-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
Acute pancreatitis as a complication of childhood cancer treatment
Acute pancreatitis (AP) is now well recognized as a possible complication of childhood cancer treatment, interrupting the chemotherapy regimen, and requiring prolonged hospitalization, possibly with intensive care and surgical intervention, thereby compromising the effect of chemotherapy and the remission of the underlying malignant disease. This review summarizes the current literature and presents the various etiological factors for AP during chemotherapy as well as modern trends in the diagnosis and therapy of AP in children.
Keywords:
Acute pancreatitis, childhood cancer, L-asparaginase, diagnosis, management
Authors: Milica Stefanović 1,*; Janez Jazbec 1; Fredrik Lindgren 2; Milutin Bulajić 3,4; Matthias Löhr 5
Authors place of work: Division of Pediatrics, Unit of Hemato-oncology, University Medical Centre Ljubljana, Ljubljana, Slovenia 1; Department of Pediatric, Karolinska University Hospital, Stockholm, Sweden 2; Department of Gastroenterology, University Hospital Center “Santa Maria della Misericordia”, Udine, Italy 3; Faculty of Medicine, University of Belgrade, Belgrade, Serbia 4; Department of Clinical Science, Intervention and Technology, Karolinska Institutet, Stockholm, Sweden 5
Published in the journal: Cancer Medicine 2016; 5(5)
Category: Review
doi: https://doi.org/10.1002/cam4.649© 2016 The Authors. Cancer Medicine published by John Wiley & Sons Ltd.
This is an open access article under the terms of the Creative Commons Attribution License, which permits use, distribution and reproduction in any medium, provided the original work is properly cited.Summary
Acute pancreatitis (AP) is now well recognized as a possible complication of childhood cancer treatment, interrupting the chemotherapy regimen, and requiring prolonged hospitalization, possibly with intensive care and surgical intervention, thereby compromising the effect of chemotherapy and the remission of the underlying malignant disease. This review summarizes the current literature and presents the various etiological factors for AP during chemotherapy as well as modern trends in the diagnosis and therapy of AP in children.
Keywords:
Acute pancreatitis, childhood cancer, L-asparaginase, diagnosis, managementIntroduction
Acute pancreatitis (AP) is defined as the histological inflammation of the pancreatic parenchyma, presenting clinically as a sudden onset of abdominal and back pain accompanied by the elevation of pancreatic enzymes in the blood and urine [1, 2]. It is a reversible process characterized by the presence of inflammatory cells and varying degrees of cellular apoptosis, necrosis, and hemorrhage [3]. AP is seen relatively rarely in children in whom the underlying etiologies differ greatly from those in adults [4]. However, recent reports in the literature have confirmed an increasing number of patients with pancreatitis in the pediatric population [2, 4-6].
Children undergoing treatment for hemato-oncological diseases comprises a specific patient population with certain characteristic in common: they have systemic disease, they are undergoing treatment with chemotherapeutics, they are immunocompromised, they have more surgical procedures under general anesthesia and they frequently receive antimicrobial treatment. This puts them at greater risk of developing AP. Currently, there are no published reviews on AP in this specific group of pediatric patients.
The purpose of this review was to present a systematic account of the various etiological factors for developing AP during childhood cancer treatment and to highlight modern trends in the diagnosis and treatment of AP in the pediatric population.
Background
Etiology
Various etiologies have to be considered when AP is suspected in children (Table 1) [7]; however, some etiologies are more frequent then others in oncological pediatric patients as described below.
Tab. 1. Etiologies of pancreatitis in children and adolescents. 
EBV, Epstein–Barr virus; 5-ASA, 5-aminosalicylic acid; SPINK-1, serine protease inhibitor, Kazal type-1; CFTR, cystic fibrosis transmembrane conductance regulator; ERCP, endoscopic retrograde cholangiopancreatography. Drugs
Numerous medications causing drug-induced pancreatitis have been indicated in the literature [8]. Although some have been reported to cause severe pancreatitis, it is important to stress that the etiology for AP does not determine its severity [9]. Once the process of AP has been initiated, its severity is determined by the propagation of proinflammatory mediators, like IL-1β, IL-6, and TNF-α [10].
L-Asparaginase is the key chemotherapeutic agent used for remission induction and consolidation therapy in acute lymphoblastic leukemia (ALL) and it is also an important component of the therapy for some non-Hodgkin lymphomas (NHL) [11, 12]. Cases of L-asparaginase-associated pancreatitis (AAP) were first reported in the 1970s with an incidence ranging from 0.7% to 24% [13-16] and mortality rates of 2–5% [14]. In a retrospective study by Knoderer et al. of 254 patients receiving L-asparaginase, 48 patients developed pancreatitis, of whom 33 (68.7%) were indicated as having AAP; however, the authors did not succeed in predicting which patients would develop pancreatitis. All 33 patients with AAP had mild cases of pancreatitis [11]. And other authors have also reported that patients with AAP presented with mild symptoms [14, 15]. In contrast, several other case reports have described various complications associated with AP [17-20], some with fatal results [21]. Chronic complications rarely occur, but when they do, they can take the form of chronic pancreatitis or diabetes mellitus [16]. AAP has been reported after treatment with all three asparaginase formulations [11-13] and Alvarez et al. suggested that the incidence of PEG-asparaginase-induced pancreatitis was higher than that induced by E. coli-asparaginase (18% vs. 1.9%) [12]. However, Kearney and colleagues reported that, in a cohort of 403 patients, the only predisposing factor for developing AAP was age, as older children had a higher risk of developing the disease. Also, the patients who experienced AAP had a significantly higher risk of a relapse of their cancer [14]. In addition to asparaginase, other chemotherapeutic agents have also been indicated in the literature as possible causes for inducing pancreatitis (Table 2).
