-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
Irradiation-Induced Genome Fragmentation Triggers Transposition of a Single Resident Insertion Sequence
Stress-induced transposition is an attractive notion since it is potentially important in creating diversity to facilitate adaptation of the host to severe environmental conditions. One common major stress is radiation-induced DNA damage. Deinococcus radiodurans has an exceptional ability to withstand the lethal effects of DNA–damaging agents (ionizing radiation, UV light, and desiccation). High radiation levels result in genome fragmentation and reassembly in a process which generates significant amounts of single-stranded DNA. This capacity of D. radiodurans to withstand irradiation raises important questions concerning its response to radiation-induced mutagenic lesions. A recent study analyzed the mutational profile in the thyA gene following irradiation. The majority of thyA mutants resulted from transposition of one particular Insertion Sequence (IS), ISDra2, of the many different ISs in the D. radiodurans genome. ISDra2 is a member of a newly recognised class of ISs, the IS200/IS605 family of insertion sequences.
Published in the journal: Irradiation-Induced Genome Fragmentation Triggers Transposition of a Single Resident Insertion Sequence. PLoS Genet 6(1): e32767. doi:10.1371/journal.pgen.1000799
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1000799Summary
Stress-induced transposition is an attractive notion since it is potentially important in creating diversity to facilitate adaptation of the host to severe environmental conditions. One common major stress is radiation-induced DNA damage. Deinococcus radiodurans has an exceptional ability to withstand the lethal effects of DNA–damaging agents (ionizing radiation, UV light, and desiccation). High radiation levels result in genome fragmentation and reassembly in a process which generates significant amounts of single-stranded DNA. This capacity of D. radiodurans to withstand irradiation raises important questions concerning its response to radiation-induced mutagenic lesions. A recent study analyzed the mutational profile in the thyA gene following irradiation. The majority of thyA mutants resulted from transposition of one particular Insertion Sequence (IS), ISDra2, of the many different ISs in the D. radiodurans genome. ISDra2 is a member of a newly recognised class of ISs, the IS200/IS605 family of insertion sequences.
Introduction
Stress-induced transposition has been an attractive notion for some time since it is potentially important in creating diversity to facilitate adaptation of the host to severe environmental conditions. One common major stress is DNA damage. This induces a variety of responses including changes in expression of numerous genes [1]–[4], cell cycle arrest [5],[6], induction of bacterial prophages [7]–[8] and, by generating diversity, can also aid development of processes such as bacterial pathogenicity and virulence [9].
Several studies have focused on DNA damage-induced transposition in bacteria but have not yet provided a coherent mechanistic scenario. This interest presumably stemmed directly from capacity of UV-irradiation to promote lysogenic induction [7]. Indeed, although IS10 transposition was shown to be induced by UV light in an SOS-dependent pathway [10], the precise mechanism has not been elucidated. A complex relationship between the SOS response and Tn5 transposition has emerged from contradictory reports [11]–[12]. More recently, activation of Tn7 transposition into regional hotspots by double-strand breaks, has suggested a relationship between Tn7 transposition and DNA repair [13], but direct evidence is still missing. Finally, numerous host factors can modulate transposition in E. coli in response to stress [14], but their specific roles are presently unknown. Here we identify and demonstrate the molecular basis of a strong radiation-stimulated response of transposition in the irradiation resistant Deinococcus radiodurans.
D. radiodurans has an exceptional ability to withstand the lethal effects of DNA-damaging agents, such as ionizing radiation, UV light and desiccation (for reviews, see [15],[16],[17]). High radiation levels result in genome fragmentation and reassembly in a process which generates significant amounts of single stranded (ss) DNA [18]. In addition to this extraordinary ability to reassemble its genome, the capacity of D. radiodurans to withstand irradiation also raises important questions about the mechanisms involved in the response to and repair of radiation-induced mutagenic lesions.
A recent study analysed the mutational profile in the thyA gene following doses of 10 kGy of γ - or 600 J m−2 of UV-irradiation. The majority of thyA mutants were due to a single insertion of one particular IS belonging to the IS200/IS605 family: ISDra2 [19](originally named IS8301 [20]). While some mutants, presumably resulting from point mutations or small insertions or deletions, retained the length of the wild-type gene, the many other resident ISs unrelated to ISDra2 made only small contributions to the mutant pool despite their presence in significant numbers (see www-IS.biotoul.fr) in the D. radiodurans R1 genome sequence [21]. The importance of the contribution of ISDra2 to mutagenesis is further underlined by its low genomic copy number in the standard R1 ATCC 13939 strain used in our studies as judged by a combination of whole genome hybridization and sequencing [19]: 1 complete and 1 inactive degenerate ISDra2 copy (in contrast to the published D. radiodurans genome sequence which revealed 7 complete and one partial ISDra2 copy [21]). Since another member of the IS200/IS605 family, IS608 from Helicobacter pylori, uses obligatory ssDNA intermediates [22], it seemed possible that the signal which triggers ISDra2 transposition is the very event which leads to genome reassembly: the formation of ssDNA.
We have explored the properties and behaviour of ISDra2 (ISDra2F; Figure 1A) in D. radiodurans and have identified the mechanism by which ISDra2 transposition is triggered by radiation. Using a genetic system to detect two principal transposition steps, transposon excision and insertion, we show that ISDra2, like other IS200/605 family members, requires TnpA but not TnpB for both and that insertion occurs 3′ to a specific pentanucleotide (as deduced from genome analyses [20]). We demonstrate genetically that both steps are significantly increased following host cell irradiation. We also show that the entire TnpA-catalysed transposition cycle including excision and insertion depends strictly on single strand DNA substrates in vitro. Finally, using a PCR-based approach, we demonstrate that, in vivo, exposure to γ-irradiation stimulates excision of the single genomic copy of ISDra2 from the genome in the form of a DNA circle. These events are closely correlated with the initiation of the process leading to genome reassembly from chromosomal fragments, which occurs mainly through a mechanism generating long stretches of single stranded DNA [18].
