-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
Risk of Streptococcus pneumoniae-associated haemolytic uraemic syndrome in industrialised nations: a systematic review of the literature
Risk of Streptococcus pneumoniae-associated haemolytic uraemic syndrome in industrialised nations: a systematic review of the literature
Background and Aim: Haemolytic uraemic syndrome (HUS) is a triad of haemolytic anaemia, thrombocytopaenia, and acute kidney injury. It is a leading cause of acute kidney injury in children and has a high rate of long-term sequelae. Streptococcus pneumoniae–associated HUS (SpHUS) is a rare complication from pneumococcal disease. This article aims to systematically review SpHUS following the global introduction of pneumococcal conjugate vaccines (PCVs).
Material and Methods: A comprehensive literature search was conducted in MEDLINE, EMBASE, and the Cochrane library from 1st January 2000 to 13th April 2022.
Results: Thirteen studies were included in this review, involving a total of 7,177 children with HUS, of which 336 cases were associated with Streptococcus pneumoniae. SpHUS accounted for 4.8% of all HUS cases, in which most patients were younger than 24 months old. Nine studies (80.4%, 281) were during the country’s PCV era, whereas 4 studies (19.6%, 66) were before the introduction of PCV into the national vaccination programme. Pneumonia was the commonest clinical presentation (77.3%; 75/97), followed by septicaemia (33.0%; 32/97), and meningitis (29.9%; 29/97). Most cases presenting with pneumonia were complicated by empyema or pleural effusion (54.4%, n=49/90). Only 5 studies reported the isolated serotypes, with the most prevalent serotype being 19A (44.4%, n=20/45), followed by serotype 3 (17.8%, n = 8/45) and 7F (6.7%, n = 3/45). Of those reporting fatality, there were 12 deaths with a fatality rate of 9.8% (n = 12/122).
Conclusion: SpHUS is rare, but commonly presents in children younger than 2 years old. There remains a high risk of long-term complications and relatively high mortality rate even in the era of conjugate vaccines.
Authors: C. Tung Ho Lok 1; A. Jien Ting Ser 2,3; P. Oligbu 4; M. Pervaiz 5; G. Oligbu 6
Authors place of work: University College London Hospital, London, UK 1; Oxford University Clinical Academic Graduate School, University of Oxford, Oxford, UK 2; Oxford University Hospitals NHS Foundation Trust, Oxford, UK 3; Department of Family Medicine, University of Benin Teaching Hospital, Nigeria 4; Department of Paediatrics, Dumfries and Galloway NHS Hospital, Scotland, UK 5; Department of Paediatrics, Dr Gray’s Hospital, NHS Grampian, Scotland, UK 6
Published in the journal: Epidemiol. Mikrobiol. Imunol. 72, 2023, č. 4, s. 213-220
Category: Původní práce
Summary
Background and Aim: Haemolytic uraemic syndrome (HUS) is a triad of haemolytic anaemia, thrombocytopaenia, and acute kidney injury. It is a leading cause of acute kidney injury in children and has a high rate of long-term sequelae. Streptococcus pneumoniae–associated HUS (SpHUS) is a rare complication from pneumococcal disease. This article aims to systematically review SpHUS following the global introduction of pneumococcal conjugate vaccines (PCVs).
Material and Methods: A comprehensive literature search was conducted in MEDLINE, EMBASE, and the Cochrane library from 1st January 2000 to 13th April 2022.
Results: Thirteen studies were included in this review, involving a total of 7,177 children with HUS, of which 336 cases were associated with Streptococcus pneumoniae. SpHUS accounted for 4.8% of all HUS cases, in which most patients were younger than 24 months old. Nine studies (80.4%, 281) were during the country’s PCV era, whereas 4 studies (19.6%, 66) were before the introduction of PCV into the national vaccination programme. Pneumonia was the commonest clinical presentation (77.3%; 75/97), followed by septicaemia (33.0%; 32/97), and meningitis (29.9%; 29/97). Most cases presenting with pneumonia were complicated by empyema or pleural effusion (54.4%, n=49/90). Only 5 studies reported the isolated serotypes, with the most prevalent serotype being 19A (44.4%, n=20/45), followed by serotype 3 (17.8%, n = 8/45) and 7F (6.7%, n = 3/45). Of those reporting fatality, there were 12 deaths with a fatality rate of 9.8% (n = 12/122).
Conclusion: SpHUS is rare, but commonly presents in children younger than 2 years old. There remains a high risk of long-term complications and relatively high mortality rate even in the era of conjugate vaccines.
INTRODUCTION
Haemolytic uraemic syndrome (HUS) is a triad of haemolytic anaemia, thrombocytopaenia, and acute kidney injury. It is a rare form of thrombotic microangiopathy and a leading cause of acute kidney injury in children [1]. HUS is most commonly infection-induced as infections may be responsible for up to 86% of HUS cases [2]. Other rarer causes of HUS include disorders of complement regulation, ADAMTS13 deficiency, defective cobalamin metabolism, malignancy, pregnancy, medications, and autoimmune conditions [3].
