Clinical course of patients with hepatorenal tyrosinemia treated with nitisinone – a 10-year prospective cohort study
Klinický průběh u pacientů s hepatorenální tyrosinemií léčených nitisinonem – desetiletá prospektivní kohortová studie
Úvod: Neléčená tyrosinemie I. typu, pokud se projeví již v kojeneckém věku, způsobuje výraznou hepatomegalii. Později v prvním roce života pak poškozuje funkce jater a renálních tubulů, což narušuje vývoj organizmu. Léčba nitisinonem (NTBC) zabraňuje poruše funkce jater a extrahepatálním projevům nemoci. Naše studie měla za cíl posoudit vliv nitisinonu na klinický průběh u pacientů postižených tyrosinemií I. typu. Metody: Tato desetiletá prospektivní kohortová studie se zaměřila na pacienty s tyrosinemií 1. typu přijatých na Shiraz Pediatric Gastroenterology Clinic mezi 20. březnem 2010 a 20. březnem 2018. Hodnocen byl klinický průběh léčby nitisinonem. Statistická analýza byla provedena pomocí softwaru SPSS 20. Výsledky: Studie zahrnovala celkem 145 pacientů. Výsledky ukázaly jasný vztah mezi závážností komplikací tyrosinemie a správným užíváním léků. Mezi pacienty užívajícími nitisinone (95 %) mělo 52,6 % dobré výsledky, 12,6 % jich čekalo na transplantaci, 32,6 % transplantaci podstoupilo a 2,1 % zemřelo. Naproti tomu z 54 pacientů, kteří NTBC neužívali, jich pouze 12 % mělo dobré výsledky, 6 % čekalo na transplantaci, 56 % podstoupilo transplantaci a 26 % zemřelo. Závěr: Výsledky této studie potvrzují roli nitisinonu při snižování závažností komplikací tyrosinemie I. typu, zlepšování klinických symptomů a snižování úmrtnosti pacientů.
Klíčová slova:
transplantace jater – tyrosinemie I. typu – nitisinon (NTBC) – tyrosin
Authors:
S. M. Dehghani 1
; L. Salarian 2; I. Shahramian 1
; F. Parooie 3
; M. Salarzaei 3
; M. Tahani 3
; F. Samiee 2; A. Amirhakimi 2
; M. Delaramnasab 3
Authors‘ workplace:
Shiraz Transplant Research Center, Shiraz University of Medical Science, Shiraz, Iran
1; Department of Pediatrics, Shiraz University of Medical Science, Shiraz, Iran
2; Pediatric Gastroenterology and Hepatology Research Center, Amir-Al-Momemnin hospital, Zabol university of medical sciences, Zabol, Iran
3
Published in:
Gastroent Hepatol 2023; 77(2): 97-102
Category:
Hepatology: original article
doi:
https://doi.org/10.48095/ccgh202397
Overview
: Introduction: Untreated type 1 tyrosinemia, when manifesting in infancy, causes severe liver enlargement, or, later in the first year, damages the hepatic and renal tubular function, leading to developmental impairment. Nitisinone (NTBC) treatment has been able to control liver dysfunction in patients and the extrahepatic manifestations of HT1. The present study aimed to evaluate the effect of nitisinone on the clinical course of patients with tyrosinemia type 1. Methods: This prospective 10-year cohort study focused on patients with type 1 tyrosinemia referred to Shiraz Pediatric Gastroenterology Clinic between March 20, 2010, and March 20, 2018. The clinical course of patients treated with nitisinone was evaluated. Statistical analysis was performed using SPSS 20 software. Results: This study included 145 patients. The results showed clear correlation between the severity of clinical complications of tyrosinemia and the proper use of the drug. The outcome among the patients taking the medications (95%) was observed to be good in 52.6%, while 12.6% of patients were on the waiting list, 32.6% had undergone transplantation, and 2.1% passed away. On the other hand, out of 54 patients who did not take NTBC, only 12% showed good outcomes, 6% were on the waiting list, 56% had undergone transplantation, and 26% died. Conclusion: The results of this study support the role of nitisinone in decreasing the complications of type 1 tyrosinemia and improving clinical symptoms, as well as reducing mortality in these patients.