Fig. 1. A genetic assay for ISDra2 transposition. 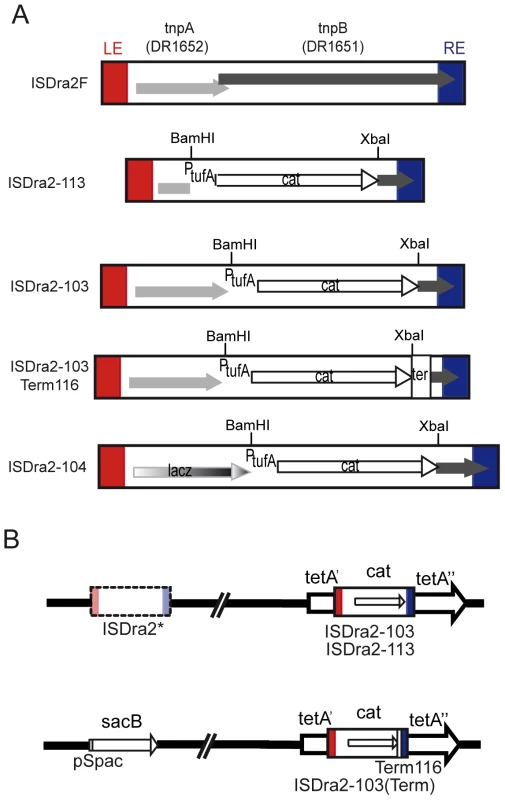
(A) Derivatives of ISDra2 (1736 bp). Orfs are indicated as boxes with arrowheads showing the direction of translation; LE and RE, red and blue boxes, respectively. In ISDra2-113 (1509 bp) both tnpA and tnpB were replaced by a CamR cassette, while ISDra2-103 (1778 bp) was deleted only of tnpB and expresses tnpA from its natural promoter. ISDra2-104 results from replacement of the tnpA coding region of ISDra2-103 by the lacZ coding region. ISDra2-103Term116 (1889 bp) carries the Deinococcal transcription terminator Term116 [45] downstream of the cat gene. (B) In vivo genetic assay to measure excision and insertion events of a derivative of ISDra2. The ISDra2F copy was first replaced by a TetR cassette and ISDra2-113 (or ISDra2-103) was inserted at the unique TTGAT target site present in the tetA gene. The second inactive copy, ISDra2*, was replaced by the sacB gene and an accompanying hygromycin (hyg) resistance cassette as a selective marker. TnpA-dependent ISDra2 excision restores a functional tetA gene, giving rise to TetR colonies while insertion into the reporter sacB gene confers resistance to sucrose. See Figure S1 and its legend. Results
ISDra2 excision and insertion in vivo in D. radiodurans
IS200/IS605 family members often carry 2 genes, tnpA, encoding the transposase and tnpB, a gene of unknown function (Figure 1A). As shown for the related IS608 [23], the transposition cycle occurs in two principal steps: excision from the donor backbone in the form of a single strand circle and subsequent insertion into a suitable DNA target [22]. These steps were monitored for ISDra2 transposition in D. radiodurans using a genetic assay. TnpA and/or TnpB were expressed in trans from a plasmid, pGY11559, under control of the IPTG-inducible Pspac promoter [19] (Table S1). For excision, the unique active ISDra2 copy (ISDra2F: D. radiodurans loci DR1651-DR1652) was replaced either by a derivative, ISDra2-113, retaining functional IS ends but in which tnpA and tnpB were replaced by a CamR cassette, or by ISDra2-103, similar to ISDra2-113 but retaining tnpA controlled by its own promoter (Figure 1A). The resident ISDra2F was first replaced by a TetR cassette and ISDra2-113 (or ISDra2-103) was inserted by homologous recombination at the unique 5′TTGAT3′ target site (see below) present in the tetA gene. IS excision restores a TetR phenotype (Figure 1B). These constructions are described in detail in Figure S1.
Ectopic TnpA expression in this system induced ISDra2-113 excision at a frequency of 2×10−3 (Table 1). This was not increased when IPTG was added to the medium, to induce TnpA expression. Similar experiments in which Pspac was used to drive a lacZ gene in plasmid pGY11556 (Figure S2) showed that addition of IPTG resulted in a 143-fold increase in β-galactosidase activity. This suggests that sufficient transposase would be produced by escape synthesis from Pspac to ensure transposition and that activity is limited by the supply of a correct DNA substrate. Activity obtained with ectopic TnpB alone (<1×10−9) was similar to the background levels observed with the empty vector plasmid (<4.8 10−9) clearly demonstrating that TnpA is absolutely required for ISDra2 excision while TnpB is dispensable. Similar results were obtained with ISDra2-103 (Figure 1A), in which tnpA was in its natural location in the IS sequence and expressed from its own promoter (Table 1).
Tab. 1. Excision frequencies of ISDra2 derivatives. 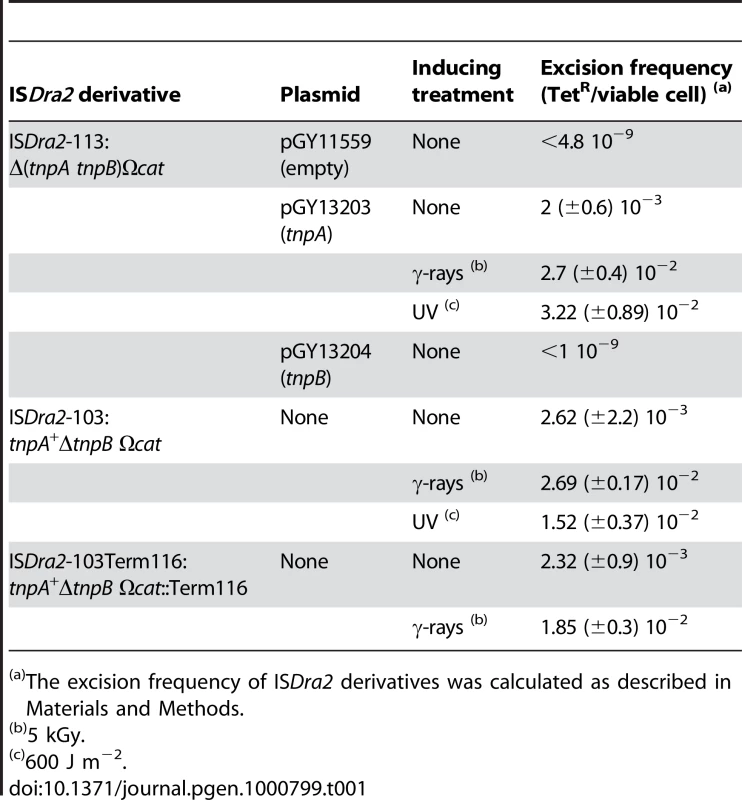
The excision frequency of ISDra2 derivatives was calculated as described in Materials and Methods. To measure transposon insertion, both non-targeted and targeted approaches were used. In both cases, we measured insertion in the subpopulation of cells in which the transposon ISDra2-113 had excised from its initial locus and had inserted elsewhere in the genome as judged respectively by the reconstitution of the TetR gene and retention of the CamR marker carried by the IS derivative.
In the non-targeted approach, the proportion of CamR among TetR clones, reflecting the frequency of spontaneous insertion, was about 10−2 in strain GY13120 (Figure 1B, top) expressing TnpA ectopically, compared to <10−9 in the absence of TnpA. These insertions were true transposition events since they had occurred 3′ to the pentanucleotide, 5′TTGAT3′ (Table S2), the sequence preceding the left end of ISDra2 in all genomic loci identified [20].
In the targeted approach, the degenerate ISDra2 copy (ISDra2* loci DR0177-DR0178) was replaced with the target sacB gene from Bacillus subtilis, coupled to a hygromycin resistance cassette to assist the construction (Figure 1B, bottom, and Figure S1). As in other bacteria [24],[25], sacB expression in the presence of sucrose is lethal for D. radiodurans (data not shown) and inactivation of sacB, for example, by IS insertion, confers a sucrose resistant phenotype. Moreover, sacB contains 10 copies of the insertion site 5′TTGAT3′ (9 on one strand and 1 on the complementary strand; Figure S3A). For this analysis, it was considered prudent to include a transcriptional terminator (Term116) downstream of the cat gene of ISDra2-113 to avoid possible interference of sacB transcription from the strong Pspac promoter with expression of the cat gene (Figure 1A and 1B; Figure S1). By imposing a triple selection for TetR, CamR and SucR, we were able to directly collect clones in which ISDra2-103Term116 had excised from its resident site (TetR) and inserted into sacB (SucR). The nucleotide sequence of the SucR mutants confirmed that each had ISDra2-113 inserted into one of the 5′TTGAT3′ target sequences (Figure S3A and Figure 3B).
Together, these results demonstrate that TnpA alone is sufficient for both transposon excision and insertion.
Effect of UV and γ-radiation on excision and insertion
Using this genetic system, we then measured the excision frequency of ISDra2-113 and ISDra2-103 following exposure to UV - (600 J m−2) or γ-irradiation (5 kGy). Both treatments increased excision frequencies about 10-fold (Table 1).
