Tofacitinib induction efficacy and safety in ulcerative colitis at week 8 – results from clinical practice
Účinnost a bezpečnost tofacitinibu u ulcerózní kolitidy v týdnu 8 – výsledky z klinické praxe
Úvod: Tofacitinib je léčivo ze skupiny inhibitorů Janusovy kinázy v perorální formě, které je určeno pro léčbu ulcerózní kolitidy (UC – ulcerative colitis). Účinnost přípravku byla prokázána v registračních studiích, avšak data o účinnosti a bezpečnosti z klinické praxe jsou dosud zřídkavá. Cílem této práce bylo posoudit klinickou odpověď na 8týdenní indukční léčbu tofacitinibem u pacientů s UC.
Metodika: Do hodnocení byli zařazeni konsekutivní pacienti, u kterých byla zahájena léčba tofacitinibem v dávce 2 × 10 mg denně. Aktivita onemocnění byla posuzována indexem aktivity Mayo vč. endoskopického subskóre na počátku léčby a v týdnu 8, spolu s hodnocením zánětlivých parametrů v podobě C-reaktivního proteinu (CRP) a fekálního kalprotektinu (FC – fecal calprotectin). Jako odpovídající na léčbu byli v týdnu 8 hodnoceni pacienti s celkovým indexem Mayo 0–5 spolu s endoskopickým subskóre 0–1. Při každé návštěvě byly hodnoceny nežádoucí účinky léčby.
Výsledky: Hodnocení proběhlo u 24 pacientů (41,7 % muži, 58,3 % ženy) o průměrném stáří 35,3 ± 11,8 let. Průměrná doba trvání choroby byla 8,3 ± 5,2 let. Průměrný pacient souboru byl v minulosti léčen dvěma biologiky, nicméně 25 % bylo zcela naivních k biologické léčbě. V týdnu 0 byla souběžná léčba kortikoidy přítomna u 41,7 % pacientů, jiná imunosupresvní nebo biologická léčba podávána nebyla. Po 8 týdnech odpovědělo na léčbu 52,9 % pacientů. Celkové Mayo skóre pokleslo u respondérů z hodnoty 5,9 ± 3,5 na 1,1 ± 1,3 (p = 0,01), zatímco u nonrespondérů došlo k nesignifikantní změně z 8,0 ± 2,5 na 8,9 ± 2,1 (p = 0,86). Zlepšení endoskopického skóre z 2,0 ± 1,0 na 0,6 ± 0,7 (p = 0,02) u respondérů kontrastuje se zcela nezměněnou hodnotou u pacientů bez odpovědi (2,9). Významný pokles hodnoth CRP (6,7 ± 6,2 vs. 2,0 ± 2,2 mg/l; p = 0,04) a FC (1 195 ± 1 189 vs. 578 ± 654 μg/g; p = 0,05) byl zaznamenán rovněž pouze v kohortě s klinickou odpovědí. Všechny hodnocené parametry v týdnu 0 byly v obou kohortách srovnatelné, s výjimkou triacylglycerolů, jejichž vstupní hodnota byla u nonrespondérů vyšší. Léčba byla do 8 týdnů ukončena u 23,5 % pacientů, u všech z důvodu nedostatečné odpovědi. Byly zaznamenány dvě stížnosti na bolesti hlavy po zahájení léčby a po jednom výskytu cytomegalovirové kolitidy, klostridiové kolitidy a orální kandidózy.
Závěr: Účinnost indukční fáze léčby tofacitinibem byla pozorována přibližně u poloviny pacinetů s UC. S ohledem na omezený vzorek pacientů a délku terapie nutno získat dlouhodobá data o účinnosti a bezpečnosti léku.
Klíčová slova:
tofacitinib citrát – ulcerózní kolitida – JAK inhibitory
Authors:
Kolář M. 1; Lukáš M. 1; Malíčková K. 1,2; Procházková L. 1; Bortlík M. 1,3,4; Ďuricová D. 1; Hrubá V. 1; Machková N. 1; Mitrová K. 1,5; Vašátko M. 1; Lukáš M. 1,2
Authors‘ workplace:
IBD clinical and research centre ISCARE a. s., Prague, Czech Republic
1; Institute of Medical Biochemistry and Laboratory Medicine, General University Hospital and 1st Faculty of Medicine, Charles University, Prague, Czech Republic
2; Department of Internal Medicine, 1st Faculty of Medicine, Charles University and Military University Hospital, Prague, Czech Republic
3; Institute of Pharmacology, 1st Faculty of Medicine, Charles University, Prague, Czech Republic
4; Department of Paediatrics, 2nd Faculty of Medicine, Charles University and University Hospital Motol, Prague, Czech Republic
5
Published in:
Gastroent Hepatol 2020; 74(1): 28-34
Category:
doi:
https://doi.org/10.14735/amgh202028
Overview
Introduction: Tofacitinib is an oral Janus kinase inhibitor approved for the treatment of ulcerative colitis (UC). Its efficiency was proven in registration trials, however data from real clinical practice are still sparse. Our aim was to evaluate efficacy and safety of tofacitinib in UC patients within 8-week induction period.
