Radiofrequency ablation in pancreatic cancer
Radiofrekvenční ablace karcinomu pankreatu
Úvod: Klinická studie hodnotící efekt peroperační radiofrekvenční ablace karcinomu pankreatu.
Metody: Do studie byli zařazeni pacienti s histologicky verifikovaným karcinomem pankreatu. U poloviny z nich (n=24) byla provedena peroperační radiofrekvenční ablace nádoru pankreatu. V kontrolní skupině (n=24) byly provedeny bypasové operace (gastro-entero a hepatiko-jejuno anastomóza). Žádný pacient nepodstoupil neoadjuvantní onkologickou léčbu. V rámci studie byla v obou skupinách hodnocena tříměsíční morbidita a mortalita, celkové přežití, kvalita života, analgetický efekt a radiologická odpověď.
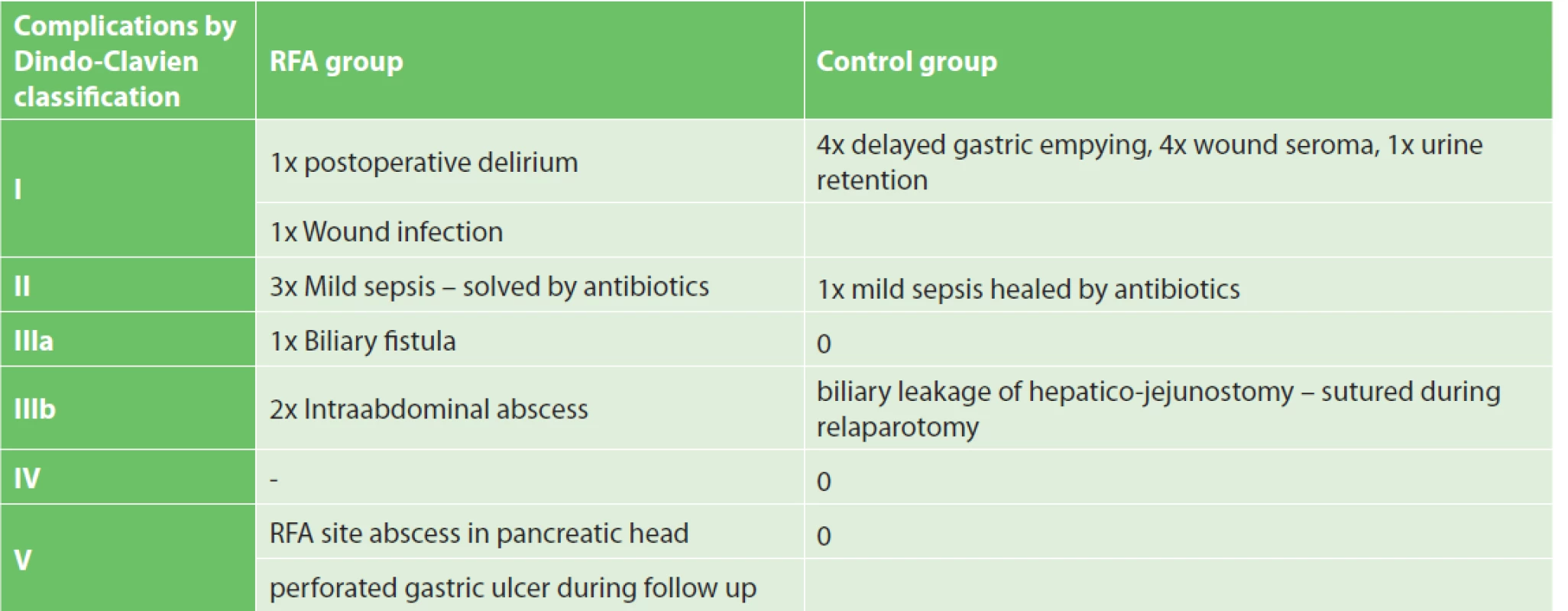
Výsledky: Tříměsíční morbidita a mortalita v RFA skupině dosáhla 41,7 % a 8,3 %. Morbidita a mortalita jako důsledek RFA byly zaznamenány u 16,6 % a 8,3 % nemocných. Medián celkového přežití činil 9,9 měsíce u pacientů RFA skupiny a 8,3 měsíce u pacientů kontrolní skupiny. Rozdíl v přežití nebyl statisticky signifikantní (p=0,758). Očekávané zlepšení kvality života ani analgetický efekt RFA nebyly prokázány. Radiologická odpověď hodnocená dle RECIST kritérií byla následující: progrese onemocnění – 41,6 %, stabilní onemocnění – 45,8 %, částečná odpověď – 8,3 % a kompletní odpověď – 0 % případů.
Závěr: Peroperační radiofrekvenční ablace karcinomu pankreatu je proveditelná metoda. Přestože u některých pacientů s lokalizovanými dobře diferencovanými nádory byly výsledky slibné, jasný pozitivní vliv peroperační RFA na celkové přežití pacientů s karcinomem pankreatu prokázán nebyl. V průběhu tříměsíčního pooperačního období RFA nezhoršila kvalitu života, jasné zlepšení kvality života ale prokázáno nebylo. Analgetický efekt RFA rovněž prokázán nebyl.
Klíčová slova:
pankreas – rakovina – radiofrekvenční ablace
Authors:
J. Hlavsa 1; V. Procházka 1
; T. Andrasina 2; T. Pavlík 3; I. Penka 1
; Z. Kala 1
Authors‘ workplace:
Department of Surgery, University Hospital Brno Bohunice and Faculty of Medicine, Masaryk University, Brno
1; Department of Radiology, University Hospital Brno Bohunice and Faculty of Medicine, Masaryk University, Brno
2; Institute of Biostatistics and Analysis, Faculty of Medicine and Faculty of Science, Masaryk University, Brno
3
Published in:
Rozhl. Chir., 2019, roč. 98, č. 11, s. 441-449.
Category:
doi:
https://doi.org/10.33699/PIS.2019.98.11.441–449
Overview
Introduction: Clinical study evaluating the impact of intraoperative radiofrequency ablation in pancreatic cancer.
Methods: Patients with histologically proved pancreatic cancer were included. Two groups were defined. In the RFA group (n=24) intraoperative RFA of the pancreatic tumour was performed. In the control group (n=24) only the bypass procedure was indicated (gastroenteric and hepaticojejunal anastomosis). No patient received neoadjuvant chemotherapy. Three-month morbidity and mortality, overall survival, quality of life, pain relief and radiological response were studied.
Results: Overall three-month morbidity and mortality were 41.7% and 8.3%, respectively. RFA related morbidity and mortality reached 16.6% and 8.3%, respectively. The overall median survival time was 9.9 and 8.3 months in the RFA group and in the control group, respectively. The survival difference was not of statistical significance (p=0.758). QoL improvement after RFA was not proved. There was no statistically significant analgesic effect of RFA. Postoperative CT scan assessed as per RECIST criteria displayed progressive disease, stable disease, partial response and complete response in 41.6%, 45.8%, 8.3% and 0% cases, respectively.
Conclusion: Intraoperative RFA of locally advanced and metastatic pancreatic cancer is a feasible palliative method. A survival benefit of this method remains doubtful, even though some positive results have been achieved in patients with localized, well-differentiated tumours. Although RFA was not associated with any impairment of the quality of life, no convincing evidence of a positive impact thereof on QoL was shown, either, during the three-month postoperative period. Pain relief was not achieved during the first 3 months after RFA.
