Benefits of hybrid methods (PET/CT, PET MRI) in the diagnosis of abdominal aortic pathology
Přínos hybridních metod (PET/CT, PET MRI) v diagnostice patologických nálezů břišní aorty
Úvod: Ultrasonografie a CT angiografie jsou běžné diagnostické zobrazovací metody používané k diagnostice patologií břišní aorty. V posledních letech se objevují často indikace k hybridním metodám (PET/CT, PET/MRI) v rámci tohoto diagnostického algoritmu. Původně byly tyto metody indikovány u malignit a zánětlivých procesů. V současnosti je snaha zobrazit lokalizované zánětlivé změny ve stěně aorty a hodnotit tak určitou „disease activity“, která by mohla předpovídat další vývoj patologického nálezu na aortě. Cílem naší práce bylo analyzovat potencionální benefit těchto hybridních metod ve smyslu predikce progrese patologií na břišní aortě.
Metody: Do naší prospektivní observační studie jsme zahrnuli 75 nemocných, u kterých bylo indikováno PET/CT (N=61) resp. PET/MRI (N=14) vzhledem k patologickému nálezu na břišní aortě v období 2015–2017. Jednalo se o pacienty s aneuryzmatem břišní aorty (AAA) (N=48; 64%), aortitidou (N=5; 6,7 %), aortální disekcí (N=4; 5,3 %), po endovaskulárním řešení AAA (EVAR) (N=6; 8%), s excesivní aterosklerózou (N=7; 9,3 %), nemocné s konkomitantně přítomným AAA a retroperitoneální fibrózou (N=4; 5.3%) a pacienty s intramurálním hematomem (N=1; 1,3 %). Minimální doba sledování byla 6 měsíců, maximální 2,5 roku. Během follow-up byly sledovány klinické symptomy, rozměr aorty, rychlost růstu AAA, hladina CRP a vše bylo korelováno s nálezem na PET/CT event. PET/MRI.
Výsledky: Zvýšená metabolická aktivita v aortě byla zaznamenána u 25 nemocných (33,3 %). Na základě statistického hodnocení nebyla nalezena souvislost mezi nálezem na PET/CT event. PET/MRI a symptomy onemocnění či jeho progrese.
Závěr: Na základě našich výsledků jsme nenalezli důkaz, že hybridní metody mohou předpovídat další vývoj patologického nálezu na břišní aortě. Aktivita na PET/CT či PET/MRI nekorelovala ani se symptomy onemocnění, ani s rychlostí progrese AAA či disekce. Naše výsledky podporují rovněž některá recentní literární data.
Klíčová slova:
PET/CT – PET/MR – Aorta – aneuryzma – disekce
Authors:
J. Molacek 1,3; J. Baxa 2,3; V. Opatrný 1; V. Treska 1,3; I. Hollan 4; J. Ferda 2,3,5,6
Authors‘ workplace:
Department of Surgery, University Hospital Pilsen
1; Department of Imaging Methods, University Hospital Pilsen
2; School of Medicine in Pilsen, Charles University
3; Hospital for Rheumatic Diseases, Department of Rheumatology, 2609 Lillehammer
4; Department of Research, Innlandet Hospital Trust, Brumunddal
5; Division of Cardiology, Department of Medicine, Brigham and Women’s Hospital, Boston
6
Published in:
Rozhl. Chir., 2019, roč. 98, č. 11, s. 450-456.
Category:
doi:
https://doi.org/10.33699/PIS.2019.98.11.450–456
Overview
Introduction: Ultrasound and CT angiography are common diagnostic methods of abdominal aortic pathologies. In the last decade, hybrid methods (PET/CT, PET/MRI) have become more common in this diagnostic algorithm. Originally they were indicated in malignancies or inflammatory processes. Currently, efforts are developed to visualize possible local inflammatory activity in the aortic wall and thus to assess a certain “disease activity” with the goal to anticipate further development of aortic pathology. The aim of our study was to analyze potential benefits of hybrid methods in predicting abdominal aortic pathology progression.
Methods: In this prospective, open-label, observational study we examined 75 patients referred to PET/CT (N=61) or PET/MRI (N=14) due to any aortic pathology in 2015–2017. The patients included those with abdominal aortic aneurysm (AAA) (N=48; 64%), aortitis (N=5; 6.7%), aortic dissection (N=4; 5.3%), patients undergoing EVAR (N=6; 8%), patients with excessive atherosclerosis (N=7; 9.3%), patients with concomitant AAA and retroperitoneal fibrosis (N=4; 5.3%) and patient with an intramural hematoma (N=1; 1.3%). The minimum follow-up period was 6 months (0.5–2.5 years). Clinical symptoms, aortic diameter, growth rate and CRP levels were analyzed during the follow-up and correlation with PET/CT or PET/MRI findings was evaluated.
Results: Increased metabolic activity in the aorta was found in 25 of the 75 examined patients (33.3%). Based on statistical analysis there were no associations between increased activity based on PET/CT or PET/MRI in the aortic wall and disease symptoms or progression.
Conclusion: Our results provide no evidence that hybrid methods can predict further development of pathological findings in the abdominal aorta. PET/CT - or PET/MRI-based activity did not correlate with disease symptoms, AAA progression rate or dissection, either. Our results are also supported by some recent literature data.
Keywords:
dissection – Aorta – PET/CT – PET/MRI – aneurysm
Introduction
At present, ultrasound scanning (USG) and CT angiography (CTA) are fully adequate for the diagnostic algorithm of abdominal aortic pathology in a great majority of cases, and both are routinely used in clinical practice. CTA in particular has high sensitivity and specificity for pathological conditions occurring in the aorta [1].
The question is what is the reason for indicating new hybrid techniques – positron emission tomography combined with CT scanning (PET/CT), and positron emission tomography combined with magnetic resonance imaging (PET/MRI). In addition to commonly used indications of these methods for inflammatory or rarely tumorous involvement of the aorta, hybrid methods are increasingly being used for “functional diagnosis” in abdominal aortic aneurysms (AAA) as well as other pathologies [2]. The aim is to try to visualize possible local inflammatory activity and thus certain “disease activity” in the aortic wall with the intention to anticipate further development of AAA.
