlých úmrtí