Methods: Data from consecutive UC patients who started tofacitinib 10 mg twice a day were evaluated. Disease activity was assessed by Mayo score including endoscopic Mayo at baseline and week 8 together with C-reactive protein (CRP) and fecal calprotectin (FC). At week 8, patients with total Mayo ≤5 with endoscopic subscore ≤ 1 were considered responders. Adverse events were registered at each visit.
Results: A total of 24 patients (41.7% males), mean age 35.3 ± 11.8 years were included. The mean disease duration was 8.3 ± 5.2 years. In median, the patients were previously treated with two biologic agents, however 25% of the patients were naïve to any biologic therapy. Systemic corticosteroids were present in 41.7% of patients at baseline and no patient had concomitant biologic or immunosuppressive therapy. At week 8, 52.9% of patients responded to treatment. The mean total Mayo decreased in responders from 5.9 ± 3.5 to 1.1 ± 1.3 (p = 0.01), while non-responders it changed from 8.0 ± 2.5 to 8.9 ± 2.1 (p = 0.86). Endoscopic subscore decreased from 2.0 ± 1.0 to 0.6 ± 0.7 (p = 0.02) in responders, however remained stable in non-responders (2.9). CRP and FC dropped significantly in responders (6.7 ± 6.2 vs. 2.0 ± 2.2 mg/L, p = 0.04; 1,195 ± 1,189 vs. 578 ± 654 μg/g, p = 0.05), but not in non-responders. Non-responders had significantly higher baseline triglycerides compared to responders. Tofacitinib was stopped in 23.5% of patients until week 8 due to insufficient response. Two patients reported headaches after treatment initiation and single events of Cytomegalovirus colitis, Clostridium difficile colitis and oral candidiasis occurred.
Conclusion: Tofacitinib was efficient in inducing clinical response with mucosal healing in about half of UC patients after 8 weeks of therapy. A need for long-term outcomes and for safety data with emphasis on infectious complications warrant further investigation.
Keywords:
Ulcerative colitis – tofacitinib citrate – JAK inhibitors
Introduction
The treatment of moderate to severe ulcerative colitis (UC) relies on several modalities including systemic corticosteroids, immunosuppressants and biologic therapy. Tofacitinib, a novel oral preparation, is Janus kinase (JAK) inhibitor targeting intracellular signalling pathway, preferentially blocking JAK1 and JAK3 activation with subsequent inhibition of signal transduction [1]. Tofacitinib was firstly authorised by European Medicines Agency in March 2017 to treat adult patients with moderate to severe rheumatoid arthritis and subsequently in 2018 the approval was extended to treatment of psoriatic arthritis and UC. The approval for UC was based on the results of Tofacitinib as Induction and Maintenance Therapy for Ulcerative Colitis trials (OCTAVE Induction 1, OCTAVE Induction 2 and OCTAVE sustain).
Data on efficiency and safety of tofacitinib from real world clinical practice in UC patients are only slowly emerging. Due to overall limited experience with the drug, our aim was to evaluate short-term efficacy and safety of tofacitinib in unselected patients with UC from a tertiary inflammatory bowel diseases (IBD) center using a thorough follow-up protocol.
Methods
Patients
Consecutive patients with active UC from a single tertiary IBD center with age between 18 and 65 years were included. Patients were either naïve to biologic therapy, in whom tofacitinib was considered as an alternative to immunosuppressive therapy with thiopurines or failed on single or more biologics with limited alternative treatment options.
Exclusion criteria included pregnancy and breastfeeding, active tuberculosis, acute infections including localized ones, severe liver disease (Child-Pugh C), absolute lymphocytes count less than 0.75 × 109/L, absolute neutrophils count less than 1.00 × 109/L and haemoglobin below 90g/L.
Treatment and follow-up
All included patients received tofacitinib 10 mg twice a day (b.i.d.) until week 8 of the follow-up, when the efficiency was evaluated. The dosing was then either decreased to 5 mg b.i.d. or, in case of insufficient efficacy, remained at 10 mg b.i.d. until week 16. Concomitant UC therapy was managed according to treating gastroenterologist experienced in IBD.