This hypothesis can also be formulated in other aortic pathologies such as dissection, intramural hematoma, inflammation, etc. The aim of this paper was to try to find a correlation between activity in the aortic wall based on PET/CT or PET/MRI imaging and the behavior, symptoms and progression of potential AAA or non-dilated aorta.
Methods
Patients
In this prospective, open-label, observational study we have included 75 patients referred to PET/CT or PET/MRI at the University Hospital due to any aortic pathologies in 2015–2017. PET/CT was performed in 61 and PET/MRI in 14 patients. All were Caucasians, >18 years old. The indications for the hybrid methods included: AAA (N=48; 64%), suspected aortitis (N=5; 6.7%), aortic dissection (N=4; 5.3%), condition after endovascular AAA treatment (N=6; 8%), excessive atherosclerosis (N=7; 9.3%), concomitant AAA and suspected retroperitoneal fibrosis (N=4; 5.3%) and intramural hematoma (N=1; 1.3%). The study was approved by the institutional ethical review board and the patients signed an informed consent form.
Assessment and follow-up
All patients were examined repeatedly; the minimum follow-up period was 6 months (0.5–2.5 years). Assessments performed at the first visit included PET/CT or PET/MRI, physical examination and evaluation of the circulating biomarker C-reactive protein (CRP). Elevated CRP levels were defined as >20 mg/L. Additional examinations included any imagine technique to compare the aortic size (ultrasound, CTA, PET/CT, PET/MRI), and also physical examination.
Imaging methods
The PET/CT was performed using an instrument with a 128-line CT subsystem and a four-ring PET subsystem (Biograph mCT 128, Siemens Healthineers, Knoxville, TN, USA) following administration of 18F-FDG (fluorodeoxyglucose, UJV, Rez u Prahy, Czech Republic) at 2.5 MBq/kg. CT data were acquired using a 100 kV protocol with automatic voltage reduction down to 80 kV in sthenic patients. Data were reconstructed in diagnostic images using iterative SAFIRE data reconstruction at a 0.75mm image width with 0.6mm reconstruction increment and a soft tissue algorithm. PET data were reconstructed in a 400x400 point matrix in a 46cm diameter transaxial field-of-view using the ultraHD algorithm that combines the TOF (time-of-flight) and PSF (point spread function) algorithms with 2mm FWHM. Data acquisition was performed by the step-and-shoot technique using 5 to 7 positions, with the data acquisition time of 1.5 minutes per position. The CT scan was performed after intravenous administration of iodinated contrast agent (Ultravist 370, Bayer Pharma, Berlin, Germany) at a quantity of 60–100 mL according to the weight of the examined person. The examination was performed in the early arterial phase throughout the body and in the late venous phase in the abdomen and pelvis.
PET/MRI scanning was performed using an integrated PET/MRI system (Biograph mMR, Siemens Healthineers, Erlangen, Germany) with the administration of 18F-FDG at 2.5 MBq/kg. As part of the examination, whole-body scanning was performed using T1 VIBE Dixon two-point, TIRM T2 STIR and DWI sequences following administration of gadolinium contrast agent. The reconstruction of PET images was done using the VIBE sequence to correct attenuation; OSEM reconstruction algorithm was used. The dose of 4 mL of 1-molar gadobutrol (Gadovist, Bayer Pharma, Berlin Germany) was used. PET data acquisition was performed over 4 minutes in each scanning position of 5 to 7 positions.
Evaluation of imaging methods
The evaluation was performed using the dedicated syngo.via MM software (Siemens Healthineers, Forchheim, Germany). The analysis was carried out by consensus of two experienced specialists double boarded in radiology and nuclear medicine who used ROI (region of interest) analysis to determine the maximum accumulation of FDG in the vascular wall and, at the same time, performed an overall assessment of changes in the abdominal aortic wall.
Terminology
In consistence with recent literature, we use the term “mycotic AAA” for AAA clearly caused by infections, in most cases with confirmation of infectious agents in the aortic wall or blood culture [3,4]. On the other hand, we save the term “inflammatory AAA” for AAA without clear infectious etiology where an autoimmune component is likely to be important [5].
Statistical analysis:
The log-rank test was used for the analysis, and associated p values <0.05 were defined as significant.
Results
Increased metabolic activity in the non-dilated aorta either in the AAA wall, atherosclerotic plaque, or an intramural hematoma on PET/CT or PET/MRI was found in 25 of the 75 examined patients (33.3%).
Overall, in terms of increased activity in PET/CT or PET/MRI, we found an atypical positive finding in these patients (15 cases of AAA, 1 after EVAR), 3 cases of aortitis, 6 cases of atherosclerotic changes, 1 case of intramural hematoma). In the remaining 50 patients (66.7%), we did not observe increased metabolic activity in the aortic area.
Data on patients with increased activity in the aortic wall are shown in Tab. 1.
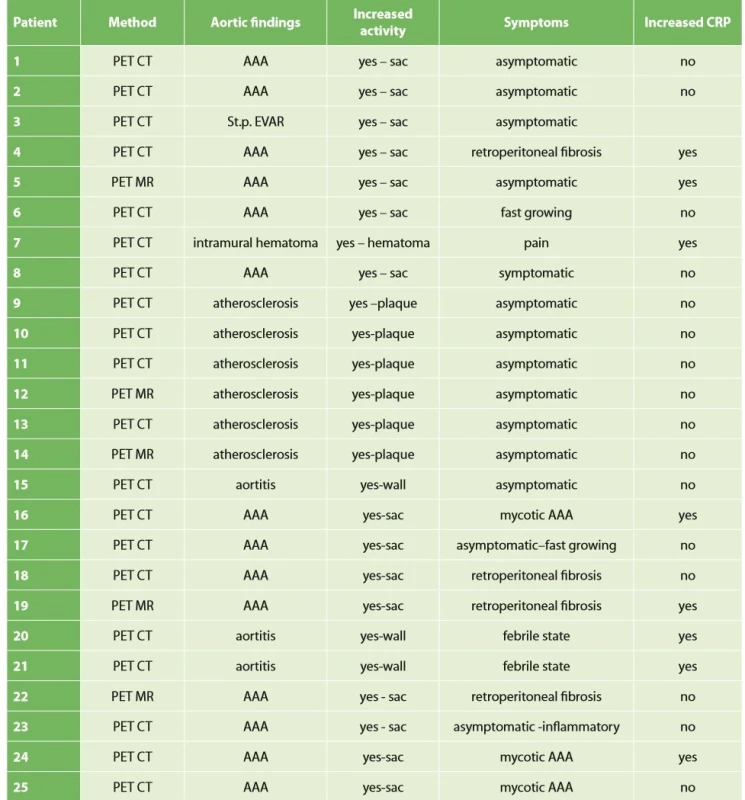
A summary of all patients examined, their findings in the aorta and potential correlation of symptoms with positive PET/CT or PET/MRI findings and correlation of CRP levels with positive PET is presented in Tab. 2.