Tab. 2. Frequently used drugs during childhood cancer treatment that are implicated in AP etiology. 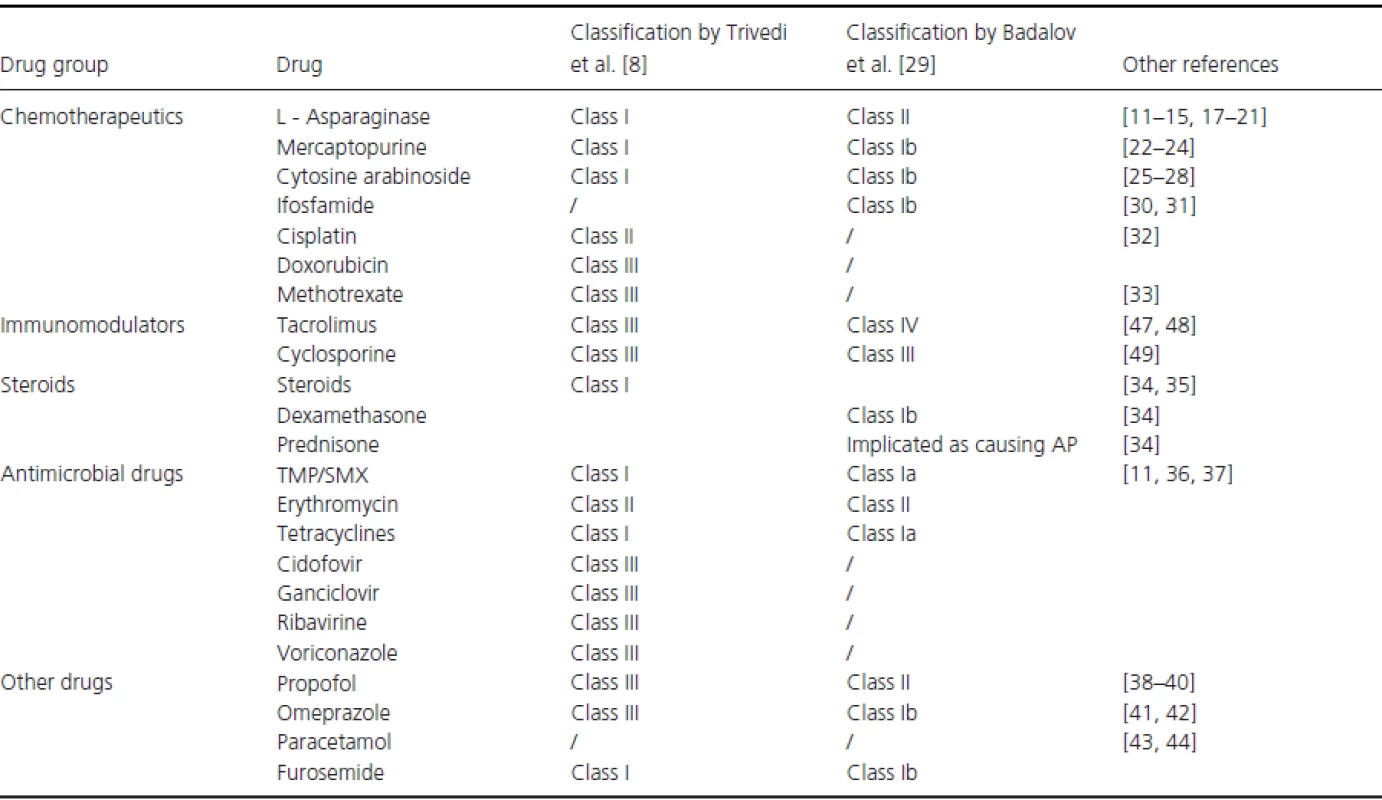
TMP/SMX, trimethoprim/sulfomethoxazole; AP, acute pancreatitis. Steroids are known to cause pancreatitis when used in the therapy of malignant disease as well as other medical conditions. In a meta-analysis comparing the corticosteroids, dexamethasone, and prednisone, used for induction therapy in childhood ALL, Teuffel et al. found that the risk ratio for AP induced by these two corticosteroids was 3.78% and that there was no significant difference between the two drugs[34]. Bai et al. reported that corticosteroids, along with valproic acid, were the two drugs most associated with drug-induced pancreatitis in children [35].
Due to frequent episodes of febrile neutropenia, sepsis and other bacterial, viral and fungal infections during chemotherapy, antimicrobial agents are frequently used. Those associated with AP are listed in Table 2. The most controversial are trimethoprim/sulfomethoxazole (TMP/SMX) since this combination is used universally in patients undergoing chemotherapy as prophylaxis against Pneumocystis jiroveciiinfection. It has been noted as the drug associated with AP in many studies, with reported recurrence of AP after rechallenge, although this is mostly seen in adults [29, 36, 37]. However, Knoderer et al. did not observe a role for TMP/SMX in causing AP in a pediatric ALL population [11]. No controlled study has been performed to examine the role of TMP/SMX in the pathogenesis of AP; it is possible that the pathogenesis depends on the dosage.
Certain other drugs, probably used more frequently in hemato-oncological patients than in the general patient population, have been listed as possible causes of AP (Table 2). Propofol, which is the most frequently used anesthetic, is reported to cause AP [8, 29, 38, 39]. However, in a retrospective study in which five of 479 children receiving chemotherapy for ALL developed AP, Crawford et al. reported that no episode of AP occurred within the latency period of propofol and that all five patients received propofol anesthesia after episodes of AP without complications [40]. In our experience, it seems that episodes of AP in hemato-oncological pediatric patients are unlikely caused by propofol.
During corticosteroid therapy most children need proton pump inhibitors (PPIs), such as omeprazole, which is reported to cause AP in the adult population. In addition, other PPIs and antacids such as cimetidine and ranitidine are also reported as possible causes of AP [8, 29, 41]. Eland et al. conducted a retrospective cohort study in which they concluded that there is no association between AP and the use of acid-suppressing drugs [42]. However, there is no published data from the pediatric population to support this claim.
