-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
Histopathological autoptic findings in 8 patients with pandemic influenza A (H1N1) pneumonia
Rozbor histopatologických pitevních nálezů u 8 pacientů s chřipkovou (H1N1) pneumonií
V letech 2009 a 2010 virus chřipky A podtypu H1N1 způsobil první chřipkovou pandemii po 41 letech. V České republice vrcholila v listopadu a prosinci roku 2009 a v jejím průběhu bylo zaznamenáno 101 úmrtí u pacientů s laboratorně potvrzenou chřipkovou infekcí H1N1. Další jednotlivé případy přibyly koncem roku 2010 a začátkem 2011. Zde dokumentujeme 8 nekroptických případů pacientů, kteří zemřeli v letech 2009 až 2011 ve Všeobecné fakultní nemocnici v Praze s potvrzenou H1N1 chřipkovou infekcí a byla u nich provedena pitva na Ústavu patologie 1. lékařské fakulty Univerzity Karlovy a Všeobecné fakultní nemocnice. Tato skupina pacientů se od jiných publikovaných liší jednak vyšším věkem zemřelých a také zejména vysokým zastoupením pacientů se závažným chronickým interním, často maligním onemocněním. U všech pacientů se rozvinula atypická pneumonie s následným respiračním selháním. V tomto článku jsme se zaměřili přednostně na histopatologické změny v respiračním traktu, které jsme doplnili o související nálezy v jiných orgánech a klinická data. Dominantním nálezem bylo difúzní alveolární poškození doprovázené různě rozsáhlými intraalveolárními hemoragiemi a zánětlivou infiltrací. U části pacientů byly nalezeny cytopatické změny pneumocytů a jejich deskvamace do alveolů, reaktivní změny bronchiálního epitelu, intraalveolární fibrinózní exudát, drobné nekrózy, reziduální nekrotizující bronchitida, ložiska granulační tkáně a počínající fibrózy. V jednom případě byly zastiženy neobvyklé vaskulární změny nejistého původu. Přestože se histopatologický nález přibližně shoduje s jinými publikovanými studiemi a kazuistikami, našli jsme i pozoruhodné rozdíly a celkově lze říci, že námi popsaný soubor pacientů je v porovnámí s ostatními mírně atypický. Jeho zdokumentováním bychom chtěli rozšířit počet popsaných případů a tím napomoci lepšímu porozumění a rozpoznání patogeneze tzv. nové chřipky.
Klíčová slova:
virus chřipky A (H1N1) – virová pneumonie – difúzní alveolární postižení
Authors: H. Skálová; C. Povýšil; J. Hofmanová; B. Goldová; R. Jakša; K. Jandová; J. Galko
Authors place of work: Czech Republic ; Institute of Pathology, 1st Faculty of Medicine, Charles University and General University Hospital in Prague
Published in the journal: Čes.-slov. Patol., 48, 2012, No. 3, p. 161-164
Category: Původní práce
Summary
In the years 2009 and 2010 a novel influenza A (H1N1) caused the first influenza pandemic after 41 years. In the Czech Republic it culminated in November and December 2009 and there were 101 laboratory-confirmed deaths. Another few cases occurred later in the year 2010 and at the beginning of 2011. Here we report 8 autoptic cases of patients who died between 2009 and 2011 with confirmed H1N1 influenza and underwent a post mortem examination at the Institute of Pathology, General University Hospital in Prague, Czech Republic. This group differs from the others reported in literature by having a higher age as well as a higher percentage of patients with pre-existing severe comorbidities including malignant diseases. All 8 patients developed atypical pneumonia with subsequent respiratory failure. In this article we present these cases with related clinical data and findings in other organs, but we focus primarily on the findings in the respiratory tract which were shown to be approximately similar to those in the other studies and case reports. Nevertheless there were also some noteworthy variations. The most prominent feature observed was diffuse alveolar damage accompanied by intraalveolar haemorrhage and inflammatory infiltrate of variable extent. Less frequent features included cytopathic changes of pneumocytes and their desquamation, reactive changes of bronchial epithelium, intraalveolar fibrinous exudate, minor necroses, residual necrotizing bronchitis, focal granulation tissue and incipient fibrosis. In one case we found an extraordinary vascular change of uncertain origin. In conclusion, this group of patients is slightly atypical and differ in some features from those in other published studies and case reports concerning novel pandemic influenza. By reporting them we wish to extend the number of described cases, which may contribute to a better understanding of the pathogenesis of novel influenza infection.
