Aorto-iliac endarterectomy: Old-fashioned or re-newed method?
Aorto-ilická endarterektomie: staro-nová metoda?
Úvod:
Aorto-ilická ateroskleróza je nejlépe léčena endovaskulárně angioplastikou/stentem nebo chirurgicky bypassem, podle závažnosti postižení. Aorto-ilická endarterektomie byla často prováděna až do 80. let, nicméně může být stále použita v případě selhání či kontraindikace standardních metod. Cílem práce je retrospektivní zhodnocení zkušeností jednoho cenra s pánevní endarterektomií.
Metodika:
Sedm pacientů o průměrném věku 60±8 let (57−68 let) podstoupilo pánevní endarterektomii v letech 2013−2018. Kategorie končetinové ischemie podle Rutherforda byly 2 (střední klaudikace) 3x, 3 (těžké klaudikace) 2x, 4 (klidová bolest) a 5 (gangréna prstů). Důvodem k endarterektomii bylo: pozdní in-stent pánevní okluze u onkologického pacienta, neúspěch nebo komplikace předchozí endovaskulární léčby krátké pánevní stenózy 2x, vysoké riziko infekce protézy u dlouhé pánevní okluze a krátký pánevní uzávěr 3x. Dva pacienti byli po předchozí orgánové transplantaci na imunosupresi.
Výsledky:
Technický uspěch byl 100 %. Nedošlo k perioperační (≤30 days) mortalitě nebo amputaci. Průměrné sledování je 17 měsíců (1,1 měsíce−3,3 roky). Jeden pacient potřeboval tibiální bypass 1 měsíc po endarterektomii pro zhojení gangrény. Jedna pacientka podstoupila pánevní stenting pro symptomatickou restenózu po 8 měsících. U všech došlo ke klinickému zlepšení a zotavení z končetinové ischémie. Dva pacienti zemřeli na nádorové onemocnění se zachovanou končetinou 1,1 měsíce a 3,1 roku po operaci. Pět ostatních dosud žije bez obtíží s průchodnou rekonstrukcí.
Závěr:
Aorto-ilická endarterektomie je vitální revaskularizační technika u vybraných pacientů v případě nevhodnosti ostatních metod.
Klíčová slova:
endarterektomie – ischemická choroba dolních končetin – pánevní tepna – břišní aorta
Authors:
J. Chlupac 1; T. Marada 1; F. Thieme 1,3; S. Malý 1,2; R. Novotný 1
; T. Pantoflicek 1; K. Sutoris 1
; R. Vysohlid 1; K. Lipár 1; L. Janousek 1,2; J. Froněk 1,3
Published in:
Rozhl. Chir., 2018, roč. 97, č. 11, s. 493-498.
Category:
Overview
Introduction:
Aorto-iliac occlusive disease is best treated with endovascular angioplasty/stenting or surgical bypass, depending on disease severity. Aorto-iliac endarterectomy was frequently used until the 1980s. However, it can still be performed in cases of previous failure or contraindication of standard methods. The aim was a retrospective evaluation of a single-center case series of aorto-iliac endarterectomy.
Methods:
Seven patients at mean age 60±8 years (57−68 years) were treated by aorto-iliac endarterectomy between 2013 and 2018. Rutherford categories of leg ischemia were 2 (moderate claudication) 3x, 3 (severe claudication) 2x, 4 (rest pain) and 5 (toe gangrene). The reasons for endarterectomy approach were: late in-stent iliac occlusion in an oncology patient, failure or complication of previous endovascular treatment of short iliac stenosis 2×, high infection risk of prosthesis use in long iliac-femoral occlusion, and short iliac occlusions 3x. Two patients after previous organ transplant were on immunosuppression.
Results:
Technical success rate was 100%. There was no peri-operative (≤30 days) death or amputation. Mean follow-up was 17 months (1.1 month−3.3 year). One patient required additional tibial bypass 1 month after endarterectomy to heal foot gangrene. One patient developed symptomatic re-stenosis which was treated with iliac stenting 8 months after procedure. All patients clinically improved and recovered from leg ischemia. Two patients died of tumor with preserved limb 1.1 month and 3.1 years after procedure, respectively. Five remaining patients are asymptomatic with patent revascularization to date.
Conclusion:
Aorto-iliac endarterectomy is a vital alternative technique for revascularization in selected patients when other methods seem inappropriate.