Keywords:
Pancreas – cancer – radiofrequency ablation
Introduction
Pancreatic cancer represents an important health and socio-economic problem as it is the fourth and sixth most common cause of cancer-related mortality in the USA and Europe, respectively [1,2,3,4]. Surgery is still the main treatment option with the potential to cure [5,6,7,8]. It has been estimated that less than 20% of patients with pancreatic cancer are candidates for potentially curative resection at the time of diagnosis, with less than 10% of patients undergoing R0 resection. This is reflected in an overall 5-year survival rate in pancreatic cancer of less than 5% [9,10,11]. If a potentially curative resection is not achievable, the patient needs to receive palliative treatment with the aim of reducing the symptoms and improving the quality of life (QoL) [12]. The most frequent symptoms related to pancreatic cancer are jaundice, pain, anorexia, vomiting and fatigue, which are also considered to be one of the major QoL determinants. More than 50% of patients have pain in the early phase of the disease, but virtually all patients suffer significant pain at some later stage [13,14].
Several studies have been done since 2000, suggesting radiofrequency ablation (RFA) is the treatment modality of choice for locally advanced pancreatic cancer. Most of them were phase two clinical studies describing feasibility and safety of the procedure [15,16,17].
The only comparative study focusing on overall survival suggests that RFA seems to provide a survival benefit especially in stage III patients with pancreatic cancer [18].
The goal of our study was to evaluate the clinical effect of intraoperative RFA of pancreatic cancer in Czech patients.
Methods
A comparative clinical study with 48 pancreatic cancer patients was performed at the Department of Surgery of Masaryk University Hospital Brno between 2008 and 2014. The study was carried out according to the principles of the Helsinki Declaration for human studies and was approved by the local ethics committee. Patients with histologically verified ductal adenocarcinoma were enrolled. Preoperative staging using AJCC 2010 TNM classification system was obtained by computed tomography (CT) and endoscopic ultrasound (EUS) guided fine needle aspiration biopsy (FNAB). The indication for surgery was authorized by a multidisciplinary oncological indication board. All of the patients underwent laparotomy. Radical resection of the tumour was not indicated either because it was a locally advanced (T4) tumour or due to a low performance status. The criteria used to define the tumour as unresectable were applied uniformly and included the combination of the following parameters: infiltration of the superior mesenteric artery, coeliac trunk, common hepatic artery, and a low performance status. Multiple hepatic and peritoneal metastases were considered to be an absolute contraindication for RFA, and these patients were excluded from the study. High operation risk patients (low Karnofsky Performance Status and ASA III-IV score) even with resectable, stage IIb tumours were indicated for RFA. In five patients RFA of the primary tumour was performed despite the incidental finding of solitary liver metastases during laparotomy. The patients were divided in two groups: RFA and control group. Clinical characteristics are summarized in Tab. 1. RFA of the primary pancreatic head tumour was accompanied by a bypass procedure (gastro-enterostomy and hepatico-jejunostomy) if feasible. In the control group, only the bypass procedure was performed.
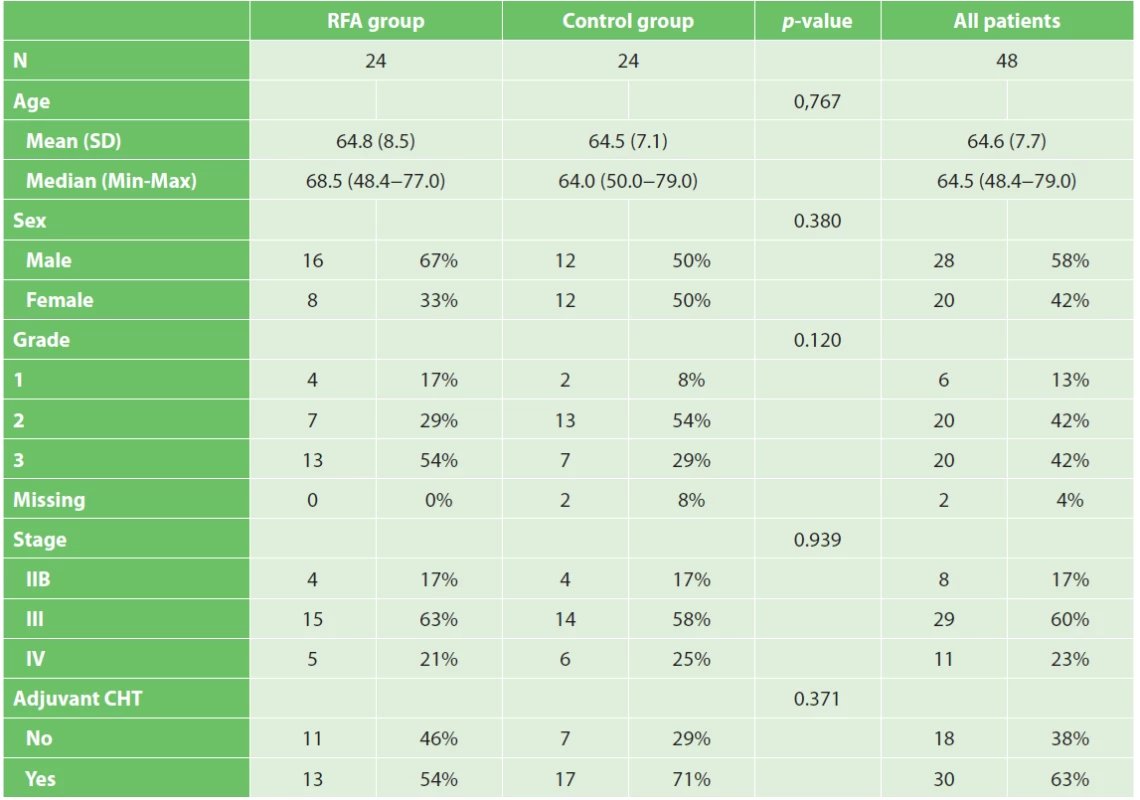
Randomisation was not carried out. The first five patients were indicated for RFA in 2008. Patients in the control group had the bypass surgery (accordingly) and were included in the interim analysis. In this time RFA was not performed and thus the surgeon did not have to choose between RFA a the bypass procedure. The interim analysis results were published in 2008 [19]. After this period, the subsequent 19 patients were indicated for RFA. Analysis of the control group was performed retrospectively. That is why QoL, radiological response and pain score were not measured in this group. All the procedures were performed uniformly by a team of three experienced pancreatic surgeons.
