Multi-electrode microfluidic platform for protein detection using electrochemical impedance spectroscopy
An impedimetric sensor was developed based on parallel detection at two identical microfluidic channels with 3 pairs of interdigitated electrodes in each. Preparation and cleaning of the gold electrodes were examined and optimized. A self-assembling monolayer of 11-mercaptopropionic acid was deposited to form a base for later covalent binding of antibody proteins via EDC/NHS chemistry. Two immunoassays containing immune reaction of a 214D4 antibody and a corresponding MUC1ex antigen and a prostate antigen antibody immunoassay with positive and negative controls were performed in two parallel microfluidic channels. Presented platform enables a simultaneous analysis of the protein reactions on multiple electrodes leading to an improvement in on-time diagnostics and point-of-care.
Keywords:
Impedance sensor, protein adhesion, antigen – antibody interaction
Authors:
Jaroslav Lazar 1; Magdalena Bialon 2; Christiane Püttmann 2; Christoph Stein 2; Wilfred Germeraad 3; Uwe Schnakenberg 1
Authors‘ workplace:
Institute of Materials in Electrical Engineering 1, RWTH Aachen University, Sommerfeldstr. 4, Aachen, Germany
1; Helmholtz Institute for Biomedical Engineering, Institute of Applied Medical Engineering, Dept. of Experimental Medicine and Immunotherapy, RWTH Aachen University, Pauwelsstr. 20, 52074 Aachen, Germany
2; Department of Immunology, Maastricht University Medical Center, Medical Center, Universiteitssingel 50, 6229 Maastricht, the Netherlands
3
Published in:
Lékař a technika - Clinician and Technology No. 4, 2015, 45, 105-109
Category:
Original research
Overview
An impedimetric sensor was developed based on parallel detection at two identical microfluidic channels with 3 pairs of interdigitated electrodes in each. Preparation and cleaning of the gold electrodes were examined and optimized. A self-assembling monolayer of 11-mercaptopropionic acid was deposited to form a base for later covalent binding of antibody proteins via EDC/NHS chemistry. Two immunoassays containing immune reaction of a 214D4 antibody and a corresponding MUC1ex antigen and a prostate antigen antibody immunoassay with positive and negative controls were performed in two parallel microfluidic channels. Presented platform enables a simultaneous analysis of the protein reactions on multiple electrodes leading to an improvement in on-time diagnostics and point-of-care.
Keywords:
Impedance sensor, protein adhesion, antigen – antibody interaction
Introduction
Electrochemical impedance spectroscopy (EIS) measures the electrical current response to an AC potential applied between electrodes, being extremely sensitive to adsorptions on the electrode surface in the frequency region below 1 kHz in the presence of an oxidation reduction system [1]. Among SPR [2], surface acoustic waves (SAW) and quartz crystal microbalance (QCM) [3, 4] EIS is widely used as a detection method for label-free molecular binding processes (e.g. antigen-antibody, protein-DNA, DNA hybridization, cellular processes) [5, 6, 12]. EIS utilizing interdigital microelectrodes is sensitive to surface modifications in the vicinity of the electrode surface, as well as on the space between the electrodes [7]. A simple electrical equivalent circuit model consisting of double layer capacity in series with solution resistance can be used to describe the non-faradaic system [8]. The surface impedance can be calculated at the applied frequencies [12].
Antigen – antibody reactions are frequently used in medical diagnostics and commonly examined by Enzyme Linked Immunosorbent Assay (ELISA) tests which are costly and time inefficient. The combination of microfluidic and biochemical immunoassay technologies allow the development of a cheap and portble microfluidic biosensors. The immobilization of the biochemical active compound plays a crucial role in the stability and the reproducibility of the sensing mechanism. Furthermore, covalent coupling enables a possibility of chip regeneration since the biochemical compound is fixed on the surface and the protein – protein coupling can be dissociated by a change of pH [13].
In this study we demonstrate a biosensing platform based on the electrochemical impedance spectroscopy on multiple interdigitated electrodes incorporated in a microfluidic channel as an approach to detect antibody – antigen interaction.
Materials and Methods
Reagents and Stock Solutions
All reagents are analytical grade unless stated otherwise. Destiled water was used for preparation of all aqueous solutions. Phosphate Buffered Saline (PBS) solution, pH 7.4 with 5mM potassium ferri/fer-rocyanide (HCF) was prepared from stocks purchased from Carl Roth (Karlsruhe, Germany. Bovine Serum Albumin (BSA) powder purchased from Carl Roth was solved to 0.1% and 1% in PBS. 11-mer-captoundecanoic acid (MPA), N-hydroxysuccinimide (NHS), 1-ethyl-3 - (dimethyxlaminopropyl) carboiimide was purchased by Sigma-Aldrich (Munich, Germany).
The monoclonal antibody (mAb) M2D11 [10] (conc. 45 µg/ml, murine IgG) recognizing a non-related antigen was used as a negative control and the breast-cancer specific mAb 214D4 [11] (conc. 45 µg/ml, murine IgG) as a positive probe. Both antibodies were produced in house.
The recombinant protein MUC1ex (extracellular domain of Mucin 1, conc. 10 µg/ml, mw = 65 kDa), which is recognized by mAb 214D4 was also produced in-house. Prostate specific antigen-antibody assay kit was purchased from Sigma-Aldrich.
