Medical consequences of Chernobyl with focus on the endocrine system: Part 1
Zdravotní dopady černobylské katastrofy se zaměřením na endokrinní systém: část 1
Atomové katastrofy se udály v posledních 70 letech několikrát. Výbuch nukleárního zařízení v roce 1986 v severní částí střední Ukrajiny byl mimořádnou zkušeností proto, že radiační zátěž dopadla na všechny věkové kategorie populace. Následné studie pak přinesly velké množství informací o účinku záření na lidský organismus. Vzhledem k tomu, že probíhá globální zhoršující se bezpečnost a současně slyšíme názory některých světových lídrů, získávají nový rozměr nejen znalosti o biologickém dopadu ionizujícího záření, ale i preventivní navržená opatření ke snížení zhoubných účinků záření. A navíc – týkají se nás všech. Tento přehled se zaměřuje na dlouhodobé důsledky černobylské katastrofy, zvláště pak na dopad na endokrinní systém u dětí a dospělých. Přehled zahrnuje souhrn preventivních opatření pro případ atomové katastrofy.
Klíčová slova:
černobylská atomová katastrofa – jonizující záření – endokrinní systém – štítná žláza – rakovina –mamma – těhotenství – děti
Authors:
Thomas P. Foley Jr. 1; Zdeňka Límanová 2; Eliška Potluková 3
Authors‘ workplace:
Division of Endocrinology & Metabolism, School of Medicine, Graduate School of Public Health, University of Pittsburgh, Children’s Hospital of Pittsburgh, USA
1; Third Department of Medicine, First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic
2; Division of Medicine, University Hospital Basel, Switzerland
3
Published in:
Čas. Lék. čes. 2015; 154: 227-231
Category:
Review Articles
Overview
In the last 70 years, atomic disasters have occurred several times. The nuclear power plant accident at Chernobyl in 1986 in North-Central Ukraine was a unique experience in population exposures to radiation by all ages, and ongoing studies have brought a large amount of information on effects of radiation on human organism. Concerning the deteriorating global security situation and the strong rhetoric of some of the world leaders, the knowledge on the biological effects of ionizing radiation and the preventive measures designed to decrease the detrimental effects of radiation gains a new dimension, and involves all of us. This review focuses on the long-term effects of Chernobyl catastrophe especially on the endocrine system in children and in adults, and includes a summary of preventive measures in case of an atomic disaster.
Keywords:
Chernobyl atomic disaster – ionizing radiation – endocrine system – thyroid – cancer – breast – pregnancy – children
INTRODUCTION
Concerning the new global security situation and the strong rhetoric of some of the world leaders, the knowledge on the biological effects of ionizing radiation gathered in the past decades gains a new dimension. There have been several large scale disasters causing radiation exposure since the atomic bomb detonations in Japan at Hiroshima and Nagasaki almost seven decades ago (1). Exposures in the past four decades were primarily from nuclear power plants and fallout from nuclear bomb testing (2) that have been reviewed in great depth (1–7). This review will focus on the medical consequences of radiation exposure on the endocrine system.
Most of the serious medical effects from radiation were caused by exposures from the nuclear power plant disaster at Chernobyl, Ukraine, twenty-eight years ago. This catastrophe affected large populations of young children who lived in close proximity to the reactor and had long outdoor exposures because the accident was kept secret by authorities of the Soviet Union (4–7). There were few medical effects among those exposed as adults with the exception of the workers who came directly to the site of the disaster.
IONIZING RADIATION – HUMAN EXPOSURE
Unstable atoms emit energy in the form of ionizing radiation in order to return to a stable state (1). The high frequency particles and electromagnetic energy cause various adverse biological effects, depending upon the type and dose of the energy released (4). Adverse biological effects include the production of free radicals, damage to DNA, production of new macromolecules and disruption of chemical bonds (1, 4). The types, characteristics and sources of ionizing radiation were reviewed recently (1, 2, 4–8).