RFA technique
Under general anaesthesia transverse laparotomy was performed facilitating an intraoperative staging mentioned above. As regards pancreatic head tumours, the Kocher manoeuvre was performed to expose the pancreatic head. Subsequently, the surgeon’s left hand was placed over the inferior vena cava to protect it from heat, and the duodenum was perfused continuously with cold saline solution through a nasogastric tube placed in the second part of the duodenum. According to the tumour size, RFA was performed by 2–4 insertions of a bipolar needle (© Olympus, Key MEd, UK – WB 990129 Celon Pro Surge 150-T20) with a 2cm electrode length using the Celon Power System RFA generator (© Olympus, Key MEd, UK). Needle insertions were guided by intraoperative ultrasonography (IOUS) to prevent adjacent vessels and duodenum injury. Ablation time was 2–3 minutes for each insertion. The power settings were adjusted to 30 W for the whole procedure. The coagulation effect was monitored by IOUS. The bypass procedure was performed in patients with pancreatic head tumours. Gastro-enterostomy in pancreatic body tumours was performed only in the case of duodenal infiltration. Finally, the lesion site was covered by a Tachosil mesh (© Nycomed, Switzerland 2011) to prevent bleeding, and drained using a silicone tube.
Postoperative care
After the procedure patients were screened daily for acute pancreatitis, postoperative pancreatic fistula (using ISGPF criteria), bleeding and infection. Somatostatin analogs were not used. Antibiotic prophylaxis for surgical site and ablation zone infection (Amoxycillin – clavulanate 1.2 g three times a day) was administered during the first postoperative week. Stress gastro-duodenal ulcer (omeprazole 20 mg once a day) and thromboembolism prophylaxis (LMWH) was applied in the recommended dosage. Enteral nutrition via naso-jejunal tube was started on the first postoperative day. Abdominal drains were removed on day 3–5 provided that no criteria for pancreatic fistula were met [20].
Follow-up
Three months morbidity and mortality were evaluated using Dindo-Clavien classification. On days 1, 7, 30 and 90, and 6 months and 12 months from the surgery CT scanning was performed to evaluate the radiological response using “RECIST” criteria. Quality of life EORTC QLQ-C30 version 3.0 and PAN26 questionnaires and pain scores were obtained at the time of admission and three months after surgery. The overall survival in both groups was estimated using Kaplan-Meier analysis. All patients were referred to an oncologist for adjuvant chemotherapy.
Statistical methods
Standard descriptive statistics, i.e. the mean, standard deviation, median, minimum, and maximum, as well as frequency tables were used to summarize patients´ characteristics. The Kaplan-Meier method was used to estimate the survival curves that were further accompanied with median survival time and overall survival probability estimates corresponding to 6 and 12 months from surgery. Statistical significance of pain score difference before and three months after RFA was assessed using the Wilcoxon test. Pearson chi-square test and Fisher exact test were used to evaluate the relationship between categorical variables, whereas the Mann-Whitney test was used to assess any difference in age of the patients. The standard level of statistical significance α=0.05 was used. QoL questionnaire answers were evaluated according to the scoring manual. Basic summarization of the QoL scores was performed using standard descriptive statistics, i.e. the mean, standard deviation, median, minimum, and maximum. A bar chart was used to visualize the mean scores. The difference in the single domains of QoL before and after RFA was assessed using the non-parametric Wilcoxon paired test. The standard level of statistical significance α=0.05 was used.
Results
Morbidity and mortality
Overall three-month morbidity and mortality were 41.7% and 8.3%, respectively. RFA related morbidity and mortality reached 16.6% and 8.3%, respectively. An intra-abdominal abscess developed in three patients. In one patient, the abscess was drained under CT guidance and the patient was discharged 23 days after RFA. In the second case, despite re-laparotomy and drainage, the patient died due to sepsis 10 days after RFA. In the last patient, the sub-fascial abscess was drained spontaneously through laparotomy. This patient was discharged on the postoperative day 32. Biliary leakage from the common bile duct appeared in one case and was healed using a percutaneous transhepatic drain combined with an abdominal drain. One patient was readmitted to the emergency room 2.5 months after RFA due to a perforated gastric ulcer. Explorative laparotomy revealed a perforated ulcer near the gastro-enterostomy site. The perforation site was sutured but the patient died several days after the surgery due to sepsis. The mean hospitalisation length was 18 days (min. 9, max. 32). See Tab. 2 for a summary of complications in the RFA and control groups.
Survival
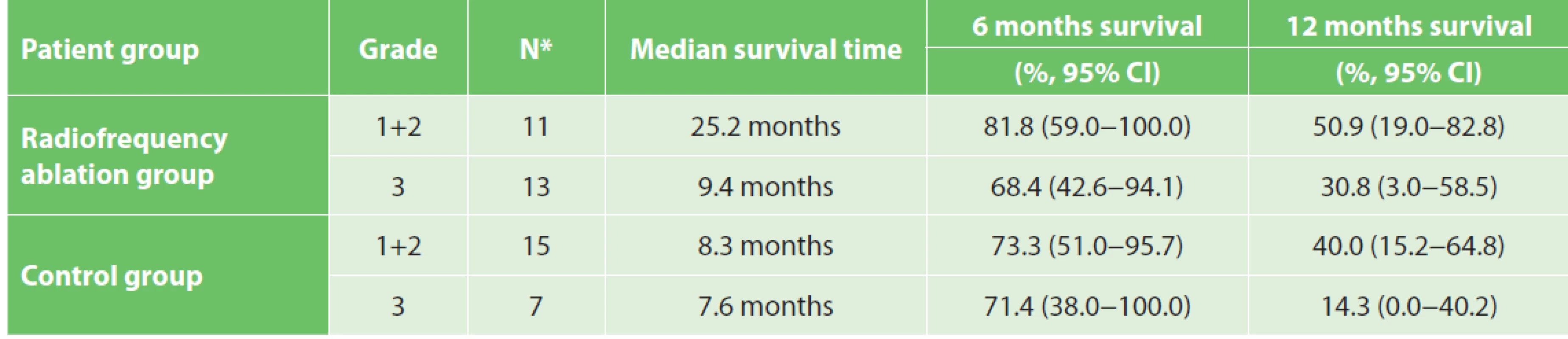
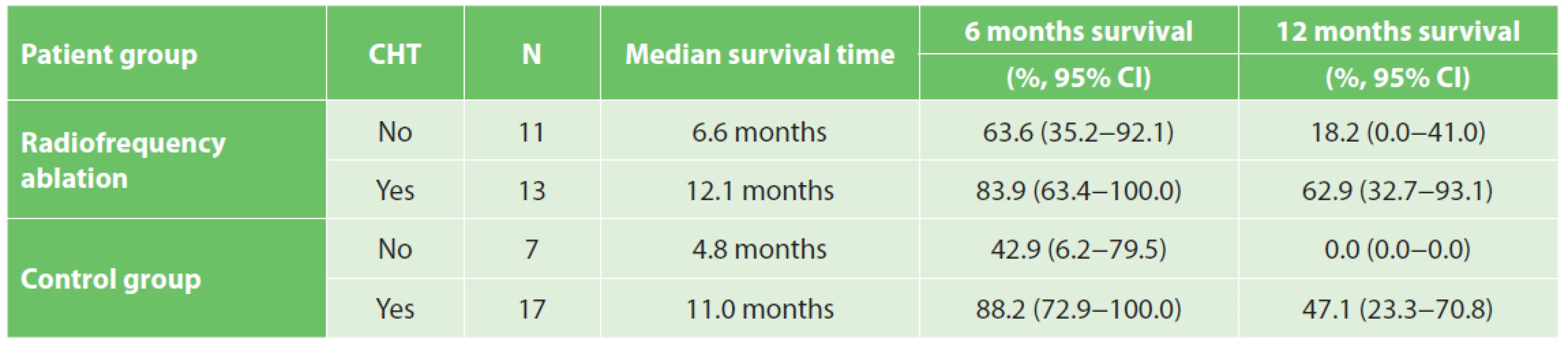
At the time of evaluation all patients were dead. The overall median survival time was 9.9 and 8.3 months in the RFA group and in the control group, respectively (Graph 1). The median survival for stage IIB, III, IV tumours was 4.3 months, 12.1 months, 5.8 months and 6.5 months, 10.7 months, 2.5 months in the RFA group and in the control group, respectively. A significant impact of the tumour grade on survival was noticed in both groups (Tab. 3). Adjuvant chemotherapy was confirmed as a factor influencing survival. The median survival time in patients with or without chemotherapy was 12.1 and 6.6 months in the RFA group and 11.0 and 4.8 months in the control group (Tab. 4).

