-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
Collapse of Telomere Homeostasis in Hematopoietic Cells Caused by Heterozygous Mutations in Telomerase Genes
Telomerase activity is readily detectable in extracts from human hematopoietic stem and progenitor cells, but appears unable to maintain telomere length with proliferation in vitro and with age in vivo. We performed a detailed study of the telomere length by flow FISH analysis in leukocytes from 835 healthy individuals and 60 individuals with reduced telomerase activity. Healthy individuals showed a broad range in average telomere length in granulocytes and lymphocytes at any given age. The average telomere length declined with age at a rate that differed between age-specific breakpoints and between cell types. Gender differences between leukocyte telomere lengths were observed for all cell subsets studied; interestingly, this trend could already be detected at birth. Heterozygous carriers for mutations in either the telomerase reverse transcriptase (hTERT) or the telomerase RNA template (hTERC) gene displayed striking and comparable telomere length deficits. Further, non-carrier relatives of such heterozygous individuals had somewhat shorter leukocyte telomere lengths than expected; this difference was most profound for granulocytes. Failure to maintain telomere homeostasis as a result of partial telomerase deficiency is thought to trigger cell senescence or cell death, eventually causing tissue failure syndromes. Our data are consistent with these statements and suggest that the likelihood of similar processes occurring in normal individuals increases with age. Our work highlights the essential role of telomerase in the hematopoietic system and supports the notion that telomerase levels in hematopoietic cells, while limiting and unable to prevent overall telomere shortening, are nevertheless crucial to maintain telomere homeostasis with age.
Published in the journal: Collapse of Telomere Homeostasis in Hematopoietic Cells Caused by Heterozygous Mutations in Telomerase Genes. PLoS Genet 8(5): e32767. doi:10.1371/journal.pgen.1002696
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1002696Summary
Telomerase activity is readily detectable in extracts from human hematopoietic stem and progenitor cells, but appears unable to maintain telomere length with proliferation in vitro and with age in vivo. We performed a detailed study of the telomere length by flow FISH analysis in leukocytes from 835 healthy individuals and 60 individuals with reduced telomerase activity. Healthy individuals showed a broad range in average telomere length in granulocytes and lymphocytes at any given age. The average telomere length declined with age at a rate that differed between age-specific breakpoints and between cell types. Gender differences between leukocyte telomere lengths were observed for all cell subsets studied; interestingly, this trend could already be detected at birth. Heterozygous carriers for mutations in either the telomerase reverse transcriptase (hTERT) or the telomerase RNA template (hTERC) gene displayed striking and comparable telomere length deficits. Further, non-carrier relatives of such heterozygous individuals had somewhat shorter leukocyte telomere lengths than expected; this difference was most profound for granulocytes. Failure to maintain telomere homeostasis as a result of partial telomerase deficiency is thought to trigger cell senescence or cell death, eventually causing tissue failure syndromes. Our data are consistent with these statements and suggest that the likelihood of similar processes occurring in normal individuals increases with age. Our work highlights the essential role of telomerase in the hematopoietic system and supports the notion that telomerase levels in hematopoietic cells, while limiting and unable to prevent overall telomere shortening, are nevertheless crucial to maintain telomere homeostasis with age.
Introduction
At least a few hundred nucleotides of telomere repeats must “cap” each chromosome end in order to suppress DNA damage signals and avoid the activation of DNA repair pathways [1]–[3]. Critically short or “uncapped” telomeres may be repaired by the enzyme telomerase [4] or by recombination [5]. However, the capacity of these telomere repair processes appears limited in most human somatic cells [6]. Apoptosis or cellular senescence is triggered when too many “uncapped” telomeres accumulate [7], posing a barrier to tumor growth, but also contributing to loss of cells with age [8].
Despite increasing evidence that telomere homeostasis is important in human aging, cancer and disease states, detailed and comparative information regarding the telomere length in different human cell subtypes of healthy individuals in relation to their age is surprisingly modest. Apart from being technically challenging [9] such studies are complicated because at birth and throughout life, telomere length is highly variable between chromosomes [10], [11], between cells [12], [13] and between individuals. Studies of identical twins have shown that individual differences in average telomere length appear to be largely genetically determined [14], [15].