Key words:
endarterectomy – peripheral arterial disease – iliac artery – abdominal aorta
Introduction
Endarteretectomy (EA) is a well-established method of surgical treatment of atherosclerotic steno-occlusive disease of the peripheral arterial system. Direct open technique consists in exposure of the diseased segment of the artery, opening it and removing sclerotic intima-media tissue including potential luminal thrombus (thrombo-endarterectomy, TAE) [1]. Intimal irregularities especially at the distal endpoint of the endarterectomy can be fixed by tack sutures to prevent downstream dissection and/or thrombosis of the arterial wall. Direct closure with vascular stitches or patch plasty of the arteriotomy follows. Blood supply to the diseased region is restored by virtually re-opening the native artery. Moreover, risk of arterial thrombo-embolism from the stenosis is reduced. Interestingly, the endarterectomized arterial wall, which is sometimes as thin as the adventitia, does not tend to form aneurysm or rupture [2].
The EA is a method of choice in surgery of the internal carotid artery and of the common femoral artery (AFC). Standard revascularization methods for lower extremity ischemia in the aorto-iliac region are percutaneous transluminal angioplasty (PTA) with iliac stenting or anatomical bypass graft (aorto-bi-femoral, aorto-iliac, iliac-femoral) or extra-anatomic bypass graft (femoro-femoral, iliac-femoral or axillo-femoral) [3]. However, in some cases it may be reasonable to perform an “old-fashioned” aorto-iliac endarterectomy (AIEA) of the atheromatous native arterial bed especially when standard methods fail or are contraindicated. In a selected group of cases this method may offer durable results comparable to bypass grafting with minimal risk of graft infection [4].
The aim was a retrospective evaluation of single-center case series of aorto-iliac endarterectomy procedure.
Methods
Seven patients at median age 60±7 years (57−68 years) were treated by AIEA in our tertiary referral hospital between 2013 and 2018. Patient data were retrospectively evaluated from a database. Patient demographics, Society for Vascular Surgery (SVS) comorbidity score [5], length of hospitalization, category of leg ischemia and clinical statuses are shown in Tab. 1. Mean SVS score (0−24 points) was 7.7±2.1. Rutherford categories of leg ischemia [5] were 2 (moderate claudication) 3x, 3 (severe claudication) 2x, 4 (rest pain) and 5 (toe gangrene).

Preoperative CT angiogram was done in all cases. The reasons for endarterectomy approach were: late in-stent iliac occlusion in an oncology patient 1x, failure or complication of previous endovascular treatment of short iliac stenosis 2x, high infection risk of prosthesis use in long iliac-femoral occlusion 1x, and short iliac occlusions 3x. Two patients after previous kidney and heart transplant were on immunosuppression thus having an increased risk of infection. All cases were discussed by a multidisciplinary team. The final decision to perform the EA was left at the discretion of a specialized vascular surgeon during the operation.
All subjects were regularly followed up in an out-patient clinic. Patency of the EA segment was evaluated by the presence of groin pulses, clinical assessment of leg ischemia symptoms and no restenosis >50% on Doppler ultrasound. The outcome measures were patency, clinical improvement, limb salvage and survival.
Operative technique
The patient is laid in supine position under general anesthesia. Epidural anesthesia is sometimes also used for perioperative and postoperative pain relief. Cefazoline is administered for infectious prophylaxis. Pararectal or oblique skin incision is performed in the lower abdomen (our cases 2−7). Distal external iliac artery (AIE) can be reached by inguinal incision (our case 1). Iliac arteries and/or abdominal aorta are exposed via retroperitoneal approach and encircled with rubber loops. The ureter is pulled laterally. Heparin is administered. The artery is cross-clamped or balloon-occluded proximally and distally to the diseased region. Longitudinal arteriotomy is performed and the lumen is carefully cleared of any sclerotic and thrombotic material. The open endarterectomy is performed with spatula and the atheroma is removed by dividing vessel walls in outer media od even close to the adventitial layer. The aim is to re-open the lumen and to achieve satisfactory blood inflow. The endarterectomy may produce a step-like proximal and distal plaque endpoint which is usually fixed by tacking sutures distally. This prevents dissection of the layers of the artery or thrombosis downstream of the procedure. Inflow and back-bleeding is tested by shortly releasing the clamps. The newly formed lumen is flushed with saline with heparin and the arteriotomy is closed by running polypropylene suture. The artery is de-clamped and heparin may be reversed by protamin-sulphate. Drain is introduced, and the wound is closed layer by layer. The patient is monitored in the intensive care unit for 1−2 days and discharged from the hospital on day 4. Physical exercise should be avoided for 4−6 weeks. Antiplatelet and statin therapy is mandatory.