Quality of life
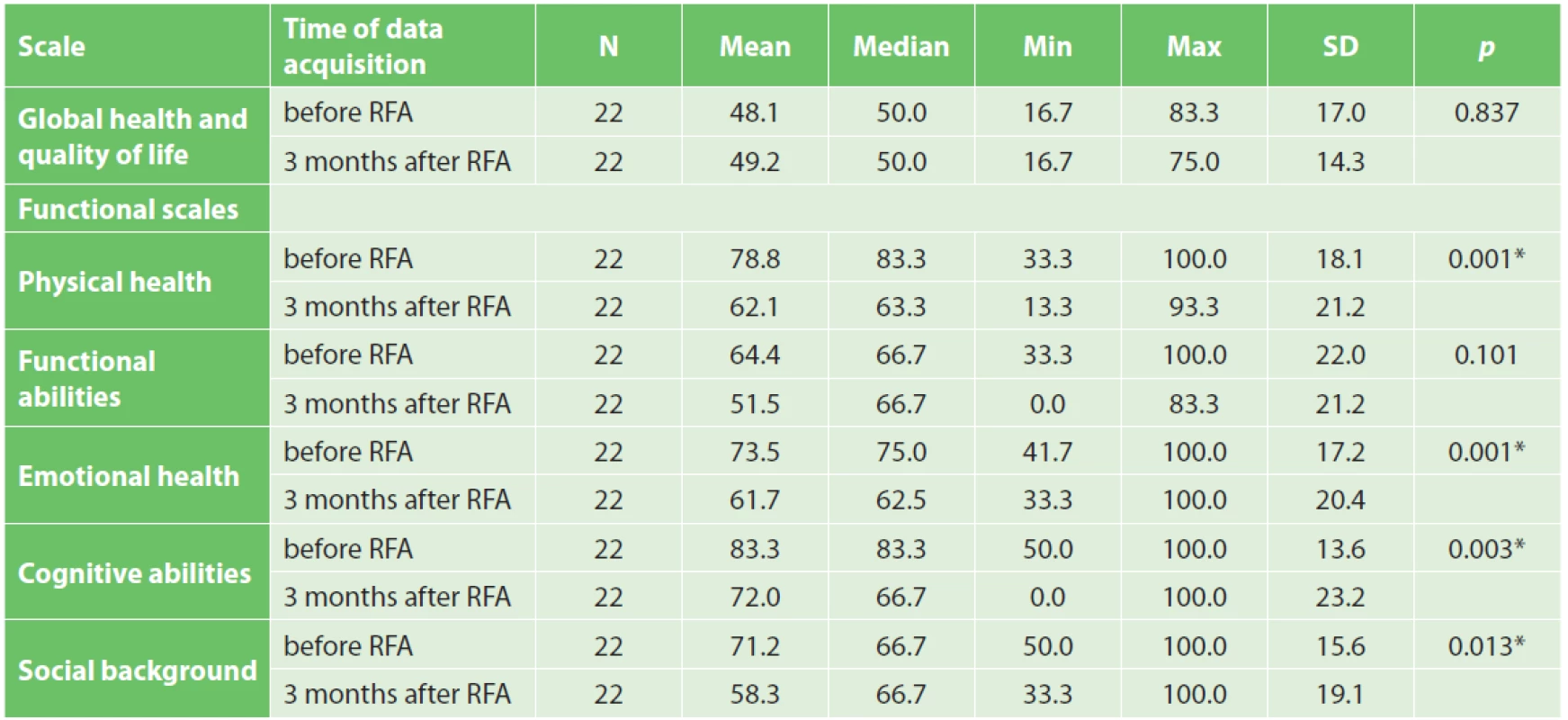
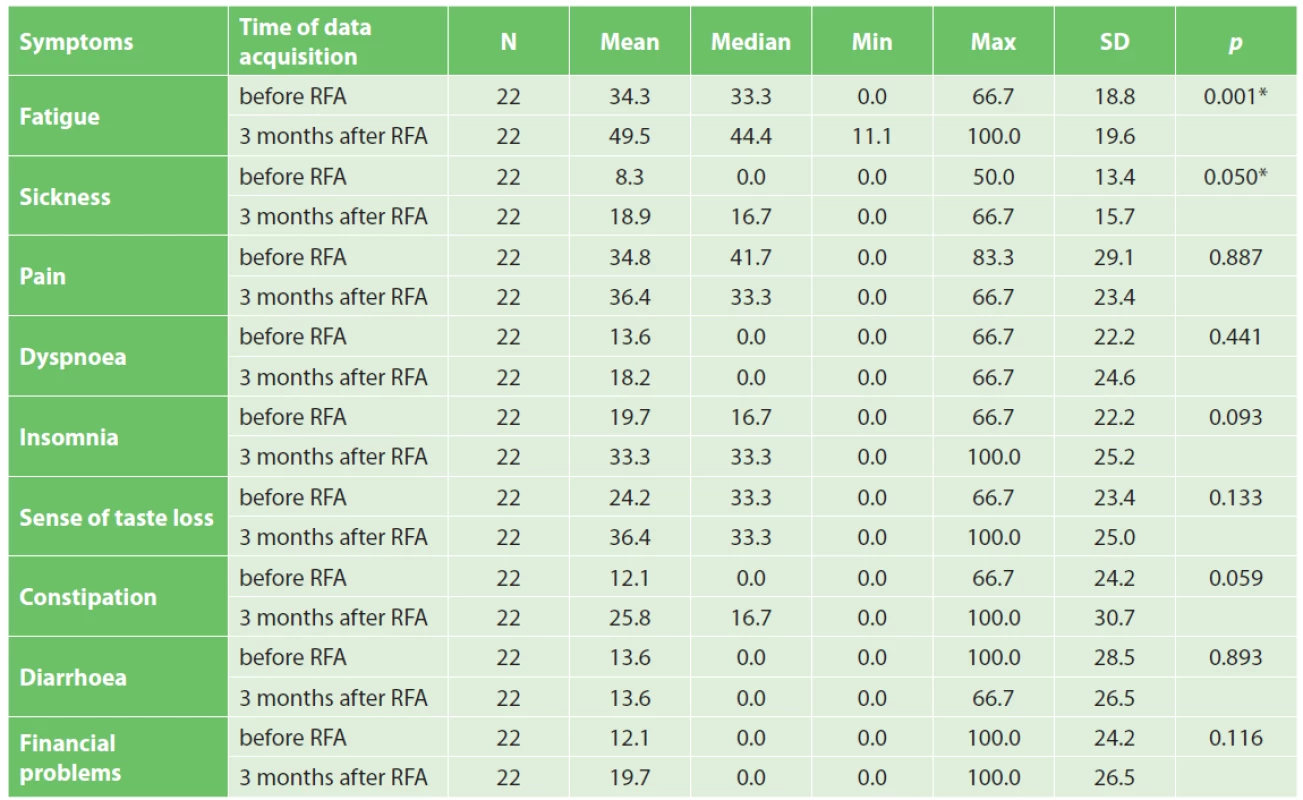
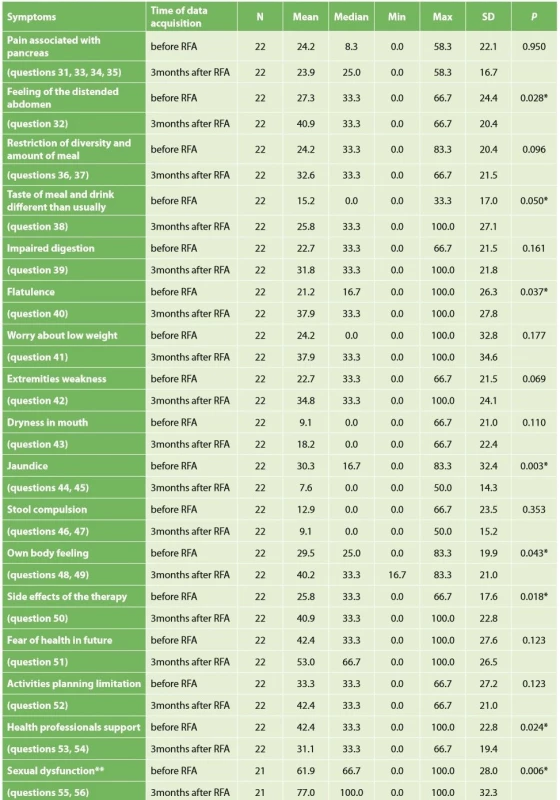
Quality of life was evaluated in 22 patients. Two patients died before the time of QoL questionnaire completion. Based on QLQ-C30 questionnaire evaluation, a statistically significant worsening of physical abilities, emotional health, cognitive functions and social background after RFA was noted. Surprisingly the overall quality of life was not impaired three months after surgery (Tab. 5). Worsening of fatigue and sickness was statistically significant, as well (Tab. 6). Improvement was not seen in any parameter. QLQ-PAN26 questionnaire evaluation proved statistically significant worsening of feeling of distended abdomen, sense of taste, and personal sexual life three months after RFA, as well as problems with flatulence (Tab. 7).
Pain score
The pain score was obtained from all 24 patients before RFA. During follow-up in one case the pain score was not recorded due to early death. In one patient it was gained during an outpatient