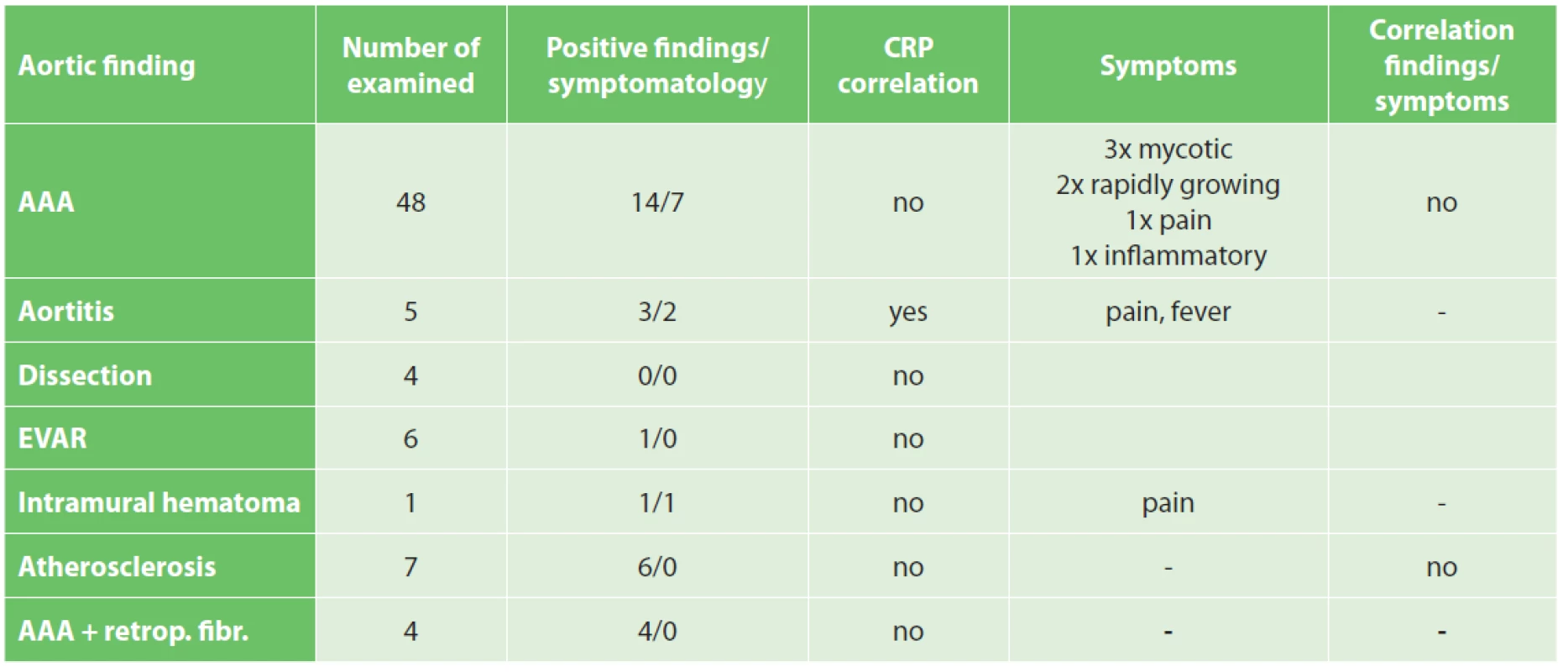
There were no associations between increased activity during PET/CT or PET/MRI in the AAA wall and its symptoms (pain, fever…) or growth rate (growth rate was analyzed as a increase diameter of AAA in the follow-up period).
Similarly, we found no association between atherosclerotic aortic wall activity and clinical manifestations or further progression of these findings.
We found an association between CRP levels and positive findings in PET/CT o PET/MRI in aortitis but the number of patients was insufficient for statistical analysis.
Discussion
For years, many research teams have been trying to develop a method that could predict the “behavior” of AAA in clinical practice. This includes in particular a prediction of progression of the aneurysm size, which may serve as an indication for surgical or endovascular intervention. Rutherford postulated the relationship between the AAA size and the risk of its rupture many years ago [6]. In recent years, the aneurysm volume has been reported, in addition to the maximum dimension, as an additional marker of rupture risk [7]. On the other hand, a number of papers describe rupture of small aneurysms (smaller than 5 cm), while large AAAs can be stable [8]. It is clear that the AAA size may not be the only factor playing a role in the risk of rupture. The ideal goal of research in this field would be to obtain a method predicting the potential progression of AAA. This would be especially useful for small AAAs where open or endovascular management is not yet indicated or for patients who have large aneurysms but a high operation risk.
Many teams have been involved in modeling AAA rupture risk based on numerical simulation [9–11]. Another potential method studied aneurysm wall “distensibility”, performing an evaluation of a certain “stretching capacity” of the aneurysm wall during the pulse wave [12,13]. All of these methods bring promise for the future but are not routinely used in clinical practice.