Radiation exposure occurs by internal radiation through inhalation, ingestion or injection, and external (whole or partial body) radiation. The health effects are mediated by direct radiation to target tissues, or indirectly through the production of free radicals or other harmful molecules (1–5) that mediate the carcinogenic effect on cells in the exposed tissues (7). The sensitivity of the thyroid is inversely related to age – the fetus and young infants are very sensitive to the carcinogenic effect of radiation whereas the adult is much less sensitive (8–10). The highest radiation doses cause thyroid cell death; moderate radiation doses are associated with an increased risk for thyroid neoplasia (papillary thyroid carcinoma, thyroid adenoma) (11–12) and breast cancer (13–14). Lower doses induce autoimmune diseases, such as thyroiditis, (12) most likely in predisposed individuals, and diabetes type 1 that is triggered in children and adolescents with genetic predisposition (15).
CHERNOBYL NUCLEAR POWER PLANT ACCIDENT
The worst nuclear accident in history and the largest peacetime exposure to radiation occurred at the Chernobyl Nuclear Power Plant in the former Union of Soviet Socialistic Republics (USSR).
On April 26, 1986, at 01 : 23, during an experiment scheduled to test a potential safety emergency core cooling feature, the reactor number four suffered a catastrophic power increase, leading to explosions in its core. This ignited the combustible graphite moderator and dispersed large quantities of radioactive fuel and core materials into the atmosphere. The burning graphite moderator increased the emission of radioactive particles, carried by the smoke, as the reactor had not been encased by any kind of hard containment vessel. The explosion and fire threw hot particles of the nuclear fuel and the dangerous fission products – radioactive isotopes such as 137Cs (cesium), 131I (iodine), 90Sr (strontium) and other radionuclides, into the air (5, 6).
The Russian authorities failed to inform the inhabitants of the area about the catastrophe. The nearby city of Pripyat was not immediately evacuated after the incident. The life in the town continued as if nothing had happened. However, within a few hours of the explosion, dozens of people fell ill. Later, they reported severe headaches and metallic tastes in their mouths, along with uncontrollable fits of coughing and vomiting (16). The general population of the USSR was first informed of the disaster on 28 April, two days after the explosion, with a 20 second announcement in the TV news program.
The prevailing weather conditions caused most of the radioactive contamination to affect the southeastern and southern areas of Belarus, western Russia and north/northwestern Ukraine (Figure 1).

Radioisotopes of iodine and cesium are present in large quantities in nuclear reactors and released into the atmosphere when a meltdown and explosion occur (5). Radioactive isotopes of iodines and cesium are the most potent isotopes that cause endocrine pathology, especially for the thyroid gland (5–6, 8–10), breast (5–6, 13–14), and other tissues that are susceptible to autoimmune diseases, such as Type 1 diabetes mellitus (15) and autoimmune thyroiditis (11–12).
The Chernobyl catastrophe affected more than five million people in three different degrees of intensity. (16) Firstly, about 600,000 workers (liquidators or emergency and recovery operations workers) were affected, of whom 240,000 worked at the reactor site surrounding 30 km zone in 1986 and 1987 when the doses were highest. Secondly, about 336,000 inhabitants who were evacuated or relocated from the contaminated areas during and after 1986; and thirdly, five million people continue to live in areas of Belarus, Ukraine and Russia that were contaminated by the accident (17).
Whereas only 31 workers died immediately after the accident, the costs in terms of the human lives are difficult to assess. According to an estimation of Cardis et al (8), the risk projections suggest that by now Chernobyl may have caused about 1,000 cases of thyroid cancer and 4,000 cases of other cancers in Europe, representing about 0.01% of all incident cancers since the accident. Models predict that by 2065 about 16,000 cases of thyroid cancer and 25,000 cases of other cancers may be expected due to radiation from the accident, whereas several hundred million cancer cases are expected from other causes (8).