visit one month after RFA. In all other 22 cases, the study protocol was adhered to and the pain score was obtained three months after RFA. There was no statistically significant analgesic effect of RFA. The median pain score measured preoperatively and three months after surgery reached 1.5 and 2.0, respectively (p=0.535). (Graph 2).

Radiological response
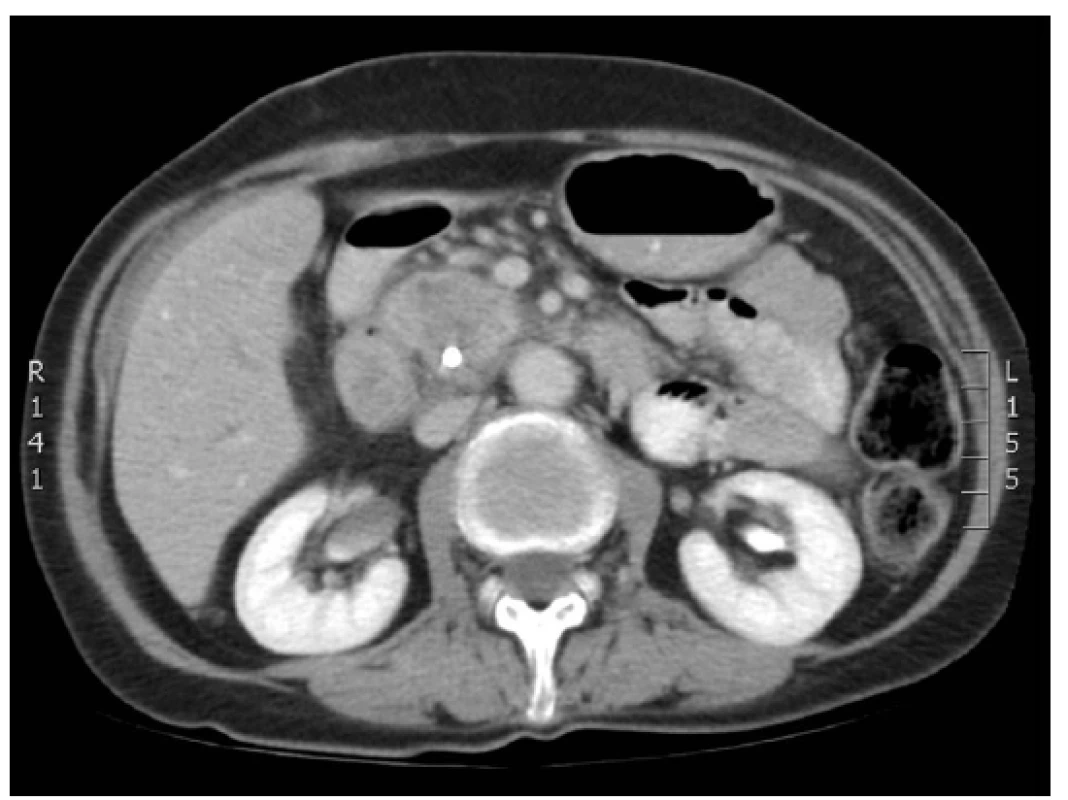
Ablation necrosis of the pancreatic tumour was viewed as a hypodense area in the arterial and portal phase of the CT scan. Changes in the ablation zone were apparent already from the first postoperative day. An identical CT image was visible on days 7, 14 and 30 after RFA (Figs. 1, 2, 3). The CT scan after 3 months from RFA was different. In most of the cases the central hypodense area disappeared. Progressive disease, stable disease, partial response and complete response were noticed in 41.6%, 45.8%, 8.3% and 0% of patients, respectively. In four patients progressive disease was manifested by local growth of the tumour. Multiple distant metastases occurred in five cases.