Results
Anatomy of lesions treated (Trans-Atlantic Inter-Society Consensus, TASC II categories), operative details, outcomes and follow up are shown in Tab. 2. Technical success rate was 100%. Prosthetic patch plasty was used once. Otherwise the arteriotomy was directly sutured. Bilateral disease was operated on once (case 7). The EA was extended to femoral bifurcation twice (cases 1 and 4). There was no involvement of internal iliac in any case.

There was no peri-operative (≤30 days) death or amputation. Mean follow up was 17 months (32 days−3.3 years). One patient sustained worsening of pre-existing chronic renal dysfunction resulting in hemodialysis. This was partly also due to tumor cachexia (case 1). One patient required additional posterior tibial bypass 1 month after endarterectomy to heal foot gangrene (case 4). One patient developed symptomatic re-stenosis at the distal endpoint of the EA of the AIE 8 months after procedure which was treated percutaneously with iliac stenting (case 3). All patients clinically improved and recovered from leg ischemia. Two patients died of tumor with preserved limb 1.1 month (case 1) and 3.1 years (case 2) after the procedure, respectively. Five remaining patients are asymptomatic with patent revascularization to date.
Discussion
We present a series of 7 patients who underwent aorto-iliac endarterectomy at our institution as an alternative technique to other standard revascularization methods in the aorto-iliac region. All procedures were technically successful. All patients clinically recovered from leg ischemia and mean follow up is 17 months. Limitations of our present study are the retrospective nature, small sample size and only short to mid-term follow-up.
Endarterectomy was first introduced on superficial femoral artery in 1947 by dos Santos for the treatment for atherosclerosis of peripheral arteries and applied for the aorto-iliac segment by Wylie et al. in 1952 [4]. It was the first method of direct revascularization for aorto-iliac occlusive disease and was frequently used during the 1950s and the 1960s. Aorto-iliac EA was gradually replaced by prosthetic graft materials and endovascular methods in most cases. Thus, the AIEA has become a “lost art” [2] of “few surgeons trained in the past” [4].
Several techniques for AIEA have been described: 1) the classic open method via longitudinal arteriotomy with or without patch closure (autologous, xenologous or synthetic patch), 2) semi-closed removal of the atheroma through proximal and distal transverse arteriotomies using a ring stripper pushed through the unopened vessel part [6], 3) hybrid remote endarterectomy using the ring stripper from femoral access with subsequent balloon angioplasty/stent of proximal transection zone [7], 4) the eversion technique with transection and reimplantation of the artery, usually the AIE [8], 5) pulsion endarterectomy in which the atheromatous plaque is extruded from the intact artery through femoral or internal iliac arteriotomy by external digital fragmentation and manipulation [9].
Aorto-iliac EA offers several advantages and indications: no prosthetic material use, better inflow to internal iliac artery (for gluteal claudication or erectile dysfunction in men), very low rate or virtually no prosthetic graft infection, which is a predominant advantage [10] no formation of anastomotic false aneurysms (the risk of false aneurysm increases with patch closure) [1], possible use in contaminated field or in case of prosthetic infection [4], direct anatomical revascularization with linear flow, no need for contra-lateral inflow or risk of steal syndrome as in extra-anatomic femoro-femoral bypass, avoidance of groin access for possible future percutaneous angioplasty or in case of groin sepsis [11], cost-effectiveness (no cost for the prosthetic graft), preservation of collaterals (lumbar, internal iliac, circumflexes) and thus less severe ischemia in case of thrombosis of the EA compared to thrombosis of a bypass graft [1], significantly lower morbidity and mortality rate in comparison with aorto-iliac-femoral bypass grafting and excellent and durable results [6].