The effective dose estimates for individuals in the general population accumulated over the 20 years following the accident were dependent on location, age and lifestyle factor. It ranged from a few mSv to some hundred mSv (17). The mean effective dose accumulated in the strict control zones reached about 50 mSv and in the less contaminated areas about 10 mSv (natural background radiation being about 1 mSv/year) (17). The highest organ-specific dose was to the thyroid gland, as discussed later.
RADIATION EXPOSURE AND HEALTH RISKS
There clearly are distinct differences of opinion about the effects of radiation on health (11–12,17–57), especially in children (32–38). There are many conflicting interpretations of radiation risk based on several studies with differences in the origin of radiation, dose, and type of exposures, susceptibility of specific sensitive tissues and organ targets, and magnitude/diversity of the populations studied (35–46). Cases of thyroid cancer increased as early as four years after the accident in those who were exposed as infants and children (5, 18). The specific tissues that concentrate iodine receive the largest radiation doses that induce molecular changes associated with the increased incidence of thyroid adenomas and carcinomas (5, 8–11, 28). Breast cancer in women statistically increased in atomic bomb survivors after a 20 year latent period (13–14, 17, 19). The time after the Chernobyl accident is as yet insufficient to ascertain if there will be a significant increase in breast cancer among those whose breast tissue was exposed to the greater doses of radiation during a hyperplastic stage of the gland such as in utero, early post-natal life, puberty, pregnancy and lactation (5, 13–14, 21–22).
THYROID DISEASES FOLLOWING CHERNOBYL RADIATION EXPOSURE
From reports prior to the Chernobyl accident, it was quite evident that the younger the child, the greater the sensitivity to ionizing radiation. This sensitivity relates in part to the rate of cell division and inversely with the degree of cell differentiation (1). Thus, the fetus is the most sensitive human subject, and the sensitivity declines with increasing age (4). Rapidly dividing cells are the most vulnerable to radiation. Though cell damage when the doses of radiation are less than 1.0 Gy are not severe in general, cells may be more susceptible to subsequent carcinogenesis which is dose related (1). Additional factors influencing the consequences of radiation exposure are the doses received by specific tissues and the types of radiation (1, 4, 7).
The crucial factor in the pathogenesis of the thyroid disease after nuclear power plant accidents is the radioactive isotope 131I. It is a major uranium and plutonium fission product, comprising nearly 3% of the total products of the fission. Its radioactive decay half-life is about eight days. Due to its mode of beta decay, 131I is notable for causing mutation and death in cells that it penetrates, and other cells up to several millimeters away. Therefore, higher doses of 131I may be less dangerous than the lower doses: causing death of the thyrocytes instead of mutations – this is used in the therapeutic radioiodine administration with the goal of thyreoelimination in the thyroid cancer treatment. The thyroid and breast are two major organs that concentrate iodine through a sodium-iodide symporter (47). The thyroid represents the most notably damaged tissue after exposure to radioactive iodine; in case of the normal breast, NIS expression occurs only during pregnancy and lactation. For the fetus during late gestation, maternal estrogen may cause NIS expression, and for the neonate, estrogen withdrawal may stimulate lactation (neonatal galactorrhea) and cause NIS expression. In both cases, high doses of – 131I could be concentrated into perinatal breast tissue to increase the subsequent risk for breast cancer. However, there are no research studies to confirm or refute these hypotheses (47).
There are specific thyroid diseases that occur at a greater frequency following exposure to ionizing radiation: thyroid adenomas which may or may not become malignant, and papillary thyroid carcinomas (48). Furthermore, no evidence exists to suggest any decline in radiation-related risk of thyroid cancer two decades after the accident (49–52). The data regarding an increased incidence of autoimmune thyroid disease, hypothyroidism and goiter are conflicting and will be discussed later in the text.