Chip Fabrication
Gold interdigital electrodes were fabricated by standard thin-film technology with a finger width of 4 µm, a gap between the fingers of 3 µm and a finger lenght of 3885 µm, as shown in figure 1 A.
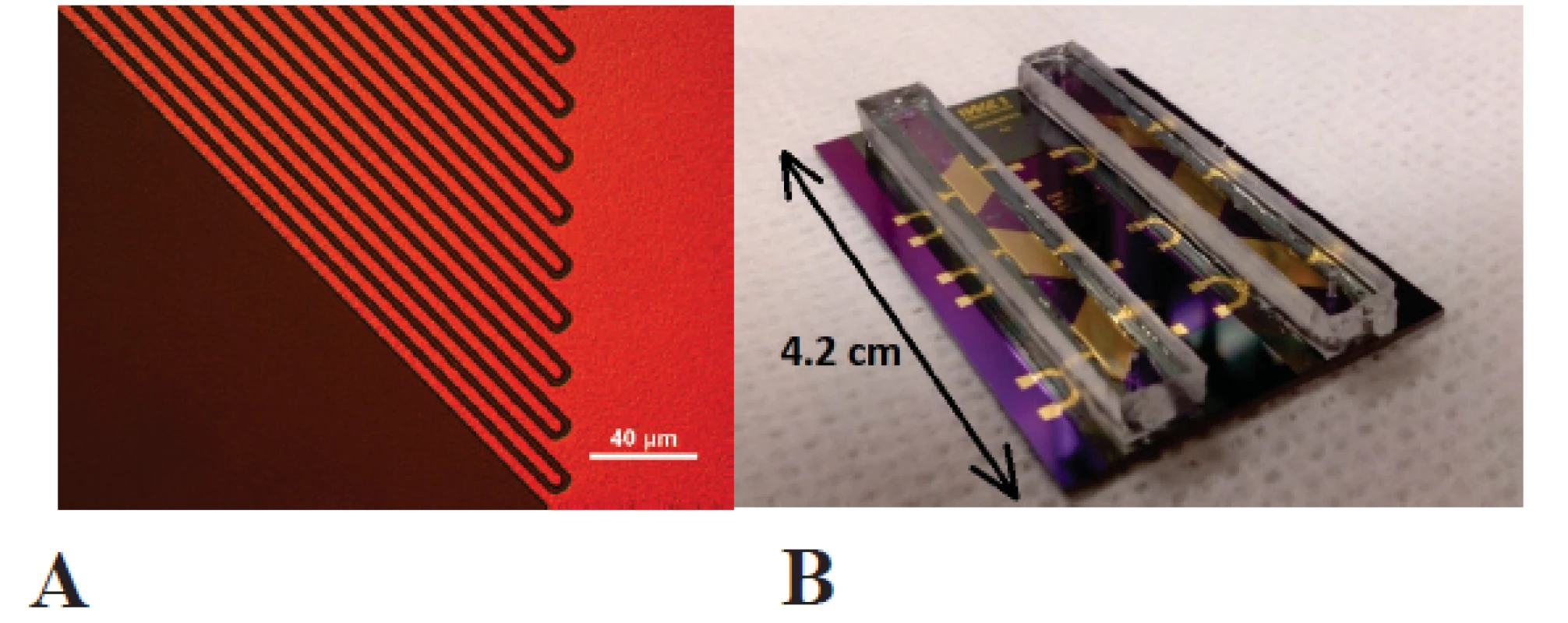
First a nLOF 2035 negative photoresist was spincoated on thermally oxidized silicon wafers RC 8 spin coater (SÜSS MicroTec AG, Germany) and photolithographically structured in MA 6 mask aligner (SÜSS MicroTec AG, Germany), followed by sputtering of 200 nm gold layer in a Nordiko NS 2550 magnetron sputtering tool. Lift-off acetone process and ultrasound bath was utilized to form the wanted structures. The chips were diced out finally.