At baseline all patients underwent thorough examination. This included complete blood count, C-reactive protein (CRP), serum lipids, liver enzymes, serology for hepatitis B virus, herpes simplex virus, and varicella zoster virus (VZV), and Interferon Gamma Release Assay (IGRA). In stool, fecal calprotectin (FC) was measured and Clostridium difficile toxins A and B were tested. All patients had also abdominal ultrasound and endoscopic examination with tissue sampling and Mayo endoscopic scoring. Disease activity was assessed using total Mayo score. In addition, patients naïve to biologic therapy had a chest X-ray.
All patients were followed at week 2, 4 and 8. At each visit partial Mayo score was calculated, blood samples were taken for assessment of CRP, blood count, serum lipids and liver enzymes (week 4 and 8 only) and stool sample was obtained for measurement of FC. Furthermore, abdominal ultrasound and endoscopic examination were repeated at week 8 of treatment.
Primary outcome was clinical response at week 8 defined as total Mayo score ≤ 5 together with Mayo endoscopic subscore ≤ 1, corresponding with mucosal healing.
Statistical analysis
Standard descriptive statistical analyses were performed, including frequency distributions for categorical data and calculation of mean and standard deviation and/or range for continuous variables. The differences between continuous variables at baseline were analysed by t-test or Mann-Whitney test and between week 0 and week 8 by the Wilcoxon test. Categorical variables were compared by Fisher’s exact test. Univariate logistic regression was used to identify independent baseline factors associated with response at week 8. The statistical tests were performed using GraphPad Prism (version 8.3.1). A p-value < 0.05 was considered statistically significant.
Results
Patient characteristics
In total, 24 UC patients (41.7% males) were included, 17 of them reached week 8 of treatment at the time of this assessment. Mean age at baseline was 35.3 ± 11.8 (19–62) years. Mean disease duration was 8.3 ± 12.7 (9–54) years and the majority of patients had extensive UC (Tab. 1). Two patients suffered from additional extraintestinal manifestations (both erythema nodosum) and one patient had previous subtotal colectomy and ileo-rectal anastomosis