Shiga and Shiga-like toxin-producing bacteria, including Shiga toxin-producing Escherichia coli (STEC) and Shigella dysenteriae type 1, represent the most common cause of infection-induced HUS. STEC infection accounts for more than 80% of all paediatric HUS cases [4]. However, other than Shiga and Shiga-like toxin-producing bacteria, there are less common causes of infection-induced HUS. Notably, as a complication of invasive pneumococcal disease (IPD, such as pneumonia, meningitis, or sepsis), Streptococcus pneumoniae–associated HUS (SpHUS) may develop. SpHUS is the second most common cause of infection-induced HUS and accounts for 40% of all paediatric non-STEC HUS cases [4]. Other rarer causes of infection-induced HUS include influenza A and Capnocytophaga canimorsus [5].
Compared to STEC-associated HUS, SpHUS has a more severe clinical course associated with poorer outcomes. SpHUS patients have a greater degree and longer duration of renal failure and haematological involvement as indicated by higher rates of blood product transfusions and dialysis [4, 6]. The average duration of dialysis for SpHUS was 10 days compared to 3.6 days for STEC-associated HUS [4]. Long-term renal and neurological sequelae can be expected in 26–40% of SpHUS patients [2].
The mechanism by which S. pneumoniae causes this damage is not fully understood. It is classically thought to be via the secretion of neuraminidases, leading to the exposure of the Thomsen-Friedenreich antigen (T-antigen) by breaking down N-acetylneuraminic acid (desialylation) on the surface of erythrocytes, glomerular endothelial cells, and platelets [7]. Pre-formed host IgM antibodies react to these antigens to produce an immunological reaction; this damages tissues that harbour this antigen [8]. The desialylation may also trigger complement activation via the alternative pathway by reducing Factor H activity, contributing to the pathogenesis of SpHUS [6]. There have also been suggestions that genetic variations in complement pathway regulation may play a role in the development of SpHUS [9]. Recently, an alternative mechanism for pathogenesis, independent of neuraminidase, involving increased activation of plasminogen leading to a disturbance in local complement homeostasis causing endothelial cell damage, has been proposed [10].
It is likely that the incidence of SpHUS is underestimated and there is commonly a delay in identifying and diagnosing SpHUS owing to the overlap in symptoms with disseminated intravascular coagulation (DIC) and streptococcal sepsis, the coexistence of DIC and SpHUS, and unfamiliarity with this rare entity [4, 5, 11]. In addition, there are limited observational studies, which were fraught with small sample sizes, and therefore the exact prevalence of SpHUS is unknown. SpHUS is considered an uncommon cause of HUS in industrialised countries [12]. In contrast, in Asia, SpHUS causes a substantial proportion of HUS cases [13]. Consequently, the prevalence of SpHUS should be determined separately by country. This study therefore focuses on SpHUS in industrialised countries of Europe, United States of America (USA), Canada, Australia, and New Zealand. The USA was the first country to license the pneumococcal conjugate vaccine (PCV) in 2000, which changed the incidence and serotypes causing IPD and consequently SpHUS [6]. This systematic review sought to review the incidence, morbidity, and mortality of SpHUS following the global introduction of pneumococcal conjugate vaccines (2000). To our knowledge, this is the first systematic review to determine the risk of SpHUS in industrialised nations. The findings of this study will highlight the importance of early diagnosis and management, given the associated morbidity and mortality.
METHODS
Information sources and search strategy
A search strategy was designed to identify studies reporting SpHUS in industrialised nations, including Europe, Canada, Australia, New Zealand, and the USA. In this review, all cases confirmed and suspected for SpHUS were included. We searched MEDLINE, EMBASE, and the Cochrane library from 1st January 2000 to 13th April 2022. The medical subject headings (MeSH) terms used included “pneumoniae”, “HUS”, “hemolytic-uremic syndrome”, “haemolytic uremic syndrome”, and “gasser syndrome”. These MeSH terms were used in different combinations. The full search strategies are shown in Appendix 1. We only included studies published in English language and where full text was available in our review. In addition, we screened reference lists of selected papers to retrieve relevant studies.
Selection of studies
Inclusion criteria required the study to report the total number of SpHUS and HUS cases in hospitals located in either Europe, Canada, Australia, New Zealand, or the USA. This was done to compare the rate of SpHUS cases in industrialised nations with similar sociodemographic factors. SpHUS was defined as having proven invasive pneumococcal infection with features suggestive of HUS. Studies were excluded if they were laboratory, experimental, or animal studies. Letters to the editor and commentaries were also excluded due to reasons including high probability of bias and low level of evidence. Studies needed to be in the English language to prevent misinterpretation. The Food and Drug Administration (FDA) licensed the first pneumococcal conjugate vaccine (PCV7) in 2000. Although some countries did not introduce PCV into their routine vaccination schedules until later, only data obtained after the global availability of PCV were used in the analysis, since PCV could be given through private medical clinics or in specific circumstances. PCVs were effective in reducing IPD and changed the serotype, mortality, and incidence of SpHUS, thus studies prior to 2000 were excluded.