Transplantation
Hematopoietic stem cell transplantation (HSCT) is an important means of treating some hemato-oncological diseases. AP was first recognized as a possible complication of HSCT in the early 1990s [44]. Nowadays, pancreatitis is well recognized as a complication of HSCT and it has been linked to many factors involved in the transplantation procedure. Werlin et al. reported the incidence of AP as 3.5% among 202 children treated with HSCT [45] with a similar incidence of post-HSCT pancreatitis (7 of 180 or 3.9%) in a pediatric population with pancreatitis due to various etiologies seen during a six-year period [4]. Ko et al. found pancreatitis at autopsy in 28% of patients treated with HSCT (51 of 184), and in five of these, pancreatitis was the cause of death. An increase in the number of survival days and any graft-versus-host disease (GVHD) found at autopsy were cited as risk factors for developing AP in these patients [46].
Some case reports have shown that drugs used in the stem cell transplantation process are also possible causes of AP [47, 48], for example, the immunosuppressive drugs, cyclosporine and tacrolimus, and the antiviral drugs, cidofovir, foscarnet, and ganciclovir, have all been associated with pancreatitis [8, 29]. Using a rat model, Ito et al. showed that therapeutically recommended doses of cyclosporine can induce AP [49].
There is also some evidence that infection with cytomegalovirus (CMV) or adenovirus can cause AP [50, 51]. Bateman et al. reported that five out 95 (5,3%) patients treated with HSCT experienced pancreatitis, one following treatment with tacrolimus [47] and four after adenoviral infection [52]. In addition, a mouse model has been used to show that adenovirus infection can cause AP [53]. Salomone et al. suggested that in patients with post-HSCT complications, particularly acute hepatic/hepatointestinal GVHD and CMV infection, the possibility of AP should be considered [54]. The reactivation of the varicella-zoster virus, which is likely to occur in post-HSCT patients, has also been described as a possible cause of AP [55, 56].
Diagnostics
Acute pancreatitis should always be included in the differential diagnosis of a child presenting with abdominal pain during cancer treatment!
The definition and diagnostic criteria for pediatric AP given by the INSPPIRE Project (International Study Group of Pediatric Pancreatitis: In Search for a Cure) are outlined in Table 3 [57].
Tab. 3. Clinical definition of AP in children [7]. ![Clinical definition of AP in children [7].](https://www.prelekara.sk/media/cache/resolve/media_object_image_small/media/image/9d8f5a24001eca0de36ece9536206d44.png)
US, transabdominal ultrasonography; CECT, contrast-enhanced computerized tomography; EUS, endoscopic ultrasonography; MRI/MRCP, magnetic resonance imaging/magnetic resonance cholangiopancreatography. Abdominal pain in children has variable characteristics but is still the most common symptom of AP, occurring in 87% of cases, followed by vomiting, abdominal distress and tenderness [58], thus AP should be strongly suspected when sudden abdominal pain is accompanied by nausea and vomiting. Generally, levels of amylase and lipase elevated to three times the normal levels will confirm the diagnosis. Since the simultaneous elevation of both pancreatic enzymes in pediatric patients increases the sensitivity of the test to 94%, the analysis of both enzymes is recommended, especially in very young children [1, 58-61]. Table 4 illustrates the main nonpancreatic causes of increased pancreatic enzyme levels. Additionally, in a population of adult patients, it has been demonstrated that an elevated creatinine level during a 48-hour period, despite adequate hydration, is associated with the development of pancreatic necrosis with a positive predictive value of 93% [62].
Tab. 4. Main nonpancreatic causes of increased pancreatic enzyme levels [1, 2]. ![Main nonpancreatic causes of increased pancreatic enzyme levels [1, 2].](https://www.prelekara.sk/media/cache/resolve/media_object_image_small/media/image/366a1385d82a05d6226588e4c15cfaec.png)
In addition to clinical symptoms and laboratory tests, diagnostic imaging plays a critical role in the evaluation of AP by determining its severity and identifying potential complications [2, 63]. The recommended imaging modalities in pediatric population are similar to those for adult patients, but pediatric experts are more likely to select tests that limit radiation and recognize that certain modalities may be more difficult to perform because of patient size and sedation need [57].
Abdominal ultrasound (US) and computed tomography (CT) scans remain the most commonly used imaging methods. US is performed most frequently due to its simplicity and the absence of radiation; however, about one-third of US findings are normal [11, 12]. According to UK guidelines it is not current practice to perform early CT scans for the detection and staging of severe cases of AP, due to its low sensitivity [64]. On the other hand, Tsuji et al. showed that perfusion CT was superior to angiography in predicting pancreatic necrosis in early SAP and that its sensitivity and specificity were 100% and 95.3%, respectively [65-67]: it is also a useful tool for predicting systemic complications [67].
Magnetic resonance imaging associated with cholangiopancreatography seems to be an excellent alternative in the evaluation of the pancreas, due to the absence of radiation and lack of invasiveness [59].
Finally, when infected pancreatic necrosis is suspected, fine-needle aspiration is recommended as a diagnostic procedure for identification of the pathogen with a possibility of making the right diagnosis in 89–100% of cases [68].
Complications
In the majority of AP cases the course of the disease is mild and self-limiting and complications are relatively rare. For example, Knoderer et al. reported that 43 patients of 48 who developed AP during treatment for ALL, acute myeloid leukemia (AML), or NHL were classified as mild cases and complications such as necrosis or pseudocysts documented by imaging methods, were present in only 10% of patients [11]. Kearney et al. reported that only 18% of patients with AAP developed complications such as pseudocysts [14].