Keywords:
influenza A virus, subtype H1N1 – viral pneumonia – diffuse alveolar damageIn March 2009, novel influenza A (H1N1) virus infection was first reported in Mexico, then subsequently in the United States and then it spread rapidly worldwide. In June 2009, the World Health Organisation declared the first global pandemic since 1968 (1). In the Czech Republic, the pandemic culminated in November and December 2009 and subsided in March 2010 (2). In May 2009, the Czech Republic had the first laboratory-confirmed infection in a pilot who had recently returned from New York (3). The total number of infected is not known because the disease usually had a mild course without any complications requiring medical care. The first death of a patient with a confirmed infection occurred in our republic in October 2009 and by the time the pandemic abated there had been 101 confirmed deaths (4). Another few cases were added later in 2010 and the beginning of 2011 in the post-pandemic phase.
Compared to seasonal influenza, the mortality rate of novel influenza is approximately equal (estimate 0.08 %) and is much lower than the reported mortality rate for the 1918 pandemic (2.1 %), the most severe influenza pandemic of the last century, which was also caused by influenza A virus, subtype H1N1 (5). In the Czech Republic seasonal influenza causes approximately 2000 deaths per year (6).
However, novel and seasonal influenza significantly differ in many features. While seasonal infections affect and endanger mostly elderly polymorbid people, the majority of those infected by novel influenza were young people up to 24 years of age and most deaths occurred in middle aged people between 24 and 49 years old. The risk factors of developing a complicated course turn out to be chronic diseases, especially respiratory diseases (e.g. asthma), then also immunodeficiency, obesity and pregnancy (7).
The symptoms of novel influenza include not only the typical ones, such as fever, fatigue, myalgia, arthralgia, headache, sore throat and cough, but also symptoms unusual for seasonal influenza, e.g. vomiting and diarrhoea in mild courses and dyspnoea, respiratory insufficiency, hypotension, and signs of sepsis in severe cases. Whereas seasonal infection dominates in the upper respiratory tract and the virus can be isolated from tracheal and bronchial epitelium, the novel virus also attacks lower airways, where it can be isolated from pneumocytes of both I and II type and from intraalveolar macrophages and it can give a rise to pneumonia (8).
In this report, we describe 8 cases of death associated with H1N1 2009 infection. All patients were hospitalized with severe respiratory insufficiency requiring artificial ventilation and 2 of them (25 %) also required extracorporal membrane oxygenation (ECMO). Six patients (75 %) received oseltamivir in therapy. The autopsy revealed viral pneumonia in all 8 cases and in 7 (88 %) the respiratory insufficiency was determined as a cause of death.
MATERIALS AND METHODS
The eight patients, who died between November 2009 and January 2011 at the General University Hospital in Prague, Czech Republic and had pre-mortem confirmed influenza A (H1N1) infection, underwent autopsy including gross, microscopic and microbiologic inquiry. Histologic examination was performed on routine hematoxylin-eosin staining on formalin-fixed, paraffin-embedded sections. PAS, Giemsa and Gram stains were used to uncover possible bacterial and mycotic superinfection. Immunohistochemistry comprised the assessment of pancytokeratins (CKAE 1-3) expression to verify damaged and changed pneumocytes. Tissue samples and postmortem swabs were sent for microbiological tests to the National Institute of Public Health, Czech Republic, where isolation, cultivation, electron microscopy and polymerase chain reaction (PCR) were performed for detection of influenza A virus, subtype H1N1 as well as other viruses and bacteria.