Microfluidic Fabrication and Instrumentation Setup
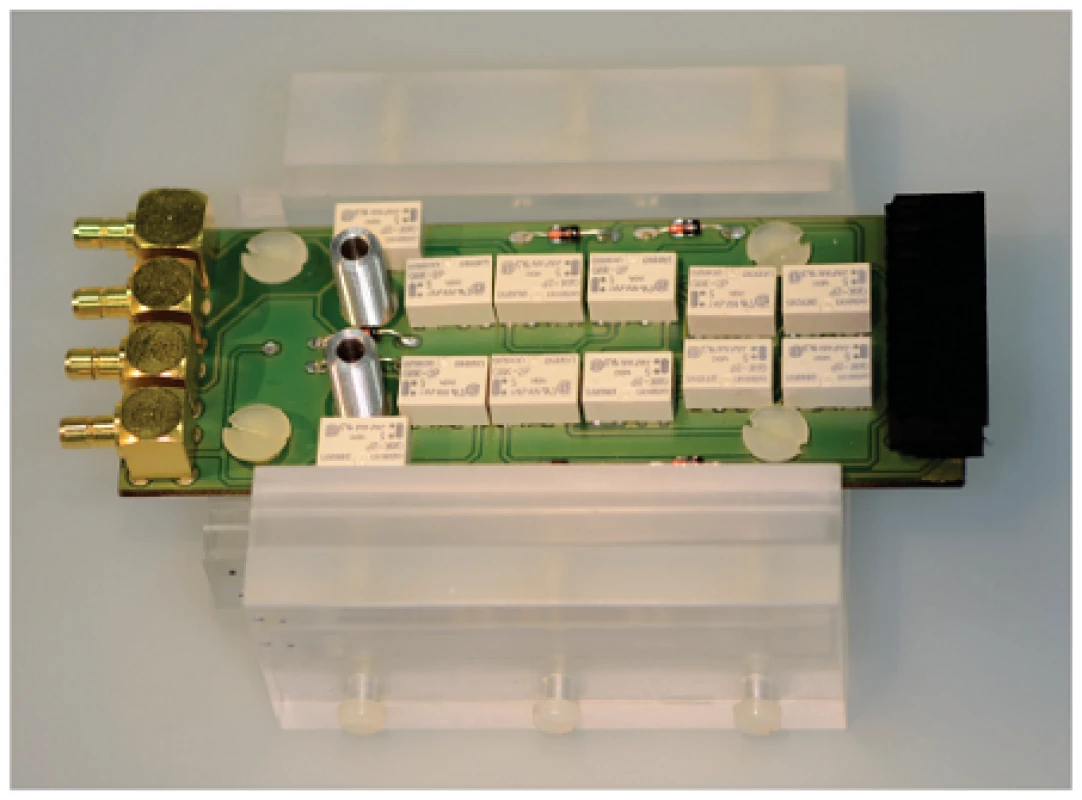
Microfluidic channel with a width of 2.5 mm was fabricated by structuring a SU8 negative photoresist side walls (Microchemicals, Ulm, Germany) and poly(dimethylsiloxane) cover lid, as shown in figure 1 B. Interdigital electrodes were contacted by the springcontact probes soldered on a PCB and fixed in a custom-made PMMA carrier (figure 2). A syringe pump (LA-100, Landgraaf Laborsysteme HLL GmbH, Langenhagen, Germany) and flexible teflon tubes were utilized for analyte application.
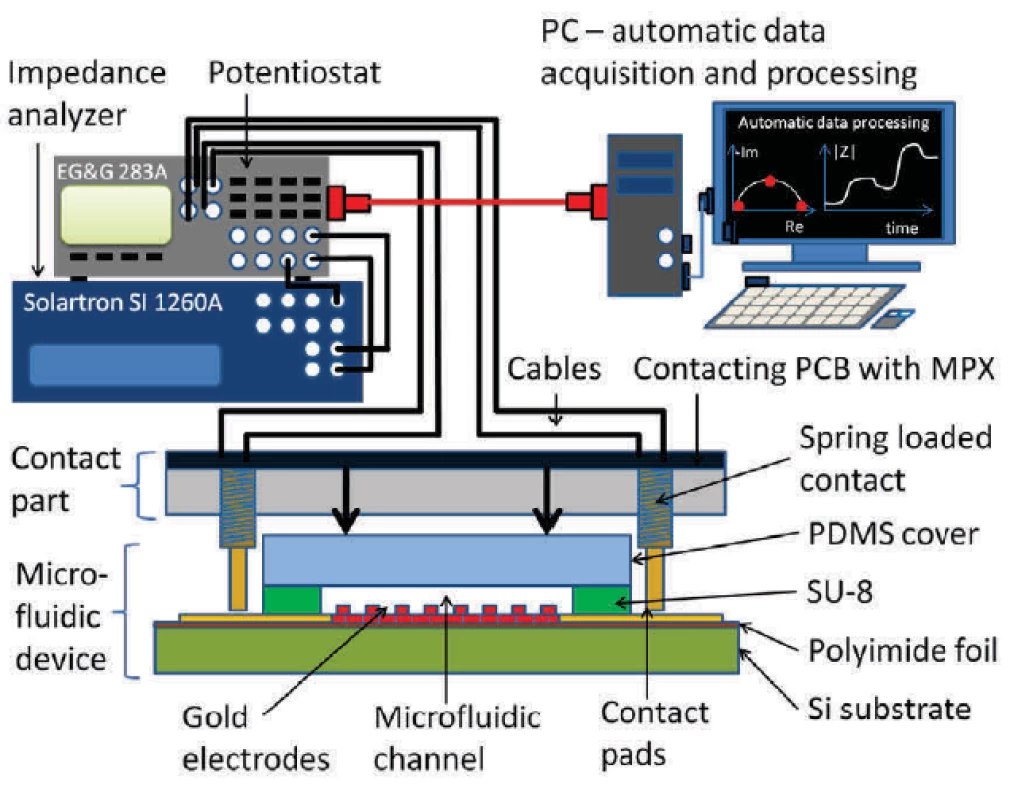
Solartron SI 1260A (Solartron Co, USA) impedance analyzer in combination with EG&G 283 (Princeton Applied Research, USA) potentiostat were used to obtain electrical impedance spectroscopy signal. Custom-made Python software extracted the equivalent circuit parameters. Spectra between 10 Hz and 100 kHz and signal amplitudes of 10 mV were evaluated. Schematics of the complete measurement setup is shown in figure 3.