due to UC. One-quarter of patients (6/24) were naïve to any biologic therapy at baseline, while 18 patients already failed on at least one biologic drug. Previous biologic therapy included variety of drugs with infliximab, vedolizumab and adalimumab being the most frequent (Fig. 1). Concomitant treatment with systemic corticosteroids was present in 10 (41.7%) patients at baseline.
Tab. 1. Základní charakteristika souboru.
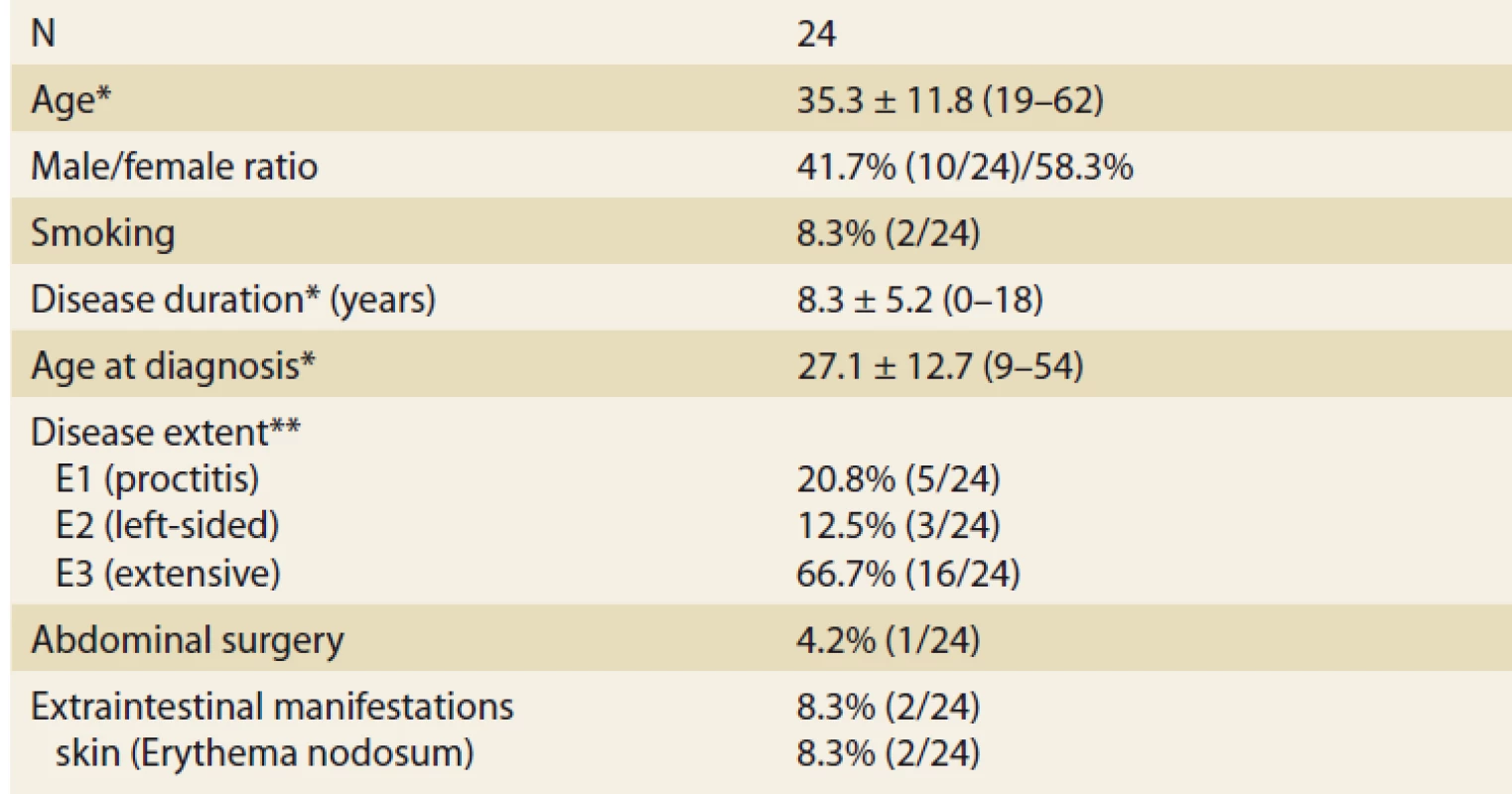
Obr. 1. Biologická léčba v minulosti.
Efficacy
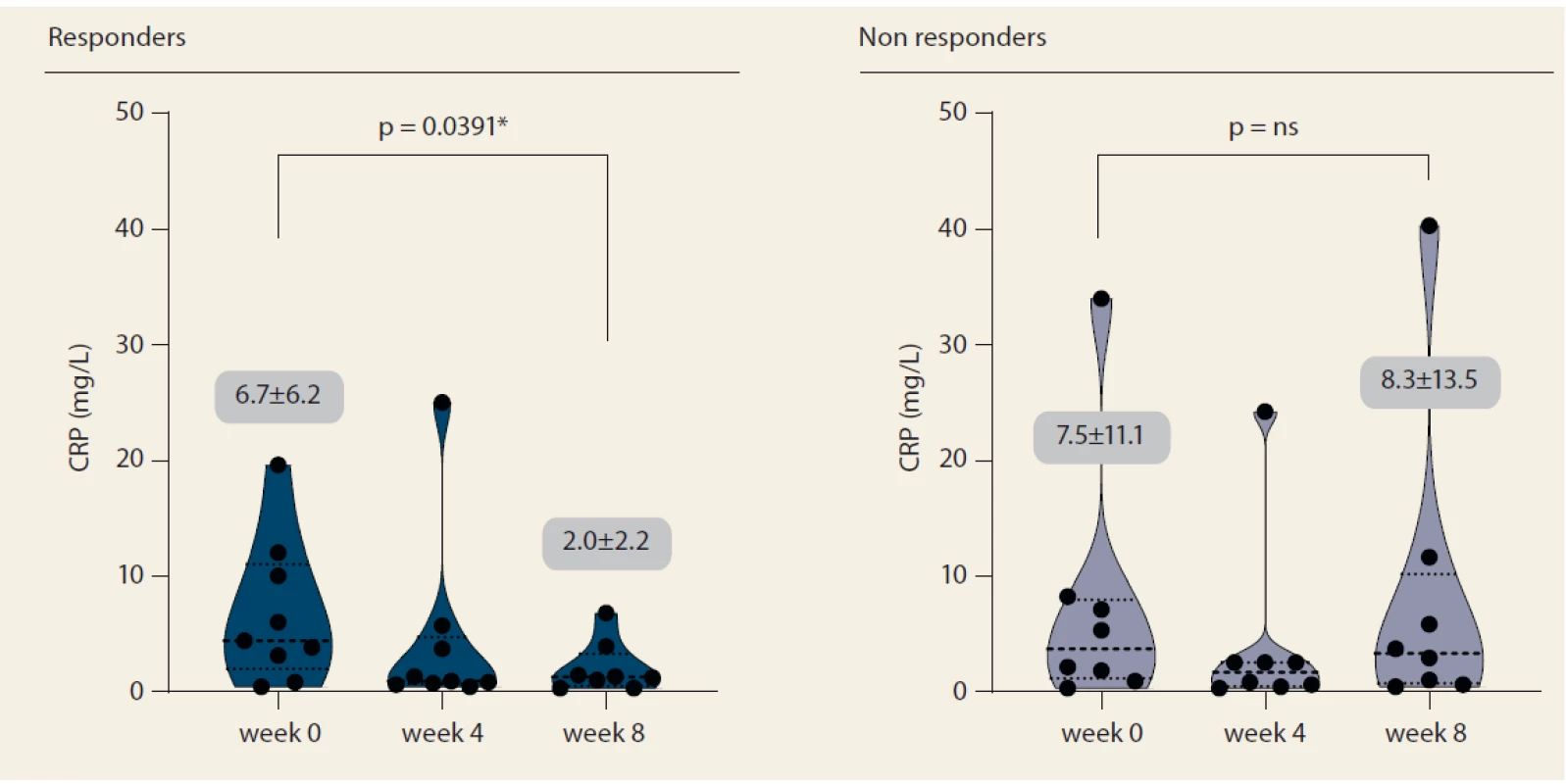
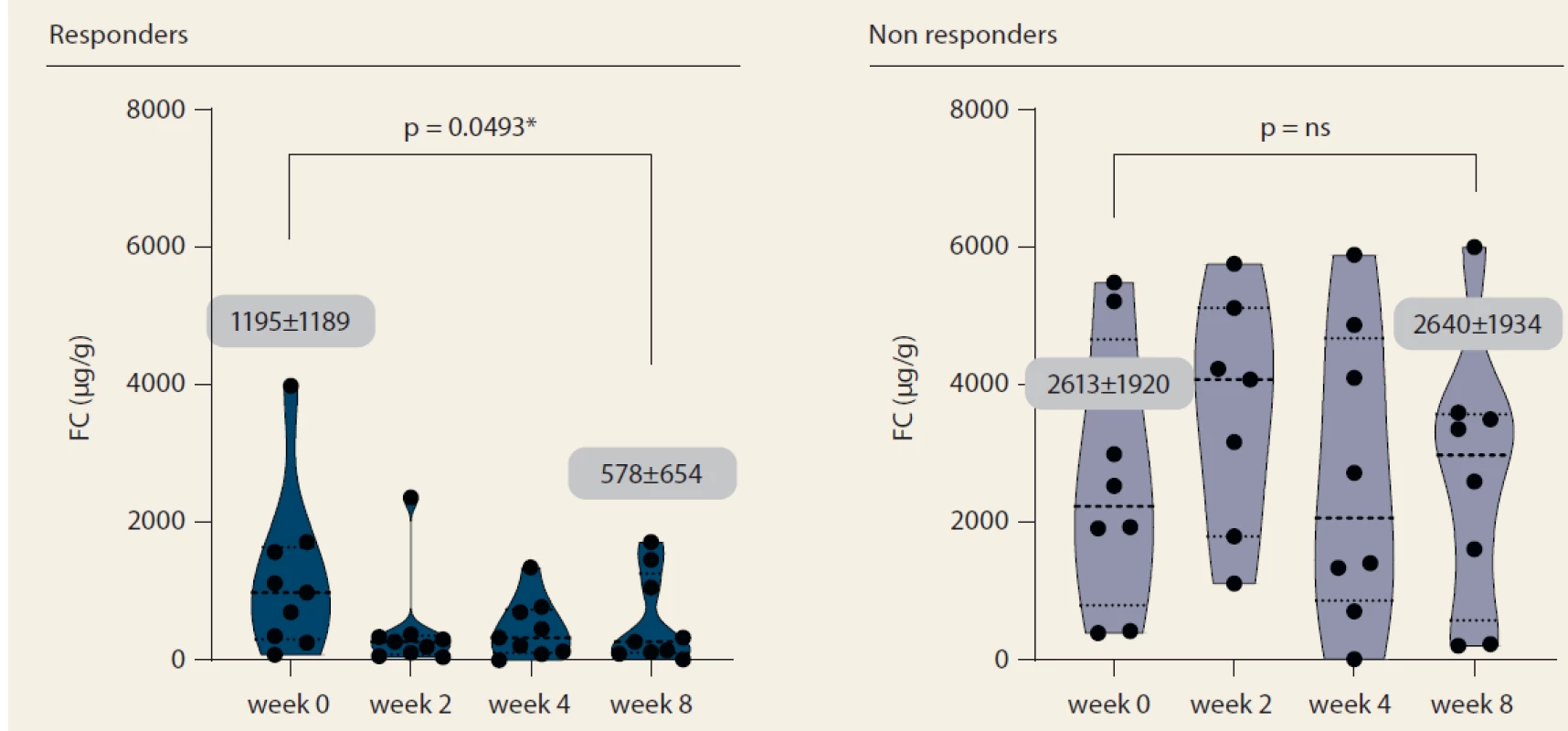
Nine patients (52.9%) responded to tofacitinib treatment at week 8 (Fig. 2). There was a significant decrease in partial Mayo score in responding patients (from 3.9 ± 2.7 to 0.6 ± 0.9, p = 0.0027) with no change in non-responders (5.1 ± 2.5 vs. 6.0 ± 2.1; p = ns). Mean endoscopic Mayo subscore improved from 2.0 ± 1.0 to 0.4 ± 0.5 (p = 0.0033) in responders, however, it remained unchanged in non-responders (Fig. 3). Similar trends with respect to clinical response at week 8 were observed also in laboratory markers such as CRP and FC (Fig. 4 and 5).