Quality assessment and data extraction
Two reviewers (C.H. and G.O.) independently screened the title and abstract of papers identified by the electronic searches, evaluating inclusion and exclusion criteria for all papers. Full articles of included publications were retrieved, and each publication was then independently reviewed for eligibility by reviewing the methodological quality, comparability of case and controls, and outcomes. Discrepancies were resolved by discussion with a third author (P.O.). The specific variables extracted from the publications included: study design, country, age of cases, year of study, method of data collection, clinical presentation, duration of hospitalisation, management, serotype isolated and outcomes. The study quality was assessed using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement [9]. All authors were involved in writing and reviewing the final manuscript.
Data analysis
All studies included in the review were summarised using descriptive analyses to provide an overview of the risk on SpHUS in industrialised nations, their demographics, clinical presentations, complications, and outcomes.
RESULTS
Study characteristics
The initial search identified 313 potential studies, of which 9 were duplicates. Of the remaining 304 studies, 220 studies were excluded based on their title and abstracts, and a further 71 articles did not meet the inclusion criteria (Figure 1). Therefore, 13 studies [2,12,14–24] Salt Lake City, UT, with IPD from 1997 to 2008 and all children in Utah with HUS since 1971. Results: We identified 435 Utah children with culture-confirmed IPD (1997–2008 were eligible for inclusion in the final analysis. Of the included studies, all were retrospective case studies except the studies from Proulx et al. and Lynn et al., which were prospective surveillance studies. All identified studies were from industrialised nations: 4 studies from USA, 3 from Europe (Germany=1, Portugal=1, Spain/Hungary=1), 2 from Canada, and 1 study each from Australia and New Zealand. An overview of the demographics of the study subjects, clinical features and outcomes are presented in Tables 1, 2, and 3.
Fig. 1. PRISMA flow diagram demonstrating identification and selection of eligible studies 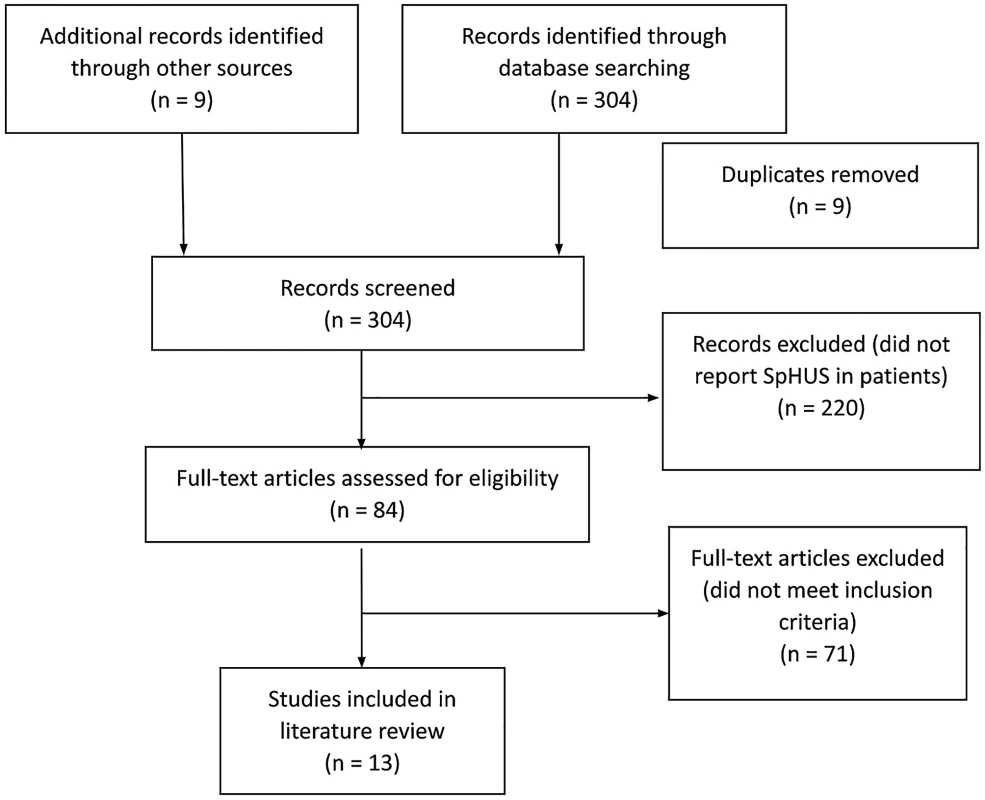
Table 1. Study design and characteristics of the studies included in the literature review
Study
Year of publication
Study population
Year of PCV introduction
Study period
Study period before/after PCV
Study design
Number of HUS cases
Number of SpHUS cases
SpHUS patients with PCV
Age of pHUS patients
Constantinescu et al.
2004
USA
2000
1997–2001
both
retrospective cohort
247
9
N/A
mean 17 ± 10 months
Cochran et al.
2003
USA
2000
July 1994–July 2001
both
retrospective cohort
15
6
mean 20 months
Proulx et al.
2004
Canada
2006
April 2000–
March 2002
before
prospective surveillance
140
4
median 15.5 months
(range: 6–31 months)
Waters et al.
2007
UK
2006
January 1998–
May 2005
before
retrospective cohort
315
43
median 13 months
(range: 5–39 months)
Copelovitch et al.
2009
Canada
2001
April 1988–
May 2009
both
retrospective cohort
179
14
mean 27 months
(range: 4–56 months)
Bender et al.