To determine whether γ-ray irradiation also stimulates the insertion step of transposition, we measured the insertion frequency of ISDra2-103 (Term116) expressing TnpA from its own promoter (to remain as close to natural conditions as possible) (Figure 1A) into sacB. The frequency of TetR colonies and of SucR CamR TetR colonies in cells irradiated with 5 kGy of γ-rays and in non-irradiated cultures was measured in the tester strain GY13174 carrying ISDra2-103 (Term116). The TetR frequency, which monitors the excision step, rose from 2.32×10−3 to 1.85×10−2 after γ - irradiation (Table 1; average values of 10 independent experiments). The frequency of SucR CamR TetR colonies, representing the overall transposition frequency into the reporter gene, also increased from 3.76×10−10 to 1.8×10−8 after γ - irradiation (Table 2).
Tab. 2. Insertion frequencies of ISDra2-103Term116 into sacB. 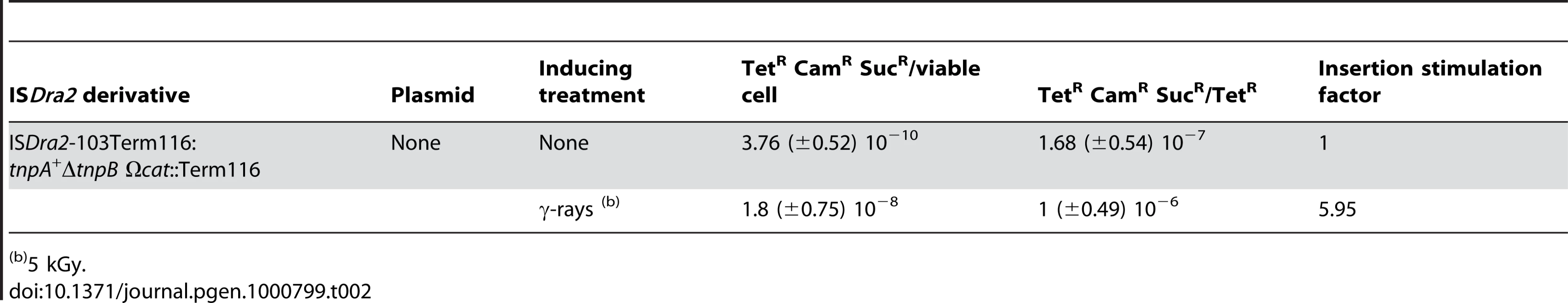
5 kGy. Thus, both excision and insertion of the ISDra2 derivatives were stimulated by irradiation. This stimulation is unlikely to result from an increase in tnpA expression after irradiation since strain GY14310 in which the coding region of tnpA was replaced by the coding region of lacZ in ISDra2-104 (Figure 1A) showed no detectable increase in β-galactosidase activity at 0, 30, 60, 120 or 180 min post-irradiation (Figure S2 and data not shown).
In vitro cleavage, strand transfer, and insertion of ISDra2
Since the related TnpA from IS608 uses obligatory single-stranded DNA substrates in in vitro transposition reactions [22], we suspected that the stimulation of ISDra2 excision and insertion might be linked to the formation of single-stranded DNA during repair of DNA damage which would supply the appropriate substrate.
To confirm that ISDra2 TnpA is active only on single-strand DNA substrates, we used an in vitro system, developed for the related IS608, to investigate TnpA-catalysed cleavage and strand transfer [22],[26]. IS608 transposition reactions are strand specific and use, by definition, the top strand. Recombination reactions recapitulating transposon excision and donor joint formation were performed. For this, the ISDra2 tnpA gene was cloned with a C-terminal His6 tag under control of a plac promoter (Materials and Methods) and the protein was purified as described previously [23]. The C-terminal His-tagged TnpA from D. radiodurans was active since it catalysed ISDra2-113 excision in vivo in a tester strain expressing TnpA-His6 under the control of Pspac promoter in plasmid pGY13505 (data not shown).
The DNA substrate was a 59 nt single strand DNA fragment including the first 39 nt of ISDra2 LE carrying a subterminal secondary structure which serves as the TnpA binding site (Burgess-Hickman et al., in prep) and a 20 nt 5′ flank with the conserved pentanucleotide target sequence (5′TTGAT3′) (Figure 2A). Incubation of the 5′ end-labelled fragment (Figure 2B, lane 1) with purified TnpA in the presence of Mg2+ generated a cleaved 5′ end-labelled donor flank fragment of 20 nt (Figure 2B, lane 2). When mixed with an unlabelled 63 nt ss DNA fragment composed of the terminal 43 nt of RE (including the secondary structure) and a 3′ 20 nt flank, an additional fragment of 40 nt representing the joined donor flanks was generated (Figure 2B, lane 3). In contrast, double stranded LE (Figure 2B, lane 4) was neither cleaved nor underwent a strand transfer reaction with the unlabelled RE (Figure 2B, lane 5).
Fig. 2. ISDra2 TnpA-catalyzed cleavage and strand transfer in vitro. 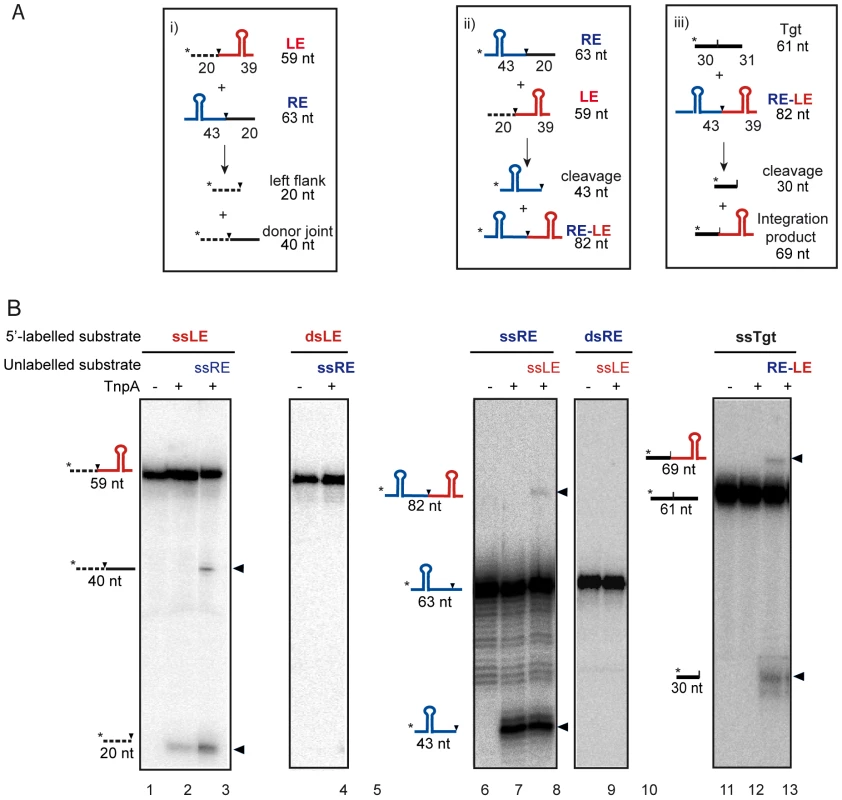
(A) Oligonucleotides used as DNA substrates. Length of cleavage products is indicated. The potential secondary structure in both LE and RE is indicated. Black dotted and black lines: left and right DNA flanks cleavage sites are shown as vertical black arrows. Asterisk (*) indicates radioisotope position. (B) Excision in vitro: donor joint formation and single-versus double-strand substrates. The 5′-32P-labelled oligonucleotide used was the 59-base LE composed of 39 nt of LE and 20 nt 5′ to the 5′TTGAT3′ and the unlabelled 63-base oligonucleotide RE. Lane 1: no-protein control; lane 2: TnpA alone; lane 3: TnpA and unlabelled RE; lane 4: dsLE, no-protein; lane 5: dsLE, TnpA and ssRE. Similar results were obtained using 5′ end-labelled RE and unlabelled LE: cleavage and strand transfer were strictly dependent on ss subtrates (Figure 2B). We were also able to recapitulate the integration reaction in vitro using an end-labelled target DNA and an unlabelled RE-LE junction (Figure 2B).