RESULTS
Patients’ characteristics and clinical data
The patients age range between 32 to 74 years (median 51 years), there were 4 men (50 %) and 4 women (50 %). Seven of them (88 %) had pre-existing comorbidities: 5 (63 %) suffered from haematological neoplasms (multiple myeloma, T-cell lymphoma, diffuse large B-cell lymphoma, hairy cell leukaemia, acute myeloid leukaemia). Four of them recently underwent chemotherapy, 2 of them with subsequent bone marrow transplantation. One of them received long-term corticoid therapy. Besides haematological patients, 1 (13 %) had rapidly progressive glomerulonephritis with the necessity of haemodialysis. One (13 %) was obese suffering from diabetes mellitus, hypertension and chronic cardiac arrhythmia with an artificial pacemaker. Only 1 patient (13 %) had no comorbidity. The time from the onset of influenza symptoms to death was between 4 and 29 days (median 10.5 days). After the report of H1N1 infection, 6 patients (75 %) received antiviral therapy (oseltamivir). One patient (13 %) did not receive the therapy and in one case this information is not available.
All 8 patients developed atypical pneumonia with subsequent respiratory failure requiring artificial ventilation and in 2 (25 %) of them extracorporal membrane oxygenation (ECMO) was also required for 7 and 15 days. Four patients (50 %) had during hospitalization reported coinfection with Klebsiella pneumoniae, Pseudomonas aeruginosa, Aspergillus fumigatus, Pneumocystis carinii, Stenotrophomonas maltophilia, Acinetobacter spp., Staphylococcus hominis.
Gross autoptic findings
The prominent finding was diffusely edematous and haemorrhagic lungs without any visible focal changes. In the trachea and bronchi a variable degree of mucosal edema and usually erythema were also seen. Two patients (25 %) had strongly congested intestinal mucosa with haemorrhages and plaques. In the first case the changes were observed almost diffusely in the small and large intestine, while in the second case they were limited to the rectum. Gross findings in other organs were related basically to the underlying chronic diseases.
Microscopic findings
Microscopic investigation of lung parenchyma in all patients demonstrated diffuse dense intraalveolar edema with prominent hyaline membranes lining the alveolar walls (Fig. 1A,B). Fibrin strands or more dense fibrin exudate were less frequent and focal. In some patients desquamated pneumocytes and macrophages diffusely filled the alveolar spaces (Fig. 1C). Cytopathic changes were observed both on desquamated and non-desquamated pneumocytes (Fig. 1D). In all samples we found not only congestion but also intraalveolar haemorrhage of variable extent, in some cases massive with foci of apoplexia. Inflammatory infiltration was usually sparse including mostly lymphocytes, occasionally together with neutrophils, in a single case with formation of small abscesses. In 2 cases there were foci of necrosis without remarkable inflammatory infiltration (Fig. 1E). In the 2 patients with the longest survival (15 and 29 days from the first signs of pneumonia) a dominant feature was intra-alveolar granulation tissue and incipient fibrosis. In the bronchioli and smaller bronchi in most patients there were reactive changes of epithelial cells with sporadic formation of hyaline membranes. Only in one patient with the longest survival did we find residual necrotizing bronchitis.