A lot of evidence supports the key role of inflammation in the AAA etiopathogenesis [14–17]. Options for monitoring inflammatory activity in vessel walls are limited and include measurements of circulating markers such as MMPs, inflammatory cell activity markers and cytokine levels. Despite extensive research in this field, none of these markers have not integrated into clinical practice as their circulating levels do not necessarily reflect their levels in the aortic wall. Indeed, a clear correlation between any of the circulating cytokines and AAA symptoms has not been confirmed by extensive studies.
In the last decade, the benefits of hybrid imaging techniques have been considered a promising option. These imaging techniques, which were primarily developed for the detection of inflammation or malignancy, may be promising, according to a number of papers, in detecting this inflammatory process in the aortic wall to predict the behavior of aneurysms, as well as atherosclerotic ulcers or aortic dissection [18–20]. The anticipated benefit of PET/CT scanning was the detection of an active ongoing inflammatory process in the aortic wall or even aortic blebs, described many years ago, which are local deformities often infiltrated by polymorphonuclear cells [21].
A number of clinical trials have demonstrated selective binding of transporters used in hybrid techniques to microcalcifications in coronary or carotid arteries, visualizing a “vulnerable” plaque with a higher risk of rupture [22]. Further work has shown a correlation between a higher uptake of 18F-FDG and higher AAA wall stress [23]. This is probably also related to a correlation between 18F-FDG activity and higher long-term growth of AAA [24,25]. The ability of hybrid techniques to detect inflammatory activity in the AAA wall, in particular the activity of macrophages, has been demonstrated [26]. In general, a number of papers show a higher activity in the AAA wall in PET/CT scanning as a marker of disease activity [27]. On the other hand, some studies have rejected this hypothesis [26,28]. One of the largest meta-analyzes dealing with this issue was published in 2015 with contradictory conclusions of individual studies (29).
The basic question we asked in our study is whether PET/CT or PET/MRI can be effectively used to monitor the development, progression and potential symptoms of AAA and other pathological conditions in the aorta. In our group of patients, we found an increased FDG uptake on PET/CT or PET/MRI in 14 of 48 cases of AAA (29.2%), but only 7 patients had clear symptoms (pain) or a fast-growing, mycotic or inflammatory AAA. On the other hand, hybrid imaging did not reveal higher activity in the remaining 34 cases of AAA in our population, which also included patients with rapidly growing or symptomatic aneurysms (N=8; 23.5%). Therefore, we have not found a clear correlation between higher activity in PET/CT (PET/MRI) and the symptoms or growth rates in patients with AAA. Similarly, we found no correlation between positive findings in excessive aortic atherosclerosis and potential symptoms or further progression of the aortic pathology. The numbers of patients with aortic dissection or aortitis are too small for any statistical evaluation.