Sensor Surface Preparation
All electrode surfaces were pre-treated by oxygen plasma (60 seconds, 30 sccm at 100 W). Electrodes were additionally cleaned in a mixture of 2% H2O2 and 2% NH3 for at least 15 minutes with 10 minutes DI water cleaning step afterwards. Bare gold electrodes were incubated for 12 hours in 4 mM 11-mercaptopropion acid in ethanol (0,1 ml/h) immediately after the cleaning step if not mentioned otherwise.
Measurement and Results
Covalent Coupling Test
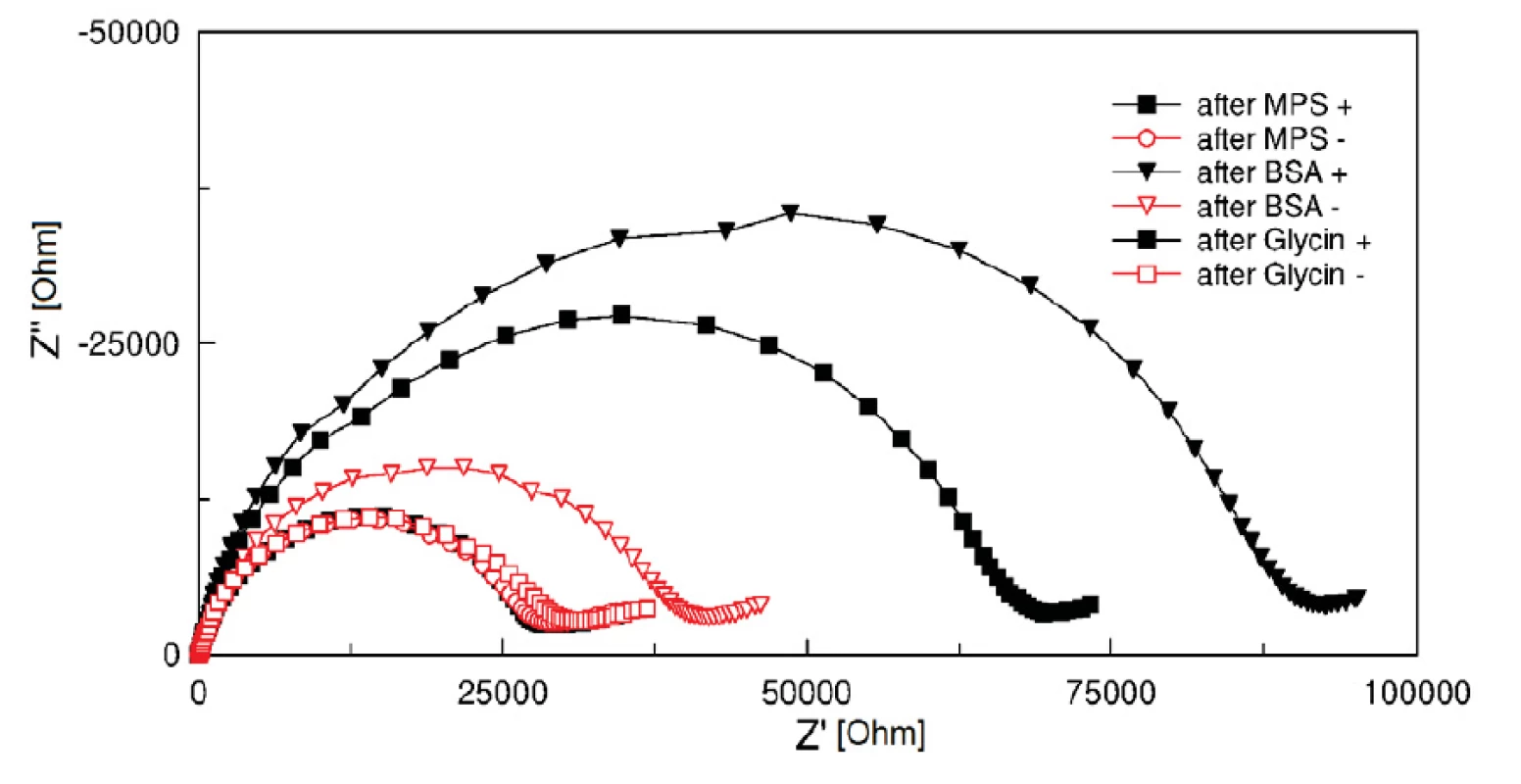
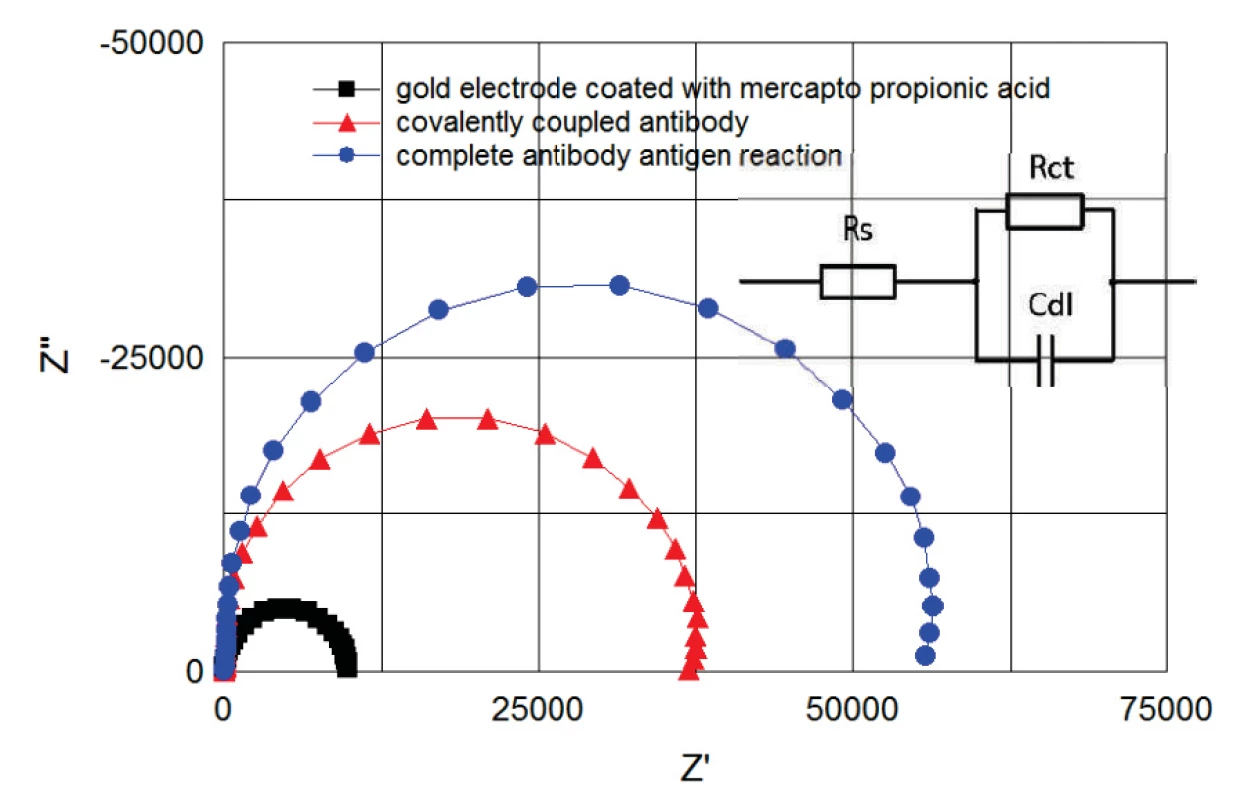
Two groups of electrodes were tested. Electrodes of the first group (‘+’ group) were activated by a 1 : 1 fresh mixture of 400 mM EDC and 100 mM NHS coupling chemistry for 10 minutes. Subsequently, 0.1% BSA solution in 5 mM HCF in PBS, pH 7.4 was applied on the electrodes for 30 minutes. Glycin of pH 2.3 (HCl titrated) was used to clean the electrodes of remaining BSA. EIS measurement in HCF solution was performed before and after BSA incubation and after cleaning process with the glycin solution. The result was compared with