2010
Utah, USA
2000
1971–2008
both
retrospective cohort
460
7
Yes= 3,
Uncertain= 2,
No= 2
median 16 months
(range: 9–84 months)
Prestidge et al.
2009
New Zealand
2008
1998–2007
before
retrospective cohort
100
11
N/A
median 8.5 months
Veesenmeyer et al.
2013
USA
2000
1997, 2000,
2003, 2006
and 2009
both
retrospective cohort
5186
201
N/A
Lawrence et al.
2018
Australia
2005
1997–2016
both
retrospective cohort
66
11
PCV7 = 4,
PCV13 = 5,
No vaccine = 2
median age 12 months (range: 7–20 months)
Lynn et al.
2005
UK
2006
February 1997 to January 2001
before
prospective surveillance
413
8
N/A
N/A
Holle et al.
2021
Germany
2006
1996 to 2019
both
retrospective cohort
7
7
PCV13 = 3
median age 12 months (range 3–28 months)
Vilardouro et al.
2022
Portugal
2015
January 1996 and March 2020
both
retrospective cohort
25
2
N/A
median age 24 months (2 months–17 years)
Gómez Delgado et al.
2021
Spain and Hungary
2015 and
2009
2006–2019
both
retrospective cohort
24
24
PCV13 = 2
PPSV23 = 1
median age 21 months (5 months – 47 years)
Abbreviations: PCV, pneumococcal conjugate vaccine; SpHUS, Streptococcus pneumoniae-associated haemolytic uraemic syndrome; HUS, haemolytic uraemic syndrome; UK, United Kingdom; USA, United States of America; N/A, not available.
Table 2. Characteristics and clinical features of cases with Streptococcus pneumoniae-associated haemolytic uraemic syndrome
Study
Co-morbidities
Clinical features
Number of deaths
Follow-up
Constantinescu et al.
N/A
N/A
0
all recovered renal function
Cochran et al.
N/A
N/A
1
ESKD = 2 (renal transplant = 1)
Proulx et al.
N/A
pneumonia = 3 (pleural effusion = 2), meningitis = 2
0
N/A
Waters et al.
N/A
pneumonia = 35 (empyema = 23),
bacteraemia = 26, meningitis = 13
5
proteinuria = 8, hypertension = 5, renal dysfunction = 10, dialysis dependent = 1
Copelovitch et al.
N/A
pneumonia =12 (empyema = 10),
meningitis = 2, DIC = 4
CNS injury =1
decreased GFR = 5 (CKD II = 2, CKD IV = 1 ESKD with transplant = 1), home dialysis = 2. pericardial effusion = 1, dilated left ventricle
Bender et al.
N/A
pneumonia = 6 (empyema = 5), meningitis and seizures = 2, hearing loss = 1, hypertension = 3
0
dialysis = 43%, long-term renal sequelae = 33%
Prestidge et al.
prematurity=2
pneumonia and pleural effusion = 7 (necrotic pneumatocoeles = 2), empyema = 1
meningitis with seizures = 4 (intracranial haemorrhage = 3, infarction = 1)
meningitis=1
ESKD = 1, long-term renal sequelae = 2, neurological impairment = 3 (hearing loss = 1, global developmental delay with seizures = 1, cerebral infarction with hydrocephalus = 1)
Veesenmeyer et al.
N/A
N/A
N/A
N/A
Lawrence et al.
N/A
pneumonia = 9 (bacteraemia = 4, empyema = 8) and meningitis with bacteraemia = 2
0
follow-up data = 7, CKD = 4 hypertensive = 6
Lynn et al.
N/A
hypertension = 3,
haemorrhage = 2, seizures = 4
2
full renal recovery = 5, CKD = 1
Holle et al.
N/A
meningitis = 4, pneumonia = 3
1
full renal recovery = 2, CKD = 5
Vilardouro et al.
N/A
N/A
N/A
N/A
Gómez Delgado et al.
N/A
N/A
N/A
N/A
Abbreviations: ESKD, end-stage kidney disease; CKD, chronic kidney disease; HUS, haemolytic uraemic syndrome; DIC, disseminated intravascular coagulation; MRI, magnetic resonance imaging; N/A, not available.
Table 3. Serotypes isolated in cases with pneumococcus-associated haemolytic uraemic syndrome
Study
Serotypes isolated = number of cases
Constantinescu et al.
N/A
Cochran et al.
N/A
Proulx et al.
N/A
Waters et al.
3 = 2, 6A = 1, 12F = 1, 14 = 2, 19A = 6
Copelovitch et al.
6A = 1, 9V = 1, 14 = 2, 19A = 8
Bender et al.
1 = 1, 3 = 2, 7F = 2, 14 = 1, 22 = 1
Prestidge et al.
N/A
Veesenmeyer et al.
N/A
Lawrence et al.
3 = 2, 7F = 1, 10A = 1, 14 = 1, 19A = 6
Lynn et al.
N/A
Holle et al.
3 = 2, 15A = 1
Vilardouro et al.
N/A
Gómez Delgado et al.