In our study we did not confirm certain conclusions of other authors about the contribution of hybrid techniques as a functional examination able to predict potential development of a degenerative aortic disease [29]. Our conclusions are supported by other recent publications (40).
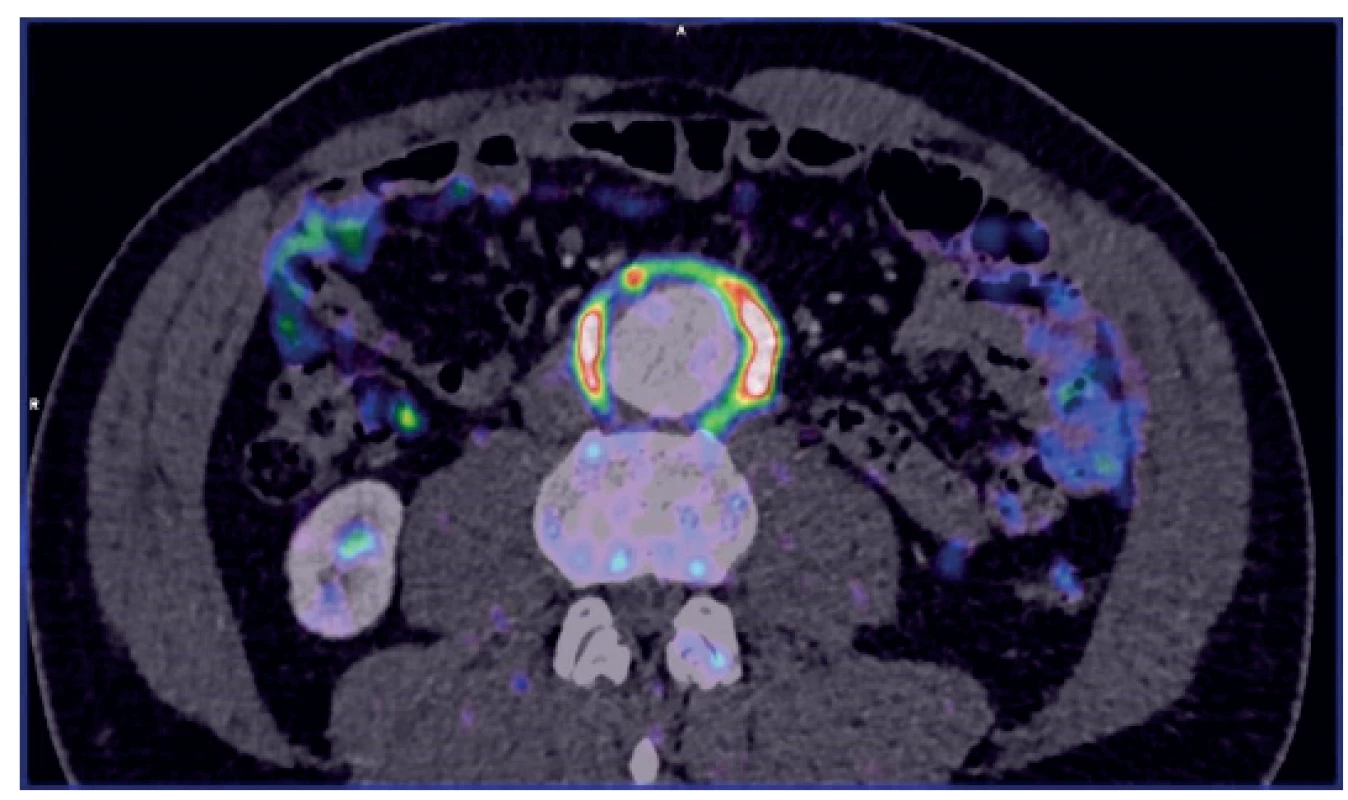
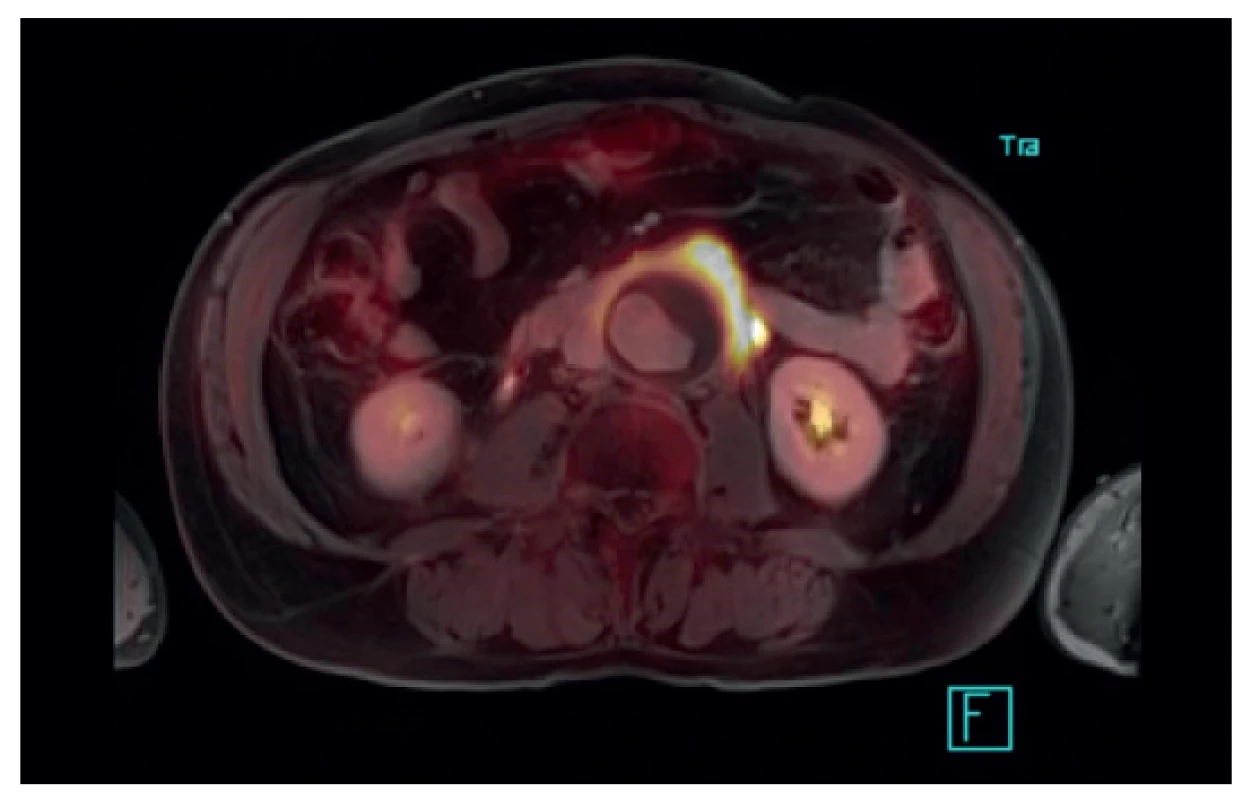
Many situations or diagnoses have been reported in the abdominal aorta where hybrid techniques have played an important role. In case of aneurysms or undilated aorta where we assume an inflammatory etiology (whether infectious or non-infectious), there is usually no doubt about the contribution of hybrid techniques. PET/CT and PET/MRI are able to reliably detect active infectious inflammation in the aortic wall (Fig. 1) [30]. In our study we observed this in 6 patients. It is also possible to recognize the extent of the inflammatory process, its location and potential transfer from another primary source (e.g., spondylodiscitis, pancreatitis), and the effect of potential conservative antibiotic therapy may be monitored (e.g., in Salmonella aortitis or Salmonella-induced AAA). Likewise, PET/CT may trigger the suspicion of inflammatory AAA, an aneurysm with wall inflammation and thickening which lacks infectious etiology (Fig. 2). The etiopathogenesis of these aneurysms has not yet been fully clarified. Knowing about the presence of this type of aneurysm before surgery is very beneficial as open surgery is quite difficult and very risky, sometimes impossible. The endovascular approach is therefore strictly preferred in these patients. Hybrid techniques are very well able to reveal retroperitoneal fibrosis concomitantly present with AAA, which again has an impact on treatment strategy decisions (Fig. 3). PET/CT (PET/MRI) is also indicated for suspected infectious complications both after open surgery and after EVAR. A hybrid technique with high sensitivity and specificity reveals this serious complication [31]. However, it cannot be assumed that increased activity in the AAA sac wall after EVAR or in the left sac after resection will automatically suggest an infectious complication. In our study, we also observed a case (after EVAR) of increased activity in the aortic wall, but neither clinical nor laboratory findings suggested infectious complications. Similarly, we have repeatedly observed increased activity in the atherosclerotic aorta at the site of the plaque, but no progression occurred during longer follow-up periods (Fig. 4). Some papers address the benefits of hybrid techniques in the diagnosis of late endoleak after EVAR, also without clearly convincing results [32].