a second electrode group (‘-‘ group), in which the EDC/NHS coupling was not carried out and therefore no covalent binding occurs. Curves after MPA, after BSA and glycin treatment show clearly the successful function of activated SAM (see figure 4). The BSA protein was fully washed out from the electrode surface (hollow points in figure 4), while BSA on EDC/NHS activated electrode surfaces stayed covalently bonded (filled points in figure 4). The surface treatment with EDC/NHS improves significantly the absorption.
Simplified Immunoassays
A simplified immunoassay was performed. The following compounds were successively applied: mAb 214D4 (conc. 45 µg/ml) in 0.1% BSA in HCF buffer and the corresponding protein MUC1ex (conc. 10 µg/ml, mw = 65 kDa) in 0.1% BSA in HCF buffer. The flow rate in all presented measurements was adjusted to 0.5 ml/hour.
At first, the chip was pre-treated as described in section 2.4 and shown in figure 5 A and 5 B.
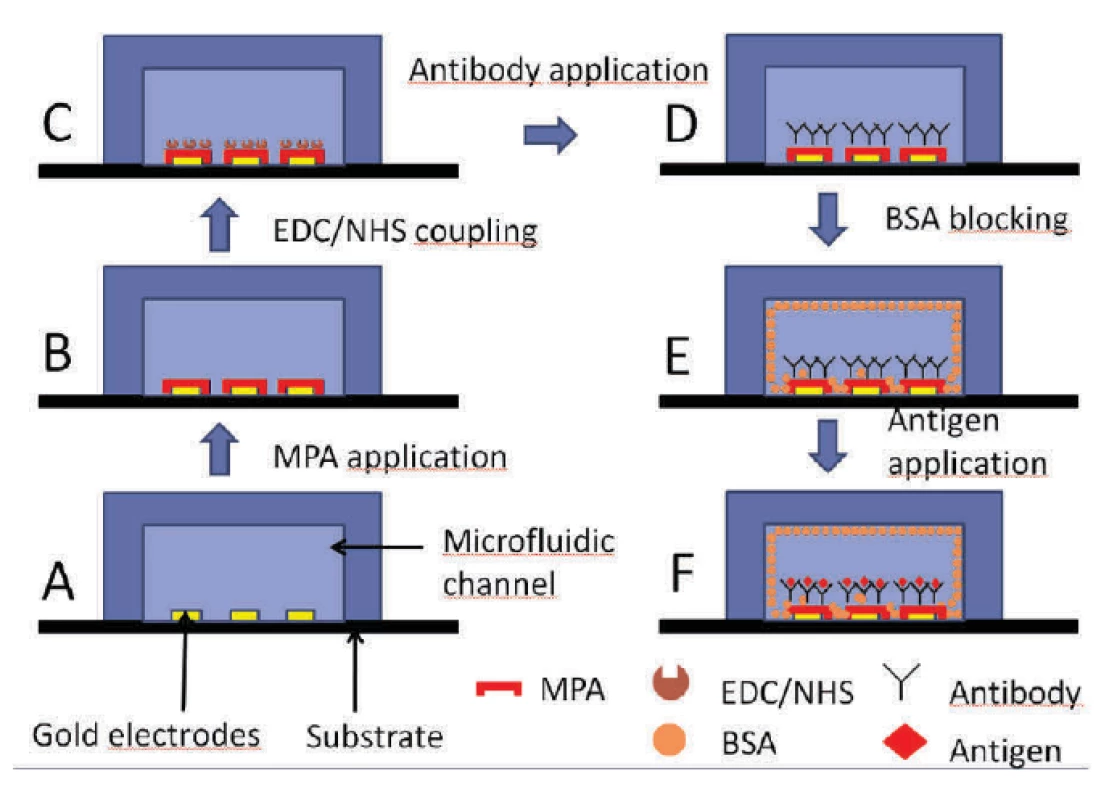