Obr. 2. Klinická odpověď na tofacitinib v týdnu 8.

Obr. 3. Endoskopická odpověď na tofacitinib

Obr. 4. Vývoj C-reaktivního proteinu do týdne 8.
Obr. 5. Vývoj fekálního kalprotektinu do týdne 8.
Tofacitinib had to be discontinued in 4 (23.5%) patients until week 8. All cases were due to treatment failure.
Two patients received salvage therapy with infliximab 10 mg/kg, one patient was transferred to ustekinumab and one patient was referred to the surgeon.
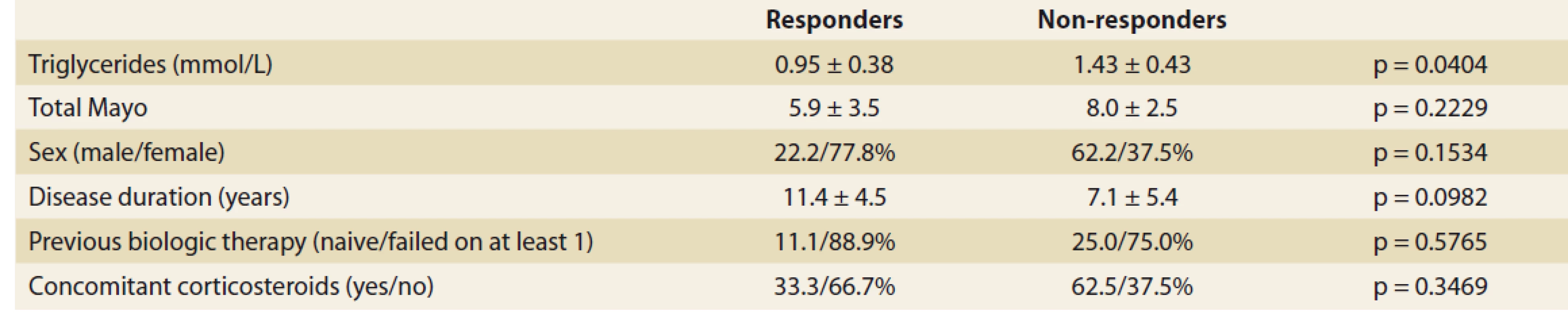
The only baseline factor significantly associated with treatment response at week 8 were triglycerides which were significantly higher in non-responders (1.43 ± 0.43mmol/L vs. 0.95 ± 0.38 mmol/L; p = 0.0404). No impact of gender, disease duration, laboratory markers, disease activity, previous biologic therapy or concomitant corticosteroids was found (Tab. 2).
Tab. 2. Vstupní faktory hodnocené ve vztahu k odpovědi v týdnu 8.
Safety
Adverse events were registered in 8 (25%) of patients. One patient experienced cytomegalovirus (CMV) colitis; the other one C. difficile colitis and one patient reported oral candidiasis. There was also a single case of unspecified respiratory infection. Two patients complained of headaches and sleep disturbances and one patient reported feelings of pressure in their eyes. No patients required hospitalization and no case of VZV was observed.
Discussion
In this investigator-initiated, real clinical practice study on tofacitinib efficacy and safety in patients with active UC, about one-half of patients responded within initial 8 weeks of treatment. Both clinical and endoscopic parameters were assessed in our cohort at week 8. Treatment was well tolerated, with no case of VZV infection observed.