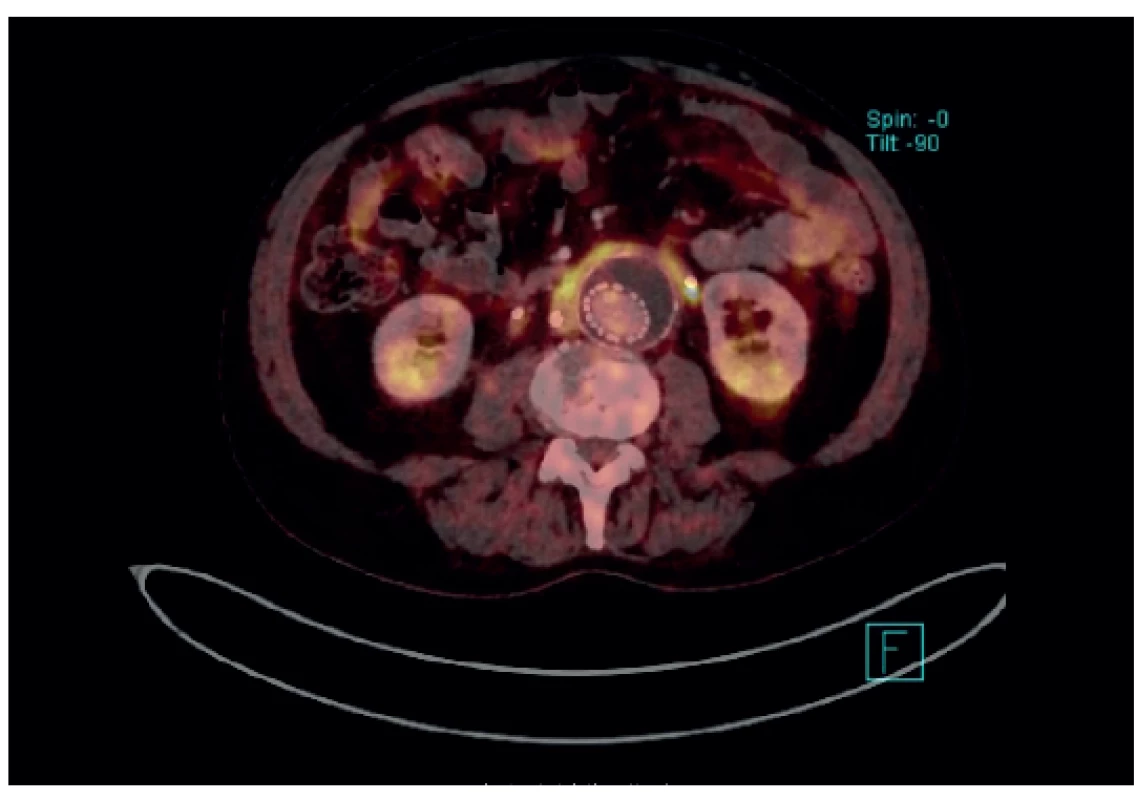
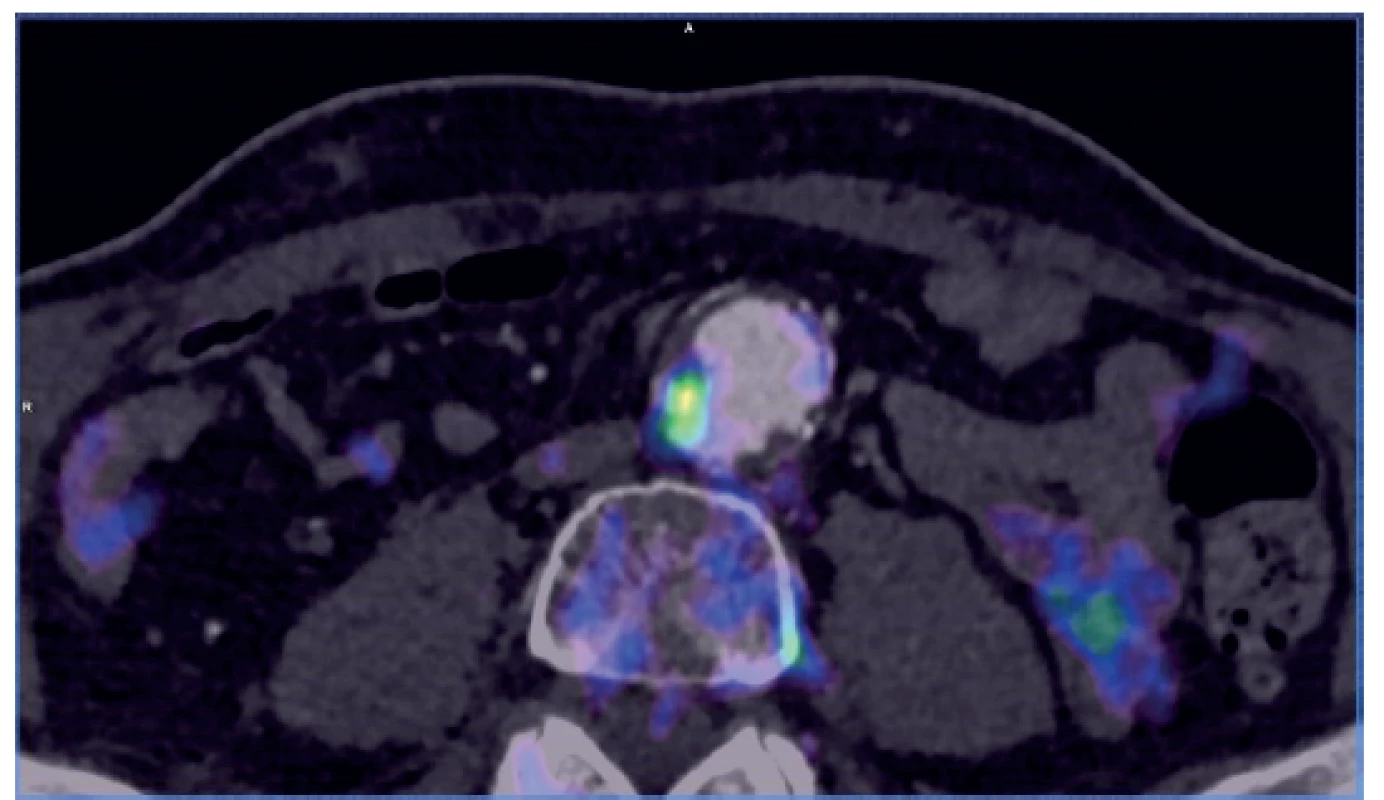
The above mentioned indications, especially in cases of a suspected infectious process in the aortic wall, are usually indisputable, and there are no doubts about the benefits of hybrid techniques in these cases, either. According to recent papers, the incidence of aortitis of various etiologies has been increasing, probably with an increasing number of differently immunosuppressed individuals (33), so we can expect more frequent indications of hybrid techniques in these patients. A discussion is still ongoing over the advantages and disadvantages of PET/CT and PET/MRI, and in which situations to choose a particular method. Due to the slightly different course of the scanning, a clear consensus is needed as to the indications of the respective techniques between angiologists or vascular surgeons and radiologists. Due to the relatively low experience with PET/MRI scanning in vascular surgery, it cannot be ruled out that different conclusions may be reached in larger patient populations.