Keywords:
Tyrosine – : tyrosinemia type 1 – nitisinone (NTBC) – liver/hepatic transplantation
Introduction
Hereditary Tyrosinemia Type 1 (HT1) (OMIM 276700) or hepatorenal tyrosinemia is an autosomal recessive hepatic metabolic disorder. In this disorder, Fumarilacetoacetate Hydrolase Enzyme (FAH) impairments, which are caused by genetic problems and defective functionality of FAH, disrupt tyrosine degradation [1,2]. Tyrosine is a nonessential aromatic amino acid synthesized from phenylalanine via hydroxylation. Tyrosine metabolism occurs mostly in the cytoplasm of the hepatocytes and to a lesser extent in the kidneys [3].
Worldwide, the prevalence of HT1 is about 1 in 100,000, but in some areas, such as Turkey, India, and Quebec, the prevalence has been reported to be higher [4]. The human FAH cDNA is located on chromosome 15q, and more than 100 mutations have been reported in FAH cDNA [4–6]. Untreated type 1 tyrosinemia, when manifesting in infancy, causes severe liver enlargement, or, later in the first year, damages the hepatic and renal tubular function and leads to developmental impairment and Vitamin D-resistant rickets [7,8]. In untreated children, unknown neurological crises may recur for up to a week, including respiratory failure needing mechanical ventilation, abdominal pain, or peripheral neuropathy. Left untreated, HT1 is usually fatal before age ten due to liver failure, neurological crisis, or liver cancer [7,9].
Until the early 1990s, the only available methods to manage HT1 symptoms were protein-restricted diets (usually reduced phenylalanine, methionine, and tyrosine) and liver transplantation. In 1992, a compound with the chemical formula 2- (2-nitro-4-trifluoromethyl benzoyl) -1, 3-cyclohexanedione, known as nitisinone or NTBC, was used to treat HT1. This treatment inhibits the upstream enzyme 4-hydroxyphenylpyruvate dioxygenase, thereby preventing the formation of toxic metabolites, fumarilacetoacetate and succinyl-acetoacetate. Given that NTBC treatment significantly increases plasma tyrosine concentrations, this treatment should be supplemented with a diet of restricted tyrosine and its precursors, such as phenylalanine [4,10,11].
Liver transplantation is the only cure for HT1, but it is associated with lifelong immune suppression and a relatively high rate of transplant loss in the paediatric population, and there is a shortage of donated organs [1]. NTBC treatment has been able to control liver dysfunction in patients and the extrahepatic manifestations of HT1. Furthermore, the diet has been greatly improved [11]. Early and timely treatment with NTBC is essential in preventing the consequences of the disease and it is ideal to start in infancy [4]. Research has shown that combination treatment of nitisinone and a low-tyrosine diet can increase survival to more than 90%, lead to normal growth and better liver function, and prevent cirrhosis, renal tubular acidosis, and secondary rheumatism [7,9]. The risk of hepatocellular carcinoma (HCC) as one of the major complications of HT1 has been significantly reduced after the introduction of NTBC, although its occurrence has also been reported in some HT1-treated NTBC patients [4]. Also, neurological deficits can be a problem in long-term management [4]. This indicates the need for further research on the effect of NTBC treatment on the HT1 patients. Our study aimed to evaluate the effect of nitisinone treatment on the clinical course of patients with type 1 tyrosinemia.
Materials and methods
This prospective cohort study evaluated 163 patients with HT1 who were referred to Shiraz Pediatric Gastroenterology Clinic of Shiraz University of Medical Sciences from March 20, 2010, to March 20, 2018. Purposeful sampling was selected. Finally, 145 HT1 patients referred to the clinic for follow-up were examined regarding the effect of nitisinone.
Inclusion and exclusion criteria
To be eligible, our patients had to fulfill the following criteria: under 18 years old, the diagnosis of HT1, and symptoms not consistent with other disorders. Patients were excluded if they had herpetic corneal ulcers, ataxia, right, and upper abdominal pain, or were not referred for follow-up.
Between 2010 and 2018, 163 children under 18 with HT1 were included. The diagnosis was made based on high levels of succinyl acetone in urine or blood, as well as the presence of clinical symptoms. The patient‘s clinical course was recorded until transplantation, death, or the end of March 20, 2018 (whichever occured earlier). The dose of nitisinone in all subjects was initially 1 mg/kg once or twice a day, with the dose increasing depending on the patient‘s condition or age. Restrictions on phenylalanine and tyrosine were also considered in these children, the aim was to maintain plasma tyrosine levels of 200–400 μmol/L.