N/A
Abbreviations: N/A, not available
Thirteen studies were included in this review, involving a total of 7,177 patients with HUS, of which 347 cases were associated with S. pneumoniae. Thus, the rate of SpHUS from all HUS cases was 4.8%. Most patients with SpHUS were younger than 24 months in age. Nine studies (80.4%, 281 cases of SpHUS) were during the country’s PCV era, whereas 4 studies (19.6%, 66 cases of SpHUS) were performed before the introduction of PCV into the specified country’s national vaccination programme. Common clinical presentations included pneumonia (77.3%; 75/97), septicaemia (33.0%; 32/97), and meningitis (29.9%; 29/97). Most cases presenting with pneumonia were complicated by empyema or pleural effusion (54.4%, n = 49/90). A relatively common chronic complication was long-term renal dysfunction (44.0%; 48/109) which included endstage kidney disease or chronic kidney disease. Only 5 studies reported the isolated serotypes, with the most prevalent serotype being 19A (44.4%, n = 20/45), followed by serotype 3 (17.8%, n = 8/45) and 7F (6.7%, n = 3/45), as shown in Table 3. Of those reporting fatality, there were 12 deaths with a fatality rate of 9.8% (n = 12/122).
Two studies investigated the role of complement. Holle et al. reported alternative pathway activation in both SpHUS patients who underwent complement analysis [14]. These two patients did not have definite pathogenic variants in complement-regulating genes but the MCP-H2 risk haplotype was identified in both patients [14]. Gómez Delgado et al. identified 5 patients of their cohort of 24 SpHUS patients with rare complement variants. They particularly highlighted risk haplotypes in the CFH-CFHR3-CFHR1 region, which may predispose patients to SpHUS [15]. Of the two patients whose plasma samples were taken in the acute phase of disease, both revealed low C3 and C4 levels [15] and could have a worse prognosis than HUS associated to E. coli infections. It has been assumed that complement genetic variants associated with primary atypical HUS cases (aHUS). The other patients’ plasma samples were taken more than 1 month after disease onset; this showed normal C3 and C4 levels [15].
DISCUSSION
A systematic review of the available literature identified a relatively high rate of SpHUS in industrialised countries. Children with SpHUS accounted for 4.8% of total HUS cases and almost half of the reported serotypes were due to 19A. Pneumonia was the most common clinical presentation, and of the studies that measured mortality as an outcome, there was a crude case-fatality rate of 9.8% in cases with SpHUS.
Population-based studies have described an increase in the incidence of SpHUS over time [12, 23], although, this may be attributable to the greater awareness of SpHUS amongst clinicians. Between 1993 and 2008, there has been an increasing incidence of childhood empyema, and in the majority of cases, these were due to Streptococcus pneumoniae [25, 26]. HUS is a known potential complication of childhood empyema; this may therefore contribute towards the reported increase in incidence of SpHUS.
The PCV7 vaccine was introduced to the routine vaccination schedule in the USA, Australia, UK, Canada, Germany, New Zealand, Hungary, Portugal, and Spain in 2000, 2001, 2006, 2006, 2006, 2008, 2009, 2015 and 2015 respectively. As a result, there has been a noticeable decline in the rate of disease caused by PCV7 vaccine serotypes (4, 6B, 9V, 14, 18C, 19F, and 23F) [27, 28]. However, the incidence of non-PCV7 serotypes rose and subsequently higher valent vaccines, such as PCV10 and PCV13, were introduced in 2010 in most industrialised nations to replace PCV7. One of the major serotypes that was implicated in serotype replacement was serotype 19A. This serotype has been particularly known to cause pneumonia/empyema [29, 30], and it is not surprising that the incidence of SpHUS rose following the introduction of PCV7. The replacement of PCV7 by PCV13 in most industrialised nations in 2010 resulted in significant reduction in serotypes contained in PCV13 except serotype 19A and 3. One of the factors attributed to this is the low vaccine effectiveness against these serotypes. It is estimated that higher serum immunoglobulin G (IgG) concentrations (> 2.83 μg/mL) are required for protection against invasive pneumococcal disease, compared to the internationally accepted threshold of 0.35 μg/mL [31]. Serotype 19A was also reported to be responsible for a significant number of vaccine failure cases, perhaps because this serotype is the least immunogenic of the vaccine serotypes in infants and toddlers [32].
Another interesting finding from this review was that most children with SpHUS were under the age of 2 years. A possible explanation was that this age group was more susceptible to IPD [33, 34]. Consequently, these infants were more vulnerable to SpHUS as a complication from IPD. In a population study in Sweden, the overall mean estimated incidence of IPD was 15.1 per 100,000 inhabitants per year. In contrast, the incidence was 23 per 100,000 in infants between 0 and 23 months [34]. This group of children are yet to develop protective antibodies following their routine vaccination. Although, SpHUS is known to be a rare condition, SpHUS has been associated with poor outcomes such as high morbidity and high mortality. Impaired kidney function, and consequently chronic kidney disease, are some of the potential complications. This highlights the need for early suspicion and meticulous management to ameliorate these complications.