Thus, the ISDra2 transposase is active on single - but not double-stranded substrates and is capable of cleavage of both LE and RE and of strand transfer to generate the donor joint and the RE-LE junction.
Kinetics of irradiation-induced ISDra2 excision
To investigate the relationship between irradiation and induction of ISDra2 transposition, we analysed the kinetics of γ-irradiation-triggered ISDra2-113 excision directly from the D. radiodurans chromosome. For this, we isolated genomic DNA before and at different times after γ-irradiation and subjected samples to PFGE analysis following NotI digestion (Figure 3B) and to PCR analysis (Figure 3A, 3C and 3D). Primer pairs P1+P2, complementary to the tetA flanks of ISDra2-113 (Figure 3A), should generate a 2120 bp fragment when ISDra2-113 is inserted into tetA and a 500 bp fragment when the donor backbone is sealed following ISDra2-113 excision. IS circle junction formation was monitored using primers P3+P4, complementary to the subterminal IS region (Figure 3A), by the appearance of a 260 bp product representing abutted LE and RE.
Fig. 3. Physical evidence for irradiation-induced ISDra2 excision. 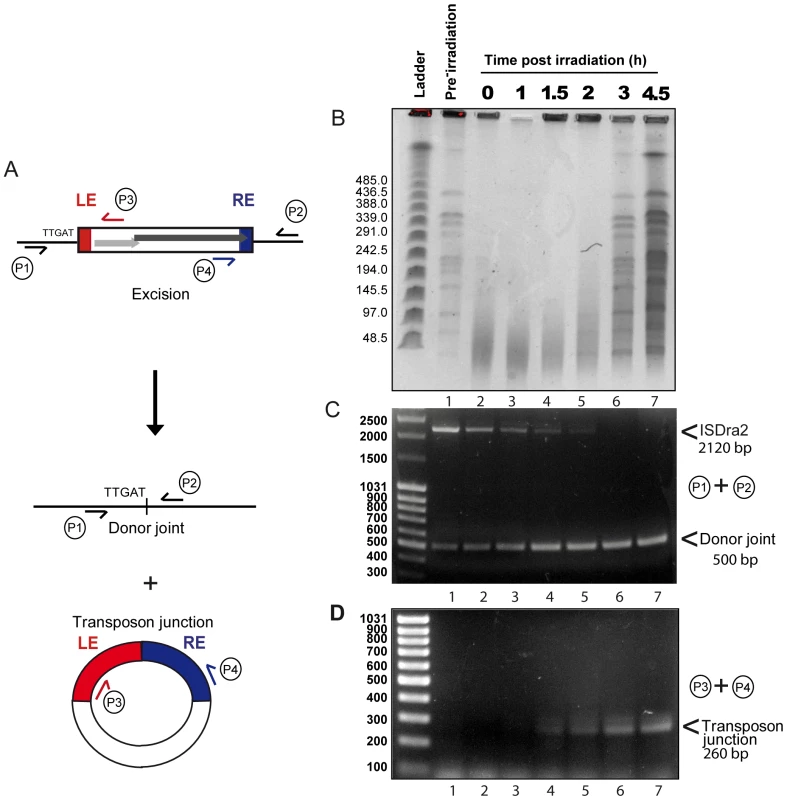
Strain GY13120 (tetAΩISDra2-113 expressing tnpA in trans) received 5 kGy of γ–irradiation and aliquots were taken to isolate genomic DNA used to prepare DNA agarose plugs and as template for PCR analysis. The time following irradiation is shown in hours above each lane. (A) Schematic representation of IS excision products. The excision products are shown together with the position of primers used in PCR analysis. Note that the transposon circle including the transposon junction is single stranded. (B) Kinetics of double-strand-break repair. DNA agarose plugs were digested with NotI prior to PFGE analyses and loaded onto a 1.06% agarose gel. L: λ Ladder. (C) Kinetics of donor joint appearance. PCR reactions were performed with primer pair P1 and P2 and loaded onto a 1% agarose gel; L: MassRuler DNA Ladder. (D) Kinetics of IS circle junction appearance. PCR reactions were performed with primer pair P3 and P4 and loaded onto a 2% agarose gel; L: O'GeneRuler 100 bp DNA Ladder. Total NotI-digested genomic DNA (Figure 3B) showed complete fragmentation immediately following irradiation (compare lanes 1 and 2). Reassembly, evidenced by the gradual reappearance of distinct NotI fragments, could be detected at about 2 h post-irradiation while the complete regenerated banding pattern exhibited by the non-irradiated sample (lane 1) occurred after 3 h (lane 6). PCR analysis of these samples using the P1+P2 primer pair revealed the presence of the full length IS together with a low but significant quantity of donor joint prior to irradiation resulting from rejoining of the DNA flanks following IS excision (Figure 3C, lane 1). This indicates that IS excision occurs during normal growth of the host strain as might be expected as tnpA expression is under control of Pspac. Importantly, a significant increase in the level of the donor joint (Figure 3C) occurred over the 4.5 h post-irradiation period. We note that the progressive disappearance of the full length IS does not necessarily reflect its excision. In order to visualize the donor joint product, the PCR conditions were adjusted (short extension times; see Materials and Methods) to favour amplification of shorter donor joint fragment. It is probable that the reduction in the intensity of full length IS is due to competition in the reaction mixture from the increasing concentration of the short donor joint. In addition, the P3+P4 primer pair revealed the gradual appearance between 1 and 1.5 h post irradiation of the IS junction species (Figure 3D). The identity of the donor joint and the IS junction was verified by sequencing. The IS junction generated by P3+P4 was cloned into a plasmid carrying CamR (unable to replicate in D. radiodurans) which was then introduced by transformation into a strain expressing ectopic TnpA. Ten independent CamR clones were analysed using inverse PCR and each was shown to carry an insertion 3′ to a 5′TTGAT3′ target sequence, demonstrating that the IS junction generated by P3+P4 is active in transposition (data not shown).
Although we did not use quantitative PCR, care was taken to include identical quantities of DNA in each reaction and, to a first approximation, the results indicate that both the donor joint and the transposon junction were formed with very similar kinetics. Products started to accumulate at a time which coincided with the end of DNA degradation and probably with the start of the ESDSA pathway generating long stretches of single stranded DNA [18]. These results therefore strongly suggest that the factor which triggers ISDra2 transposition is the formation of single stranded DNA during D. radiodurans genome assembly.
Discussion
D. radiodurans has been the object of much interest due to its astonishing capacity to resist high levels of radiation [15],[17] and to the genome fragmentation and reassembly processes essential for its survival after irradiation [18]. Clearly such exceptional properties might influence the behavior of mobile genetic elements within the genome and perhaps reveal new and interesting regulatory mechanisms. One hint that this might be the case came from the observation that transposition of one IS, ISDra2, into the thyA gene was apparently increased by high levels of γ - or UV-irradiation [19]. ISDra2 was the only insertion sequence of the 12 different IS family members present in the D. radiodurans genome to behave in this way.