Patients‘ records and history were collected. Several follow-up visits were scheduled and the patients who did not attend them were excluded from the study. Complete physical examination reports and demographic information such as the patients’ age at diagnosis, age at the time of symptoms, sex, patient weight, and disease symptoms were recorded. Biochemical tests, imaging, diet, drug use, and disease outcome were collected and used for statistical analysis.
Shiraz University made ethical approval of Medical Sciences, and the information of individuals and their outcomes was kept confidential (IR.SUMS.MED.REC.1397.459).
Statistical methods
The data was analyzed using an independent T-test, chi-square test, logistic regression test, and Pearson and Spearman correlation coefficients. Statistical analysis was performed using SPSS 20 software, and the level of significance (P <0.05) was considered.
Results
A total of 162 patients was included, out of which 144 were taking NTBC (good use 94 and poor use 50). The mean age was 2.20 among the patients with a good outcome, 3.13 for the ones on the waiting list, 3.78 for the transplanted and 2.18 among the deceased group. The patients who had received a transplanted liver had the highest mean weight (13.9 kg), followed by those waiting for transplantation (12.5 kg), the deceased group (10.7), and the good outcome group (10.1 kg).
Tab. 1 shows the prevalence of different clinical complications associated with HT1 disease, including ascites, cyanosis, encephalopathy, gastrointestinal bleeding, hepatocellular carcinoma, infection, jaundice, and bacterial peritonitis, which all differed significantly among the outcome groups.
Tab. 1. Prevalence různých komplikací v souvislosti s výsledkem pacienta.
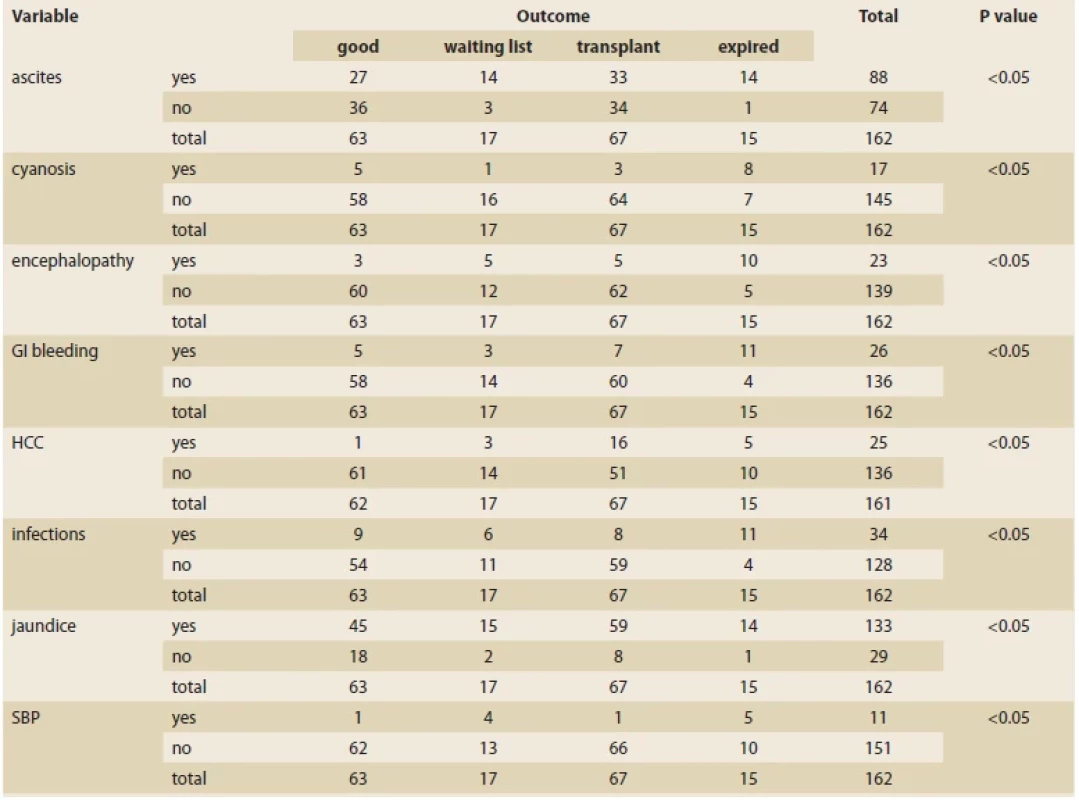
There was no significant relationship between the presence of rickets and the outcome of patients. In addition, gender and family history had no effect on the outcome, either.