Fig. 1. Histological findings associated with influenza A (H1N1) pneumonia: (A) intraalveolar edema with haemorrhages, hyaline membranes lining alveolar spaces, HE, 100x, (B) highlighted hyaline membranes in Masson’s trichrome, 100x, (C) expression of pancytokeratin AE1/AE3 in desquamated pneumocytes, immunohistochemistry, 100x, (D) cytopathic changes of pneumocytes, HE, 200x, (E) necrosis, HE, 40x, (F) atypical acute vasculitis with nearly obturating intimal proliferation, HE, 100x. 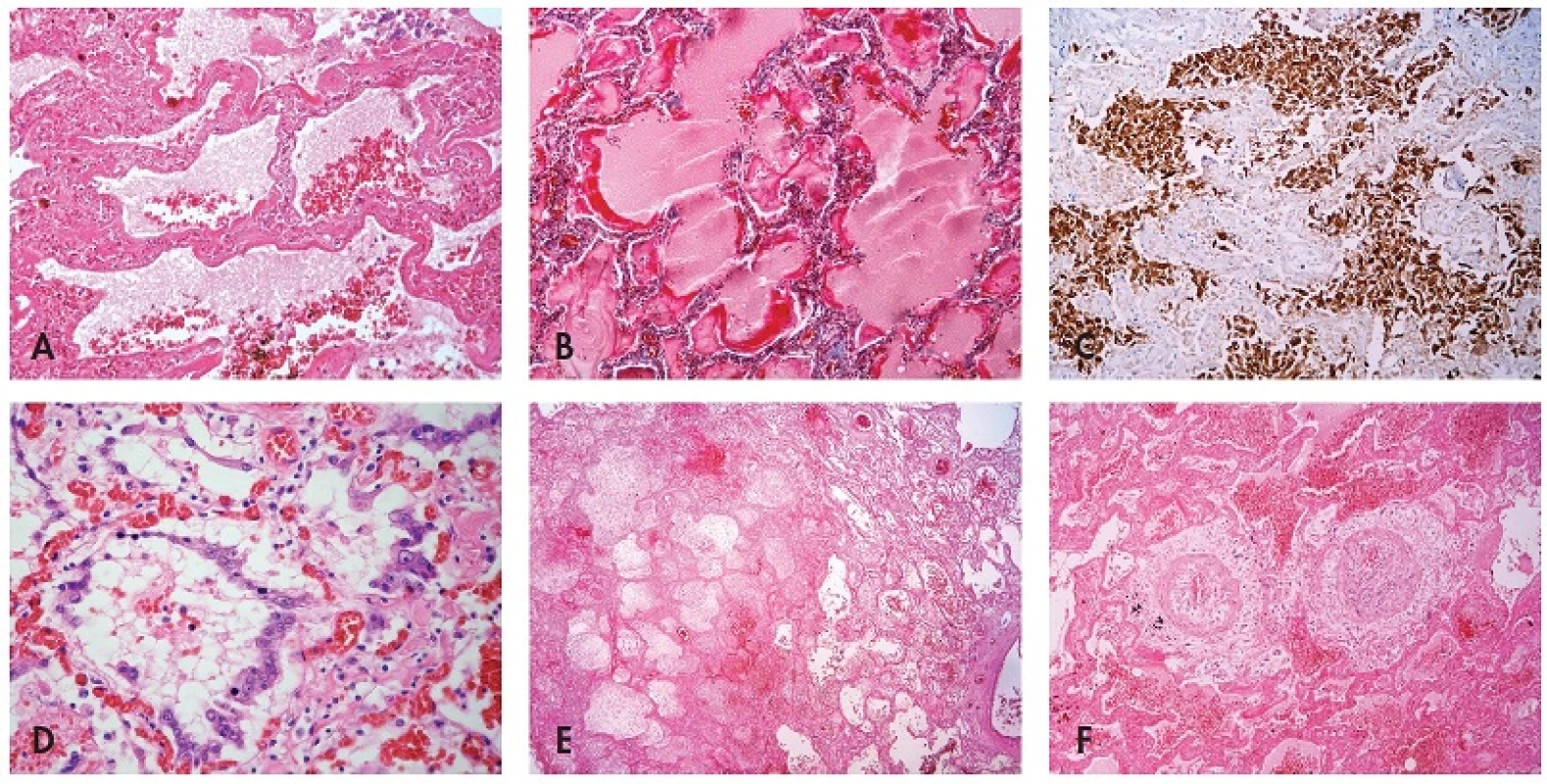
In the trachea and major bronchi of approximately half of the cases we found mild inflammation in mucosa, submucosa and submucosal glands with reactive changes of epithelial cells and occasional submucosal haemorrhage.
In one case in sections from lungs we found an unusual feature that resembled atypical acute vasculitis with extensive, sometimes nearly obturating intimal proliferation with focal periadventitial giant cell reaction. Nonetheless, lamina elastica interna and externa remained intact (Fig. 1F). Detailed examination of this patient showed that these vascular changes did not occur in any other organ. Anyway, thrombosis of the pulmonary arteries was not found in this patient as well as in any others.
