Electrogastrography in experimental pigs
The power analysis
Elektrogastrografie u pokusných prasat
Analýza amplitud
Elektrogastrografie (EGG) je spolehlivá diagnostická metoda, která poskytuje informace o četnosti a relativní amplitudě kontrakcí antra žaludku pomocí elektrod umístěných na kůži. Cílem této studie bylo analyzovat poměr amplitud získaný při záznamu EGG. Do studie bylo zařazeno osm dospělých prasat. Záznam EGG byl proveden pomocí zařízení Digitrapper EGG (Synectics Medical AB, Stockholm, Švédsko). Experimenty byly provedeny v celkové anestezii. Provedli jsme tři samostatné záznamy každého zvířete. První bylo bazální měření EGG v celkové anestezii po 24hodinovém lačnění. Druhé bylo provedeno po podání 100 mg itopridu a třetí záznam byl po objemovém zatížení žaludku (360 ml vody na jedno zvíře). Každý záznam trval 30 min. Analýza amplitud neposkytla další zlepšení diferenciace mezi jednotlivými EGG vyšetřeními (základní, objemové zatížení a itoprid) ve srovnání s procentuálním vyjádřením dominantní frekvence. Motorický index žaludečního antra a index komplexní myoelektrické aktivity byly vzájemně v těsné korelaci. Ani motorický index žaludečního antra ani index komplexní myoelektrické aktivity však nejsou při konečném hodnocení jednotlivých EGG zásadně přínosné. Lze nalézt pouze nevýznamné tendence (bazální EGG měla nejvyšší hodnoty obou indexů). Dospěli jsme k závěru, že procentuální vyjádření dominantní frekvence (pomalých žaludečních vln) zůstává nejdůležitějším kritériem hodnocení EGG u experimentálních prasat.
Klíčová slova:
elektrogastrografie – experimentální prasata – dominantní frekvence – analýza amplitud – pomalé žaludeční vlny
Authors:
S. M. Ali 1; J. E. Varayil 1; I. Tachecí 1,2 1,2 1,2 1,2
Authors‘ workplace:
University Department of Gastroenterology, Charles University in Prague, Faculty of Medicine at Hradec Králové, Hradec Králové, Czech Republic, 2nd Department of Internal Medicine, Charles University in Prague, Faculty of Medicine at Hradec Králov
1; Institute of Experimental Biopharmaceutics, Joint Research Centre of Czech Academy of Sciences and PRO. MED. CS Prague a. s., Hradec Králové, Czech Republic
3
Published in:
Gastroent Hepatol 2011; 65(6): 325-330
Category:
Clinical and Experimental Gastroenterology: Original Article
Overview
Electrogastrography (EGG) is a reliable diagnostic technique providing information on the frequency and relative amplitude of antral contractions of stomach using cutaneous electrodes. The aim of this study was to analyze the power ratio obtained during the EGG recording. Eight mature pigs entered our study. EGG was recorded using a Digitrapper EGG equipment (Synectics Medical AB, Stockholm, Sweden). The experiment was carried out under general anaesthesia. We performed three separate recordings in each animal. First was a baseline measurement of EGG under general anaesthesia, after 24 hours of fasting. The second was after administration of 100 mg itopride and the third recording was after intragastric volume challenge (360 mL of water per animal). Each recording lasted for 30 minutes. Power analysis did not provide further improvement of differentiation between particular EGG tests (basic, volume challenge and itopride) compared to the running spectrum percent activity of amplitude. The antral motor index and complex myoelectrical activity index correlate closely with each other. However, neither antral motor index nor complex myoelectrical activity index are essentially helpful in final evaluation of particular EGGs. Only non-significant trends could be found (basic EGG had the highest values of both indices). We conclude that running spectrum percent activity (dominant frequencies of gastric slow waves) remains the most important criterion for EGG evaluation in experimental pigs.