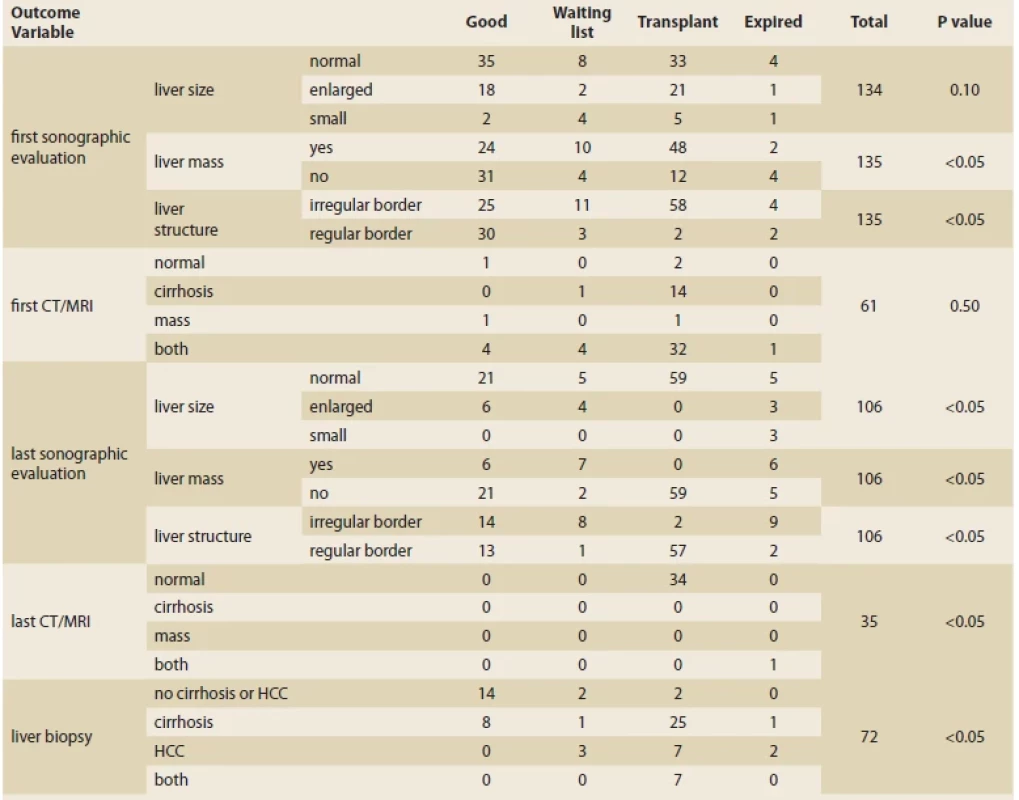
Tab. 2 shows the cases studied in the first and last imaging and biopsy results and their relationship with the outcome. Patients undergoing transplantation had 100% normal liver size, 100% no mass, and 96.61% normal parenchyma at the last ultrasound compared to the first. There was no cirrhosis or mass in children transplanted on the last MRI and CT scan (unlike the first imaging, where 65.3% of people in this category had cirrhosis and liver mass). There was a significant relationship between liver biopsy and treatment outcome. The patients with no cirrhosis or HCC showed a significantly higher rate of good outcomes. At the same time, transplantation was mostly seen among patients who developed cirrhosis.
Tab. 2. Vztah mezi nálezy ultrazvuku, CT, MR a biopsie a výsledkem pacienta.