Subsequently, EDC/NHS (4 : 1) was applied in both channels for 10 minutes (figure 5 C) followed by a 214D4 antibody treatment (figure 5 D). The rest of the channel was blocked with BSA (1 mg/ml) in buffer, see figure 5 E. To avoid the non-specific binding of proteins and to ensure an additional blocking effect of the surface in each step, BSA (1 mg/ml) in HCF buffer (BSA + HCF) was used as a protein dilution buffer in further steps. Finally, the corresponding protein MUC1ex antigen (figure 5 F) was introduced onto the channel.
The immunoassay was monitored in real time by EIS (figure 6). The recorded data were continuously fitted to the Randles equivalent model consisting of series resistance Rs, Cdl double layer capacity and Rct charge transfer (polarization resistance), see figure 6.
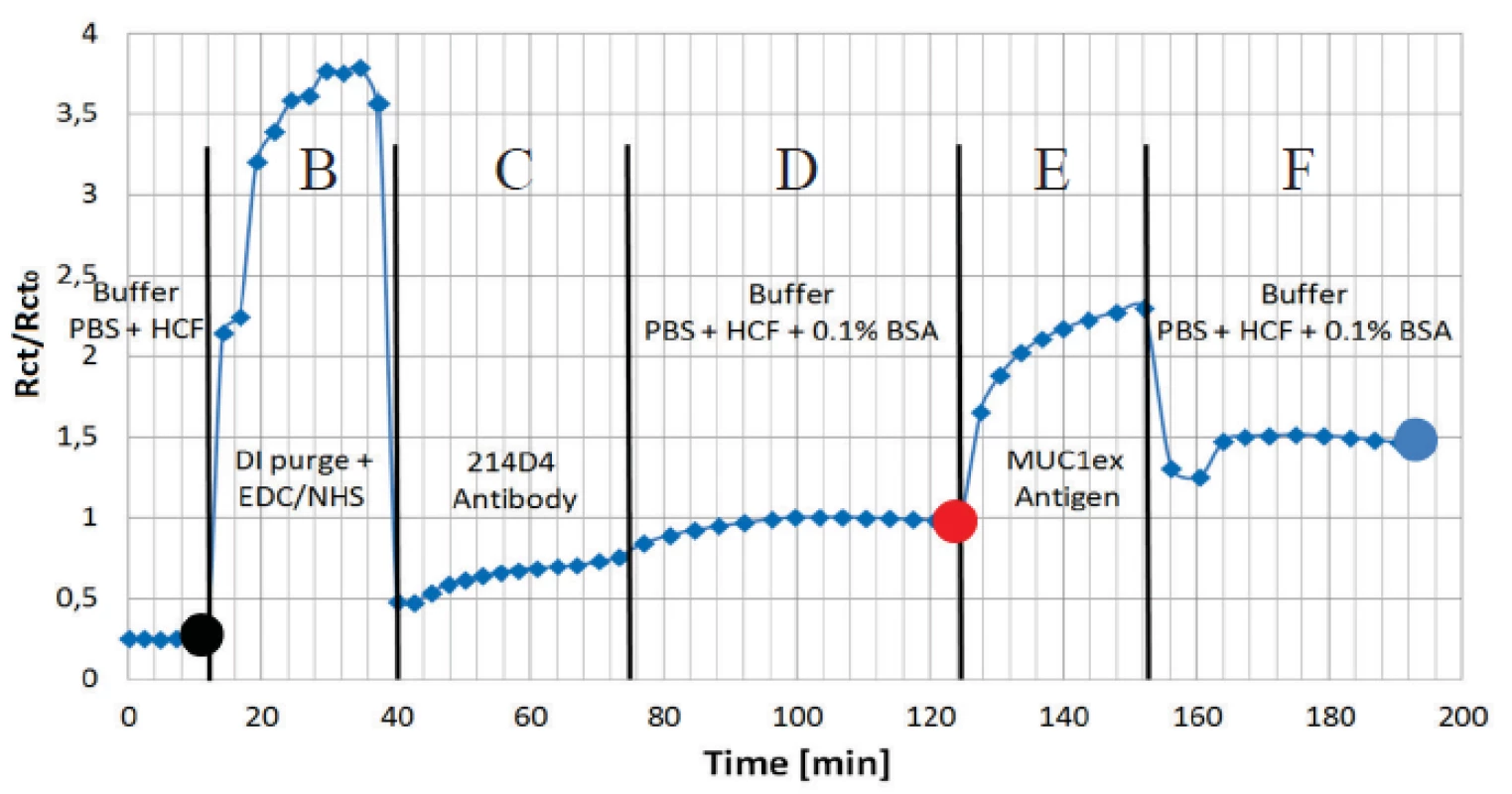
The charge transfer resistance Rct has appeared as the most sensitive parameter for the protein adhesion. The continuous curve of the normalized Rct for all the applied immunoassay is presented in figure 7. Data was normalized to the buffer level (Rct0) before antigen application. The increases in amplitude clearly show the functionality of the assay.