The complications observed in children with AP can be immediate or delayed. Immediate complications include hypovolemic and septic shock, renal dysfunction, cavity effusions, and acute respiratory distress syndrome. The most common delayed complications include pancreatic necrosis and the formation of pseudocysts [1, 59]. Pancreatic necrosis has an infection rate of between 30% and 70%, and the immediate identification of this complication is critical for the child's prognosis since it often progresses to multisystem failure [59]. It has been shown that 80% of all AP deaths are due to infected necrosis of the pancreas and consequently to septic complications [69, 70]. With respect to pseudocysts, there are numerous case reports showing pseudocyst development during cancer treatment in children, most of them during L-asparaginase treatment [18-20, 71].
Treatment
While the etiology of childhood AP differs from AP in adult patients, its treatment, like its diagnostics, is based on current adult therapy strategies. Since there is no specific treatment for AP, various different guidelines have been published and these are not always in agreement [64, 72, 73]. For example, administration of prophylactic antibiotics for the prevention of pancreatic infection in SAP is contentious. Two controlled, double-blind studies were published: Isenmann et al. [74] using ciprofloxacin/metronidazole and Garcia-Barrasa et al. [70] using ciprofloxacin, both showing no difference in mortality or the incidence of pancreatic infection between placebo and the studied medication groups. In contrast, regarding necrotizing AP, two separate meta-analyses concluded that antibiotic prophylaxis is superior to antibiotic treatment on demand and that the patients with proven pancreatic necrosis should receive either imipenem or meropenem prophylaxis [69, 75]. On the contrary, a third meta-analysis performed in 2008 concluded that the use of prophylactic antibiotics for acute necrotizing pancreatitis had no effect on infected pancreatic necrosis and no effect on the mortality rate among these patients [76].
The use of protease inhibitors, applied either intravenously or by continuous regional arterial infusion (CRAI), to treat patients with AP remains controversial and is recommended only in Japanese (JPN) and Italian national guidelines [73, 77]. Morimoto et al. published a report describing five pediatric patients with severe AAP who were successfully treated using CRAI with protease inhibitors and antibiotics resulting in a swift resumption of chemotherapy and allowing the patients to remain in complete remission with further chemotherapy that excluded L-asparaginase [78].
Despite initially encouraging results in large randomized studies, antisecretory agents such as octreotide [79], and anti-inflammatory drugs such as lexipafant, have proved disappointing as therapies for AP [64, 77]. On the other hand, more recent studies report on the use of ocreotide as a means of preventing AP during L-asparaginase readministration [80].
However, the available guidelines do agree on other areas of AP treatment [64, 72, 73]. At the onset of symptoms, and before the disease severity is known, supportive care is the most important factor. Critical components in the care of patients with AP include oxygen supplementation and fluid resuscitation. Although pain control is mentioned only in the JPN guidelines, it is crucial during the care of a patient with AP. Currently, enteral rather than total parenteral nutrition is recommended in cases where nutrition support is necessary. The current data do not support nasogastric suction, gut decontamination or the use of H2 blockers in AP, unless other indications are present. Furthermore, all patients with predicted SAP should be monitored closely and prompt transfer to an intensive care unit should take place in the case of sustained organ failure.
Opinions regarding the treatment of infected pancreatic necrosis are generally in accord in the current literature and the treatment of choice is surgical debridement. As an alternative, minimally invasive approaches may be used in selected circumstances with the appropriate expertise. On the other hand, the approach for sterile necrosis is conservative at the onset of the disease, and if surgical treatment is needed it should be delayed as long as possible. Debridement should only be considered if abdominal pain persists and prevents oral intake. Conservative treatment is also recommended for pancreatic pseudocysts but occasionally a patient may require surgical, endoscopic or radiological intervention. The condition should be managed on an individual basis according to how long the cyst has persisted, its symptoms, accompanying complications or an increase in diameter.
At the Karolinska University Hospital the initial treatment of AP consists of “pancreatic rest” by fasting followed by limited pancreatic stimulation, pain management, antiemetics, intravenous fluid therapy, and careful monitoring for early detection and treatment of possible complications. It is also important to determine the etiology of the pancreatitis in order to determine whether the cause itself is treatable. Upon admission, the patient is usually rehydrated (12.5 mL/kg/h, for 4 h), and this is often followed by fluid maintenance 120–150% of the continuous basal rate. This fluid therapy is managed on an individual basis and is based on objective measurements and the monitoring of electrolytes and fluid balance aiming for a urine output of at least 0.5–1 mL/kg per hour). In most cases of AP enteral nutrition, which stabilizes the gut barrier, stimulates the motility and prevents the overgrowth of bacteria, can be introduced within 48 hours. At Karolinska University hospital, we have great experience with nasojejunal tubes as an enteral route for feeding in more complicated cases, were oral nutrition or nasogastric tube have failed. Parenteral nutrition is rarely required, though it could be necessary if longer or more complicated episodes of pancreatitis occur. Although certain adult studies have shown increased risk of complications, such as infections [81] and mortality [82] with parenteral nutrition, it is still used in over 40% of the pediatric patients [58].
Pain management is important for comfort and for reducing energy expenditure. To achieve this, the intravenous administration of analgesics such as morphine or other opioids and nonopioid drugs, such as paracetamol, is often required. In the INSPPIRE study [56], 94% of the pediatricians used opioids and it seems safe to use them without worsening the pancreatitis by inducing spasms in the sphincter of Oddi [83]. With respect to the management of infection, at the Karolinska University Hospital, antibiotics are administrated only if sepsis, concomitant bacterial infection or infected necrotizing pancreatitis occurs. Therapeutic pediatric endoscopic retrograde cholangiopancreatography may be required in complicated cases involving duct-rupture, main pancreatic duct (MPD) stones, or in pancreatic pseudocysts that compress the MPD. Some cases of AP with severe complications due to chemotherapy agents need repeated treatment with endoscopic transgastric and/or transpappillary stents. Such procedures are best performed with the patient under general anesthesia, which provides a good opportunity for introducing a nasogastric jejunal tube beyond the ligament of Treitz. In very rare and complicated cases of AP invasive surgery is needed.
What type of aftercare?