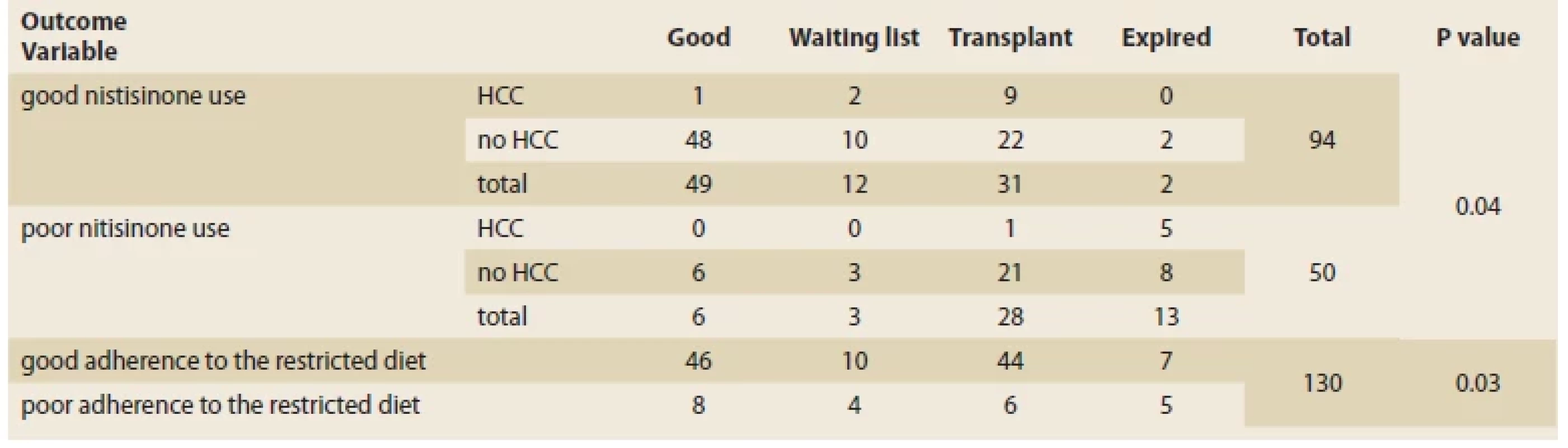
NTBC use
Of the 94 patients who used the drug properly, 52.6% had a good outcome, 12.6% were ot the transplant waiting list, 32.6% were transplanted, and 2.1% died. Forty-four patients used NTBC poorly, of which 12% had a good outcome, 6% were waiting to for a transplant, 56% received a transplant, and 26% died. Good adherence to the diet lead to significantly better outcomes among the patients. Patients who took their NTBC regularly were more likely to have a good outcome and lower mortality (2.1% vs. 26%, P <0.05) (Tab. 3).
Tab. 3. Vztah mezi užíváním NTBC, omezenou dietou a výsledkem pacientů.
Discussion
Our results indicated a better outcome (49/94 vs. 6/50) and lowered mortality (2.1% vs. 26%) for patients who regularly used NTBC compared to those who did not. In their study, Larochelle et al reported dramatically lower morbidity and mortality rates for patients who used NTBC. In their study, patients were treated from neonatal age and none of the patients treated with NTBC were reported to have developed the liver disease during the five-year follow-up. While among 28 patients who did not take NTBC, 20 (70%) required liver transplantation in their twenties [12]. In their 20-year study of patients with HT1, Larochelle et al also reported that patients not treated with NTBC spent 7.1% of their lives in hospital, of whom 3.7% were in active neurological crisis.
In contrast, none of the patients treated with NTBC experienced acute complications from the disease. In the group of patients who did not take NTBC, 29% died at an average age of 16 months prior to liver transplantation [12]. Liver disorders and death due to liver failure are consequences of poor treatment of HT1 [7]. As seen in HT1 and other chronic liver illnesses, stress-induced failure of cell death systems may result in a buildup of damaged cells and increase the risk of cancer [13]. The liver is heavily impacted by the disease in the first weeks and months of life, the result of which can be acute liver failure with coagulopathy, ascites, and edema due to albumin and other plasma protein deficiency [7]. The present study showed a lower level of liver transplantation in the NTBC group (65% vs. 100%). The last ultrasound in patients undergoing liver transplantation was normal and without abnormal liver size, and 96.61% had normal parenchyma. Liver transplantation is a definitive treatment for the defective metabolic pathway and other manifestations of serious diseases such as liver failure and HCC in HT1 [14]. Arikan et al also reported in a study that HCC is primarily seen in older children and is most often associated with liver cirrhosis [15]. In patients who died, liver mass, abnormal liver size and parenchyma were more frequent. These results indicate the functional role of NTBC and appropriate early treatment with NTBC. Hepatic complications were reduced in these patients. According to the present study, with appropriate drug use, liver transplants and morbidity decreased, especially when nitisinone was initiated in the first weeks after birth, thus preventing premature liver and kidney damage. Simonsley et al, in a study of the effect of nitisinone on HT1, found that the cost of using a variety of healthcare resources was significantly reduced. The frequency of hospitalizations to paediatric critical care units, the length of hospital stays, and the need for liver transplants are all significantly reduced with nitisinone therapy [16].