Our study has some limitations, including the small number of patients and a relatively short observational time. However, compared to other available studies, our group of patients is comparable. Heterogeneity of the patient population is another limitation – which at the same time is an advantage, though, as it gives an opportunity to compare different diseases. A benefit of our study consists in using not only PET/CT but also PET/MRI.
Conclusion
From our point of view, the indications for PET/CT and PET/MRI remain questionable in small aneurysms, in high-risk patients with AAA and in patients with dissection or excessive atherosclerosis. Of course, unless there is another reason for a hybrid imaging technique. Our hypothesis expected to find a benefit in terms of prediction of further behavior of the aortic pathology. We were unable to demonstrate the intended benefit, i.e. obtaining information about “disease activity” and therefore predicting the progression of the findings in our study population. We are aware of the many study limitations, yet we tend to agree in our conclusions with those authors who have also failed to demonstrate the benefits of hybrid imaging techniques in the above situations.
This article was supported by the project UK Progres Q39 and AZV 15-32727A
Conflict of interests
The authors declare that they have no conflict of interest in connection with this paper and that the article has not been published in any other journal.
doc. MUDr. Jiří Moláček, Ph.D.
Chirurgická klinika FN a LF v Plzni
Alej Svobody 80
304 60 Plzeň
e-mail: molacek@fnplzen.cz
Sources
1. Manna C, Silva M, Cobelli R, et al. High-pitch dual-source CT angiography without ECG-gating for imaging the whole aorta: intraindividual comparison with standard pitch single-source technique without ECG gating. Diagn Interv Radiol Ank Turk. 2017;23 : 293–9. doi: 10.5152/dir.2017.16617.
2. McBride OMB, Joshi NV, Robson JMJ, et al. Positron emission tomography and magnetic resonance imaging of cellular inflammation in patients with abdominal aortic aneurysms. Eur J Vasc Endovasc Surg. 2016;51 : 518–26. doi: 10.1016/j.ejvs.2015.12.018.
3. Ross JDW, Ura M, Kruger A, et al. Surgical management of mitral valve infective endocarditis with annular abscess and calcification in the setting of a leaking mycotic infrarenal abdominal aortic aneurysm: a case report. J Cardiothorac Surg. 2014;9 : 154. doi: 10.1186/s13019-014-0154-0.
4. Stokes W, Janvier J, Vaughan S. Chronic Q fever in Alberta: A case of Coxiella burnetii mycotic aneurysm and concomitant vertebral osteomyelitis. Can J Infect Dis Med Microbiol. 2016;7456157. doi: 10.1155/2016/7456157.
5. Sakai K, Watanabe T, Yoshida T. Endovascular treatment of immunoglobulin G4-related inflammatory abdominal aortic aneurysm. J Vasc Surg Cases Innov Tech. 2018;4 : 189–92. doi: 10.1016/j.jvscit.2018.03.012.
6. Rutherford’s vascular surgery. 3th ed. Philadelphia, WB Saunders 2015 : 912.
7. Renapurkar RD, Setser RM, O’Donnell TP, et al. Aortic volume as an indicator of disease progression in patients with untreated infrarenal abdominal aneurysm. Eur J Radiol. 2012;81:e87-93. doi: 10.1016/j.ejrad.2011.01.077.
8. Nicholls SC, Gardner JB, Meissner MH, et al. Rupture in small abdominal aortic aneurysms. J Vasc Surg. 1998;28 : 884–8. doi: 10.1016/s0741-5214(98)70065-5.
9. Doyle BJ, Morris LG, Callanan A, et al. 3D reconstruction and manufacture of real abdominal aortic aneurysms: from CT scan to silicone model. J Biomech Eng. 2008;130 : 34501. doi: 10.1115/1.2907765.
10. Volokh KY, Vorp DA. A model of growth and rupture of abdominal aortic aneurysm. J Biomech. 2008;41 : 1015–21. doi: 10.1016/j.jbiomech.2007.12.014.
11. McGloughlin TM, Doyle BJ. New approaches to abdominal aortic aneurysm rupture risk assessment: engineering insights with clinical gain. Arterioscler Thromb Vasc Biol. 2010;30 : 1687–94. doi: 10.1161/ATVBAHA.110.204529.
12. Molacek J, Baxa J, Houdek K, et al. Assessment of abdominal aortic aneurysm wall distensibility with electrocardiography-gated computed tomography. Ann Vasc Surg. 2011;25 : 1036–42. doi: 10.1016/j.avsg.2011.05.034.
13. de Beaufort HWL, Nauta FJH, Conti M, et al. Extensibility and distensibility of the thoracic aorta in patients with aneurysm. Eur Soc Vasc Surg. 2017;53 : 199–205. doi: 10.1016/j.ejvs.2016.11.018.
14. Treska V, Kocova J, Boudova L, et al. Inflammation in the wall of abdominal aortic aneurysm and its role in the symptomatology of aneurysm. Cytokines Cell Mol Ther. 2002;7(3):91–7. doi: 10.1080/13684730310001652.
15. MA3RS Study Investigators. Aortic wall inflammation predicts abdominal aortic aneurysm expansion, rupture, and need for surgical repair. Circulation 2017;136 : 787–97. doi: 10.1161/CIRCULATIONAHA.117.028433.
16. Rabkin SW. The role matrix metalloproteinases in the production of aortic aneurysm. Prog Mol Biol Transl Sci. 2017;147 : 239–65. doi: 10.1016/bs.pmbts.2017.02.002.