The disadvantages include more technically demanding procedure, sometimes higher blood loss [6] and possible hypogastric plexus injury with sexual dysfunction in men. Contraindications to EA that favor bypass grafting are: aneurysmal disease, total aortic occlusion up to renal arteries, more distal extension of atherosclerosis to the groin, i.e. to the AIE and the AFC. Extended aorto-iliac(-femoral) EA have been abandoned and replaced with simple and faster bypass grafting. Extension of the AIEA distally to longer, thinner, tortuous and more muscular external iliac artery is documented to have a higher risk of early thrombosis and late stenosis [4]. However, isolated EA of the AIE with bovine pericardial patch closure [11] or aorto-iliac-femoral EA [6] are also feasible methods.
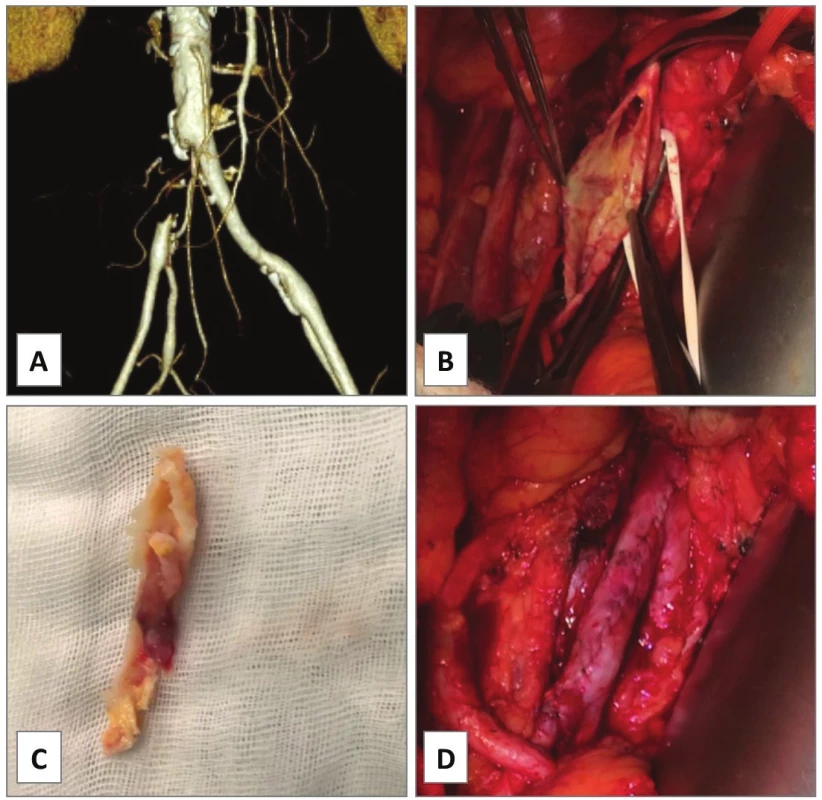
In our series, EA of the AIE was performed in cases 1−4. In case 1, occluded AIE stent was extracted and the left AIE and the AFC were endarterectomized from groin access only as a semi-invasive procedure for severe rest pain of the leg in a comorbid patient.Unfortunately, he died of rapidly progressing pancreatic cancer on postoperative day 32. In case 2, PTA of simple stenosis of the left AIE was converted to open EA with prosthetic patch plasty due to severe bleeding from vessel perforation with a guidewire. Primary patency was 3.1 years until death of lung cancer. In case 3, an endovascular specialist failed to cross an excentric complex lesion of the left AIE with guidewire (Fig. 1A) in a patient with renal transplant (located in the right iliac fossa). Open EA with direct closure followed (Fig. 1B−D). Indeed, symptomatic re-stenosis occurred at the distal endpoint after 8 months (Fig. 1E). Iliac stenting was performed (Fig. 1F) thus the EA having primary assisted patency for 3.3 years to date. In case 4, chronic total occlusion of the left common iliac artery (AIC), AIE and AFC was encountered (Fig. 2A). Such extent of the disease would normally require bypass grafting [4]. However, comorbidity and infection risks were high: presence of toe gangrene, concomitant radiotherapy for prostate cancer, permanent urinary catheter, chronic urinary infection and a mega-ureter due to sub-vesical obstruction. Thus, an extensive ilio-femoral EA with outflow to profunda femoris artery (APF) was carried out with good technical success (Fig. 2B). Persistent gangrene (Fig. 2C) required additional posterior tibial bypass with saphenous vein graft after 1 month resulting in healed trans-metatarsal amputation (Fig. 2D) and primary patency of the EA at 1.9 years.