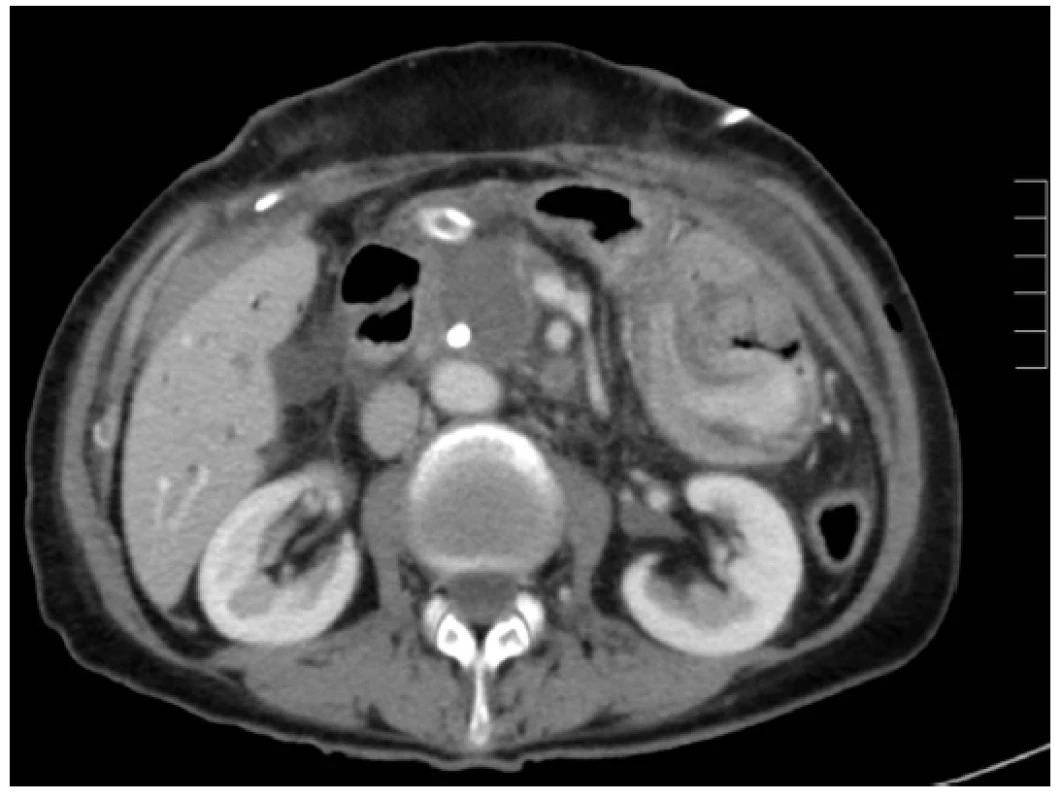
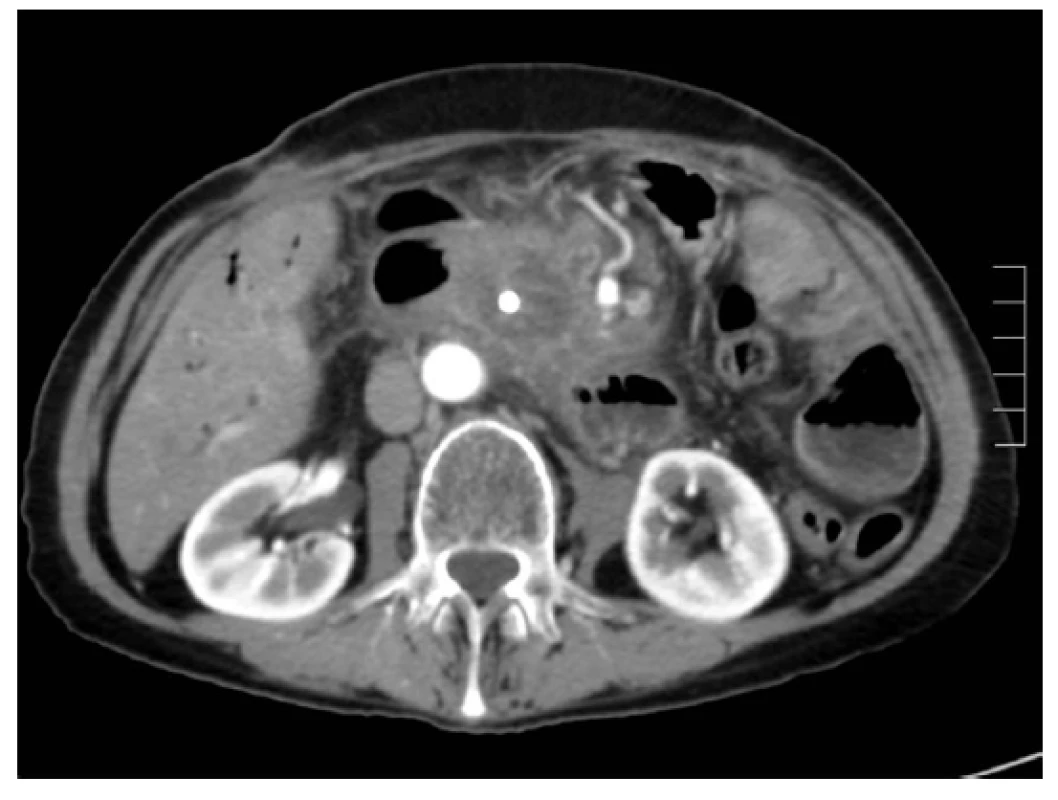
Discussion
RFA is a modified electrocautery technique for local tumour destruction, which is produced by frictional heating of tissues with high-frequency alternating current. It has been used in liver, lung, breast, adrenal gland, prostate, bone, kidney, brain and intrahepatic bile duct tumours. [21–30]. Thanks to the dismal prognosis of locally advanced pancreatic cancer, there has been an effort to use RFA in this indication since 2000. The crucial difficulty of RFA seemed to be the procedure-related complications including bleeding, acute pancreatitis, duodenal, biliary and pancreatic fistulae. Recent studies have described RFA-related morbidity and mortality up to 25% and 22%, respectively. The authors suggested IOUS navigation, duodenal perfusion with cold saline and a proper ablation protocol as the possibilities to increase the safety of RFA [15,16,17,18,31]. The Celon Power System RFA generator (© Olympus, Key MEd, UK) was used for pancreatic cancer ablation in our study for the first time. In this machine only power and time of ablation are adjusted without the possibility to measure the temperature in the ablated zone. The applied methodology was supported by our own experiments in normal porcine pancreatic tissue suggesting duodenal perfusion was most effective in prevention of duodenal wall thermal injury, and confirming the results of Girelli’s and Date’s studies [17,32,33]. In our series the three-month RFA-related morbidity did not exceed 17% and was comparable with Girelli’s study even though a different ablation protocol was used. Although duodenal cooling was applied, RFA-related complications observed were most probably caused by thermal damage of the duodenal wall, pancreatic tissue and common bile duct. That led to an infected necrosis of the burned area. In one case an intraabdominal abscess developed as a consequence of duodenal leakage. In another two patients a subfascial abscess came out from infected necrosis of the ablated zone. In one case thermal damage of the common bile duct led to bile leakage described above. Taking into account these complications, non-thermal ablation methods minimizing thermal damage of the surrounding structures, e.g. irreversible electroporation, seem to be justified in this field in future [34]. The effect of RFA on overall survival was evaluated by Spiliotis in a retrospective comparative study published in 2007. Data on 25 consecutive patients undergoing palliative therapy with or without RFA for locally advanced pancreatic cancer were included. Thirteen patients received palliative therapy alone, whereas 12 patients received palliative therapy plus RFA. Overall median survival in patients receiving palliative therapy alone was 13 months. Where radiofrequency ablation was applied, the median survival increased to 33 months. Although the follow-up has not been closed in this study, the authors conclude that RFA compared to palliative therapy seems to provide a survival benefit especially for stage III pancreatic cancer patients [18]. Another study from Japan evaluating the efficacy of RFA was performed in the cohort of 20 pancreatic cancer patients and did not prove any statistically significant difference in survival [16]. Regarding our study, although no statistically significant difference in overall survival between RFA and the control group was proven, a certain positive tendency did occur in stage III patients. In this group the median survival reached 12.1 months and 10.7 months for RFA and the control group, respectively, suggesting, in agreement with Spiliotis’ study, that RFA could provide a survival benefit especially for stage III patients with grade 1–2 pancreatic cancer. In metastatic disease RFA did not prolong survival at all (median survival 2.5 months). Compared to well-differentiated tumours, poor differentiation was associated with worse prognosis (25.2 months and 9.4. months for grade 1+2 and grade 3 tumours, respectively). The unexpected short survival in stage IIB patients in our study can be explained by the poor health status and small cohort (n=4). In conformity with other studies we found that adjuvant chemotherapy was an important factor influencing overall survival. Although neoadjuvant chemotherapy was not applied in our study, this theme is very current nowadays. Martin et al. have described a survival benefit of irreversible electroporation of pancreatic cancer in a group of patients selected based on neoadjuvant chemotherapy. The principle of this “selection” is to eliminate patients with aggressive tumours where any local ablation procedure is not justifiable [34]. The quality of life and survival time belong to the most followed parameters in pancreatic cancer. There is a relationship between these. Higher QoL scores are associated with longer survival [35]. There is evidence that non-radical, palliative resections in locally advanced or metastatic pancreatic cancer compared to double loop bypass impair QoL, do not prolong overall survival, and should not be indicated routinely [36]. Since no study evaluating QoL after RFA in pancreatic cancer has been published yet it is difficult to compare. The question is whether intraoperative RFA compared to other alternative therapeutic modalities (e.g. palliative pancreatoduodenectomy, bypass surgery) improves QoL of locally advanced PC