One of the main questions when childhood cancer treatment is complicated with AP is what course of treatment to follow after an episode of AP, especially when it is drug-related. When there is an adequate replacement for an AP-inducing drug it should be considered. Sastry et al. reported replacing tacrolimus with cyclosporine in a patient with tacrolimus-induced AP without recurrence of AP symptoms [47]. In general, the main uncertainty reported in the current literature is the question of whether or not to restart L-asparaginase treatment after an episode of AAP, as L-asparaginase is an important drug for remission maintenance in ALL treatment. Secondary episodes of AP after L-asparaginase re-challenge have been reported but these generally occur without life-threatening complications [11, 14]. There have also been reported cases of patients that stayed in remission even when L-asparaginase was omitted from chemotherapy [19, 77]; however, other cases have been reported of patients who relapsed and died [19]. The main factors affecting the decision of whether or not to restart L-asparaginase treatment include the time of AP onset during chemotherapy and the severity of the episode of AP.
Wu et al. reported replacing L-asparaginase with methotrexate after AP. In this study, four patients, treated with octreotide for AP, stayed in remission and one, who did not receive octreotide, died due to relapse of ALL [79]. In contrast, Hung et al. reported a case of ifosfamide-induced AP during treatment of osteosarcoma in which a second episode of AP occurred after ifosfamide re-challenge; however, the symptoms of AP were resolved and the patient stayed in remission [31]. Therefore, until the predisposing factors for AP development have been identified, cases reported in the literature suggest that one episode of mild pancreatitis may not be an absolute contraindication to the administration of further doses of the chemotherapeutic agent suspected of causing the AP [14]. For clinicians, it seems essential to find a balance for each separate case between the risk of AP recurrence and the potential therapeutic benefits of restarting a chemotherapeutic agent that is potentially vital in ensuring that the patient stays in remission.
Conclusions
The risk of developing AP during childhood cancer treatment is significant; therefore, it is of critical importance to be able to recognize the symptoms of AP, and to try, if possible, to determine its etiology and to treat the disease in the best possible way according to its severity, complications and using the available guidelines. Fast resolution of AP symptoms and continuation with chemotherapy is vital to achieve remission of the underlying malignant disease.
Conflict of Interest
None declared.
Funding Information
Sponsored by EPC (European Pancreatic Club) fellowship and Ministry of education Science and Sports of Republic Slovenia research grants J3 4220 and P3-0343.
Received: 9 October 2015
Revised: 4 December 2015
Accepted: 29 December 2015
Version of Record online: 13 February 2016* Correspondence:
Milica Stefanović
Division of Pediatrics
Unit of Hemato-oncology
University Medical Centre Ljubljana
Bohoričeva 20
1000 Ljubljana, SloveniaTel: +386 1 522
Fax: +386 1 522
E-mail:micastrbacki@gmail.com
Zdroje
1 Mekitarian Filho, E., W. B. Carvalho, and F. D. Silva. 2012. Acute pancreatitis in pediatrics: a systematic review of the literature. J. Pediatr. 88 : 101–114.
2 Velasco-Benítez, C. A. 2011. Pancreatitis in children. Rev. Col. Gastroenterol. 26 : 48–53.
3 Bradley, E. L., 3rd. 1993. A clinically based classification system for acute pancreatitis: summary of the International Symposium on Acute Pancreatitis, Atlanta, GA, September 11 through 13, 1992. Arch. Surg. 128 : 586–590.
4 Werlin, S. L., S. Kugathasan, and B. C. Frautschy. 2003. Pancreatitis in children. J. Pediatr. Gastroenterol. Nutr. 37 : 591–595.
5 Tiao, M. M., J. H. Chuang, S. F. Ko, H. W. Kuo, C. D. Liang, and C. L. Chen. 2002. Pancreatitis in children: clinical analysis of 61 cases in southern Taiwan. Chang Gung Med. J. 25 : 162–168.
6 Park, A., S. U. Latif, A. U. Shah, J. Tian, S. Werlin, A. Hsiao, et al. 2009. Changing referral trends of acute pancreatitis in children: A 12-year single-center analysis. J. Pediatr. Gastroenterol. Nutr. 49 : 316–322.
7 Löhr, M., and Å. Andrén-Sandberg. eds. 2011. Pancreatitis - diagnosis and therapy. UNI-MED Science, Uni-Med isbn: 978-3-8374-1317-5.
8 Trivedi, C. D., and C. S. Pitchumoni. 2005. Drug-induced pancreatitis an update. J. Clin. Gastroenterol. 39 : 709–716.
9 Gullo, L., M. Migliori, A. Olah, G. Farkas, P. Levy, C. Arvanitakis, et al. 2002. Acute pancreatitis in five European countries: etiology and mortality. Pancreas 24 : 223–227.
10 Steer, M. 2002. Pancreatitis severity: who calls the shots? Gastroenterology 122 : 1168–1172.
11 Knoderer, H. M., J. Robarge, and D. A. Flockhart. 2007. Predicting asparaginase-associated pancreatitis. Pediatr. Blood Cancer 49 : 634–639.
12 Alvarez, O. A., and G. Zimmerman. 2000. Pegaspargase-induced pancreatitis. Med. Pediatr. Oncol. 34 : 200–205.
13 Sikorska-Fic, B., E. Stan'czak, M. Matysiak, and A. Kamin'ski. 2000. Acute pancreatitis during chemotherapy of acute lymphoblastic leukaemia complicated with pseudocyst. Med. Wieku Rozwoj. 12 : 1051–1055.
14 Kearney, S. L., S. E. Dahlberg, D. E. Levy, S. D. Voss, S. E. Sallan, and L. B. Silverman. 2009. Clinical course and outcome in children with acute lymphoblastic leukemia and asparaginase-associated pancreatitis. Pediatr. Blood Cancer 53 : 162–167.