Our cases 5−7 had a typical proximal type I of atherosclerosis of the aorto-iliac segment [4]. Cases 5 and 6 were similar: heavily calcified TASC C chronic total occlusions of the AIC. Such lesions may newly be treated by endovascular recanalization/stenting with satisfactory results. In our department, management with aorto-iliac bypass is preferred. However, isolated EA of the AIC is an equal alternative technique (Fig. 3) especially in younger patients (case 5, age 48 years).
In case 7, CT angiogram showed bilateral stenosis of the origin of the AIC with heavy mural calcifications causing ultra-short claudication of 5 m in a heart transplant patient (Fig. 4A). Kissing stents procedure was intended, however, conventional angiography revealed propagation of the stenotic process up to the distal aorta (Fig. 4B). Due to the immune-compromised status, open EA of the aortic bifurcation but no prosthetic grafting was accomplished with good immediate technical and clinical success (Fig. 4C−F).

There is no direct randomized comparison among different AIEA techniques or with bypass procedures [9]. A retrospective review of open surgical management of aorto-iliac occlusive disease by Chiu et al. (2010) compared aorto-femoral bypass (AFBP), iliac-femoral bypass (IFBP) and the AIEA [12]. The systemic peri-operative morbidity was 16.0% for AFBP, 18.9% for IFBP and 12.5% for AIEA, respectively (p<0.05). Mortality rate was 4.1% for AFBP, 2.7% for IFBP and 2.7% for AIEA, respectively (p<0.05). Overall 5-year primary patency rates were 86% for AFBP, 85% for IFBP and 88% for AIEA, respectively (p=n.s.). Thus, the AIEA showed significantly lower morbidity and mortality rates with similar long-term patency compared with aorto-femoral bypass. Patency rates of extra-anatomic bypasses are somewhat inferior having 5-year patency of 50−75% [3].
Current guidelines recommend treating localized aorto-iliac occlusive disease (TASC A and B) by endovascular methods and extensive atherosclerotic disease (TASC C and D) by surgical revascularization [3]. A systematic review of endovascular treatment of extensive aorto-iliac occlusive disease (TASC C and D) reported 5-year primary patency rates ranging 60−86% being lower than those for surgical repair. Mortality was described from 1.2% to 6.7%. However, reinterventions can often be performed percutaneously with secondary patency (5-year: 80−90%) comparable to surgical repair [13].
We believe that all modalities for the management of aorto-iliac disease (endovascular treatment, anatomic or extra-anatomic bypass, endarterectomy) are complementary and vital methods. Moreover, they can complement each other in cases of failure or contraindication of one or another method, recurrent disease and redo procedures. Each of them may be suitable for various parts of the spectrum of the occlusive disease and they all should be part of the armamentarium of the vascular team.
Conclusion
Endarterectomy is an old technique that remains an important adjunctive method in the spectrum of repair modalities for aorto-iliac occlusive disease. Besides conventional catheter-based techniques and anatomic or extra-anatomic bypass grafting it can become truly innovative when these standard methods seem inappropriate. These situations include early or late failure of endovascular therapy, severe comorbidity, high infection risk of prosthetic bypass or focal steno-occlusive disease especially in young patients. Prerequisites for satisfactory results are proper patient selection and a correct operative technique. Our retrospective case series shows favorable mid-term outcomes.
Abbreviations
AA − abdominal aorta
AFBP − aorto-femoral bypass
AFC − common femoral artery
AFS − superficial femoral artery
AIC − common iliac artery
AIE − external iliac artery
AIEA − aorto-iliac endarterectomy
Amp. − amputation
APF − profunda femoris artery
bilat. − bilaterally
car − carotid status
CT − computed tomography
d − days
DM − diabetes mellitus
dx. − right-sided
EA − endarterectomy
F − female
h − hours
HLP − hyperlipoproteinemia
hosp. − hospitalization
HT − hypertension
IFBP − iliac-femoral bypass
inf. − infection
IS − immunosuppression
M − male
m − minutes
m − months
ml − milliliters
Nic − nicotinism
onco − oncologic disease
PTA − percutaneous transluminal angioplasty
pulm − pulmonary status
ren − renal status
SD − standard deviation
sin. − left-sided
SVS − Society for Vascular Surgery
TASC − Trans-Atlantic Inter-Society Consensus
TEA − thromboendarterectomy
tot. − total
Tx − organ transplant status
y − years
Conflict of interests
The authors declare that they have not conflict of interest in connection with the emergence of and that the article was not published in any other journal.references
MUDr. Jaroslav Chlupáč Ph.D.