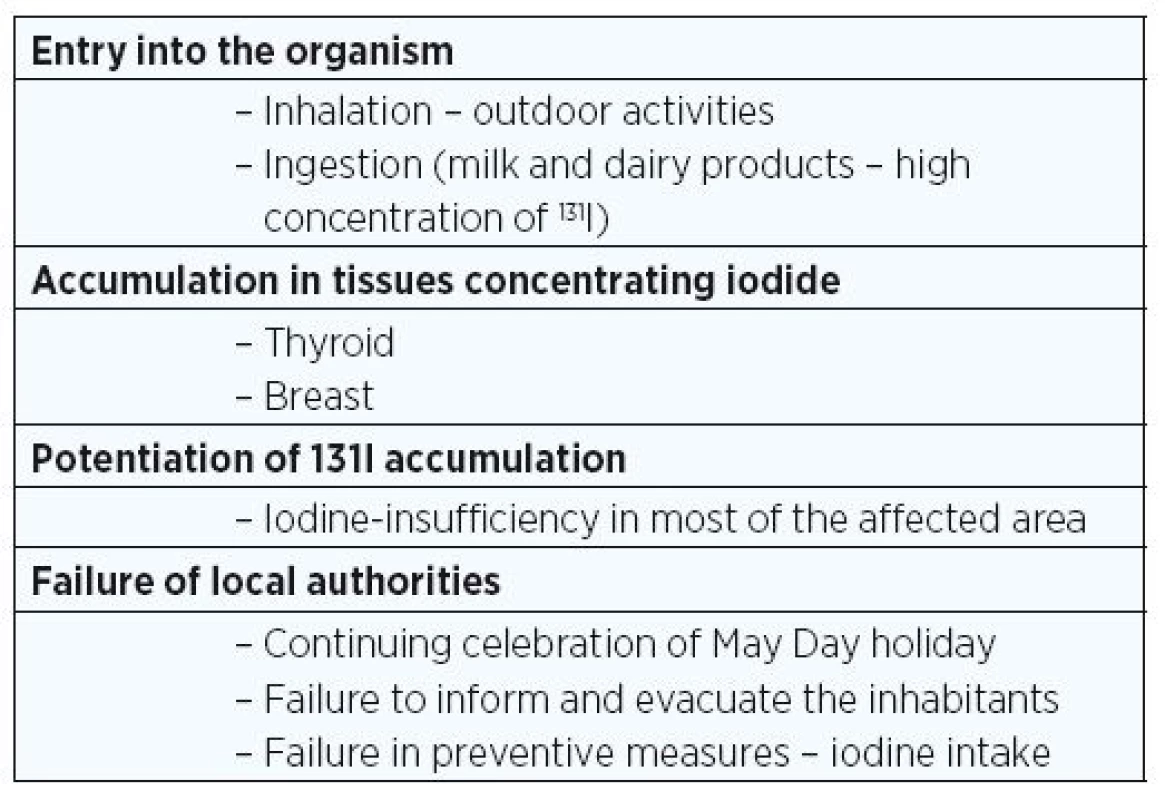
There are specific reasons why thyroid pathology was particularly prevalent after Chernobyl (Table 1).The radioactive isotopes of iodine, particularly 131I and the more potent, shorter half-life isotopes were released in large quantities into the atmosphere. Since these isotopes are volatile, they are easily inhaled and ingested. Milk was a particular source of 131I, as the lactating breast tissue concentrates iodine via the sodium-iodide symporter leading to accumulation of 131I in the milk. Other factors that increased the dose of radiation received are: 1. iodine deficiency in the regions of greatest exposure as a consequence of inadequate iodine availability (dietary supplementation of foods and dairy products) that causes a much greater uptake of iodine by the thyroid; 2. the occurrence of greater outdoor activity by children of all ages and adults. The May Day holiday was celebrated throughout the USSR at the time of peak releases of radiation while the authorities in Moscow kept the accident and its radiation contamination a secret from local authorities; and 3. children and women were not notified and not evacuated until much later after the accident even though livestock were relocated.