15 Earl, M. 2009. Incidence and management of asparaginase-associated adverse events in patients with acute lymphoblastic leukemia. Clin. Adv. Hematol. Oncol. 7 : 600–606.
16 Flores-Calderón, J., E. Exiga-Gonzaléz, S. Morán-Villota, J. Martín-Trejo, and A. Yamamoto-Nagano. 2009. Acute pancreatitis in children with acute lymphoblastic leukemia treated with L-asparaginase. J. Pediatr. Hematol. Oncol. 31 : 790–793.
17 Yu, C. H., K. H. Lin, D. T. Lin, R. L. Chen, Y. C. Horng, and M. H. Chang. 1994. L-asparaginase-related pancreatic pseudocyst: report of a case. J. Formos. Med. Assoc. 93 : 441–444.
18 Karabulut, R., K. Sönmez, C. Afşarlar, A. Can Başaklar, and N. Kale. 2005. Pancreas pseudocyst associated with L-asparaginase treatment: a case report. Acta Chir. Belg. 105 : 667–669.
19 Caniano, D. A., A. F. Browne, and E. T. Boles, Jr. 1985. Pancreatic pseudocyst complicating treatment of acute lymphoblastic leukemia. J. Pediatr. Surg. 20 : 452–455.
20 Sadoff, J., S. Hwang, D. Rosenfeld, L. Ettinger, and N. Spigland. 1997. Surgical pancreatic complications induced by L-asparaginase. J. Pediatr. Surg. 32 : 860–863.
21 McLean, R., S. Martin, and P. R. Lam-Po-Tang. 1982. Fatal case of L-asparaginase induced pancreatitis. Lancet 2 : 1401–1402.
22 Carter, M. J., A. J. Lobo, and S. P. Travis; IBD Section, British Society of Gastroenterology. 2004. Guidelines for the management of inflammatory bowel disease in adults. Gut 53(Suppl 5):V1–V16.
23 van Geenen, E. J., N. K. de Boer, P. Stassen, R. K. Linskens, M. J. Bruno, C. J. Mulder, et al. 2010. Azathioprine or mercaptopurine-induced acute pancreatitis is not a disease-specific phenomenon. Aliment. Pharmacol. Ther. 31 : 1322–1329.
24 Willert, J. R., G. V. Dahl, and N. M. Marina. 2002. Recurrent mercaptopurine-induced acute pancreatitis: a rare complication of chemotherapy for acute lymphoblastic leukemia in children. Med. Pediatr. Oncol. 38 : 73–74.
25 McGrail, L. H., L. H. Sehn, R. B. Weiss, M. R. Robson, J. H. Antin, and J. C. Byrd. 1999. Pancreatitis during therapy of acute myeloid leukemia: cytarabine related? Ann. Oncol. 10 : 1373–1376.
26 Altman, A. J., P. Dinndorf, and J. J. Quinn. 1982. Acute pancreatitis in association with cytosine arabinoside therapy. Cancer 49 : 1384–1386.
27 Kudo, K., A. Hama, S. Kojima, R. Ishii, A. Morimoto, F. Bessho, et al. 2010. Mosaic Down syndrome-associated acute myeloid leukemia does not require high-dose cytarabine treatment for induction and consolidation therapy. Int. J. Hematol. 91 : 630–635.
28 Kawasaki, H., K. Isoyama, M. Eguchi, S. Hibi, N. Kinukawa, Y. Kosaka, et al. 2001. Superior outcome of infant acute myeloid leukemia with intensive chemotherapy: results of the Japan Infant Leukemia Study Group. Blood 98 : 3589–3594.
29 Badalov, N., R. Baradarian, K. Iswara, J. Li, W. Steinberg, and S. Tenner. 2007. Drug induced acute pancreatitis: an evidence-based review. Clin. Gastroenterol. Hepatol. 5 : 648–661.
30 Garg, R., S. Agarwala, and V. Bhatnagar. 2010. Acute pancreatitis induced by ifosfamide therapy. J. Pediatr. Surg. 45 : 2071–2073.
31 Hung, M. C., G. Y. Hung, P. C. Lin, C. M. Tiu, and Y. C. Tien. 2007. Acute pancreatitis associated with ifosfamide. J. Chin. Med. Assoc. 70 : 176–179.
32 Bunin, N., W. H. Meyer, M. Christensen, and C. B. Pratt. 1985. Pancreatitis following cisplatin: a case report. Cancer Treat. Rep. 69 : 236–237.
33 Shrikiran, A. 2011. A rare case of methotrextate induced pancreatitis in acute leaukemia patient. Webmed Central Paediatr. 2:WMC002820.
34 Teuffel, O., S. P. Kuster, S. P. Hunger, V. Conter, J. Hitzler, M. C. Ethier, et al. 2011. Dexamethasone versus prednisone for induction therapy in childhood acute lymphoblastic leukemia: a systematic review and meta-analysis. Leukemia 25 : 1232–1238.
35 Bai, H. X., M. H. Ma, A. I. Orabi, A. Park, S. U. Latif, V. Bhandari, et al. 2011. Novel characterization of drug-associated pancreatitis in children. J. Pediatr. Gastroenterol. Nutr. 53 : 423–428.
36 Park, T. Y., H.-C. Oh, and J. H. Do. 2010. A case of recurrent pancreatitis induced by trimethoprim-sulfamethoxazole re-exposure. Gut. Liv. 4 : 250–252.
37 Brett, A. S., and S. V. Shaw. 1999. Simultaneous pancreatitis and hepatitis associated with trimethoprim-sulfamethoxazole. Am. J. Gastroenterol. 94 : 267–268.
38 Gottschling, S., R. Larsen, S. Meyer, N. Graf, and H. Reinhard. 2005. Acute pancreatitis induced by short-term propofol administration. Paediatr Anaesth. 15 : 1006–1008.
39 Bustamante, S. E., and E. Appachi. 2006. Acute pancreatitis after anesthesia with propofol in a child with glycogen storage disease type IA. Paediatr. Anaesth. 16 : 680–683.