We show that ISDra2 transposition is specifically triggered during the process of reassembly of the D. radiodurans genome which is associated with recovery from irradiation damage. ISDra2 belongs to the IS200/IS605 family (see www-is.biotoul.fr) and the paradigm of this family, IS608, transposes by excision of a single strand circular DNA intermediate which can then insert into a single strand DNA target [22]. Our results demonstrate that ISDra2 also exhibits a strict requirement for single stranded DNA in vitro (Figure 2). Furthermore, we show genetically that both ISDra2 excision (detected by the restoration of an intact tetA gene) and insertion (into the reporter sacB resulting in resistance to sucrose) require TnpA, and that insertion occurs 3′ to the specific pentanucleotide sequence, 5′TTGAT3′, found adjacent to the left end of all naturally occurring genomic ISDra2 copies [20]. Moreover, we observe a 50 to 60-fold increase in the overall frequency of transposition following UV or γ-irradiation resulting from stimulation of the two transposition steps: a 8-fold increase in excision and a 6-fold increase in insertion of the IS circle transposition intermediate (Table 1). The overall transposition stimulation factor is in accord with the 50 - and 100-fold induction of in vivo transposition of wild-type ISDra2 into the thyA gene by UV - and γ-irradiation respectively [19].
Monitoring tnpA promoter activity with the lacZ reporter gene demonstrated that the radiation-triggered ISDra2 transposition was not due to a specific induction of TnpA expression. Moreover, a same stimulatory effect of irradiation was observed whether TnpA was expressed from its natural promoter or from an external IPTG-inducible Pspac promoter.
Importantly, using a physical PCR-based approach, we also demonstrate that ISDra2 excision, reclosure of the chromosomal DNA donor joint and formation of a transposon joint with abutted left and right ends (consistent with a circular form of ISDra2) occur within irradiated D. radiodurans cells. Both rejoined donor DNA flanks and the LE-RE junction begin to accumulate after 90 min of post-irradiation incubation. This correlates with the end of the degradation of damaged DNA and the start of DNA double strand break repair processes such as extended synthesis dependent strand annealing (ESDSA) that generates high concentration of single stranded DNA [18],[27].
Induction of transposition by exposure to environmental stress has often been tacitly assumed. However, there has been only limited supporting evidence for the idea that transposition can be induced efficiently by environmental insults and the sparse data available are generally based on indirect genetic assays [10], [12]–[13], [28]–[32]. These data should be revisited using more powerful technologies now available.
The idea of environmentally-induced transposition has also arisen from analysis of the growing number of available complete prokaryote genome sequences. These have identified many bacterial and archaeal species in which the number of ISs is dramatically high. They include different Shigellae species [33], Bordetella pertusis [34], Yersinia pestis [35]–[36] Lactobacillus [37], Sulfolobus solfataricus [38] among many others. While it is attractive to imagine that this is the result of transposition bursts induced by an environmental trigger, a strong alternative has been to invoke stochastic transposition together with the formation of population bottlenecks produced in small isolated populations to fix such mutational events (insertions) [34].
Irradiation-triggered transposition described here is a response to an extreme set of environmental conditions which transiently generates large quantities of a substrate (single strand DNA) favoring transposition of ISDra2 but not of the other, unrelated, ISs present in the D. radiodurans genome. This response would result in movement of the single “top strand” IS copy generating mutational diversity while retaining the inactive bottom strand copy. Insertion of the single-stranded circle intermediate and replication of the bottom strand would increase the copy number of the IS and disperse it throughout the genome. Moreover, D. radiodurans possesses an efficient natural competence system whose regulation remains to be explored. In view of the fact that such processes in other bacterial species occur using single strand DNA intermediates, transformation may be instrumental in assuring spread of this single strand transposable element [39]. Thus radiation-triggered ISDra2 transposition might additionally generate diversity through participation of DNA containing newly transposed ISDra2 copies in intercellular transformation.
An increase in the ISDra2 copy number exposed to a single gamma irradiation cycle has been observed previously (see Figure S2 in [19]). Moreover, Islam et al [20] characterized the distribution of ISDra2 in different laboratory D. radiodurans strains and found that its copy number varied from strain to strain: 7 copies were identified in the published D. radiodurans R1 genome sequence, 6 in the MR1 strain, 21 in the KD8301 strain but only one in KR1, the parent strain of KD8301 and in the reference strain ATCC 13939 used here. Unfortunately, the history and the intermediate strains are not available and no conclusions can be drawn concerning the factors which contributed to the gain or loss of ISDra2 copies in the genome of these strains. The expansion of transposon copy number with accompanying ectopic sequence homology raises the question whether this would be a detriment to the extensive chromosome repair process after heavy irradiation. Precise reconstitution of a shattered genome through ESDSA involves annealing of 20–30 kb single-stranded DNA overhangs. Zahradka et al ([18]) speculated that short blocks, 1–2 kb, of dispersed repetitive sequences present in the genome of D. radiodurans would not compromise the accuracy of repair through ESDSA. These authors pointed out that annealing only a limited repeated sequence block within two long non-complementary single-stranded overhangs could not readily link two fragments together. However, such ectopic sequence homology might stimulate chromosomal rearrangements through other DNA double strand break repair pathway such as single strand annealing (SSA) with a potential of increasing the plasticity of the genome under very adverse conditions.
Since this class of newly recognized transposable elements require single stranded DNA as both substrate and target site, any process which generates single strand DNA such as replication, mismatch repair and transcription, might lead similarly to limited induction of transposition and also provide suitable insertion sites. In view of the extremely widespread occurrence of members of this IS family in nature, this type of mechanism could be important in regulating transposition activity, interfacing transposition with host physiology and cell cycle and in creating genomic diversity as has been recently shown for the IS200/605 family member, IS1541, whose insertion allows the Yersinia pestis host to escape from adaptive immune responses and plague immunity [9].
Materials and Methods
Bacterial strains, media, and growth conditions
Bacterial strains are listed in Table S1. E. coli strain DH5α was the general cloning host and strain SCS110 was used to propagate plasmids prior to introduction into D. radiodurans via transformation[40]. All D. radiodurans strains were derivatives of strain R1 (ATCC 13939). TGY2X liquid medium and TGY plates [41] were used for D. radiodurans and Luria-Bertani (LB) broth for E. coli strains. Media were supplemented with the appropriate antibiotics used at final concentrations of: chloramphenicol 20 µg ml−1 for E. coli and 3 µg ml−1 for D. radiodurans; spectinomycin 40 µg ml−1 for E. coli and 75 µg ml−1 for D. radiodurans; tetracycline 2.5 µg ml−1 for D. radiodurans; hygromycin 50 µg ml−1. 5% (w/v) sucrose was supplemented to isolate the D. radiodurans sacB inactivated mutants.
Transformation of D. radiodurans with genomic DNA, PCR products, or plasmid DNA was performed as described [41].
DNA manipulations
Plasmid DNA was extracted from E. coli using the QIAprep Spin miniprep kit (Qiagen). D. radiodurans chromosomal DNA was isolated as described previously [19]. PCR reactions were carried out with Phusion DNA Polymerase (Abgene).
Inverse PCR was performed as follows: genomic DNA was digested with NarI, purified by Phase Lock Gel procedure (Eppendorf), and then ligated using T4 DNA ligase. After ethanol precipitation, the ligated circular DNA was used as a template with primers P3 and P4 described in Table S1. The iPCR products were then directly sequenced with LEext and REext by Genome Express (Grenoble, France). Oligonucleotides used are listed in Table S3.
PCR reactions used for analysis of kinetics of irradiation-induced ISDra2 excision were performed as follows: PCR was carried out in a final volume of 50 µl with using 0.5 Units of DyNazyme EXT DNA polymerase (Finnzymes) and 200 µM of each dNTP. To detect the donor joint, PCR analysis using P1+P2 primer pair was performed under the following conditions: 94°C for 3 min, 30 cycles of 94°C for 45 s, 60°C for 45 s, and 72°C for 20 s; and finally 72°C for 10 min. To detect the RE-LE junction, PCR analysis using P3+P4 was carried out as follows: the PCR of the first round was performed with 1 µg of genomic DNA under the following conditions: 94°C for 3 min, 30 cycles of 94°C for 45 s, 55°C for 45 s, and 72°C for 10 s, and finally 72°C for 10 min. The second round of PCR used a 15 µl aliquot from round 1 as template and was done under the same conditions than round 1.