SpHUS is associated with a high rate of complications, which include end-stage kidney disease, longterm hypertension, requirement for dialysis, and renal transplant. This suggests that SpHUS may be more severe with greater morbidity than HUS cases caused by Escherichia coli. Cabrera et al. noted that SpHUS patients tended to be younger in age and had over three times the length of hospital stays than patients with HUS from other causes [35]. In a separate extensive European population-based study, it was observed that a notably larger percentage of individuals diagnosed with SpHUS necessitated dialysis, in contrast to the less than 5% incidence of dialysis requirement among individuals with STEC-associated HUS [36]. In addition, complications, such as seizures, were rare in STEC-associated HUS [36]. In contrast, this review showed that seizures were identified in 11.2% of patients out of the 9 studies that reported clinical features (shown in Table 2). Thus, patients with SpHUS appear to have a poorer prognosis than patients with STEC-associated HUS. Consequently, SpHUS patients require frequent monitoring and close follow-up to observe for signs indicative of long-term complications. Furthermore, it should be noted that the observed crude case fatality rate of 9.8% exceeds the documented mortality rate of 4.1% among patients diagnosed with STEC-associated HUS [36].
SpHUS is mainly treated by supportive care, such as mechanical ventilation, dialysis therapy, and treatment of the underlying infection with the use of antibiotics. It has been observed that patients with SpHUS were more likely to require dialysis and a longer hospital stay than patients with HUS caused by Escherichia coli [17]. Despite not being entirely evidence based, the traditional accepted practice was to use washed packed red blood cells (PRBC) if required, and to avoid the use of plasma product due to fear of introducing pre-formed anti-Thomsen-Friedenreich antibodies, which can worsen the disease process [5, 6, 22, 37]. However, recent advances in the understanding of the underlying pathogenesis of SpHUS are challenging the accepted practice of avoiding plasma containing products [10, 11]. This is supported by previous studies which have demonstrated no worsening of haemolysis or organ function when unwashed PRBC or plasma have been administered [20, 22, 38, 39].
In two of the studies included in this systematic review, pathogenic variants in complement genes and risk polymorphisms were identified [14, 15]. In the study by Holle et al., two patients had broad functional and genetic complement analysis and both patients had complement activated by the alternative pathway and risk haplotypes. Subsequently, the complement C5 inhibitor, eculizumab, was prescribed to three patients for SpHUS [14]. Gómez Delgado et al. performed genetic analyses on a small cohort of European patients and five patients presented rare complement variants, and they particularly highlighted the risk haplotypes in the CFH-CFHR3-CFHR1 region which may predispose patients to SpHUS. Furthermore, desialylation of Factor H (FH) and the FH-Related proteins by the pneumococcal neuraminidase was observed in plasma samples of 6 SpHUS patients [15]. These studies highlighted that disordered complement activation may have a role in the pathophysiology of HUS. Thus, mutations in the complement regulatory genes may predispose an individual to SpHUS. As a result, targeted therapies towards this mechanism may help reduce mortality and morbidity from SpHUS. However, the high costs and potential side effects of eculizumab should be considered when introducing this medication into national clinical guidelines. Further prospective studies are warranted to provide more definitive evidence for the feasibility of eculizumab treatment.
This is the first systematic review on SpHUS focusing on industrialised countries. However, this review is not without limitations. Most of the studies included in this review were performed before 2010, which was when most industrialised countries changed from PCV7 to PCV13. It is likely that following the introduction of PCV13, the trend of SpHUS would have changed. Further studies comparing the incidence, serotype, and complications of SpHUS following the introduction of the PCV13 vaccine would be valuable in understanding the effect of higher-valency conjugate vaccines. In addition, the vaccine coverage rate differs between different countries. A retrospective cohort study found that the PCV vaccine coverage rate for Switzerland was 4.5% [40]. This was considerably low when compared to the USA with a coverage rate above 80% [41]. It is therefore vitally important to consider the vaccine coverage rate when interpreting the impact of the PCV vaccine on SpHUS. In addition, the diagnostic method for SpHUS was variable between different countries, regions, and hospitals. This was due to the absence of a globally accepted guideline for laboratory diagnostics of SpHUS. Furthermore, the direct Coombs test (direct antiglobulin test) has been described as not adequately sensitive for the diagnosis of SpHUS [13]. Subsequently, some cases may have been left undiagnosed; this may result in an underestimation in the actual incidence.
Nonetheless, this review provides valuable information on the incidence, morbidity, and mortality of SpHUS following the global introduction of the PCV.
CONCLUSION
SpHUS is rare, but commonly presents in children younger than 2 years old. There is a persisting high risk of long-term complications and relatively high mortality rate even in the era of conjugate vaccines. Additional research is warranted to assess the epidemiological patterns and clinical characteristics of SpHUS in recent years, particularly in the wake of a decade of PCV13 utilisation in industrialised nations.
Do redakce došlo dne 30. 8. 2022.
Adresa pro korespondenci:
Carmen Lok Tung Ho
University College London Hospital
London United Kingdom
e-mail: carmen.ho16imperial.ac.uk
Zdroje
- Gerber A, Karch H, Allerberger F, et al. Clinical course and the role of Shiga toxin-producing Escherichia coli infection in the hemolytic-uremic syndrome in pediatric patients, 1997–2000, in Germany and Austria: A prospective study. J Infect Dis., 2002;186(4):493–500.