Transplant Surgery Department
Institute for Clinical and Experimental Medicine
Vídeňská 1958/9
Prague 4 − Krč
e-mail: jaroslav.chlupac@ikem.cz
Sources
1. Redox JM, Maize D, Coffin O. Long-term outcome of 121 iliofemoral endarterectomy procedures. Ann Vass Surg 2001;15 : 163−70.
2. Connolly JE, Price T. Aortoiliac endarterectomy: a lost art? Ann Vasc Surg 2006;20 : 56−62.
3. Norgren L, Hiatt WR, Dormandy JA, et al. Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II). Eur J Vasc Endovasc Surg 2007;33Suppl1:S1−75.
4. Brewster CD. Direct reconstruction for aortoiliac occlusive disease. In: Rutherford RB. Rutherford vascular surgery. 6th edition, Philadelphia, USA, Elsevier Saunders 2005 : 1106−36.
5. Rutherford RB, Baker JD, Ernst C, et al. Recommended standards for reports dealing with lower extremity ischemia: revised version. J Vasc Surg 1997;26 : 517−38.
6. Oertli D, Wigger P, Landmann J, et al. Long-term results after open and semiclosed thrombendarterectomy for aortoiliac occlusive disease. Eur J Vasc Endovasc Surg 1996;11 : 432−36.
7. Bekken JA, de Boer SW, van der Sluijs R, et al. Remote iliac artery endarterectomy: A case series and systematic review. J Endovasc Ther 2018;25 : 140−9.
8. Inahara T. Eversion endarterectomy for aortoiliofemoral occlusive disease. A 16 year experience. Am J Surg 1979;138 : 196−204.
9. Wall ML, Davies RS, Sykes TC et al. Iliofemoral pulsion endarterectomy. Ann Vasc Surg 2009;23 : 259−63.
10. van den Akker PJ, van Schilfgaarde R, Brand R, et al. Long-term results of prosthetic and non-prosthetic reconstruction for obstructive aorto-iliac disease. Eur J Vasc Surg 1992;6 : 53−61.
11. Ebaugh JL, Gupta N, Raffetto JD. Single-incision external iliac artery endarterectomy and patch angioplasty. Ann Vasc Surg 2011;25 : 1165−9.
12. Chiu KWH, Davies RSM, Nightingale PG, et al. Review of direct anatomical open surgical management of atherosclerotic aorto-iliac occlusive disease. Eur J Vasc Endovasc Surg 2010;39 : 460−71.
13. Jongkind V, Akkersdijk GJ, Yeung KK, et al. A systematic review of endovascular treatment of extensive aortoiliac occlusive disease. J Vasc Surg 2010;52 : 1376−83.
Labels
Surgery Orthopaedics Trauma surgeryArticle was published in
Perspectives in Surgery

2018 Issue 11
- Possibilities of Using Metamizole in the Treatment of Acute Primary Headaches
- Metamizole at a Glance and in Practice – Effective Non-Opioid Analgesic for All Ages
- Metamizole vs. Tramadol in Postoperative Analgesia
-
All articles in this issue
- Indications to open surgical revascularization of visceral arteries in the endovascular era
- Trauma of the extracranial cerebral arteries due to injuries of the cervical spine
- Branched pedal bypass in the treatment of critical limb ischemia – a single center experience
- Dunbar syndrome – single-center experience with surgical treatment
- Hypogastric artery aneurysm – a case report
- Early surgical and vascular complications after open repairs of aortoiliac aneurysms
- Aorto-iliac endarterectomy: Old-fashioned or re-newed method?
- Perspectives in Surgery
- Journal archive
- Current issue
- About the journal
Most read in this issue
- Dunbar syndrome – single-center experience with surgical treatment
- Hypogastric artery aneurysm – a case report
- Trauma of the extracranial cerebral arteries due to injuries of the cervical spine
- Indications to open surgical revascularization of visceral arteries in the endovascular era