Clinical remission rates reported in registration trials OCTAVE Induction 1 and 2 after 8 weeks of treatment were 18.5 and 16.6%, resp. [1]. Obviously, more stringent criteria for this primary endpoint were required, and results are thus difficult to compare. Nevertheless, clinical response rates in both trials were 60 and 55%, resp., and mucosal healing, with the same criteria as in our study (endoscopic Mayo subscore ≤ 1), occurred in approx. 30% of patients. Efficacy of tofacitinib induction in our study thus resembles results from the above-mentioned registration trials, including the fact that previous exposure to anti-tumour necrosis factor (TNF) antibodies has no impact on treatment results.
A French group recently reported real world data on 38 patients with refractory UC who failed on all previous conventional treatment modalities including anti-TNFs and vedolizumab. Their combined rate of failure and non-response at week 14 was 47.4%, while combined steroid-free clinical remission and response without remission was present in 44.8% of patients [2].
Weisshof et al. published a retrospective experience with tofacitinib in 58 IBD patients (53 UC), 93% of whom previously failed anti-TNF and 81%, anti-integrin. Clinical remission and response rate at week 8 in this cohort was 69% [3], higher than in our and previons French cohort. However, response and remission were not clearly defined in this study.
Currently available data on tofacitinib clinical response rates after 8 weeks seem to correspond with our results, considering limited size of our cohort.
Positioning of tofacitinib in current therapeutic armamentarium for UC will rely not only on clinical efficacy, but also on safety and onset of response. Hanauer et al. published post-hoc analysis of data from phase 3 trials demonstrating significant improvement of symptoms already within 3 days of treatment based on reported improvements in total number of daily bowel movements and rectal bleeding [4]. A case series published by Kotwani et al. presented four patients with acute severe UC who were successfully administered tofacitinib rescue therapy after no significant improvement of a minimum of 3 days of intravenous methylprednisolone [5]. Similar successful case series of four acute severe UC patients was reported from Michigan. In this case, all the patients were administered tofacitinib 10 mg 3× daily for 9 doses [6]. Ultimately, a case of combined rescue therapy with infliximab 10mg/kg and tofacitinib 10 mg b.i.d. was published by Griller et al [7].
Our patients experienced some infectious adverse events including CMV, C. difficile colitis and oral candidiasis. With regards to safety, an integrated safety analysis of tofacitinib UC clinical studies was published by Sandborn et al [8]. The study included data from 1,157 patients treated with tofacitinib up to 4.4 years and reported similar rates of adverse events in placebo and tofacitinib groups in both induction and maintenance cohorts and overall safety profile similar to anti-TNFs except for higher incidence rates of VZV infection with a dose relationship. In the above-mentioned real world study with median follow-up of 41.5 weeks, adverse events were reported in 37% (14) of patients, including 5 severe infections [2]. Weisshof et al. reported systemic infections in 20.1% (12) patients after median of 10.6 months of follow-up, including CMV and C. difficile colitis, Aspergillus sinusitis, Group B streptococcal sepsis or Escherichia coli gastroenteritis [3]. Majority of infections in this study occurred in patients on concomitant immunosuppressive therapy with corticosteroids or tacrolimus.
Data from cohorts of patients with rheumatoid arthritis provide more extensive information on the safety of tofacitinib. The results of a global, long-term extension study included 4,481 patients and 16,291 patient-years of exposure [9]. Observed incidence rates of serious adverse events, serious infections, malignancies, major cardiovascular events, deep vein thrombosis and pulmonary embolism in this study were consistent with previous findings and comparable to those seen with anti-TNFs. Recently, preliminary results of study A3921133 were reported. This is an ongoing open-label post-marketing surveillance study comparing safety of tofacitinib and anti-TNFs in patients with rheumatoid arthritis. Based on interim results, treatment arm with tofacitinib 10 mg b.i.d. was terminated and transferred to 5 mg b.i.d. due to increased risk of thromboembolic events and overall mortality. Pulmonary embolism hazard ratio (HR) for tofacitinib 10 mg b.i.d. vs. anti-TNF was 5.96 (1.75–20.33) and overall mortality HR was 3.28 (1.55–6.95), mainly due to cardiovascular events, infections and malignancies. Complications occurred more frequently in patients over 65 years of age. Although rheumatoid arthritis patients pose, in general, more risky cohort, using tofacitinib 10 mg b.i.d. as a maintenance therapy in UC in patients with known increased risk of thromboembolism is currently not recommended. Also, tofacitinib therapy in UC patients over 65 years of age should only be considered in case of no other alternative.
Among imitations of our study, a small sample size and heterogeneous population of UC patients should be mentioned. However, unselected cohort reflects the reality in routine clinical practice and gives the opportunity to assess potential predictors of effective treatment.