17. Piechota-Polanczyk A, Demyanets S, Mittlboeck M, et al. The influence of simvastatin on NGAL, matrix metalloproteinases and their tissue inhibitors in human intraluminal thrombus and abdominal aortic aneurysm tissue. Eur J Vasc Endovasc Surg. 2015;49 : 549–55. doi: 10.1016/j.ejvs.2015.02.011.
18. Courtois A, Nusgens BV, Hustinx R, et al. 18F-FDG uptake assessed by PET/CT in abdominal aortic aneurysms is associated with cellular and molecular alterations prefacing wall deterioration and rupture. J Nucl Med. 2013;54 : 1740–7. doi: 10.2967/jnumed.112.115873.
19. Tawakol A, Migrino RQ, Hoffmann U, et al. Noninvasive in vivo measurement of vascular inflammation with F-18 fluorodeoxyglucose positron emission tomography. Am Soc Nucl Cardiol. 2005;12 : 294–301.
20. Rudd JHF, Myers KS, Bansilal S, et al. 18Fluorodeoxyglucose positron emission tomography imaging of atherosclerotic plaque inflammation is highly reproducible: implications for atherosclerosis therapy trials. J Am Coll Cardiol. 200728;50 : 892–6. doi: 10.1016/j.jacc.2007.05.024.
21. Hunter GC, Leong SC, Yu GS, et al. Aortic blebs: possible site of aneurysm rupture. J Vasc Surg. 1989;10 : 93–9.
22. Irkle A, Vesey AT, Lewis DY, et al. Identifying active vascular microcalcification by 18F-sodium fluoride positron emission tomography. Nat Commun. 2015;6 : 7495. doi: 10.1038/ncomms8495.
23. Xu XY, Borghi A, Nchimi A, et al. High levels of 18F-FDG uptake in aortic aneurysm wall are associated with high wall stress. Eur J Vasc Endovasc Surg. 2010;39 : 295–301. doi: 10.1016/j.ejvs.2009.10.016.
24. Lee H, Paeng JC, Kim KH, et al. Correlation of FDG PET/CT findings with long-term growth and clinical course of abdominal aortic aneurysm. Nucl Med Mol Imaging. 2018;52 : 46–52. doi: 10.1007/s13139-017-0482-9.
25. Tsuruda T, Nagamachi S, Nishimura M, et al. Multiple 18F-fluorodeoxyglucose positron emission tomography scans showing progression of abdominal aortic aneurysm: A case report. Medicine (Baltimore) 2016;95:e3650. doi: 10.1097/MD.0000000000003650.
26. Kotze CW, Menezes LJ, Endozo R, et al. Increased metabolic activity in abdominal aortic aneurysm detected by 18F-fluorodeoxyglucose (18F-FDG) positron emission tomography/computed tomography (PET/CT). Eur J Vasc Endovasc Surg. 2009;38 : 93–9. doi: 10.1016/j.ejvs.2008.12.016.
27. Forsythe RO, Dweck MR, McBride OMB, et al. 18F-sodium fluoride uptake in abdominal aortic aneurysms: The SoFIA3 Study. J Am Coll Cardiol. 2018;71 : 513–23. doi: 10.1016/j.jacc.2017.11.053.
28. Barwick TD, Lyons OTA, Mikhaeel NG, et al. 18F-FDG PET-CT uptake is a feature of both normal diameter and aneurysmal aortic wall and is not related to aneurysm size. Eur J Nucl Med Mol Imaging 2014;41 : 2310–8. doi: 10.1007/s00259-014-2865-9.
29. Timur UT, van Herwaarden JA, Mihajlovic D, et al. 18F-FDG PET scanning of abdominal aortic aneurysms and correlation with molecular characteristics: a systematic review. EJNMMI Res. 2015;5 : 76. doi: 10.1186/s13550-015-0153-8.
30. Sailer AM, Bakers FC, Daemen JW, et al. 18F-FDG PET/MRI in the diagnosis of an infected aortic aneurysm. Cardiovasc Diagn Ther. 2018;8(Suppl 1):S208–11. doi: 10.21037/cdt.2017.08.05.
31. Rojoa D, Kontopodis N, Antoniou SA, et al. 18F-FDG PET in the diagnosis of vascular prosthetic graft infection: A diagnostic test accuracy meta-analysis. Eur J Vasc Endovasc Surg. 2019;57 : 292-301. doi: 10.1016/j.ejvs.2018.08.040.
32. Kim BJ, Bradley KM, Subesinghe M. 18F-FDG PET/CT detected delayed endoleak in an aortoiliac endovascular aneurysm repair. Clin Nucl Med. 2018;43 : 190–1. doi: 10.1097/RLU.0000000000001974.
33. Yuan S-M, Lin H. Aortitis presenting as fever of unknown origin. Ann Thorac Cardiovasc Surg. 2018;24 : 279–87. doi: 10.5761/atcs.ra.18-00136.
Labels
Surgery Orthopaedics Trauma surgeryArticle was published in
Perspectives in Surgery

2019 Issue 11
- Possibilities of Using Metamizole in the Treatment of Acute Primary Headaches
- Spasmolytic Effect of Metamizole
- Metamizole at a Glance and in Practice – Effective Non-Opioid Analgesic for All Ages
-
All articles in this issue
- Venous access in cancer patients
- Laparoscopic versus open liver resections for colorectal cancer liver metastases: short term results
- Congruence of histological diagnosis with imaging and operation diagnosis in acute appendicitis
- The year 1848 – a significant turning point in the history of Czech surgery
- Souručenství rukou a rolí
- Hepatic pseudolymphoma: a surprising finding in a case with suspected generalisation of lung cancer
- Radiofrequency ablation in pancreatic cancer
- Benefits of hybrid methods (PET/CT, PET MRI) in the diagnosis of abdominal aortic pathology
- Perspectives in Surgery
- Journal archive
- Current issue
- About the journal
Most read in this issue
- Venous access in cancer patients
- Congruence of histological diagnosis with imaging and operation diagnosis in acute appendicitis
- Laparoscopic versus open liver resections for colorectal cancer liver metastases: short term results
- Hepatic pseudolymphoma: a surprising finding in a case with suspected generalisation of lung cancer