Microscopical investigation of the other organs often revealed centrolobular necrosis in the liver and acute tubular necrosis in the kidneys. Intestinal samples in the 2 cases with gross signs of inflammation were heavily autolysed. However, in one case we could distinguish phlegmonous proctocolitis and haemorrhages in rectal submucosa. In another case there was only a slight congestion of autolysed mucosa without inflammatory cells. Microscopical results and their frequency are listed in Table 1.
Tab. 1. Frequency of histopathological features in autoptic samples of the patients with confirmed influenza A (H1N1) infection (n = 8). 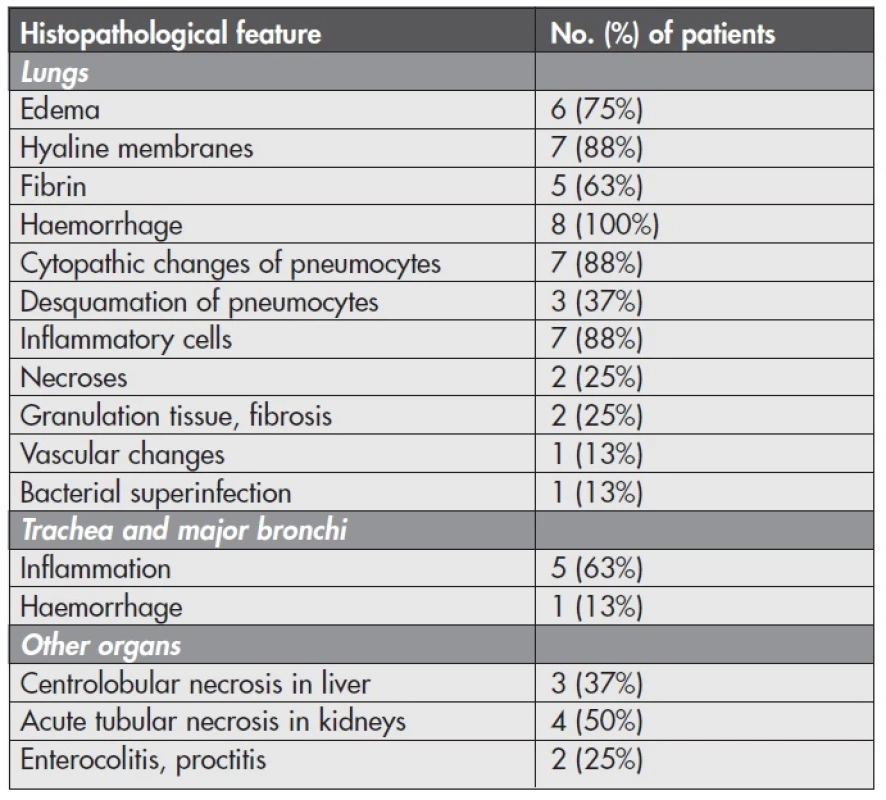
Microbiological findings
In 7 cases influenza viral RNA was proved by PCR in material from the respiratory tract and in the patient with gross signs of enterocolitis, also in the small intestine. Isolation and cultivation of the virus was not successful because of the small viral charge in autoptic material. In one case the postmortal tests were not performed.
Coinfection with other viruses, namely Picornavirus and Herpes virus, was established by electron microscopy in 5 cases (63 %). Bacterial coinfection with Staphylococci and Mycoplasma was determined by electron microscopy and isolation on tissue cultures in 2 cases (25 %). Only in one patient (13 %) was bacterial superinfection found by Gram-positive cocci and rods in histological preparations.
Cause of death
The final autoptic diagnosis determined the cause of death as respiratory insufficiency in 4 cases (50 %), respiratory insufficiency during septical shock in one case (13 %), cardiorespiratory insufficiency in 2 cases (25 %) including one patient with neutropenia and multiorgan failure during septic shock. One patient (13 %) died after severe bleeding from an intercostal artery after thoracic puncture.