Plasmids
For plasmid pGY13224 expressing tnpA and tnpB from a Pspac promoter, the coding sequences of the two genes were amplified by PCR using primers DraF (tagged with EcoRV) and DraR (tagged with XhoI). After cleavage, the PCR fragment was cloned into pGY11559 between the SwaI and XhoI sites.
For plasmid pGY13203 expressing tnpA from the Pspac promoter, the D. radiodurans ISDra2 tnpA gene was amplified by PCR using the primer pair DraF/DraX and D. radiodurans R1 genomic DNA as template. The product was cloned into plasmid pGY11559 between the EcoRV and XhoI sites.
For plasmid pGY13204 expressing tnpB from the Pspac promoter, the N-terminal part of tnpB was amplified using the primer pair 1651F/1651R, digested with NdeI and BsaI and ligated to the 10885-bp NdeI-BsaI fragment from pGY13224.
For plasmid pGY13507 expressing sacB from the Pspac promoter, the B. subtilis sacB gene was amplified with the primer pair NdeUPsacB/XhoDwnsacB and cloned into pGY11559 between the NdeI and XhoI sites.
For plasmid pGY11556 expressing the E. coli lacZ from the Pspac promoter, pGY11559 was digested with BglII and XhoI and ligated to the BglII-XhoI fragment from pGY11540 containing lacZ fused to the Pspac promoter.
Plasmids for production and in vivo analysis of His-tagged TnpA
The tnpA coding sequence was amplified with the primer pair NdeUptnpA/TnpAHISsph and was cloned at the NdeI and SphI sites of pAPT110 with a C-terminal His6 tag under the control of Plac, to generate plasmid pGY13503. The plasmid also carried the lacI gene to regulate tnpA-His6 expression. To analyse the ability of C-terminal His-tagged TnpA to catalyse in vivo transposition, the tnpA-His6 copy was amplified from pGY13503 with primer UpNde107 (tagged with NdeI) and DwntnpAHISxho (tagged with XhoI) and cloned into pGY11559 at the unique NdeI and XhoI sites to produce plasmid pGY13505, in which tnpA-His6 is expressed under the control of Pspac promoter.
Pulsed field gel electrophoresis
Irradiated cultures were diluted in TGY2X to an A650 = 0.3 and incubated at 30°C. At different post-irradiation incubation times, samples (5 ml) were taken to prepare DNA plugs as described [42]. The DNA in the plugs was digested for 16 h at 37°C with 60 units of NotI restriction enzyme. After digestion, the plugs were subjected to pulsed field gel electrophoresis for 28 hours at 10°C using a CHEF MAPPER electrophoresis system (Biorad) with the following conditions: 5.5 V/cm, linear pulse of 40 s, and a switching angle of 120° (−60° to +60°).
Measurement of in vivo spontaneous, γ-, and UV-induced excision frequencies of ISDra2 derivatives
Individual CamR TetS colonies purified from GY13111 or derivatives of GY13115 strain expressing in trans TnpA, or TnpB or no protein were inoculated into 3 ml of TGY2X supplemented with spectinomycin when required and grown to an A650 of 1–2. The bacterial cultures were washed in 10 mM MgSO4, resuspended in the same buffer to an A650 = 1. Half of the resuspension was kept on ice, and the second half was exposed to UV light at a dose rate of 3.5 J m−2 s−1 in Petri dishes. For γ-irradiation, the cultures were grown to an A650 = 1, then concentrated 30-fold in TGY2X and irradiated on ice with a 137Cs irradiation system (Institut Curie, Orsay, France) at a dose rate of 41.8 Gy min−1.
UV-, γ - or non-irradiated cells were diluted in TGY2X to an A650 = 0.3 and grown to stationary phase. Determination of the total number of viable cells was performed on TGY plates and excision of ISDra2 derivatives from tetA gene was selected on TGY plates containing tetracycline. Colonies were counted after 3–4 days of incubation at 30°C. The frequencies of the excision event per viable cell from 10 independent experiments were used to calculate the mean values and the standard deviations.
Measurement of in vivo spontaneous and γ-induced insertion frequencies of ISDra2-103 into sacB
5 individual CamR TetS colonies purified from GY13174 strain expressing ISDra2-103 were inoculated into 10 ml of TGY2X supplemented with hygromycin and grown to an A650 of 1.5. The bacterial cultures were concentrated 30-fold in TGY2X. 100 µl of the resuspension was kept on ice and the rest was γ-irradiated as described above. After dilution, γ - or non-irradiated cells were grown to stationary phase. Excision of ISDra2-103 from tetA and insertion into sacB gene were selected on TGY plates containing tetracycline, or tetracycline, chloramphenicol and sucrose, respectively. Colonies were counted after 3–4 days of incubation at 30°C. The insertion frequencies per viable cell from 5 independent experiments were used to calculate the mean values and the standard deviations.
Measurement of lacZ expression under control of PtnpA or Pspac promoters
Replacement of the tnpA coding region of ISDra2-103 with the lacZ coding region to generate ISDra2-104 (Figure 1A) was performed as follows: the PtnpA::lacZ fusion was amplified by the joining PCR method [43]; see Table S3). The resulting lacZ fusion, the accompanying chloramphenicol resistance cassette and the right end of ISDra2-103 were then inserted into the tetA gene of strain GY13109 using the tripartite ligation method [44]. The resulting strain GY14310 was selected for CamR and the insertion of the lacZ fusion by homologous recombination was confirmed by diagnostic PCR. LacZ gene was expressed under the Pspac promoter in strain GY14312 containing plasmid pGY11556. Expression of the lacZ reporter gene was detected in D. radiodurans colonies formed on TGY plates containing 5-bromo-4-chloro-3-indolyl-β-D-galactoside (X-gal) at 40 µg/ml. β-galactosidase activity was measured as previously described [41].
TnpA purification, DNA procedures, and oligonucleotide cleavage and strand transfer reactions in vitro
TnpA was purified from E. coli K12 MC1061 endA carrying TnpA-His6 expression plasmid pGY13503 (Table S1) following induction with 0.5 mM IPTG as previously described [25].
Oligonucleotide substrates for in vitro reactions
LE (59-mer):
GGCGTCTGAATGGCCTTGATGCTTGAGGGGCGCACACTCGTGACTTCAGTCATGAGTTA
LEcom (59-mer):
TAACTCATGACTGAAGTCACGAGTGTGCGCCCCTCAAGCATCAAGGCCATTCAGACGCC
RE (63-mer):
CTGCGAAGTGAGAATCACGCGACTTTAGTCGTGTGAGGTTCAAGAGTCCCTTGGCGCCCATGA
REcom (63-mer)
TCATGGGCGCCAAGGGACTCTTGAACCTCACACGACTAAAGTCGCGTGATTCTCACTTCGCAG
In vitro oligonucleotide cleavage and strand transfer reactions
Reactions were performed by 45 min incubation of 20 fmol of a 5′-32P-labelled oligonucleotide and 1 pmol of the same oligonucleotide unlabelled with or without 10 pmol unlabelled recombining oligonucleotide, 0.5 µg of poly-dIdC and 20 pmol TnpA-His6 at 37°C in a final volume of 16 µl in 20 mM HEPES (pH 7.5), 2.5% DMSO, 200 mM NaCl, 5 mM MgCl2, 1 mM TCEP, 20 µg/ml BSA and 10% glycerol. The reactions were terminated by addition of 0.1% SDS followed by 15 min of incubation at 37°C and separated on a 10% denaturing sequencing polyacrylamide gel. The gel was analysed by phosphorimaging.