Key words:
electrogastrography – experimental pigs – running spectrum percent activity – power analysis – gastric slow waves
Electrogastrography (EGG) is a reliable diagnostic technique used for recording gastric myoelectrical activity using cutaneous electrodes placed on the anterior abdominal wall overlying the stomach. Electrogastrography provides surrogate information on the frequency and relative amplitude of antral contractions [1,2]. These activities originate from the gastric pacemaker located in the mid portion of the stomach which fires at three cycles per min (cpm) with aboral propagation of spike bursts to the pylorus in humans [1,3]. Since its first application in the 1920s [4] numerous studies have demonstrated a close association of cutaneously monitored electric abnormalities with gastrointestinal motility disorders [1,3,5–19]. Many studies have shown that the EGG is an accurate measurement of the gastric slow wave frequency, and that the relative change in amplitude of the EGG signals might reflect the contractility of the stomach [2,3,8,16].
It is now well established that neuromuscular activities of the stomach generate electrical phenomena termed “gastric slow waves” [3,20]. It is also believed that the interstitial cells of Cajal originate the rhythmic depolarization of the gastric slow wave. Under quiescent conditions, slow waves partially depolarize gastric smooth muscle but do not cause contraction. Additional depolarization provided by neurohumoral stimulation is the trigger for phasic gastric contractions which follow the spread of the electrical slow waves and can result in peristalsis. Thus, gastric electrical slow waves control the maximal frequency and the direction of contractions in the distal stomach [1,21,22].
The major parameters of EGG and gastric emptying measures (e.g. half-life of elimination in 13C-octanoic acid breath test) mutually correlate in healthy humans [23]. EGG has been advocated as a diagnostic test for the clinical evaluation of patients with unexplained nausea, vomiting and other dyspeptic symptoms to gain insight into mechanisms of symptom generation [10,14,18,19,24].
Spectral analysis of the signal from EGG identifies two components of the surface electrical signal: frequency and power which is related to the amplitude of the signal [25]. Numerous studies have demonstrated that frequency is related to the gastric slow waves recorded by serosal or mucosal electrodes [13,15,26]. Conversely, the physiological significance of the power (or amplitude) and its fluctuations has not been clearly established. The increase in the amplitude of the signal observed after a meal may be due to increased contractile activity or to gastric distension [8,26].
Even though there are distinct differences between the anatomy and physiology of humans and pig [27,28], they are a very close representative in relation to gastro-intestinal functions. The shape of porcine stomach is like a pouch and the gastric cardia is close to the pylorus and they have a special transverse pyloric fold called torus pyloricus which controls the flow of contents into the duodenum. Significant amount of food remnant is found in the porcine stomach even after 36–48 hours of fasting showing that the emptying is much slower than that in humans [28].
To the best of our knowledge, there are no previous reports on EGG in pigs in available literature. Our group set up and worked out the methods for EGG in experimental pigs recently [22]. The aim of this study was to evaluate power analysis of EGG recording in experimental pigs and to compare its diagnostic yield with the running spectrum analysis (dominant frequencies of gastric slow waves) under experimental conditions.
Material and methods
Study subjects
Our study was performed on eight mature female pigs. They were 4–5 months old and weighed 30.5 ± 2.6 kg. All the animals were fed twice a day on standard diet (assorted food A1) and were allowed access to water ad libitum. The animals entered the study were after 24 hours of fasting. Sus scrofa f. domestica, the hybrid of Czech white and Landrace breeds where proved to be suitable for the experiment from our previous study.
Study protocol
The experiment was carried out under general anaesthesia. Intramuscular injection of ketamine (20 mg per kg; Narkamon, Spofa Praha, Czech Republic) was used at the beginning. A repeated dose of the drug was administered intramuscularly as and when required for maintaining the sedation to avoid any kind of motion artefacts. All the animals were in a right lateral position during the experiment. The epigastric area was shaved before application of electrodes to decrease impedance in signal conduction through the skin. Electrodes were placed at a similar site in all the animals to avoid positional errors. The first electrode was placed within 5 cm of the processus xiphoideus in the centre; the other two were then placed at a distance of 10 cm from the central electrode on left and right hypochondria.
The Project was approved by the Institutional Review Board of Animal Care Committee of the Institute of Experimental Biopharmaceutics, Academy of Sciences of the Czech Republic, Record Number 1492006.
Recording of gastric myoelectric activity
EGG was recorded using a Digitrapper EGG (Synectics Medical AB, Stockholm, Sweden).The EGG system comprises a portable unit for electronic recording, a pair of recording electrodes along with a reference electrode, all connected to the portable recording unit. In each of the eight animals, we performed three separate recordings. First was a baseline measurement under general anaesthesia, after 24 hours of fasting. Then we administered of 100 mg itopride, a prokinetic drug (Ganaton, Abbott Laboratories) and the second measurement was done. The third recording was immediately after intragastric volume challenge (360 mL of water per animal). Each recording lasted for 30 minutes.