To show the variability of the sensor, a second immunoassay based on prostate antigen – antibody reaction was also performed to prove its suitability and reliability. No HCF redox couple was used in this assay. One channel with three electrodes was devoted for a positive control and one channel with three electrodes for a negative control with a non-corresponding antigen. Pre-treatment and covalent coupling of a PSA antibody was carried out as described previously.
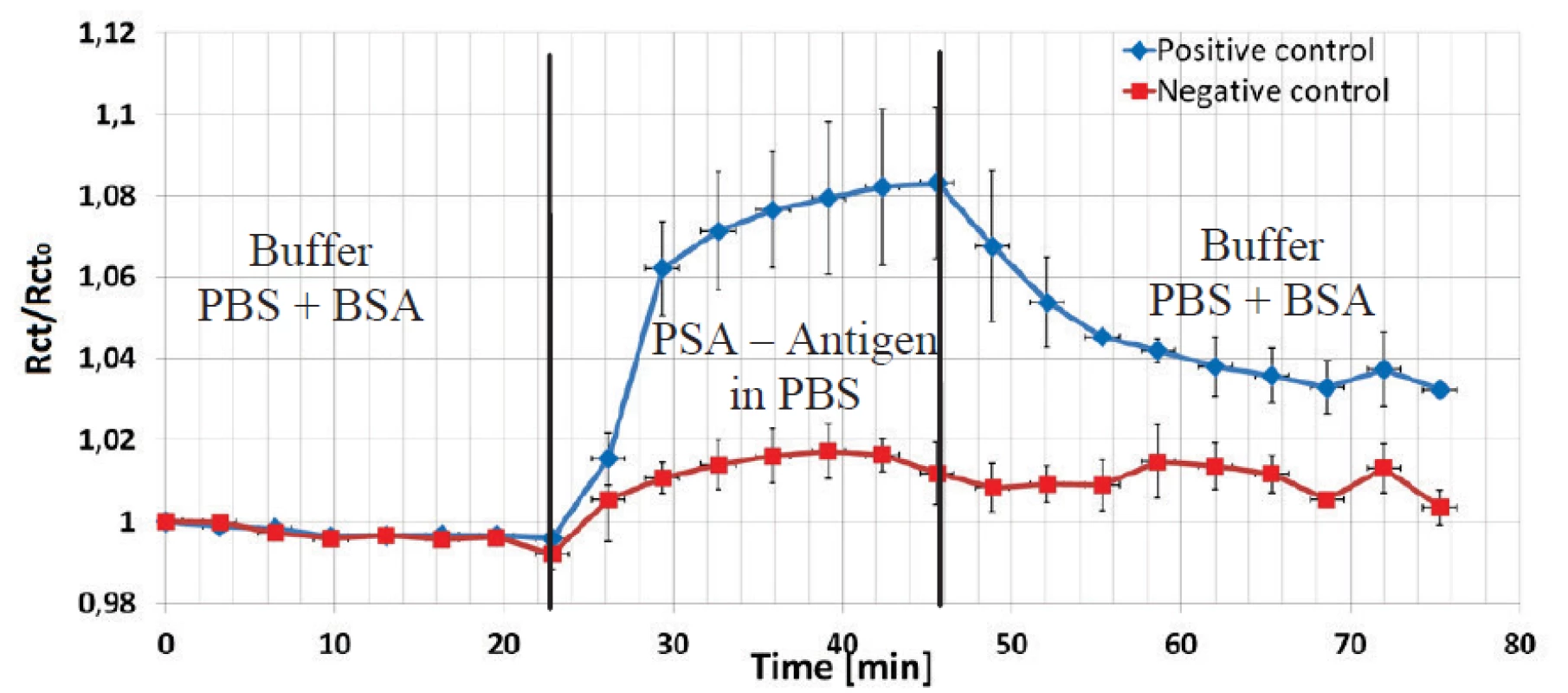
The data shown in Figure 8 were normalized to the Rct value after the PBS+BSA step. The Rct values increased for three electrodes in the positive channel by 8% due to the deposition of the antigen PSA protein in spite in the negative channel just by 2%. The final BSA+PBS purge caused a decrease of the impedance level in the positive channel, but the Rct increasewas significant higher by factor three compared to the increase in the negative control.
Conclusion
The suitability of an impedimetric biosensor based on three gold planar interdigital electrode arrays in one microfluidic channel has been demonstrated for the first time. Stability and function of the covalent biomolecule coupling by EDC/NHS chemistry was proven. Experiments have shown a sufficient sensitivity to detect two antibody - antigen immunoassays. Parallelization of the channels gives a possibility to test a positive and a negative control using the same conditions, but different antibodies.