5′-end-labelling
10 pmol of oligonucleotide was mixed with 16 pmol of [γ-32P] ATP (5000 Ci/mmol, Amersham Inc.) and 1 unit of T4 kinase (NEB Inc.) in T4 kinase buffer (70 mM Tris–HCl pH 7.6, 10 mM MgCl2, 5 mM DTT). Incubation was for 1 h at 37°C. Labelled oligonucleotides were purified by filtration through Sephadex G25. Ds substrates were obtained by hybridisation of labelled top-stranded oligonucleotide with cold bottom-stranded oligonucleotide.
Supporting Information
Zdroje
1. WalkerGC
1984 Mutagenesis and inducible responses to deoxyribonucleic acid damage in Escherichia coli. Microbiol Rev 48(1) 60 93
2. CriswellT
LeskovK
MiyamotoS
LuoG
BoothmanDA
2003 Transcription factors activated in mammalian cells after clinically relevant doses of ionizing radiation. Oncogene 22(37) 5813 5827
3. GötzD
PaytubiS
MunroS
LundgrenM
BernanderR
WhiteMF
2007 Responses of hyperthermophilic crenarchaea to UV irradiation. Genome Biol 8(10) R220
4. FuY
PastushokL
XiaoW
2008 DNA damage-induced gene expression in Saccharomyces cerevisiae. FEMS Microbiol Rev 32(6) 908 926
5. ZhouBB
ElledgeSJ
2000 The DNA damage response: putting checkpoints in perspective. Nature 408(6811) 433 439
6. IshikawaK
IshiiH
SaitoT
2006 DNA damage-dependent cell cycle checkpoints and genomic stability. DNA Cell Biol 25(7) 406 411
7. LwoffA
SiminovitchL
KjeldgaardN
1950 [Induction of the production of bacteriophages in lysogenic bacteria.]. Ann Inst Pasteur (Paris) 79(6) 815 859
8. RobertsJW
DevoretR
1983 Lysogenic induction. Cold Spring Harbor, N.Y. Cold Spring Harbor Laboratory; 123 144 In: Lambda II.
9. CorneliusCA
QueneeLE
ElliD
CilettiNA
SchneewindO
2009 Yersinia pestis IS1541 transposition provides for escape from plague immunity. Infect Immun 77(5) 1807 1816
10. EichenbaumZ
LivnehZ
1998 UV light induces IS10 transposition in Escherichia coli. Genetics 149(3) 1173 1181
11. WeinreichMD
MakrisJC
ReznikoffWS
1991 Induction of the SOS response in Escherichia coli inhibits Tn5 and IS50 transposition. J Bacteriol 173 6910 6918
12. KuanCT
LiuSK
TessmanI
1991 Excision and transposition of Tn5 as an SOS activity in Escherichia coli. Genetics 128 45 57
13. ShiQ
ParksAR
PotterBD
SafirIJ
LuoY
ForsterBM
PetersJE
2008 DNA damage differentially activates regional chromosomal loci for Tn7 transposition in Escherichia coli. Genetics 179(3) 1237 1250
14. TwissE
CorosAM
TavakoliNP
DerbyshireKM
2005 Transposition is modulated by a diverse set of host factors in Escherichia coli and is stimulated by nutritional stress. Mol Microbiol 57(6) 1593 1607
15. NarumiI
2003 Unlocking radiation resistance mechanisms: still a long way to go. Trends Microbiol 11(9) 422 425
16. CoxMM
BattistaJR
2005 Deinococcus radiodurans - the consummate survivor. Nat Rev Microbiol 3(11) 882 892
17. BlasiusM
SommerS
HubscherU
2008 Deinococcus radiodurans: what belongs to the survival kit? Crit Rev Biochem Mol Biol 43(3) 221 238
18. ZahradkaK
SladeD
BailoneA
SommerS
AverbeckD
PetranovicM
LindnerAB
RadmanM
2006 Reassembly of shattered chromosomes in Deinococcus radiodurans. Nature 443(7111) 569 573
19. MennecierS
ServantP
CosteG
BailoneA
SommerS
2006 Mutagenesis via IS transposition in Deinococcus radiodurans. Mol Microbiol 59(1) 317 325
20. IslamSM
HuaY
OhbaH
SatohK
KikuchiM
YanagisawaT
NarumiI
2003 Characterization and distribution of IS8301 in the radioresistant bacterium Deinococcus radiodurans. Genes Genet Syst 78(5) 319 327
21. MakarovaKS
AravindL
WolfYI
TatusovRL
MintonKW
KooninEV
DalyMJ
2001 Genome of the extremely radiation-resistant bacterium Deinococcus radiodurans viewed from the perspective of comparative genomics. Microbiol Mol Biol Rev 65(1) 44 79
22. GuynetC
HickmanAB
BarabasO
DydaF
ChandlerM
Ton-HoangB
2008 In vitro reconstitution of a single-stranded transposition mechanism of IS608. Mol Cell 29(3) 302 312
23. Ton-HoangB
GuynetC
RonningDR
Cointin-MartyB
DydaF
ChandlerM
2005 Transposition of ISHp608, member of an unusual family of bacterial insertion sequences. Embo J 24(18) 3325 3338
24. GayP
Le CoqD
SteinmetzM
BerkelmanT
KadoCI
1985 Positive selection procedure for entrapment of insertion sequence elements in gram-negative bacteria. J Bacteriol 164(2) 918 921
25. JagerW
SchaferA
KalinowskiJ
PuhlerA
1995 Isolation of insertion elements from gram-positive Brevibacterium, Corynebacterium and Rhodococcus strains using the Bacillus subtilis sacB gene as a positive selection marker. FEMS Microbiol Lett 126(1) 1 6
26. RonningDR
GuynetC
Ton-HoangB
PerezZN
GhirlandoR
ChandlerM
DydaF
2005 Active site sharing and subterminal hairpin recognition in a new class of DNA transposases. Mol Cell 20(1) 143 154
27. SladeD
LindnerAB
PaulG
RadmanM
2009 Recombination and replication in DNA repair of heavily irradiated Deinococcus radiodurans. Cell 136(6) 1044 1055
28. KuanCT
TessmanI
1991 LexA protein of Escherichia coli represses expression of the Tn5 transposase gene. J Bacteriol 173 6406 6410
29. KuanCT
TessmanI
1992 Further evidence that transposition of Tn5 in Escherichia coli is strongly enhanced by constitutively activated RecA proteins. J Bacteriol 174 6872 6877
30. ChowKC
TungWL
2000 Magnetic field exposure stimulates transposition through the induction of DnaK/J synthesis. Biochem Biophys Res Commun 270(3) 745 748
31. Del ReB
BersaniF
AgostiniC
MesircaP
GiorgiG
2004 Various effects on transposition activity and survival of Escherichia coli cells due to different ELF-MF signals. Radiation and Environmental Biophysics 43(4) 265 270
32. Del ReB
GaroiaF
MesircaP
AgostiniC
BersaniF
GiorgiG
2003 Extremely low frequency magnetic fields affect transposition activity in Escherichia coli. Radiation and Environmental Biophysics 42(2) 113 118
33. OhtsuboH
NymanK
DoroszkiewiczW
OhtsuboE
1981 Multiple copies of iso-insertion sequences of IS1 in Shigella dysenteriae chromosome. Nature 292(5824) 640 643
34. PrestonA
ParkhillJ
MaskellDJ
2004 The Bordetellae: lessons from genomics. 2(5) 379 390
35. ParkhillJ
WrenBW
ThomsonNR
TitballRW
HoldenMT
PrenticeMB
SebaihiaM
JamesKD
ChurcherC
MungallKL
2001 Genome sequence of Yersinia pestis, the causative agent of plague. Nature 413(6855) 523 527
36. ChainPS
CarnielE
LarimerFW
LamerdinJ
StoutlandPO
RegalaWM
GeorgescuAM
VergezLM
LandML
MotinVL
2004 Insights into the evolution of Yersinia pestis through whole-genome comparison with Yersinia pseudotuberculosis. Proc Natl Acad Sci U S A x, 101(38) 13826 13831
37. CallananM
KaletaP