During the whole experiment, the animals were closely monitored for any movement or muscle tremor. If there were signs of movement like limb shaking or restlessness, the measurement was paused and the necessary steps were taken to calm the animal down, notes were made to identify these artefacts and their subsequent removal during analysis. In the case of any such unforeseen event, intramuscular bolus of anaesthesia was administered. Our experiment was carried out in a peaceful manner, barring two events.
The amplitude of stomach slow wave is very weak, while many visceral organs also produce rhythmic electric signals, like small and large intestine, heartbeat, respiration, and even body movements. Thus noise other than the slow wave had to be carefully monitored for and removed. The best signal among all recordings was selected to compute EGG parameters based on spectral analysis. Prior to subjecting the visually recognizable waveforms to computer analysis, periods which exhibit clear artefacts were identified and excised. Motion artefacts, if included in the quantitative computer software, may lead to erroneous determinations. Obtained EGG parameters include dominant frequency, power, % normal rhythm, % bradygastria, % tachygastria, instability coefficient and power ratio. Power analysis was based on the measurement of height of all amplitudes obtained at each EGG recording independently.
Along with the evaluation of power analysis, we also calculated the antral motor index (AMI) in all our animals using the EGG readings [29]. The antral motor index is defined as the sum of height of all waves × number of waves at a single EGG recording. To get comparable results, we exclude all the artefact intervals before calculating the AMI. We have worked out our own new parameter – the complex myoelectrical activity index (the Varayil index). This was calculated as the sum (of height of all waves × number of waves at a single EGG recording) divided by total time of assessable recording (in seconds).
Statistical analysis
The data obtained after removing the visible artefacts were statistically treated. We performed descriptive statistics, Mann-Whitney rank sum test and paired t test. Because of great inter-individual variability in power in particular animals, power analyses were referred to the mean values of power at basic EGG recording and expressed in percentage.
Results
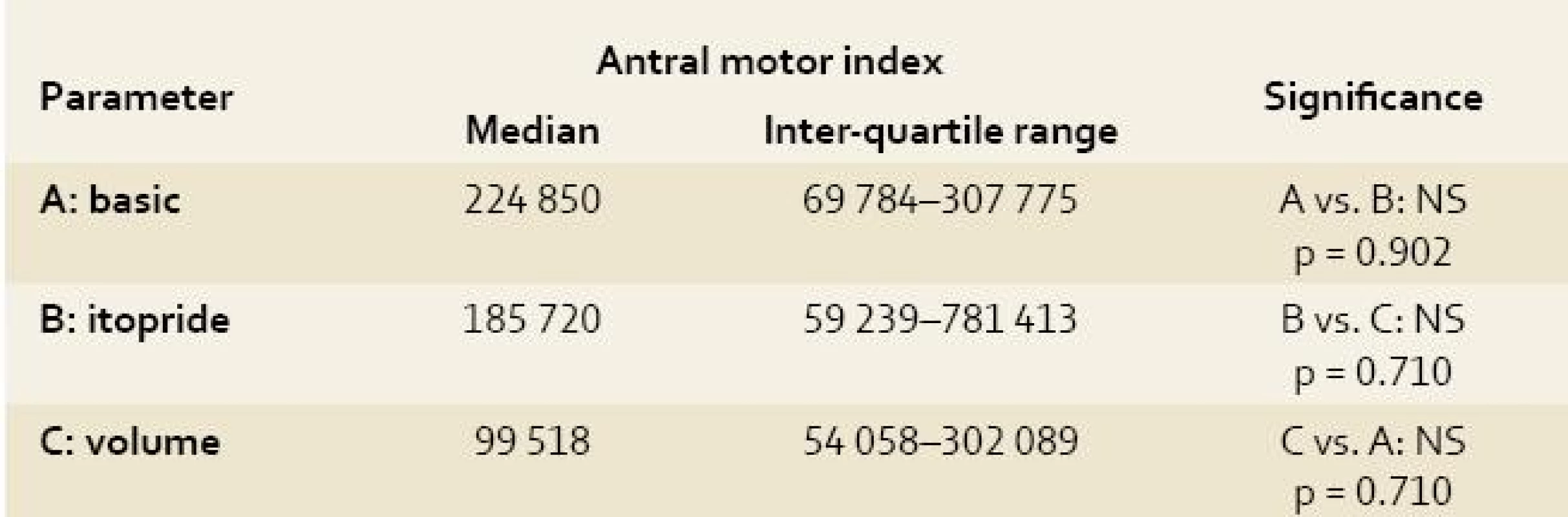
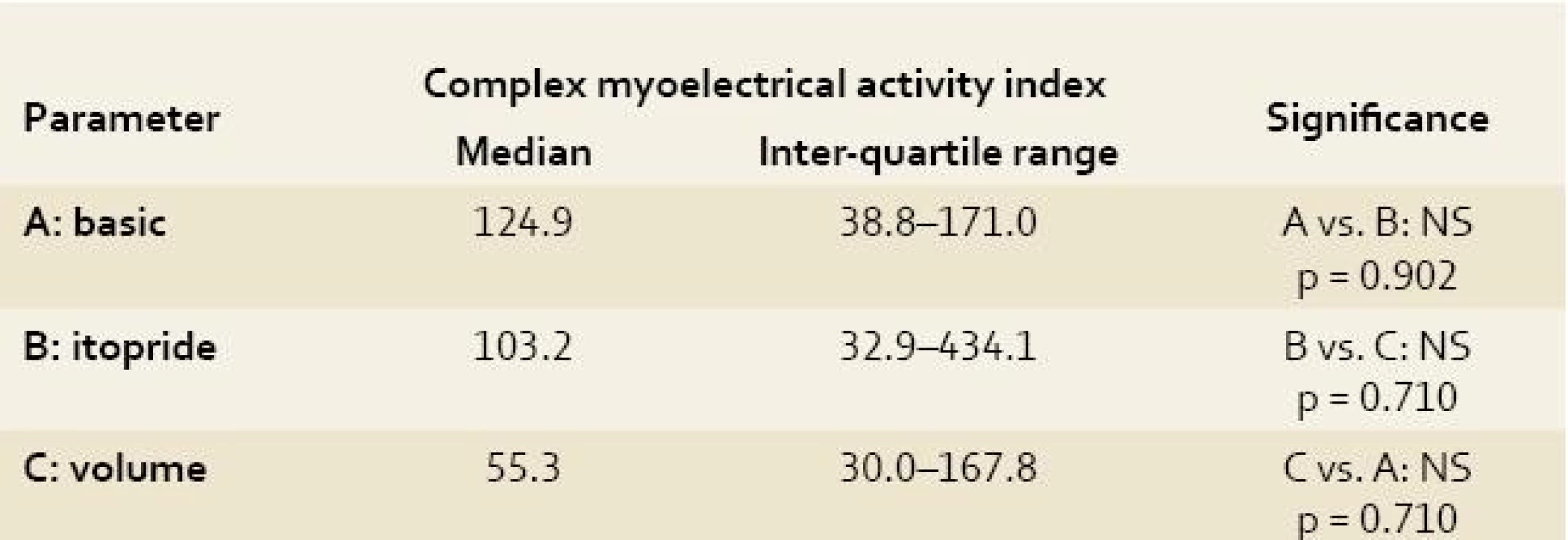
All obtained EGG recordings in all animals were fully relevant and assessable Power analyses of the EGG recordings were feasible. Both indices (AMI and CMAI) were calculated in all measurements. Results are summarised in Tables 1–3. There was no significant difference using power analysis, AMI and CMAI between three measurements (basic EGG recording, EGG after itopride administration and EGG after volume challenge).