40 Crawford, M. W., C. Pehora, and A. V. Lopez. 2009. Drug - induced acute pancreatitis in children receiving chemotherapy for acute leukemia: does propofol increase the risk? Anesth. Analg. 109 : 379–381.
41 Youssef, S. S., S. B. Iskandar, J. Scruggs, and T. M. Roy. 2005. Acute pancreatitis associated with omeprazole. Int. J. Clin. Pharmacol. Ther. 43 : 558–561.
42 Eland, I. A., C. H. Alvarez, B. H. Stricker, and L. A. Rodríguez. 2000. The risk of acute pancreatitis associated with acid-suppressing drugs. Br. J. Clin. Pharmacol. 49 : 473–478.
43 Fernandes, R. 2009. Acute pancreatitis following paracetamol overdose. BMJ Case Rep. doi: 10.1136/bcr.08.2009.2224.
44 Schmidt, L. E., and K. Dalhoff. 2004. Hyperamylasaemia and acute pancreatitis in paracetamol poisoning. Aliment. Pharmacol. Ther. 20 : 173–179.
45 Werlin, S. L., J. Casper, D. Antonson, and C. Calabro. 1992. Pancreatitis associated with bone marrow transplantation in children. Bone Marrow Transplant. 10 : 65–69.
46 Ko, C. W., T. Gooley, H. G. Schoch, D. Myerson, R. C. Hackman, H. M. Shulman, et al. 1997. Acute pancreatitis in marrow transplant patients: prevalence at autopsy and risk factor analysis. Bone Marrow Transplant. 20 : 1081–1086.
47 Sastry, J., S. Young, and P. J. Shaw. 2004. Acute pancreatitis due to tacrolimus in a case of allogeneic bone marrow transplantation. Bone Marrow Transplant. 33 : 867–868.
48 Nieto, Y., P. Russ, G. Everson, S. I. Bearman, P. J. Cagnoni, R. B. Jones, et al. 2000. Acute pancreatitis during immunosuppression with tacrolimus following an allogeneic umbilical cord blood transplantation. Bone Marrow Transplant. 26 : 109–111.
49 Ito, T., T. Kimura, H. Yamaguchi, M. Kinjo, T. Sumii, I. Nakano, et al. 1993. Acute pancreatitis induced by cyclosporin A under stimulation of pancreas by caerulein. Pancreas 8 : 693–699.
50 Niemann, T. H., M. E. Trigg, N. Winick, and G. D. Penick. 1993. Disseminated adenoviral infection presenting as acute pancreatitis. Hum. Pathol. 24 : 1145–1148.
51 Tomonari, A., S. Takahashi, K. Takasugi, J. Ooi, N. Tsukada, T. Konuma, et al. 2006. Pancreatic hyperamylasemia and hyperlipasemia in association with cytomegalovirus infection following unrelated cord blood transplantation for acute myelogenous leukemia. Int. J. Hematol. 84 : 438–440.
52 Bateman, C. M., A. M. Kesson, and P. J. Shaw. 2006. Pancreatitis and adenoviral infection in children after blood and marrow transplantation. Bone Marrow Transplant. 38 : 807–811.
53 Shifrin, A. L., N. Chirmule, G. P. Gao, J. M. Wilson, and S. E. Raper. 2005. Innate immune responses to adenoviral vector-mediated acute pancreatitis. Pancreas 30 : 122–129.
54 Salomone, T., P. Tosi, C. Raiti, M. Stanzani, G. Leopardi, F. Miglio, et al. 1999. Clinical relevance of acute pancreatitis in allogeneic hemopoietic stem cell (bone marrow or peripheral blood) transplants. Dig. Dis. Sci. 44 : 1124–1127.
55 Schiller, G. J., S. D. Nimer, J. L. Gajewski, and D. W. Golde. 1991. Abdominal presentation of varicella-zoster infection in recipients of allogeneic bone marrow transplantation. Bone Marrow Transplant. 7 : 489–491.
56 Ladrière, M., B. Bibes, C. Rabaud, P. Delaby, T. May, and P. Canton. 2001. Varicella zoster virus infection after bone marrow transplant. Unusual presentation and importance of prevention. Presse Med. 30 : 1151–1154.
57 Morinville, V. D., S. Z. Husain, H. Bai, B. Barth, R. Alhosh, P. R. Dure, et al.; On behalf of the INSPPIRE Group. 2012. Definitions of pediatric pancreatitis and survey of present clinical practices. J. Pediatr. Gastroenterol. Nutr. 55 : 261–265.
58 Bai, H. X., M. E. Lowe, and S. Z. Husain. 2011. What have we learned about acute pancreatitis in children? J. Pediatr. Gastroenterol. Nutr. 52 : 262–270.
59 Consuelo Sánchez, A., and J. A. García Aranda. 2012. Acute pancreatitis. Bol. Med. Hosp. Infant Mex. 69 : 3–9.
60 Nydegger, A., R. T. L. Couper, and M. R. Olive. 2006. Childhood pancreatitis. J. Gastroenterol. Hepatol. 21 : 499–509.
61 Frank, B., and K. Gottlieb. 1999. Amylase normal, lipase elevated: is it pancreatitis? A case series and review of the literature. Am. J. Gastroenterol. 94 : 463–469.
62 Muddana, V., D. C. Whitcomb, A. Khalid, A. Slivka, and G. I. Papachristou. 2009. Elevated serum creatinine as a marker of pancreatic necrosis in acute pancreatitis. Am. J. Gastroenterol. 104 : 164–170.
63 Darge, K., and S. Anupindi. 2009. Pancreatitis and the role of US, MRCP and ERCP. Pediatr. Radiol. 39:S153–S157.
64 UK Working Party on Acute Pancreatitis. 2005. UK guidelines for the management of acute pancreatitis. Gut 54(Suppl. III):iii1–iii9.