DISCUSSION
This report comprises post mortem pathological findings of 8 patients with confirmed influenza A (H1N1) infection, who died during the pandemic and post pandemic phase at the General University Hospital in Prague, Czech Republic. This group of patients differs from most of the published studies and case reports (5,7,9,10) in some features, not only in the higher age of the deceased but predominantly in the fact that most of these patients had underlying severe chronic and often malignant diseases. More than half of them suffered from a haemathological neoplasm and underwent immunocompromising therapy, which together led in some of them to pancytopenia. This fact can explain both the high incidence of pulmonary haemorrhage and the rather sparse or even absent inflammatory infiltration in these patients. Another two patients received ECMO, which significantly prolonged their survival and in both of them we found organizing diffuse alveolar damage (DAD) with granulation tissue and fibrosis. In the patient with the longest survival only sporadic still ventilated areas of lung parenchyma remained. The most common complication of ECMO was bleeding including pulmonary haemorrhages (11), which were present in both patients. Moreover, one patient with ECMO died in hemorrhagic shock because of uncontrollable bleeding from the injured intercostal artery after thoracic puncture.
Microscopic findings were generally similar to other studies and case reports (5,7–10,12) with dominant features of DAD. Our group of patients differs from all of these except one (12) not only due to a high incidence of pulmonary haemorrhage, which was described above, but also in a scarce bacterial coinfection and signs of neutrophilic bronchopneumonia and only limited or even missing inflammation in the trachea and major bronchi. Another remarkable difference was that none of the patients had peripheral pulmonary vessel thrombosis. However, we found an exceptional vascular change of small pulmonary arteries of uncertain origin in the obese polymorbid patient. These changes were not found in any other organ of this patient.
Mauad and others (10) in a study on lung pathology in fatal novel influenza infection divided the changes into three distinct patterns: First, with exudative DAD with reactive pneumocytes and sparse interstitial inflammation; second, with prominent necrotizing bronchiolitis and DAD in the phase of organization; and third, with exudative DAD with an intense haemorrhagic component and no cytopathic changes in pneumocytes. If we apply this scheme to our cases we can see that they correspond only partially. Just one patient had a pure third pattern. 5 others had more or less typical changes according to the first pattern but with haemorrhages. One corresponded the most to the second pattern, but also with haemorrhages. One patient had a combination of all three described patterns. If we suppose that haemorrhages represent in most patients a complication of the underlying disease or therapy, we can apply this scheme with significantly higher confidence. Nonetheless, there still remain features that overlap or do not match completely.
Regarding other organs, acute tubular necrosis in kidneys and centrolobular necrosis in the liver are common findings related to shock. However, according to the latest research it is possible that Bowman’s capsule epithelial cells and distal tubular cells are a direct target for influenza A (H1N1) virus (13). Although gastrointestinal symptoms are typical for novel influenza, they have escaped histological examination to date, most probably because they appear during the mild course. In one patient from our group the RNA of influenza virus A (H1N1) was also proved by PCR in the small intestine and she had macroscopical signs of mild enterocolitis. Unfortunately, the samples were unusable for histological evaluation because of advanced autolysis. In another patient with histologically proved phlegmonous proctitis PCR was not performed.
In conclusion, we found similar features to those described in other published studies and case reports, but also some differences particularly in the characteristics and frequency of single symptoms in the patients. Moreover, we found an unusual feature that, to the best of our knowledge, has not been described before. By reporting them we wish to extend the number of described cases, which may help to increase understanding of the pathogenesis of novel influenza infection. However, described histological changes are not specific for influenza and so evidence of influenza A (H1N1) virus from PCR or cultivation is essential for definite diagnosis.
Address for correspondence:
MUDr. Helena Skálová
Ústav patologie 1. LF UK a VFN
Studničkova 2, 12800 Praha 2
tel: +420224968711
email: helena.skalova@vfn.cz
Zdroje
1. Pandemic influenza.
[http://www.euro.who.int/en/what-we-do/health-topics/communicable-diseases/influenza/facts-and-figures/pandemic-influenza]
2. Džupová O, Havlíčková M, Helcl M, Kabelková M, Kulichová J, Roháčová H, Beneš J. Pacienti s pandemickou chřipkou A (H1N1) 2009 v intenzivní péči. Anest Intenziv Med 2010; 21(5): 251–257.