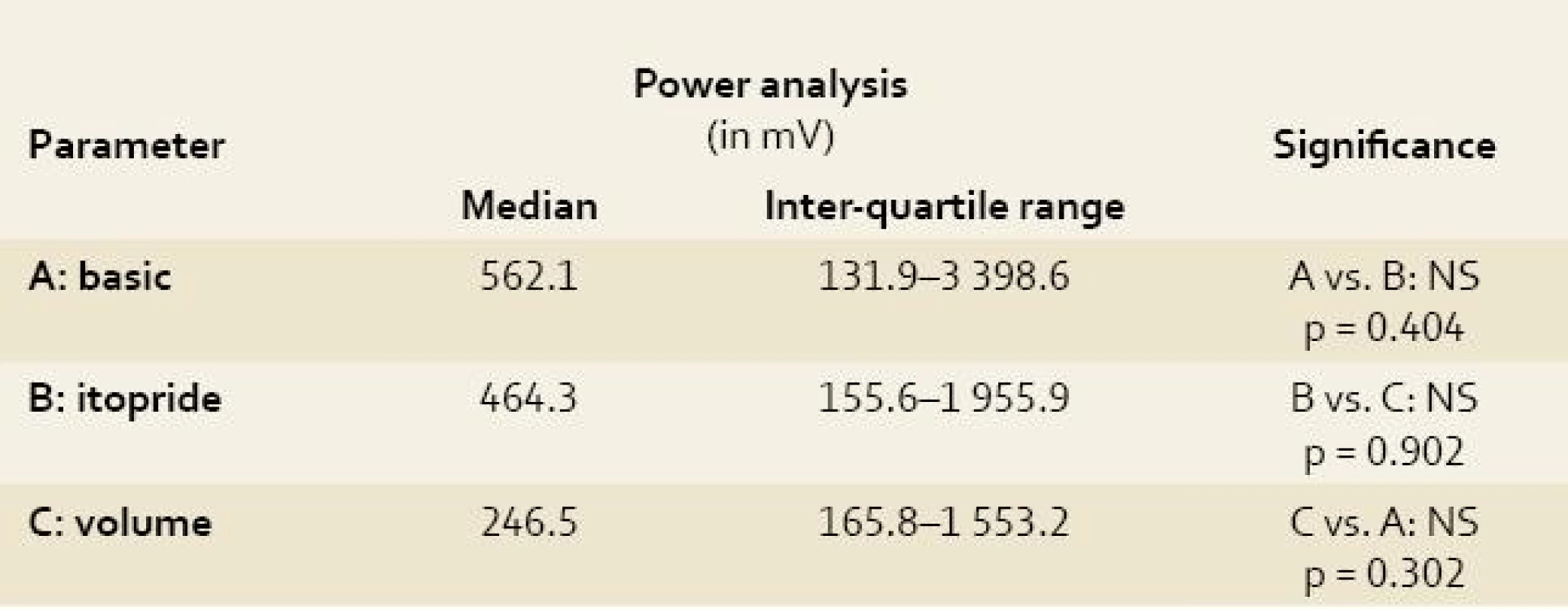
Because of great inter-individual variability in power in particular animals, power analyses after itopride and volume challenge were referred to the mean values of power at basic EGG recording and expressed in percent. Difference in delta were not significant: group A (basic recording) vs. group B (EGG after itopride administration): delta plus 2% (p = 0.710); group A vs. group C (EGG after volume challenge): delta minus 38.5% (p = 0.209); group B vs. group C: delta minus 14.2% (p = 0.572).
The antral motor index and complex myoelectrical activity index correlate closely with each other (r = 1.0; p < 0.00001), however they did not correlate with the running spectrum percent activity (dominant frequencies of gastric slow waves). Only non-significant trends could be found (basic EGG had the highest values of both indices).
Discussion
Our initial experiences of EGG with experimental animals were successful. In our previous trial, we demonstrated that EGG in experimental pigs is feasible. Analyses of dominant frequencies were comparable to the normal frequencies in humans [22]. Thus EGG in experimental pigs is a suitable model for further preclinical studies [30,31].
The aim of this project was to appraise if power analysis and motor indices could further improve the evaluation of EGG recording and thus make its diagnostic yield more precise. Based on our current results, these tools failed to provide any additional discriminating information (to the “standard” running spectrum percent activity).
In humans, there is an increase of power ratio after water ingestion (gastric power is calculated by numeral integration of the power spectrum density curve; given in μV2) [32]. We decide another approach in our experimental setting. To be able to compare the power of control group (basal recording on fasting condition with no challenging action) with those after itopride and water administration, we evaluated the height of all amplitudes obtained at particular EGG recordings (in mV). Surprisingly, we got a trend (albeit a non-significant one) of decreased power after itopride and water challenge compared to controls. This phenomenon should be further clarified. It is questionable if it can be at least partly explained by different anatomy and physiology of the porcine stomach compared to humans [28].