THYROID NEOPLASIA AND CARCINOGENESIS IN INFANTS AND CHILDREN EXPOSED TO RADIATION FROM THE CHERNOBYL ACCIDENT
Of particular interest was the short latent period between the age at exposure and the detection of thyroid cancer. After exposure to the atomic bomb, the latent period for the appearance of thyroid cancer was reported to be around 7 to 10 years after exposure. However, cases of thyroid cancer began to increase by as early as 3 to 4 years after exposure to radiation from Chernobyl. Initially it was thought to be a consequence of ascertainment and very close clinical observation, but within a very short period of time a remarkable large number of cases were found (52), and the diagnostic evaluation confirmed the accuracy of the pathologic diagnosis by pathologists with an expertise in pediatric thyroid malignancies (53) (Figure 2). The shorter latent period may relate to the prolonged radioiodine exposure time, increased radiation dose to the thyroid from iodine deficiency and the unique molecular genetics and histopathology of the thyroid cancer.

PATHOGENESIS AND MOLECULAR ABNORMALITIES
Most Chernobyl-related molecular abnormalities are chromosomal rearrangements that cause constant or constitutive activation of the tyrosine kinase (TK) receptor domain (26–28). The mechanism of radiation-induced rearrangements occurs between a TK receptor and an activating gene, the signal peptide that leads to formation of chimeric transforming sequences (26–27). Constitutive activation of the TK receptor contributes in part to transformation from normal cell replication to neoplastic transformation, associated with benign neoplasia and malignancy. The resultant “chimeric” RET proto-oncogenes associated with papillary thyroid carcinoma (PTC) are expressed in transformed thyroid cells as RET/PTC subtypes (27). Specific RET gene rearrangements, particularly RET-PTC1 and RET-PTC3, are found in PTC specimens from those exposed to radiation from the Chernobyl accident as children and adolescents (5, 28–32).
Papillary thyroid carcinomas were the most common form affecting all children, as follicular was quite uncommon and there was no proven increase in any other thyroid malignancy in those exposed to Chernobyl radiation as children. The most prevalent of these papillary thyroid cancer cases occurred in children who were exposed while in utero during mid to late gestation, or during early postnatal life. Upon molecular analysis of children detected early after the accident were found to have a specific chromosomal rearrangement known as RET-PTC 3 associated with exposure to 131Iodine which emits both gamma (γ) radiation and beta (β) particles (10, 21, 28, 30, 55). The pathology seen with this particular rearrangement also was unique, and is described as the solid variant of papillary thyroid cancer with increased aggressiveness in terms of pathologic stage (56). An increasing number of children detected after a longer latent period from exposure were found to have the RET-PTC 1 chromosomal rearrangement associated with γ-radiation emitted by both 137Cs with a very long half-life and 131I with an 8-day half-life (55). Thyroid cancer associated with RET-PTC 1 was found to have the classic, less aggressive diffuse sclerosing variant of papillary thyroid carcinoma (28, 30, 32).
AUTOIMMUNE THYROID DISEASE AND RADIATION EXPOSURE
Apart from the increased risk for thyroid neoplasia, an association of radiation exposure with the presence of thyroid autoantibodies and elevated TSH in serum has been documented. However, the latent period to onset, the magnitude and expression of the clinical disease as well as the duration of the effect are quite variable.
A study in 1998 compared children and adolescents with similar ethnic mixes from two villages in Belarus 6 to 8 years after the Chernobyl accident (12). One village in Gomel Region was heavily contaminated with radioactive fallout (~ 5.4 Ci/km2) whereas the other village in Vitebsk Region had negligible contamination (< 0.1Ci/km2). TPOAb and/or TgAb were significantly higher among those living in the contaminated region (56 of 287, or 19.5%) compared to those living in the negligibly contaminated oblast (8 of 208, or 3.8%). Serum fT4, FT3 and TSH did not differ between the two populations.