O'CallaghanJ
O'SullivanO
JordanK
McAuliffeO
Sangrador-VegasA
SlatteryL
FitzgeraldGF
BeresfordT
2008 Genome Sequence of Lactobacillus helveticus, an Organism Distinguished by Selective Gene Loss and Insertion Sequence Element Expansion 10.1128/JB.01295-07. J Bacteriol 190(2) 727 735
38. FileeJ
SiguierP
ChandlerM
2007 Insertion sequence diversity in archaea. Microbiol Mol Biol Rev 71(1) 121 157
39. Jean-Pierre Claverys
Bernard Martin
Patrice Polard
2009 The genetic transformation machinery: composition, localization, and mechanism. FEMS Microbiology Reviews 33(3) 643 656
40. MeimaR
RothfussHM
GewinL
LidstromME
2001 Promoter cloning in the radioresistant bacterium Deinococcus radiodurans. J Bacteriol 183(10) 3169 3175
41. Bonacossa de AlmeidaC
CosteG
SommerS
BailoneA
2002 Quantification of RecA protein in Deinococcus radiodurans reveals involvement of RecA, but not LexA, in its regulation. Mol Genet Genomics 268(1) 28 41
42. MattimoreV
BattistaJR
1996 Radioresistance of Deinococcus radiodurans: functions necessary to survive ionizing radiation are also necessary to survive prolonged desiccation. J Bacteriol 178(3) 633 637
43. FabretC
EhrlichSD
NoirotP
2002 A new mutation delivery system for genome-scale approaches in Bacillus subtilis. Mol Microbiol 46(1) 25 36
44. MennecierS
CosteG
ServantP
BailoneA
SommerS
2004 Mismatch repair ensures fidelity of replication and recombination in the radioresistant organism Deinococcus radiodurans. Mol Genet Genomics 272(4) 460 469
45. LecointeF
CosteG
SommerS
BailoneA
2004 Vectors for regulated gene expression in the radioresistant bacterium Deinococcus radiodurans. Gene 336(1) 25 35
Štítky
Genetika Reprodukčná medicína
Článek Activation of Mutant Enzyme Function by Proteasome Inhibitors and Treatments that Induce Hsp70Článek Maternal Ethanol Consumption Alters the Epigenotype and the Phenotype of Offspring in a Mouse ModelČlánek Genetic Dissection of Differential Signaling Threshold Requirements for the Wnt/β-Catenin PathwayČlánek Distinct Type of Transmission Barrier Revealed by Study of Multiple Prion Determinants of Rnq1
Článok vyšiel v časopisePLOS Genetics
Najčítanejšie tento týždeň
2010 Číslo 1- Gynekologové a odborníci na reprodukční medicínu se sejdou na prvním virtuálním summitu
- Je „freeze-all“ pro všechny? Odborníci na fertilitu diskutovali na virtuálním summitu
-
Všetky články tohto čísla
- Irradiation-Induced Genome Fragmentation Triggers Transposition of a Single Resident Insertion Sequence
- A Major Role of the RecFOR Pathway in DNA Double-Strand-Break Repair through ESDSA in
- Kidney Development in the Absence of and Requires
- Modeling of Environmental Effects in Genome-Wide Association Studies Identifies and as Novel Loci Influencing Serum Cholesterol Levels
- Inverse Correlation between Promoter Strength and Excision Activity in Class 1 Integrons
- Activation of Mutant Enzyme Function by Proteasome Inhibitors and Treatments that Induce Hsp70
- Postnatal Survival of Mice with Maternal Duplication of Distal Chromosome 7 Induced by a / Imprinting Control Region Lacking Insulator Function
- The Werner Syndrome Protein Functions Upstream of ATR and ATM in Response to DNA Replication Inhibition and Double-Strand DNA Breaks
- Maternal Ethanol Consumption Alters the Epigenotype and the Phenotype of Offspring in a Mouse Model
- Understanding Gene Sequence Variation in the Context of Transcription Regulation in Yeast
- miR-30 Regulates Mitochondrial Fission through Targeting p53 and the Dynamin-Related Protein-1 Pathway
- Elevated Levels of the Polo Kinase Cdc5 Override the Mec1/ATR Checkpoint in Budding Yeast by Acting at Different Steps of the Signaling Pathway
- Alternative Epigenetic Chromatin States of Polycomb Target Genes
- Co-Orientation of Replication and Transcription Preserves Genome Integrity
- A Comprehensive Map of Insulator Elements for the Genome
- Environmental and Genetic Determinants of Colony Morphology in Yeast
- U87MG Decoded: The Genomic Sequence of a Cytogenetically Aberrant Human Cancer Cell Line
- The MCM-Binding Protein ETG1 Aids Sister Chromatid Cohesion Required for Postreplicative Homologous Recombination Repair
- Genetic Dissection of Differential Signaling Threshold Requirements for the Wnt/β-Catenin Pathway
- Differential Localization and Independent Acquisition of the H3K9me2 and H3K9me3 Chromatin Modifications in the Adult Germ Line
- Genetic Crossovers Are Predicted Accurately by the Computed Human Recombination Map
- Collaborative Action of Brca1 and CtIP in Elimination of Covalent Modifications from Double-Strand Breaks to Facilitate Subsequent Break Repair
- Distinct Type of Transmission Barrier Revealed by Study of Multiple Prion Determinants of Rnq1
- Genome-Wide Association Study Identifies as a Novel Susceptibility Gene for Osteoporosis
- and Regulate Reproductive Habit in Rice
- Nonsense-Mediated Decay Enables Intron Gain in
- Altered Gene Expression and DNA Damage in Peripheral Blood Cells from Friedreich's Ataxia Patients: Cellular Model of Pathology
- The Systemic Imprint of Growth and Its Uses in Ecological (Meta)Genomics
- The Gift of Observation: An Interview with Mary Lyon
- Genotype and Gene Expression Associations with Immune Function in
- The Elongator Complex Regulates Neuronal α-tubulin Acetylation
- Rising from the Ashes: DNA Repair in
- Mis-Spliced Transcripts of Nicotinic Acetylcholine Receptor α6 Are Associated with Field Evolved Spinosad Resistance in (L.)
- BRIT1/MCPH1 Is Essential for Mitotic and Meiotic Recombination DNA Repair and Maintaining Genomic Stability in Mice
- Non-Coding Changes Cause Sex-Specific Wing Size Differences between Closely Related Species of
- Evidence for Pervasive Adaptive Protein Evolution in Wild Mice
- Evolutionary Mirages: Selection on Binding Site Composition Creates the Illusion of Conserved Grammars in Enhancers
- VEZF1 Elements Mediate Protection from DNA Methylation
- PLOS Genetics
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- A Major Role of the RecFOR Pathway in DNA Double-Strand-Break Repair through ESDSA in
- Kidney Development in the Absence of and Requires
- The Werner Syndrome Protein Functions Upstream of ATR and ATM in Response to DNA Replication Inhibition and Double-Strand DNA Breaks
- Alternative Epigenetic Chromatin States of Polycomb Target Genes
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