patients. Walter referred 42 patients with locally advanced and metastatic PC who underwent palliative pancreatoduodenectomy, and 154 treated with double loop bypass only. These groups were compared with regard to survival, postoperative morbidity, and QoL. The EORTC QLQ-C30 was used to assess QoL before surgery, at the time of discharge, three months after surgery, and six months after surgery. Preoperative QoL mean scores in this study were very similar to ours (global health 48 and 48, physical health 77 and 79, functional abilities 77 and 64, emotional health 67 and 73, cognitive abilities 83 and 83, social background 67 and 71) [36]. Comparing Walter´s and our study three months after surgery RFA does not seem to impair the quality of life. Although global health did not worsen after RFA, there were some negative changes in some particular parameters. We recognized worsening of physical abilities, emotional health, cognitive functions, social background, fatigue and sickness without any global health decrease. This discrepancy could be perhaps explained by atonement with the disease. Concerning the symptoms three months after RFA, most of the patients complained about feeling of distended abdomen, loss of taste, flatulence, and sexual dysfunction. The worsening of their own body feeling was assessed as very serious. These facts point out some concrete critical points of palliative care in locally advanced and metastatic pancreatic cancer, indicating that supplementation of exocrine pancreatic insufficiency, taste support and psychological intervention may be the essential points. Pain relief after RFA of locally advanced PC was referred by Girelli. Twenty-four of the 35 patients who complained of preoperative abdominal or back pain experienced clinical benefit 1 week after the procedure. Median pain scores were reduced from a preoperative value of 4 to 2 after RFA. One month after the procedure no patient complained of worsening of abdominal pain or new-onset pain [17]. Even though pain relief was not proven in our study, no worsening of pain was observed in the 10-degree visual-analogous pain scale or based on the EORTC QLQ-PAN26 questionnaire three months after RFA. At the time of pain evaluation only one patient needed strong opioids.
Conslusion
According to our study, intraoperative RFA of locally advanced and metastatic pancreatic cancer is a feasible palliative method. Survival benefit of this method is still doubtful, even though some positive results have been achieved in patients with localized, well-differentiated tumours. During the three-month postoperative period, RFA has not impaired global health feeling; nevertheless, its positive impact on QoL was not clearly proved. Pain relief has not been achieved during the first 3 months after RFA.
Acknowledgements
This study was supported by the Grant Agency of the Ministry of Health of the Czech Republic (IGA MZ ČR) (Projects Nos. NS10239-3/2009 and NS10419-3/2009). The authors thank Beata Hemmelová for patient follow-up, Katarína Můčková and Iva Svobodová for histo-pathological evaluation, Michal Crha and Alois Nečas for experimental support, and Barbora Garajová for postoperative intensive care. The authors declare no conflict of interest.
Conflict of interests
The authors declare that they have no conflict of interest in connection with this paper and that the article has not been published in any other journal.
Jan Hlavsa MD
Department of Surgery
University Hospital Brno Bohunice
and Faculty of Medicine Masaryk University Brno
625 00 Brno
e-mail: hlavsjan@seznam.cz
Sources
- Alexakis N, Halloran C, Raraty M, et al. Current standards of surgery for pancreatic cancer. Br J Surg. 2004;91 : 1410–27. doi:10.1002/bjs.4794.
- Tingstedt B, Andersson E, Flink A, et al. Pancreatic cancer, healthcare cost, and loss of productivity: a register-based approach. World J Surg. 2011;35 : 2298–305. doi: 10.1007/s00268-011-1208-2.
- Parkin D, Bray F, Devesa S. Cancer burden in the year 2000. The global picture. Eur J Cancer 2001;37 : 4–66. doi:10.1016/s0959-8049(01)00267-2.
- Jemal A, Siegel R, Ward E, et al. Cancer statistics. CA Cancer J Clin. 2006;56 : 106–30. doi: 10.3322/canjclin.56.2.106.
- Kuhlmann KF, Castro SM, Wesseling JG, et al. Surgical treatment of pancreatic adenocarcinoma: actual survival and prognostic factors in 343 patients. Eur J Cancer. 2006;40 : 549–58. doi: 10.1016/j.ejca.2003.10.026.
- Millikan KW, Deziel DJ, Silverstein JC, et al. Prognostic factors associated with resectable adenocarcinoma of the head of the pancreas. Am Surg. 1999;65 : 618–23.
- Sohn TA, Yeo CJ, Cameron JL, et al. Resected adenocarcinoma of the pancreas in 616 patients: results, outcomes, and prognostic indicators. J Gastrointest Surg. 2000;4 : 567–79.
- Wagner M, Redaelli C, Lietz M, et al. Curative resection is the single most important factor determining outcome in patients with pancreatic adenocarcinoma. Br J Surg. 2005;91 : 586–94. doi:10.1002/bjs.4484.
- Wray CJ, Ahmad SA, Matthews JB, et al. Surgery for pancreatic cancer: recent controversities and current practice. Gastroenterology 2005;128 : 1626–41. doi: 10.1053/j.gastro.2005.03.035.
- Schneider G, Siveke JT, Eckel F, et al. Pancreatic cancer: basic and clinical aspects. Gastroenterology 2005;128 : 1606–25. doi: 10.1053/j.gastro.2005.04.001.
- Li D, Xie K, Wolff R, et al. Pancreatic cancer. Lancet 2004;363 : 1049–57. doi: 10.1016/S0140-6736(04)15841-8.