Acknowledgement
This work was gratefully supported by INTERREG IV-A 2007-2013 EUREGIO MAAS-RIJN under grant INTERREG.EMR.INT4-1.2.-2009-11/058-MICROBIOMED.
Dr.-Ing. Uwe Schnakenberg
Institute of Materials in Electrical Engineering 1
Faculty of Electrical Engineering
RWTH - Aachen
Sommerfeldstr. 24, DE-52074 Aachen
E-mail: schnakenberg@iwe1.rwth-aachen.de
Sources
[1] DANIELS, J. S., POURMAND, N. Labell-Free Impedance Biosensors: Opportunities and Challenges. Electroanalysis, 2007, vol. 19, p. 1239–1257.
[2] SCARANO, S., MASCINI, M., TURNER, A. P. F., MINUNNI, M., Surface plasmon resonance imaging for affinity-based biosensors, Biosensors and Bioelectronics, 2010, vol. 25/5, p. 957–966.
[3] VIDAL, J. C., DUATO, P., BONEL, L., CASTILLO, J. R., Use of polyclonal antibodies to ochratoxin A with a quartz–crystal microbalance for developing real-time mycotoxin piezoelectric immunosensors, Analytical and Bioanalytical Chemistry, 2009, vol. 394/2, p. 572–582.
[4] SANGDAE, L., KI-BOK, K., YONG-IL, K., Love wave SAW biosensors for detection of antigen-antibody binding and comparison with SPR biosensor, Food Science and Biotechnolog, 2011, vol. 20/5, p. 1413–1418.
[5] TANG, X. H., et al. Direct Protein Detection with a Nano-interdigitated Array Gate MOSFET. Biosens.Bioelectronics, 2009 24.12, p. 3531–37.
[6] BERDAT, D., RODRIGUEZ, A. C. M., HERRERA, F., GIJS, M. A. M., Label-free detection of DNA with interdigitated micro-electrodes in a fluidic cell. Lab Chip, 2008, vol. 8, p. 302–308.
[7] WANG, R., et al., Interdigitated array microelectrode based impedance immunosensor for detection of avian irus H5N1. Talanta, 2009, vol. 79, p 159–164.
[8] VAN GERWEN, P. et al. Nanoscaled interdigitated electrode arrays for biochemical sensors, Sens. Actuat. B, 1998, vol. 49, p.73–80.
[9] NARAKATHU, B. B., ATASHBAR, M. Z., BEJCEK B. E., Improved detection limits of toxic biochemical species based on impedance measurements in electrochemical biosensors. Biosens.Bioelectron. 2010, 26.2, p.923–928.
[10] PUETTMANN, C., KOLBERG, K., HAGEN, S., SCHMIES, S., FISCHER, R., NAEHRING, J., BARTH, S., A monoclonal antibody for the detection of SNAP/CLIP-tagged proteins. Immunology letters, 2012, PMID 23085606.
[11] VAN ELSSEN, C. H., FRINGS, P. W., BOT, F. J., VAN DE VIJVER, K. K., HULS, M. B., MEEK, B., HUPPERETS, P., GERMERAAD, W. T., BOS, G.M. Expression of aberrantly glycosylated Mucin-1 in ovarian cancer. Histopathology, 2010, 57/4, p. 597–606.
[12] ZOU, Z., et al. Functionalized nano interdigitated electrodes arrays on polymer with integrated microfluidics for direct bio-affinity sensing using impedimetric measurement. Sensors and Actuators A: Physical, 2007, Vol. 136, Nr. 2, p. 518–526.
[13] ZHANG, X., et al. A reusable electrochemical immunosensor for carcinoembryonic antigen via molecular recognition of glycoprotein antibody by phenylboronic acid self-assembly layer on gold. Analyst, 2008, Vol 133, Nr. 4, p. 485–492.
Labels
BiomedicineArticle was published in
The Clinician and Technology Journal

2015 Issue 4
-
All articles in this issue
- MERANIE PARAMETROV ELEKTROMAGNETICKÝCH POLÍ PRI POUŽÍVANÍ PROSTRIEDKOV MOBILNEJ KOMUNIKÁCIE V ŠKOLSKOM PROSTREDÍ
- Multi-electrode microfluidic platform for protein detection using electrochemical impedance spectroscopy
- Effect of linear accelerator settings on evaluation of dosimetric verification of VMAT plans
- Spectral analysis of atrial components of ablation catheter signals during slow pathway ablation for typical atrioventricular nodal reentrant tachycardia
- The Clinician and Technology Journal
- Journal archive
- Current issue
- About the journal
Most read in this issue
- MERANIE PARAMETROV ELEKTROMAGNETICKÝCH POLÍ PRI POUŽÍVANÍ PROSTRIEDKOV MOBILNEJ KOMUNIKÁCIE V ŠKOLSKOM PROSTREDÍ
- Spectral analysis of atrial components of ablation catheter signals during slow pathway ablation for typical atrioventricular nodal reentrant tachycardia
- Multi-electrode microfluidic platform for protein detection using electrochemical impedance spectroscopy
- Effect of linear accelerator settings on evaluation of dosimetric verification of VMAT plans