A decade later in 2008, five of the same co-authors again studied serum thyroid antibodies and TSH in specimens collected from 1,433 adolescents living in paired contaminated and non-contaminated villages in Belarus, Russia and Ukraine (56). The subjects were in utero to age 4 years at the time of the accident and ages 13 to 17 years old when tested. Only in Belarus villages was a higher prevalence of TPOAb in contaminated (6.4%) vs non-contaminated (2.4%) villages found. There were no differences in TgAb and thyroid function tests in Belarus, Russia and Ukraine villages. Their data provide evidence that exposure to Chernobyl radiation fallout during childhood causes a transient, radiation-induced autoimmune reaction with an increase in serum thyroid antibodies that does not trigger an increase in autoimmune thyroid disease (57).
Another more recent study of 10,827 individuals exposed to a known dose of 131I at the time of the Chernobyl accident found an association between the 131I dose and primary hypothyroidism (48). These data are consistent with a similar study of a Ukrainian cohort and suggests that environmental exposure to 131I during childhood is associated with primary hypothyroidism, but it is not associated with any other thyroid disease (57).
Although increases in rates of other cancers have been reported, much of these increases appear to be due to improvement in diagnostic procedures. Thus, apart from the large increase in thyroid cancer incidence in young people and excluding the high radiation exposure of the liquidators (power plant cleanup workers), there are at present no clearly demonstrated radiation-related increases in cancer risk. However, based on previous experience with ionising radiation, a small increase in the relative risk of cancer is expected which may nevertheless translate into a substantial number of radiation-related cancer cases in the future, given the very large number of individuals exposed (17).
BREAST CANCER ASSOCIATED WITH RADIATION EXPOSURE
Exposure to ionizing radiation and the association with cancer has been established for decades during specific situations such as direct exposure and fallout from atomic bombs, therapeutic radiation use in children, diagnostic radiation and environmental or occupational exposures (13–14, 17, 19, 62, 67, 75–79). The carcinogenic sensitivity of breast tissue to radiation is most prevalent at specific developmental ages and during biologic hyperplasia of breast tissue, such as in utero, early post-natal life of the infant, puberty, and lactation. However, NIS expression in normal adult breast occurs when there is cell proliferation, as only during pregnancy and lactation (47). Exposure to specific forms of radiation can cause a high dose of direct radiation to the breast, such as direct external radiation to the breast (13, 14, 67) and radioactive iodine, usually 131I, for treatment of hyperthyroidism and thyroid cancer during the first two to three decades of life (13–14, 62, 67). The latter exposure has been particularly prevalent following the Chernobyl accident because of the compounding dose of exposure from 131I used to ablate residual thyroid tissue after thyroidectomy for thyroid cancer that presents during childhood in many young women at a time when their breast tissues are sensitive to external radiation in the lower dose ranges of 0.2 to 0.7 Gy. The breast cancer risk is greater for pediatric female breast tissues than the low risk as seen with adult female breast tissues (67). Data from these young women indicate that the radiation dose to breast tissues of young children, adolescents and young adults may be as high as 0.35 to 0.55 Gy. This exposure range at these young ages is associated with a lifetime risk of breast cancer that ranges between 2 to 4 cases per 100 exposed individuals and 8 to 17 solid tumors per 100 exposed individuals (62). For these reasons there are recommendations that these high risk populations of young Chernobyl thyroid cancer patients who received ablation therapy with 131I need surveillance for an as yet undetermined length of time that may be a lifetime (61).
There are two studies (62, 67) and independent discussions about breast cancer and its relationship to radiation exposure from Chernobyl (5, 13–14). Whether or not there has been a significant increase in incidence of breast cancer remains undetermined. Even if there is an increase, it may or may not be caused by radiation exposure from the fallout of the Chernobyl accident, as it may result from an increased incidence for other reasons, or even both.
Between the periods of 1979 and 1985 before the accident and periods after the accident between 1986 and 2001, the numbers of breast cancer cases were compared among women living in the most contaminated oblasts of Belarus and Ukraine (62). The average contaminated dose of radiation in the selected oblasts was 40.0 mSv or greater. In these oblasts, a significant 2-fold increase was found during the years 1997 and 2001 (10 or more years after the accident) when compared to women in the same regions whose estimated cumulated dose was < 5.0 mSv, the least radiation exposure (62). The relative risks (RR) for Belarus and Ukraine were 2.24 (95% confidence interval, or CI, was 1.51–3.32) and 1.78 (95% CI 1.08–2.93) respectively, for the two countries. The highest increase was among the younger women age < 45 at the age of exposure, and a 14% increase in breast cancer in women with a cumulated exposure dose between 5.0 and 19.9 mSv. These data were based on cancer registries that also showed increases in breast cancer incidence in all areas after the accident, reflecting improved ascertainment and cancer diagnosis.