3. První případ prasečí chřipky v ČR. [http://www.pandemiechripky.cz/mexicka-praseci-chripka]
4. SZÚ: Aktualizované informace – potvrzené případy onemocnění virem „Pandemic (H1N1) 2009“ ke dni 3.3.2010. [http://www.szu.cz/tema/prevence/aktualizovane-informace-o-potvrzenych-pripadech-onemocneni-2]
5. Harms PW, Schmidt LA, Smith LB, et al. Autopsy findings in eight patients with fatal H1N1 influenza. Am J Clin Pathol 2010; 134 (1): 27–35.
6. Kynčl J, Havlíčková M. Základní epidemiologické charakteristiky chřipkové epidemie. Klin Mikrobiol Inf Lek 2010; 16(4): 116–119
7. Gill JR, Sheng ZM, Ely SF, et al. Pulmonary pathologic findings of fatal 2009 pandemic influenza A/H1N1 viral infection. Arch Patol Lab Med 2010; 134 (2): 235–243.
8. Guarner J, Falcón-Escobedo R. Comparison of the pathology caused by H1N1, H5N1, and H3N2 influenza viruses. Arch Med Res 2009; 40 (8): 655–661.
9. Shieh WJ, Blau DM, Denison AM, et al. 2009 pandemic influenza A (H1N1): pathology and pathogenesis of 100 fatal cases in the united states. Am J Pathol 2010; 177 (1): 166–175.
10. Mauad T, Hajjar LA, Callegari GD, et al. Lung pathology in fatal novel human influenza A (H1N1) infection. Am J Respir Crit Care Med 2010; 181 (1): 72–79.
11. Park PK, Blum JM, Napolitano LM, et al. Extracorporal Membrane oxygenation (ECMO) in Patients with ARDS. Crit Care Clin 2011; 27(3):627–46.
12. Rosen DG, Polez AE, Anzalone ML, et al. Postmortem findings in eight cases of influenza A/H1N1. Mod Pathol 2010; 23 : 1449–1457.
13. Nin N, Lorente JA, Sánchez-Rodríguez C, et al. Kidney histopathological findings in fatal pandemic 2009 influenza A (H1N1). Intensive Care Med 2011; 37 (5): 880–881.
Štítky
Patológia Súdne lekárstvo Toxikológia
Článok vyšiel v časopiseČesko-slovenská patologie

2012 Číslo 3-
Všetky články tohto čísla
- Pseudotumors & MIMICKERS
- SLINNÉ ŽLÁZY VYPADAJÍ V MIKROSKOPU TAK KRÁSNĚ!
- HEMATOPATOLOGIE, UROPATOLOGIE, NEFROPATOLOGIE...
- Pseudotumors & Mimickers: Přehled vybraných pseudotumorů s uvedením nádorů, které mohou mikroskopicky imitovat
- Melanocytic pseudotumors
- Změny ve specializačním vzdělávání v patologii od roku 2012
- Differential diagnosis of the chronic pancreatitis and the pancreatic ductal adenocarcinoma
- Giant-cell lesions of bone and their differential diagnosis
- Pseudotumors of the testis and testicular adnexa
- Patologie slinných žláz
- Primary vaginal squamous cell carcinoma arising in a squamous inclusion cyst: Case report
- Sarcomatoid (metaplastic) spindle cell carcinoma arising in a phylloid tumor with massive squamous metaplasia – a case report and review of the literature
- Histopathological autoptic findings in 8 patients with pandemic influenza A (H1N1) pneumonia
- Immunoexpression of type-1 adiponectin receptor in the human intestine
- Historie a současnost tenkojehlové aspirační cytologie
- Česko-slovenská patologie
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- Giant-cell lesions of bone and their differential diagnosis
- Sarcomatoid (metaplastic) spindle cell carcinoma arising in a phylloid tumor with massive squamous metaplasia – a case report and review of the literature
- Differential diagnosis of the chronic pancreatitis and the pancreatic ductal adenocarcinoma
- Melanocytic pseudotumors
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