Lack of standardized methodology in terms of electrode positions, recording periods, test meals, analytic software and normal reference values makes the significance of EGG recording controversial [22,24,34].
Dominant power analysis has also other limitations. Even though the dominant frequencies are not affected much with the positioning of the electrodes, minor changes can have a huge effect on the determination of the dominant powers [16,20,32,35]. We are aware that there can be changes in the power data due to this.
All our recordings were performed under general anaesthesia in pigs; otherwise EGG would not be practical. In humans anaesthesia have significant effect on the myoelectrical activity of the stomach, so the measurement may be influenced and affected by the anaesthesia administered [22].
Many obstacles are required to be overcome when EGG recording system is used to evaluate the stomach dysmotility [2,36,37]. First, the amplitude of surface-recorded slow waves is very weak, in the range of 50–500 µV, compared to cardiac myoelectricity. Consequently, an amplifier is needed when this very low voltage signal is acquired by the EGG system. Second, the abdominal cavity is not silent because many visceral organs also produce rhythmic electric signals, for example heartbeat, respiration, other organs of the gastrointestinal tract and even body movements and success of the future studies also depends on how much these can be from stomach slow waves [1,8,11,17,26,32,33,36–38].
Conclusions
Power analysis and motor indices did not provide further improvement of differentiation between particular EGG tests (basic, volume challenge, itopride) compared to running spectrum percent activity (dominant frequencies of gastric slow waves).
The antral motor index and complex myoelectrical activity index (Varayil index) correlate closely with each other. However, neither antral motor index nor complex myoelectrical activity index (Varayil index) are essentially helpful in final evaluation of particular EGGs. Only non-significant trends could be found (basic EGG had the highest values of both indices).
For our further studies, more adjusted placement of electrodes should be used for each animal (to find out the highest possible amplitudes) rather than the standard placement used now before the start of EGG recording. We conclude that running spectrum percent activity (dominant frequencies of gastric slow waves) remains the most important criterion for EGG evaluation.
Acknowledgement
The study was supported by research project MZO 00179906 from the Ministry of Health of the Czech Republic and by research grant GAČR 305/080535, Czech Republic.
Autoři deklarují, že v souvislosti s předmětem studie nemají žádné komerční zájmy.
The authors declare they have no potential conflicts of interest concerning drugs, products, or services used in the study.
Redakční rada potvrzuje, že rukopis práce splnil ICMJE kritéria pro publikace zasílané do biomedicínských časopisů.
The Editorial Board declares that the manuscript met the ICMJE „uniform requirements“ for biomedical papers.
Doručeno/Submitted: 26. 9. 2011
Přijato/Accepted: 16. 10. 2011
Professor Jan Bureš, MD, PhD
2nd Department of Medicine Charles University Teaching Hospital, Sokolská 581500 05 Hradec Králové, Czech Republic
bures@lfhk.cuni.cz
Sources
1. Chen JZ, McCallum RW. Electrogastrographic parameters and their clinical significance. In: Chen JD, McCallum RW (Eds.). Electrogastrography: Principles and applications. New York: Raven Press 1994.
2. Smout AJPM, van der Schee EJ, Grashuis JL. What is measured in electrogastrography? Dig Dis Sci 1980; 25(3): 179–187.
3. Chen JD, Pan J, McCallum RW. Clinical significance of gastric myoelectrical dysrhythmias. Dig Dis 1995; 13(5): 275–290.
4. Alverez WC. The electrogastrogram and what it shows. J Am Med Assoc 1922; 78 : 1116–1119.
5. Bartolotti M, Sarti P, Barbara L. Gastric myoelectrical activity in patients with chronic idiopathic gastroparesis. J Gastrointest Motil 1990; 2 : 104–108.
6. Bures J, Kabelác K, Kopacova M et al. Electrogastrography in patients with Roux-en-Y reconstruction after previous Billroth gastrectomy. Hepatogastroenterology 2008; 55(85): 1492–1496.
7. Camilleri M, Malagelada JR. Abnormal intestinal motility in diabetics with gastroparesis syndrome. Eur J Clin Invest 1984; 14(6): 420–427.