In the present study, 46 out of 54 people with a good disease prognosis had a proper diet. When the only treatment available was a dietary modification, the survival rate among patients whose symptoms developed before two months of age was 29% [16], which reflects the fact that although the diet is beneficial to patients, the combination of NTBC and diet can restrict tyrosine precursors for the treatment of tyrosinemia complications [7]. Tyrosine levels can be lowered by consuming less protein. However, if low levels of phenylalanine drop, this quantity could be dangerous. Due to the competition between phenylalanine and tyrosine for transport to the brain, a diet high in tyrosine and low in phenylalanine may prevent phenylalanine from reaching the brain, thereby lowering the amount of phenylalanine that is available for the synthesis of neurotransmitters and proteins. Some writers claim that this insufficiency may cause a decline in cognitive development that manifests as moderate cognitive impairment later in life [17,18]. However, given the role of tyrosine and phenylalanine, restricted nutrition as a complementary treatment in addition to the use of nitisinone is mandatory.
Conclusion
Overall, the present study‘s findings showed that adherence to treatment with NTBC reduces morbidity and mortality.
Conflict of Interest: The authors declare that the article/ manuscript complies with ethical standards, patient anonymity has been respected, and they state that they have no financial, advisory or other commercial interests in relation to the subject matter.
Publication Ethics: This article/ manuscript has not been published or is currently being submitted for another review. The authors agree to publish their names and e-mails in the published article/ manuscript.
Dedication: The article/ manuscript is not supported by a grant nor has it been created with the support of any company. The Editorial Board declares that the manuscript met the ICMJE “uniform requirements” for bio-medical papers.
Konflikt zájmů: Autoři deklarují, že text článku odpovídá etickým standardům, byla dodržena anonymita pacientů a prohlašuji, že v souvislosti s předmětem článku nemají finanční, poradenské ani jiné komerční zájmy.
Publikační etika: Příspěvek nebyl dosud publikován ani není v současnosti zaslán do jiného časopisu pro posouzení. Autoři souhlasí s uveřejněním svého jména a e-mailového kontaktu v publikovaném textu.
Dedikace: Článek není podpořen grantem ani nevznikl za podpory žádné společnosti.
Redakční rada potvrzuje, že rukopis práce splnil ICMJE kritéria pro publikace zasílané do bio medicínských časopisů.
Submitted/ Doručeno: 6. 2. 2023
Accepted/ Přijato: 20. 2. 2023
Prof. Iraj Shahramian, MD
Department of Pediatrics
Pediatrics Office
University of Medical Sciences
Nemazee Hospital
Namazi Sq.
71937 11351 Shiraz
Iran
irajshahramian@gmail.com
Gastroent Hepatol 2023; 77(2): 97– 102
Sources
1. Hickey RD, Nicolas CT, Allen K et al. Autologous Gene and Cell Therapy Provides Safe and Long-Term Curative Therapy in A Large Pig Model of Hereditary Tyrosinemia Type 1. Cell Transplant 2019; 28 (1): 79–88. doi: 10.1177/0963 689718814188.
2. Xie Y, Lv X, Ni D et al. HPD degradation regulated by the TTC36-STK33-PELI1 signaling axis induces tyrosinemia and neurological damage. Nat Commun 2019; 10 (1): 4266. doi: 10.1038/s41467-019-12011-0.
3. Fernández-Lainez C, Ibarra-González I, Belmont-Martínez L et al. Tyrosinemia type I: clinical and biochemical analysis of patients in Mexico. Ann Hepatol 2014; 13 (2): 265–272.
4. Couce ML, Sánchez-Pintos P, Aldámiz-Echevarría L et al. Evolution of tyrosinemia type 1 disease in patients treated with nitisinone in Spain. Medicine (Baltimore) 2019; 98 (39): e17303. doi: 10.1097/MD.0000000000017303.