- Lawrence J, Gwee A, Quinlan C. Pneumococcal haemolytic uraemic syndrome in the postvaccine era. Arch Dis Child, 2018;103(10):957–961.
- Salvadori M. Update on hemolytic uremic syndrome: Diagnostic and therapeutic recommendations. World J Nephrol., 2013;2(3):56.
- Bitzan M, Lapeyraque AL. Pediatric kidney disease: Second edition. In: Pediatric Kidney Disease: Second Edition. 2017. p. 653 – 731.
- Copelovitch L, Kaplan BS. Streptococcus pneumoniae-associated hemolytic uremic syndrome. Pediatr Nephrol., 2008;23(11):1951–1956.
- Agarwal HS, Latifi SQ. Streptococcus pneumoniae-associated hemolytic uremic syndrome in the era of pneumococcal vaccine. Pathogens, 2021;10(6).
- Novak R, Martin C, Orsini E. Hemolytic-uremic syndrome and T-cryptantigen exposure by neuraminidase-producing pneumococci: an emerging problem? Pediatr Pathol, 1983;(1):409–413.
- Ramasethu J, Luban NLC. T activation. Br J Haematol, 2001; 112(2):259–263.
- Gilbert RD, Nagra A, Haq MR. Does dysregulated complement activation contribute to haemolytic uraemic syndrome secondary to streptococcus pneumoniae? Med Hypotheses, 2013;81(3):400–403.
- Meinel C, Spartà G, Dahse HM, et al. Streptococcus pneumoniae from Patients with Hemolytic Uremic Syndrome Binds Human Plasminogen via the Surface Protein PspC and Uses Plasmin to Damage Human Endothelial Cells. J Infect Dis, 2018;217(3):358 – 370.
- Scobell RR, Kaplan BS, Copelovitch L. New insights into the pathogenesis of Streptococcus pneumoniae–associated hemolytic uremic syndrome. Pediatr Nephrol., 2020;35(9):1585–1591.
- Bender JM, Ampofo K, Byington CL, et al. Epidemiology of streptococcus pneumoniae-induced hemolytic uremic syndrome in Utah children. Pediatr Infect Dis J, 2010;29(8):712–716.
- Lee CS, Chen MJ, Chiou YH, et al. Invasive pneumococcal pneumonia is the major cause of paediatric haemolytic-uraemic syndrome in Taiwan. Nephrology, 2012;17(1):48–52.
- Holle J, Habbig S, Gratopp A, et al. Complement activation in children with Streptococcus pneumoniae associated hemolytic uremic syndrome. Pediatr Nephrol., 2021;36(5):1311–1315.
- Gómez Delgado I, Corvillo F, Nozal P, et al. Complement Genetic Variants and FH Desialylation in S. pneumoniae-Haemolytic Uraemic Syndrome. Front Immunol., 2021;12.
- Vilardouro AS, Cachão J, Rodrigues M, et al. Hemolytic-uremic syndrome: 24 years’ experience of a pediatric nephrology unit. Brazilian J Nephrol., 2022;51–59.
- Constantinescu AR, Bitzan M, Weiss LS, et al. Non-enteropathic hemolytic uremic syndrome: Causes and short-term course. Am J Kidney Dis., 2004;43(6):976–982.
- Proulx F, Sockett P. Prospective surveillance of Canadian children with the haemolytic uraemic syndrome. Pediatr Nephrol., 2005;20(6):786–790.
- Cochran JB, Panzarino VM, Maes LY, et al. Pneumococcus-induced T-antigen activation in hemolytic uremic syndrome and anemia. Pediatr Nephrol., 2004;19(3):317–321.
- Waters AM, Kerecuk L, Luk D, et al. Hemolytic Uremic Syndrome Associated with Invasive Pneumococcal Disease: The United Kingdom Experience. J Pediatr., 2007;151(2):140–144.
- Copelovitch L, Kaplan BS. Streptococcus pneumoniae Associated hemolytic uremic syndrome: Classification and the emergence of serotype 19A. Pediatrics, 2010;125(1).
- Prestidge C, Wong W. Ten years of pneumococcal-associated haemolytic uraemic syndrome in New Zealand children. J Paediatr Child Health, 2009;45(12):731–735.
- Veesenmeyer AF, Edmonson MB. Trends in US hospital stays for Streptococcus pneumoniae-associated hemolytic uremic syndrome. Pediatr Infect Dis J., 2013;32(7):731–735.
- Lynn RM, O’Brien SJ, Taylor CM, et al. Childhood hemolytic uremic syndrome, United Kingdom and Ireland. Emerg Infect Dis, 2005;11(4):590–596.
- Nyman A, Pitchumani S, Jaffe A, et al. Pneumococcal empyema and haemolytic uraemic syndrome in children: experience from a UK tertiary respiratory centre. Arch Dis Child, 2009;94(8):645 – 646.
- Byington C, Spencer L, Johnson T, et al. An Epidemiologic Investigation of a Sustained High Rate of Pediatric Parapneumonic Empyema: Risk Factors and Microbiological Associations. Clin Pediatr (Phila), 2002;34(4):434–440.