In conclusion, induction treatment with tofacitinib was efficacious in half of our unselected UC cohort with reasonable safety consistent with currently available data. Further long-term studies from clinical practice and careful pharmacovigilance are warranted.
Submitted: 3. 2. 2020
Accepted: 10. 2. 2020
MUC. Martin Kolář
IBD Clinical and Research Centre
ISCARE a. s.
Českomoravská 2510/19
190 00 Praha 9
Czech Republic
Sources
1. Sandborn WJ, Su C, Panes J. Tofacitinib as induction and maintenance therapy for ulcerative colitis. N Engl J Med 2017; 377 : 496–497.
2. Lair-Mehiri L, Stefanescu C, Vaysse T et al. Real-world evidence of tofacitinib effectiveness and safety in patients with refractory ulcerative colitis. Dig Liver Dis 2019.
3. Weisshof R, Aharoni Golan M, Sossenheimer PH et al. Real-World Experience with Tofacitinib in IBD at a Tertiary Center. Dig Dis Sci 2019; 64 : 1945–1951.
4. Hanauer S, Panaccione R, Danese S et al. tofacitinib induction therapy reduces symptoms within 3 days for patients with ulcerative colitis. Clin Gastroenterol Hepatol 2019; 17 : 139–147.
5. Kotwani P, Terdiman J, Lewin S. Tofacitinib for rescue therapy in acute severe ulcerative colitis: a real-world experience. J Crohns Colitis 2020.
6. Berinstein JA, Steiner CA, Regal RE et al. Efficacy of induction therapy with high-intensity tofacitinib in 4 patients with acute severe ulcerative colitis. Clin Gastroenterol Hepatol 2019; 17 : 988–990.
7. Griller N, Cohen L. Rapid onset of tofacitinib induction therapy for the treatment of ulcerative colitis. Clin Gastroenterol Hepatol 2019; 17 : 1213.
8. Sandborn WJ, Panés J, D’Haens GR et al. Safety of tofacitinib for treatment of ulcerative colitis, based on 4.4 years of data from Global Clinical Trials. Clin Gastroenterol Hepatol 2019; 17 : 1541–1550.
9. Wollenhaupt J, Lee EB, Curtis JR et al. Safety and efficacy of tofacitinib for up to 9.5 years in the treatment of rheumatoid arthritis: final results of a global, open-label, long-term extension study. Arthritis Res Ther 2019; 21 : 89.
Labels
Paediatric gastroenterology Gastroenterology and hepatology SurgeryArticle was published in
Gastroenterology and Hepatology
2020 Issue 1
- Possibilities of Using Metamizole in the Treatment of Acute Primary Headaches
- Metamizole at a Glance and in Practice – Effective Non-Opioid Analgesic for All Ages
- Metamizole vs. Tramadol in Postoperative Analgesia
- Spasmolytic Effect of Metamizole
- The Importance of Limosilactobacillus reuteri in Administration to Diabetics with Gingivitis
-
All articles in this issue
- On the threshold of the next year and the anniversary of the Battle of White Mountain
- What is the cost of inflammatory bowel disease?
- Guidelines of the Slovak Work ing Group on IBD in SGS for the Treatment of IBD at Reproductive Age
- Telemedicine and inflammatory bowel disease – results of the IBD Assistant pilot project
- Direct costs for the treatment of inflammatory bowel diseases
- Ustekinumab – a new biological therapy of ulcerative colitis
- How to choose the right IBD patients for vedolizumab treatment
- Unexpected cause of death of patient with upper gastrointestinal bleeding
- Fecal microbiota transplantation – past, present, and future
- Glomerulopathies in patients with inflammatory bowel disease
- Part 7 – Time trends in the volume and proportion of different types of hospitalization of patients with IBD
- In memoriam: doc. MUDr. Václav Jirásek
- In memoriam: MUDr. Branislav Valach
- The selection from international journals
- Autodidaktický test: IBD
- Tofacitinib induction efficacy and safety in ulcerative colitis at week 8 – results from clinical practice
- Novel Large Bowel Developments prof. Jaroslaw Regula – Gastro Update Europe 2019, Budapest
- Small bowel diseases and infections prof. Gerhard Rogler – Gastro Update Europe 2019, Budapest
- Gastroenterology and Hepatology
- Journal archive
- Current issue
- About the journal
Most read in this issue
- Fecal microbiota transplantation – past, present, and future
- Ustekinumab – a new biological therapy of ulcerative colitis
- Unexpected cause of death of patient with upper gastrointestinal bleeding
- Telemedicine and inflammatory bowel disease – results of the IBD Assistant pilot project