During similar intervals of time a similar study reported breast cancer trends after the Chernobyl accident among women in two oblasts of Belarus (67). The national cancer registry was used to compare breast cancer incidence between 1979 and 2003 in all women living in Gomel Oblast, having the highest Chernobyl radiation exposure, and in Vitebsk Oblast with the least radiation exposure from Chernobyl. The comparisons included rural and urban areas in these oblasts and specific ages at exposure. Breast cancer incidences were higher and more rapidly increasing in urban than rural areas in both Oblasts among women ages 30 to 49 years. Similar breast cancer trends were seen in women from urban and rural regions, though their data do not support evidence for Chernobyl-induced breast cancer in Belarus (68). These discrepancies may relate to the inadequate duration of latent periods from exposure to disease that may require an additional 3 or more decades of observation and analysis. The incidence of breast cancer among women exposed to the higher doses of radiation could be greater than currently considered (13–14).
Belarusian registry data showed that breast cancer incidence peaked at 45–49 years old for women living in the most contaminated areas when compared to pre-Chernobyl data in the contaminated areas as well as women living in the least contaminated regions (64 years peak age) (13–14, 68). Radiation exposure prior to the age of 20 years carries the greatest risk of breast cancer for reasons previously discussed (13–14, 59, 69–70). Genetic susceptibility data, frequent monitoring of women at increased risk and fiscal support for these and other educational programs are essential for the coming decades in order to detect breast cancer at the very earliest stage (5, 13–14, 62).
ADDRESS FOR CORRESPONDENCE:
Eliška Potluková, MD, Ph.D.
Division of Medicine
University Hospital Basel
Petersgraben 4, 4056 Basel, Switzerland
e-mail: eliska.potlukova@usb.ch
Sources
References are to be found at the end of the Part 2.
Labels
Addictology Allergology and clinical immunology Angiology Audiology Clinical biochemistry Dermatology & STDs Paediatric gastroenterology Paediatric surgery Paediatric cardiology Paediatric neurology Paediatric ENT Paediatric psychiatry Paediatric rheumatology Diabetology Pharmacy Vascular surgery Pain management Dental HygienistArticle was published in
Journal of Czech Physicians

- Advances in the Treatment of Myasthenia Gravis on the Horizon
- Possibilities of Using Metamizole in the Treatment of Acute Primary Headaches
- Metamizole at a Glance and in Practice – Effective Non-Opioid Analgesic for All Ages
- Metamizole vs. Tramadol in Postoperative Analgesia
- Spasmolytic Effect of Metamizole
-
All articles in this issue
- Ultrasound elastography and its use in the head and neck imaging
- The Cryopre-servation: history and the ethical issue of storing embryos
- Serum concentration and tubular resorption of sodium and chloride in patients with chronic renal disease
- Trajectory of anaesthesiology and intensive medicine – history, presence and prospects
- Wisdom of the World Medical Association (to one forgotten anniversary)
- JEAN DAUSSET (1916–2009)
- Pharmaconutrition in intensive and perioperative care
- New psychoactive substances and their prevalence in the Czech Republic
- Medical consequences of Chernobyl with focus on the endocrine system: Part 1
- Journal of Czech Physicians
- Journal archive
- Current issue
- About the journal
Most read in this issue
- New psychoactive substances and their prevalence in the Czech Republic
- Ultrasound elastography and its use in the head and neck imaging
- The Cryopre-servation: history and the ethical issue of storing embryos
- Pharmaconutrition in intensive and perioperative care