- van der Linden M, Weiß S, Falkenhorst G, et al. Four years of universal pneumococcal conjugate infant vaccination in Germany: Impact on incidence of invasive pneumococcal disease and serotype distribution in children. Vaccine, 2012;30(40):5880–5885.
- Whitney C, Farley M, Hadler J, et al. Decline in Invasive Pneumococcal Disease after the Introduction of Protein–Polysaccharide Conjugate Vaccine. N Engl J Med, 2013;348(18):1737–1746.
- Andrews NJ, Waight PA, Burbidge P, et al. Serotype-specific effectiveness and correlates of protection for the 13-valent pneumococcal conjugate vaccine: A postlicensure indirect cohort study. Lancet Infect Dis, 2014;14(9):839–846.
- Oligbu G, Hsia Y, Folgori L, et al. Pneumococcal conjugate vaccine failure in children: A systematic review of the literature. Vaccine, 2016;34(50):6126–6132.
- Snape MD, Klinger CL, Daniels ED, et al. Immunogenicity and reactogenicity of a 13-valent-pneumococcal conjugate vaccine administered at 2, 4, and 12 months of age: A double-blind randomized active-controlled trial. Pediatr Infect Dis J, 2010;29(12):80–90.
- Balloch A, Licciardi P V., Russell FM, et al. Infants aged 12 months can mount adequate serotype-specific IgG responses to pneumococcal polysaccharide vaccine. J Allergy Clin Immunol, 2010;126(2):395–397.
- Hsiao HJ, Wu CT, Huang JL, et al. Clinical features and outcomes of invasive pneumococcal disease in a pediatric intensive care unit. BMC Pediatr, 2015;15(1):1–6.
- Backhaus E, Berg S, Andersson R, et al. Epidemiology of invasive pneumococcal infections: Manifestations, incidence and case fatality rate correlated to age, gender and risk factors. BMC Infect Dis, 2016;16(1):1–12.
- Cabrera G, Fortenberry J, Warshaw B, et al. Hemolytic uremic syndrome associated with invasive Streptococcus pneumoniae infection. Pediatrics, 1998;101 : 699–703.
- Kielstein JT, Beutel G, Fleig S, et al. Best supportive care and therapeutic plasma exchange with or without eculizumab in Shiga-toxin-producing E. coli O104:H4 induced haemolyticuraemic syndrome: An analysis of the German STEC-HUS registry. Nephrol Dial Transplant, 2012;27(10):3807–3815.
- Spinale JM, Ruebner RL, Kaplan BS, et al. Update on Streptococcus pneumoniae associated hemolytic uremic syndrome. Curr Opin Pediatr, 2013;25(2):203–208.
- Loirat C, Saland J, Bitzan M. Management of hemolytic uremic syndrome. Press Medicale, 2012;41(3 PART 2):e115–135.
- Brandt J, Wong C, Mihm S, et al. Invasive Pneumococcal Disease and Hemolytic Uremic Syndrome. Pediatrics, 2002;110(2):371 – 376.
- Zens KD, Baroutsou V, Fehr JS, et al. Pneumococcal Vaccination Coverage and Uptake Among Adults in Switzerland: A Nationwide Cross-Sectional Study of Vaccination Records. Front Public Heal, 2022;9 : 1–10.
- Hill H, Elam-Evans L, Yankey D, et al. National, State, and Selected Local Area Vaccination Coverage Among Children Aged 19–35 Months – United States, 2014. MMWR Morb Mortal Wkly Rep, 2015;64(33):889–896.
Štítky
Hygiena a epidemiológia Infekčné lekárstvo Mikrobiológia
Článok vyšiel v časopiseEpidemiologie, mikrobiologie, imunologie
Najčítanejšie tento týždeň
2023 Číslo 4- Parazitičtí červi v terapii Crohnovy choroby a dalších zánětlivých autoimunitních onemocnění
- Očkování proti virové hemoragické horečce Ebola experimentální vakcínou rVSVDG-ZEBOV-GP
- Koronavirus hýbe světem: Víte jak se chránit a jak postupovat v případě podezření?
-
Všetky články tohto čísla
- HIV-1 subtypes distribution and resistance to ART in HIV-infected persons in Slovakia (2019–2021)
- Risk of Streptococcus pneumoniae-associated haemolytic uraemic syndrome in industrialised nations: a systematic review of the literature
- Patogeneze infekcí vyvolaných virem SARS-CoV-2
- Vzteklina ve světě a program „Zero by 30“
- Analýza očkování vakcínami proti meningokokovým onemocněním u pacientů s invazivním meningokokovým onemocněním, Česká republika, 2006–2022
- Významné životní jubileum RNDr. Pavly Urbáškové, CSc.
- Pokyny pro autory a recenzenty
- Rejstřík
- Epidemiologie, mikrobiologie, imunologie
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- Patogeneze infekcí vyvolaných virem SARS-CoV-2
- Vzteklina ve světě a program „Zero by 30“
- HIV-1 subtypes distribution and resistance to ART in HIV-infected persons in Slovakia (2019–2021)
- Risk of Streptococcus pneumoniae-associated haemolytic uraemic syndrome in industrialised nations: a systematic review of the literature
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