8. Chen JZ, McCallum RW. Clinical applications of electrogastrography. Am J Gastroenterol 1993; 88(9): 1324–1336.
9. Debinski HS, Ahmed S, Milla PJ et al. Electrogastrography in chronic intestinal pseudoobstruction. Dig Dis Sci 1996; 41(7): 1292–1297.
10. Geldof H, van der Schee EJ, van Blankenstein M et al. Electrogastrographic study of gastric myoelectrical activity in patients with unexplained nausea and vomiting. Gut 1986; 27(7): 799–808.
11. Guo J-P, Bonapace ES, Parkman HP et al. The value of the second postprandial hour during electrogastrography (EGG) in symptomatic patients with and without gastroparesis (Abstract). Dig Dis Sci 1999; 44: 2150.
12. Hasler WL, Soudah HC, Dulai G et al. Mediation of hyperglycemia-evoked gastric slow wave dysrhythmias by endogenous prostaglandin. Gastroenterology 1995; 108(3): 727–736.
13. Koch KL, Stern RM, Stewart WR et al. Gastric emptying and gastric myoelectrical activity in patients with diabetic gastroparesis: effect of long-term domperidone treatment. Am J Gastroenterol 1989; 84(9): 1069–1075.
14. Leung MW, Wong BP, Chao NS et al. Electrogastrography in the management of pediatric functional dyspepsia and motility disorder. J Pediatr Surg 2006; 41(12): 2069–2072.
15. Lin Z, Chen JDZ, Schirmer BD et al. Postprandial response of gastric slow waves: correlation of serosal recordings with the electrogastrogram. Dig Dis Sci 2000; 45(4): 645–651.
16. Mantides A, Stefanides G, Kioulanis J et al. Cutaneous electrogastrography for the assessment of gastric myoelectrical activity in type I diabetes mellitus. Am J Gastroenterol 1997; 92(7): 1190–1193.
17. Rague BW, Oman CM. Use of a microcomputer system for running spectral analysis of EGG’s to predict the onset of motion sickness. Proc IEEE 9th Annual Conf of Eng in Med and Biol Society; 1987 : 87–90.
18. Riezzo G, Cucchiari S, Chiloiro M et al. Gastric emptying and myoelectrical activity in children with nonulcer dyspepsia effect of cisapride. Dig Dis Sci 1995; 40(7): 1428–1434.
19. Talley NJ, Stanghellini V, Heading RC et al. Functional gastroduodenal disorders. Gut 1999; 45 (Suppl 2): II37–II42.
20. Abell TL, Malagelada J-R. Electrogastrography: current assessment and future perspectives. Dig Dis Sci 1988; 33(8): 982–992.
21. Smout AJPM, Jebbink HJA, Samson M. Acquisition and analysis of electrographic data. The Dutch experience. In: Chen JZ, McCallum RW (Eds.). Electrogastrography: Principles and applications. New York: Raven Press 1994.
22. Varayil JE, Ali SM, Tachecí I et al. Electrogastrography in experimental pigs. Methodical design and initial experience. Folia Gastroenterol Hepatol 2009; 7 : 98–104.
23. Bureš J, Kopáčová M, Voříšek V et al. Correlation of electrogastrography and gastric emptying rate estimated by 13C-octanoic acid breath test in healthy volunteers. Folia Gastroenterol Hepatol 2007; 5 : 5–11.
24. Zhang H, Xu X, Wang Z et al. Correlation between gastric myoelectrical activity recorded by multi-channel electrogastrography and gastric emptying in patients with functional dyspepsia. Scand J Gastroenterol 2006; 41(7): 797–804.
25. van der Schee ET, Grashuis GL. Contraction-related, low frequence components in canine electrogastrographic signals. Am J Physiol 1983; 245(4): G470–G475.
26. Sha W, Pankaj JP, Chen DZ. Correlations among Electrogastrograms, Gastric Dysmotility, and Duodenal Dysmotility in Patients with Functional Dyspepsia. J Clin Gastroenterol 2009; 43(8): 716–722.
27. Bures J, Kopácová M, Kvetina J et al. Different solutions used for submucosal injection influenced early healing of gastric endoscopic mucosal resection in a preclinical study in experimental pigs. Surg Endosc 2009; 23(9): 2094–2101.