65 Tsuji, Y., K. Hamaguchi, Y. Watanabe, A. Okumura, H. Isoda, N. Yamamoto, et al. 2010. Perfusion CT is superior to angiography in predicting pancreatic necrosis in patients with severe acute pancreatitis. J. Gastroenterol. 45 : 1155–1162.
66 Tsuji, Y., H. Yamamoto, S. Yazumi, Y. Watanabe, K. Matsueda, H. Yamamoto, et al. 2007. Perfusion computerized tomography can predict pancreatic necrosis in early stages of severe acute pancreatitis. Clin. Gastroenterol. Hepatol. 5 : 1484–1492.
67 Tsuji, Y., N. Takahashi, and C. Tsutomu. 2012. Pancreatic perfusion CT in early stage of severe acute pancreatitis. Int. J. Inflam. 2012 : 497386. doi: 10.1155/2012/497386.
68 Amano, H., T. Takada, S. Isaji, Y. Takeyama, K. Hirata, M. Yoshida, et al. 2010. Therapeutic intervention and surgery of acute pancreatitis. J. Hepatobiliary Pancreat. Sci. 17 : 53–59.
69 Dambrauskas, Z., A. Gulbinas, J. Pundzius, and G. Barauskas. 2007. Meta-analysis of prophylactic parenteral antibiotic use in acute necrotizing pancreatitis. Medicina (Kaunas) 43 : 291–300.
70 García-Barrasa, A., F. G. Borobia, R. Pallares, R. Jorba, I. Poves, J. Busquets, et al. 2009. A double-blind, placebo-controlled trial of ciprofloxacin prophylaxis in patients with acute necrotizing pancreatitis. J. Gastrointest. Surg. 13 : 768–774.
71 Spraker, H. L., G. P. Spyridis, C. H. Pui, and S. C. Howard. 2009. Conservative management of pancreatic pseudocysts in children with acute lymphoblastic leukemia. J. Pediatr. Hematol. Oncol. 31 : 957–959.
72 Banks, P.A., M. L. Freeman; the Practice Parameters Committee of the American College of Gastroenterology. 2006. Practice guidelines in acute pancreatitis. Am. J. Gastroenterol. 101 : 2379–400.
73 Takeda, K., T. Takada, and Y. Kawarada. 2006. JPN Guidelines for the management of acute pancreatitis: medical management of acute pancreatitis. J. Hepatobiliary Pancreat. Surg. 13 : 42–47.
74 Isenmann, R., M. Rünzi, M. Kron, S. Kahl, D. Kraus, N. Jung, et al. 2004. Prophylactic antibiotic treatment in patients with predicted severe acute pancreatitis: a placebo-controlled, double blind trial. Gastroenterology 126 : 997–1004.
75 Heinrich, S., M. Schäfer, V. Rousson, and P. A. Clavien. 2006. Evidence-based treatment of acute pancreatitis: a look at established paradigms. Ann. Surg. 243 : 154–168.
76 Bai, Y., J. Gao, D. W. Zou, and Z. Li. 2008. Prophylactic antibiotics cannot reduce infected pancreatic necrosis and mortality in acute necrotizing pancreatitis: evidence from a meta-analysis of randomized controlled trials. Am. J. Gastroenterol. 103 : 104–110.
77 Bang, U. C., S. Semb, C. Nojgaard, and F. Bendtsen. 2008. Pharmacological approach to acute pancreatitis. World J. Gastroenterol. 14 : 2968–2976.
78 Morimoto, A., T. Imamura, R. Ishii, Y. Nakabayashi, T. Nakatani, J. Sakagami, et al. 2008. Successful management of severe L-asparaginase-associated pancreatitis by continuous regional arterial infusion of protease inhibitor and antibiotic. Cancer 113 : 1362–1369.
79 Wu, S. F., A. C. Chen, C. T. Peng, and K. H. Wu. 2008. Octreotide therapy in asparaginase-associated pancreatitis in childhood acute lymphoblastic leukemia. Pediatr. Blood Cancer 51 : 824–825.
80 Tokimasa, S., and K. Yamato. 2012. Does ocreotide prevent L-asparaginase associated pancreatitis in children with acute lymphoblastic leukaemia? Br. J. Haematol. 157 : 381–382.
81 Sax, H. C., B. W. Warner, M. A. Talamini, F. N. Hamilton, R. H. Bell Jr., J. E. Fischer, et al.1987. Early total parenteral nutrition in acute pancreatitis: lack of beneficial effects. Am. J. Surg. 153 : 117–124.
82 Petrov, M. S., R. D. Pylypchuk, and N. V. Emelyanov. 2008. Systematic review: nutritional support in acute pancreatitis. Aliment. Pharmacol. Ther. 28 : 704–712.
83 Thompson, D. R. 2001. Narcotic analgesic effects on the sphincter of Oddi: a review of the data and therapeutic implications in treating pancreatitis. Am. J. Gastroenterol. 96 : 1266–1272.
Štítky
Onkológia
Článok vyšiel v časopiseCancer Medicine
Najčítanejšie tento týždeň
2016 Číslo 5- Nejasný stín na plicích – kazuistika
- Zpracované masné výrobky a červené maso jako riziko rozvoje kolorektálního karcinomu u žen? Důkazy z prospektivní analýzy
- DESATORO PRE PRAX: Aktuálne odporúčanie ESPEN pre nutričný manažment u pacientov s COVID-19
- I „pouhé“ doporučení znamená velkou pomoc. Nasměrujte své pacienty pod křídla Dobrých andělů
- Když se ve střevech děje něco nepatřičného...
Najčítanejšie v tomto čísle- Acute pancreatitis as a complication of childhood cancer treatment
- Family history of breast cancer and its association with disease severity and mortality
- The effects of hemoglobin levels and their interactions with cigarette smoking on survival in nasopharyngeal carcinoma patients
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