- Weitz J, Kienle P, Buchler M. Bypass surgery for advanced pancreatic cancer. In: The pancreas: An integrated textbook of basic science, medicine and surgery. Blackwell Publishing, Oxford 2008 : 719–26. doi: 10.1002/9781119188421.ch110.
- Lebovits AH, Lefkowitz M. Pain management of pancreatic carcinoma: a review. Pain 1989;36 : 1–11.
- Russell RC. Palliation of pain and jaundice: an overview. Ann Oncol. 1999;10 : 165–9.
- Tang Z, Wu Y, Fang H, et al. Treatment of unresectable pancreatic carcinoma by radiofrequency ablation with ‘cool-tip needle’: report of 18 cases. [in Chinese]. Zhonghua Yi Xue Za Zhi 2008;88 : 391–4.
- Matsui Y, Nakagawa A, Kamiyama Y, et al. Selective thermocoagulation of unresectable pancreatic cancers by using radiofrequency capacitive heating. Pancreas 2000;20 : 14–20. doi: 10.1097/00006676-200001000-00002.
- Girelli R, Frigerio I, Salvia R, et al. Feasibility and safety of radiofrequency ablation for locally advanced pancreatic cancer. Br J Surg. 2010;97 : 220–5. doi: 10.1002/bjs.6800.
- Spiliotis J, Datsis A, Michalopoulos N, et al. Radiofrequency ablation combined with palliative surgery may prolong survival of patients with advanced cancer of the pancreas. Langenbecks Arch Surg. 2007;392 : 55–60. doi: 10.1007/s00423-006-0098-5.
- Hlavsa J, Kala Z, Válek V, et al. Radiofrequency ablation (RFA) of pancreatic tumors. [In Czech] Rozhl Chir. 2008;87 : 462–6.
- Bassi C, Dervenis Ch, Butturini G, et al. Postoperative panreatic fistula: An international study group (ISGPF) definition. Surgery 2005;138 : 8–13. doi: 10.1016/j.surg.2005.05.001.
- Maurizio P, Laura R, Rapaccini G, et al. Effectiveness of radiofrequency ablation of hepatocellular carcinoma. World J Surg. 2009;33 : 1103–4. doi: 10.1007/s00268-008-9863-7.
- Robert M, Neumann U, Neuhaus P, et al. Open surgical is superior to percutaneous access for radiofrequency ablation of hepatic metastases. World J Surg. 2009;33 : 804–11. doi: 10.1007/s00268-008-9905-1. doi: 10.1007/s00268-008-9905-1.
- Simon C. Current role of image-guided ablative therapies in lung cancer. Expert Rev Anticancer Ther. 2005;5 : 657–66. doi: 10.1586/14737140.5.4.657.
- Nogutchi M. Minimally invasive surgery for small breast cancer. J Surg. Oncol. 2003;84 : 94–101. doi: 10.1002/jso.10292.
- Boss A, Clasen S, Kuczyk M, et al. Magnetic resonance – guided percutaneous radiofrequency ablation of renal cell carcinoma: a pilot clinical study. Invest Radiol. 2005;40 : 583–90. doi: 10.1097/01.rli.0000174473.32130.28.
- Wood B, Abraham J, Hvizda J, et al. Radiofrequency ablation of adrenal tumors and adrenocortical carcinoma metastases. Cancer 2003;97 : 554–60. doi: 10.1002/cncr.11084.
- Shariat S, Raptidis G, Masatoschi M, et al. Pilot study of radiofrequency interstitial tumor ablation (RITA) for the treatment of radio-recurrent prostate cancer. Prostate 2005;64 : 260–7. doi: 10.1002/pros.20242.
- Martel J, Bueno A, Ortiz E. Percutaneous radiofrequency treatment of osteoid osteoma using cool tip electrode. Eur J of Radiol. 2005;56 : 403–8. doi: 10.1016/j.ejrad.2005.05.014.
- Ganaadha S, Wulf S, Morris D, et al. Safety and efficacy of radiofrequency ablation of brain: a potentially minimally invasive treatment for brain tumors. Minim Invasive Neurosurg. 2004;47 : 325–8. doi: 10.1055/s-2004-830124.
- Chiou Y, Hwang J, Chou Y, et al. Percutaneous ultrasound guided radiofrequency ablation of intrahepatic cholangiocarcinoma. Kaohsiung J Med Sci. 2005;21 : 304–9. doi: 10.1016/S1607-551X(09)70125-1.
- Hadjicostas P, Malakounides N, Varianos C, et al. Radiofrequency ablation in pancreatic cancer. HPB 2006;8 : 61–4. doi: 10.1080/13651820500466673.
- Crha M, Hlavsa J, Procházka V, et al. An evaluation of peripancreatic tissue cooling techniques utilized during radiofrequency pancreatic ablation in an ex vivo study of pigs. Acta Vet Brno 2011;80 : 407–13. doi:10.2754/avb201180040407.
- Date R, McMahon R, Siriwardena A, et al. Radiofrequency ablation of the pancreas. I: Definition of optimal thermal kinetic parameters and the effect of simulated portal venous circulation in an ex-vivo porcine model. JOP 2005;6 : 581–7.
- Martin RC 2nd, McFarland K, Velanovich V, et al. Irreversible electroporation therapy in the management of locally advanced pancreatic adenocarcinoma. J Am Coll Surg. 2012;215 : 361–9. doi: 10.1016/j.jamcollsurg.2012.05.021.
- Braun DP, Gupta D, Staren ED. Longitudinal health-related quality of life assessment implications for prognosis in stage IV pancreatic cancer. Pancreas 2013;42 : 254–9. doi: 10.1097/MPA.0b013e31825b9f56.
- Walter J, Nier A, Rose T, et al. Palliative partial pancreaticoduodenectomy impairs quality of life compared to bypass surgery in patients with advanced adenocarcinoma of the pancreatic head. Eur J Surg Oncol. 2011;37 : 798–804. doi: 10.1016/j.ejso.2011.06.017.
Labels
Surgery Orthopaedics Trauma surgeryArticle was published in
Perspectives in Surgery

2019 Issue 11
- Possibilities of Using Metamizole in the Treatment of Acute Primary Headaches
- Metamizole at a Glance and in Practice – Effective Non-Opioid Analgesic for All Ages
- Metamizole vs. Tramadol in Postoperative Analgesia
-
All articles in this issue
- Venous access in cancer patients
- Laparoscopic versus open liver resections for colorectal cancer liver metastases: short term results
- Congruence of histological diagnosis with imaging and operation diagnosis in acute appendicitis
- The year 1848 – a significant turning point in the history of Czech surgery
- Souručenství rukou a rolí
- Hepatic pseudolymphoma: a surprising finding in a case with suspected generalisation of lung cancer
- Radiofrequency ablation in pancreatic cancer
- Benefits of hybrid methods (PET/CT, PET MRI) in the diagnosis of abdominal aortic pathology
- Perspectives in Surgery
- Journal archive
- Current issue
- About the journal
Most read in this issue
- Venous access in cancer patients
- Congruence of histological diagnosis with imaging and operation diagnosis in acute appendicitis
- Laparoscopic versus open liver resections for colorectal cancer liver metastases: short term results
- Hepatic pseudolymphoma: a surprising finding in a case with suspected generalisation of lung cancer