28. Kopáčová M, Tachecí I, Květina J et al. Wireless video capsule enteroscopy in preclinical studies: Methodical design of its applicability in experimental pigs. Dig Dis Sci 2010; 55(3): 626–630.
29. Faure C, Wolff VP, Navarro J. Effect of meals and intravenous erythromycin on manometric and electrogastrographic measurements of gastric motor and electrical activity. Dig Dis Sci 2000; 45(3): 525–528.
30. Květina J, Varayil JE, Ali SM et al. Preclinical electrogastrography in experimental pigs. Interdiscip Toxicol 2010; 3(2): 53–58.
31. Tachecí I, Květina J, Kuneš J et al. Electrogastrography in experimental pigs: the influence of gastrointestinal injury induced by dextran sodium sulphate on porcine gastric erythromycin-stimulated myoelectric activity. Neuro Endocrinol Lett 2011; 32 (Suppl 1): 101–105.
32. Shimada Y, Watanabe M, Shibahara N et al. Electrogastrographic power ratio in humans is not related to changes in antrum-skin distance but to antral motility. J Gastroenterol 1998; 33(3): 310–317.
33. de Sobral Cintra RJ, Tchervensky IV, Dimitrov VS et al. Optimal wavelets for electrogastrography. Conf Proc IEEE Eng Med Biol Soc 2004; 1 : 329–332.
34. Hamilton JW, Bellahsene BE, Reicherlderfer M et al. Human electrogastrograms – comparison of surface and mucosal recordings. Dig Dis Sci 1986; 31(1): 33–39.
35. Abell TL, Malagelada JR. Glucagon-evoked gastric dysrhythmias in human shown by and improved electrogastrographic technique. Gastroenterology 1985; 88(6): 1932–1940.
36. Abell TL, Malagelada J-R, Lucas AR et al. Gastric electromechanical and neurohormonal function in anorexia nervosa. Gastroenterology 1987; 93(5): 958–965.
37. Bellahsene BE, Hamilton JW, Webster JG et al. An improved method for recording and analyzing the electrical activity of the human stomach. IEEE Trans Biomed Eng 1985; 32(11): 911–915.
38. Sanaka MR, Xing JH, Soffer EE. The effect of body posture on electrogastrogram (Abstract). Am J Gastroenterol 2001; 96: S73.
Labels
Paediatric gastroenterology Gastroenterology and hepatology SurgeryArticle was published in
Gastroenterology and Hepatology
2011 Issue 6
- Possibilities of Using Metamizole in the Treatment of Acute Primary Headaches
- Metamizole at a Glance and in Practice – Effective Non-Opioid Analgesic for All Ages
- Metamizole vs. Tramadol in Postoperative Analgesia
- Spasmolytic Effect of Metamizole
- The Importance of Limosilactobacillus reuteri in Administration to Diabetics with Gingivitis
-
All articles in this issue
- November 17th 1989 and today
-
Interview with Prof. MUDr. Julius Špičák, CSc.,
Chairman of the Czech Society of Gastroenterology at the 32nd Czech and Slovak Gastroenterological Congress in Brno (3.–5. 11. 2011) - Chronic radiation proctitis – does argon plasma coagulation (APC) resolve this therapeutic problem?
- Proton-pump inhibitors – up to date
- From drainage of the cystoid to pancreatic cancer
- Virtual simulator for digestive endoscopy
- 20th Congress of the Gastroenterology Society of Central Germany in Magdeburg from 12 to 14 May 2011
- The young Czech researcher, hepatologist MUDr. Jan Petrášek, Ph.D., has been awarded the prestigious Česká hlava prize for exceptional scientific discovery
- We regret to announce the passing of MUDr. Milan Kaláb, CSc., on 29. 11. 2011 at the age of 66
-
Electrogastrography in experimental pigs
The power analysis
- Gastroenterology and Hepatology
- Journal archive
- Current issue
- About the journal
Most read in this issue
- Chronic radiation proctitis – does argon plasma coagulation (APC) resolve this therapeutic problem?
- Proton-pump inhibitors – up to date
- From drainage of the cystoid to pancreatic cancer
- Virtual simulator for digestive endoscopy