5. Grompe M. The pathophysiology and treat- ment of hereditary tyrosinemia type 1. Semin Liver Dis 2001; 21 (4): 563–571. doi: 10.1055/ s-2001-19035.
6. Angileri F, Bergeron A, Morrow G et al. Geographical and ethnic distribution of mutations of the fumarylacetoacetate hydrolase gene in hereditary tyrosinemia type 1. JIMD Rep 2015; 19: 43–58. doi: 10.1007/8904_2014_363.
7. Sniderman King L, Trahms C, Scott CR. Tyrosinemia Type I. 2006 [online]. In: Adam MP, Everman DB, Mirzaa GM, et al (eds). GeneReviews®. Seattle (WA): University of Washington, Seattle 1993–2023.
8. Rashad M, Nasser C. P434 Tyrosinemia type 1: a case report. Arch Dis Child 2019; 104 (3): A326.
9. Abdelghaffar T, Elsayed S, Elnaghni S et al. Phenotypic characteristics of a tyrosinemia type I: an Egyptian cohort. QJM: Int J Med 2018; 111 (1): hcy200. doi: 10.1093/qjmed/hcy200. 067.
10. Lindstedt S, Holme E, Lock EA et al. Treatment of hereditary tyrosinaemia type I by inhibition of 4-hydroxyphenylpyruvate dioxygenase. Lancet 1992; 340 (8823): 813–817. doi: 10.1016/0140-6736 (92) 92685-9.
11. Couce ML, Dalmau J, del Toro M et al. Tyrosinemia type 1 in Spain: mutational analysis, treatment and long-term outcome. Pediatr Int 2011; 53 (6): 985–989. doi: 10.1111/j.1442- 200X.2011.03427.x.
12. Larochelle J, Alvarez F, Bussières JF et al. Effect of nitisinone (NTBC) treatment on the clinical course of hepatorenal tyrosinemia in Québec. Mol Genet Metab 2012; 107 (1–2): 49–54. doi: 10.1016/j.ymgme.2012.05.022.
13. Vogel A, Van Den Berg IE, Al‐Dhalimy M et al. Chronic liver disease in murine hereditary tyrosinemia type 1 induces resistance to cell death. Hepatology 2004; 39 (2): 433–443. doi: 10.1002/hep.20077.
14. Karaca CA, Yilmaz C, Farajov R et al. Live donor liver transplantation for type 1 tyrosinemia: An analysis of 15 patients. Pediatr Transplant 2019; 23 (6): e13498. doi: 10.1111/petr.13498.
15. Arikan C, Kilic M, Nart D et al. Hepatocellular carcinoma in children and effect of living‐donor liver transplantation on outcome. Pediatric transplant 2006; 10 (1): 42–47. doi: 10.1111/j.1399-3046.2005.00395.x.
16. Simoncelli M, Samson J, Bussières JF et al. Cost-consequence analysis of nitisinone for treatment of tyrosinemia type I. Can J Hosp Pharm 201; 68 (3): 210–217.
17. Bendadi F, de Koning TJ, Visser G et al. Impaired cognitive functioning in patients with tyrosinemia type I receiving nitisinone. J Pediatr 2014; 164 (2): 398–401. doi: 10.1016/j.jpeds.2013.10.001.
18. Morrow G, Tanguay RM. Biochemical and Clinical Aspects of Hereditary Tyrosinemia Type 1. Adv Exp Med Biol 2017; 959: 9–21. doi: 10.1007/978-3-319-55780-9_2.
Labels
Paediatric gastroenterology Gastroenterology and hepatology SurgeryArticle was published in
Gastroenterology and Hepatology

2023 Issue 2
- Metamizole vs. Tramadol in Postoperative Analgesia
- Metamizole at a Glance and in Practice – Effective Non-Opioid Analgesic for All Ages
- Possibilities of Using Metamizole in the Treatment of Acute Primary Headaches
- Current Insights into the Antispasmodic and Analgesic Effects of Metamizole on the Gastrointestinal Tract
- Spasmolytic Effect of Metamizole
Most read in this issue
- Autoimmune pancreatitis in childhood
- Ultra-processed food – a threat to liver health
- Neurobiology of liver diseases
- International experience with Crohn’s disease exclusion diet (CDED) with partial enteral nutrition (PEN)