In most somatic cells the telomere length declines with age and with cell division in culture, albeit at different rates [13], [16]. For example, in humans and baboons, lymphocytes show a more pronounced telomere loss with age than granulocytes [14], [17]. These two cell types represent the two major branches of the hematopoietic system, which can be further subdivided into distinct cell populations based on their phenotype and function. Within the hematopoietic hierarchy, the most primitive cells, hematopoietic stem cells (HSC), have the longest telomeres [18], [19]. HSC differentiate to produce progenitor cells of both the myeloid and lymphoid lineage that proliferate prior to differentiation into mature “end” cells. Unlike most immune cells most differentiated myeloid cells such as granulocytes are incapable of further cell divisions.
The precise role of telomerase in hematopoietic stem and progenitor cells and in lymphocytes remains poorly understood. Telomerase expression is readily detected in hematopoietic cells [20]–[22]; however, this activity appears unable to prevent telomere loss with age or proliferation. It is often assumed that telomerase is required to maintain the telomere length in various stem cells. With the exception of embryonic stem cells and abnormal tumor (stem) cells this assumption is not supported by data. Studies on the role of telomeres and telomerase in HSC from healthy individuals are challenging because HSC are very rare cells that typically reside in bone marrow. In contrast, the various nucleated blood cells that are derived from HSC are easily accessible for study. The average telomere length in granulocytes can be used as a surrogate marker for the telomere length in HSC [23], if one assumes that the number of cell divisions between HSC and granulocytes is relatively constant [18]. Individual carriers of heterozygous mutations for either the telomerase RNA gene (hTERC) or the telomerase reverse transcriptase gene (hTERT) can present with a wide spectrum of diseases [24] including dyskeratosis congenita [25], [26], bone marrow failure [24] and pulmonary fibrosis [27]. Heritable telomerase deficiencies provide an excellent model to study the role of telomerase in human hematopoietic cells.
Here we report our data on the median telomere length (MTL) in five distinct leukocyte subpopulations of over 800 healthy individuals between birth and 100 years of age as well as 60 individuals that are heterozygous for one of the telomerase genes, hTERC or hTERT. The telomere length in leukocytes from healthy individuals was found to vary over a broad range at any given age and the rate of telomere attrition also varied with age and with cell type. Strikingly, the telomeres in cells from individuals with telomerase deficiency were found to be very short in all cell types, and this deficit was found to be comparable for most cell subtypes for hTERC or hTERT deficiency. The largest (age adjusted) differences in telomere length deficits between hTERC or hTERT were seen in “naïve” T cells for hTERC deficient individuals and in NK/differentiated T cells for hTERT deficient individuals. These results demonstrate that normal telomerase levels are essential to maintain normal telomere homeostasis in HSC and lymphocytes. Our results provide valuable reference data for further studies of telomere biology in health and disease and point to a crucial rate-limiting role for telomerase in HSC and immune cells.
Results
Lymphocyte and granulocyte telomere length dynamics with age
We measured the telomere length in lymphocytes and granulocytes of 835 healthy individuals using automated multicolor flow FISH (Figure 1). On average, 7 to 8 individuals were tested for each age-year. Various best-fit models were tested to model the overall decline in telomere length with age. In view of the very rapid decline in telomere length in the first years of life in humans [14] as well as non-human primates [28], we divided the telomere length decline over three age segments. The first is between birth and one year of age when the growth rate of bones and weight in infants shows a marked deceleration (for reference curves, see http://www.cdc.gov/growthcharts/clinical_charts.htm). A second arbitrary cut-off was set at 18 years of age because the decline in telomere length in all leukocytes appeared to drop notably after puberty. Telomere length data within the three selected age segments: below 1 year (yr), 1–18 yrs and 19 yr and higher are shown in Figure 1 and Table 1. The overall age-related telomere length decline was most pronounced in lymphocytes with significant losses ranging from 1190 base pairs (bp) per year between birth and 1 year of age to 126 bp per year during childhood and 43 bp per year in adulthood. In contrast, the age-related telomere length decline in granulocytes and by extension in HSC was more modest during early life (485 bp per year), childhood (74 bp per year) and adulthood (28 bp per year).
Fig. 1. Decline in telomere length with age differs between lymphocytes and granulocytes. 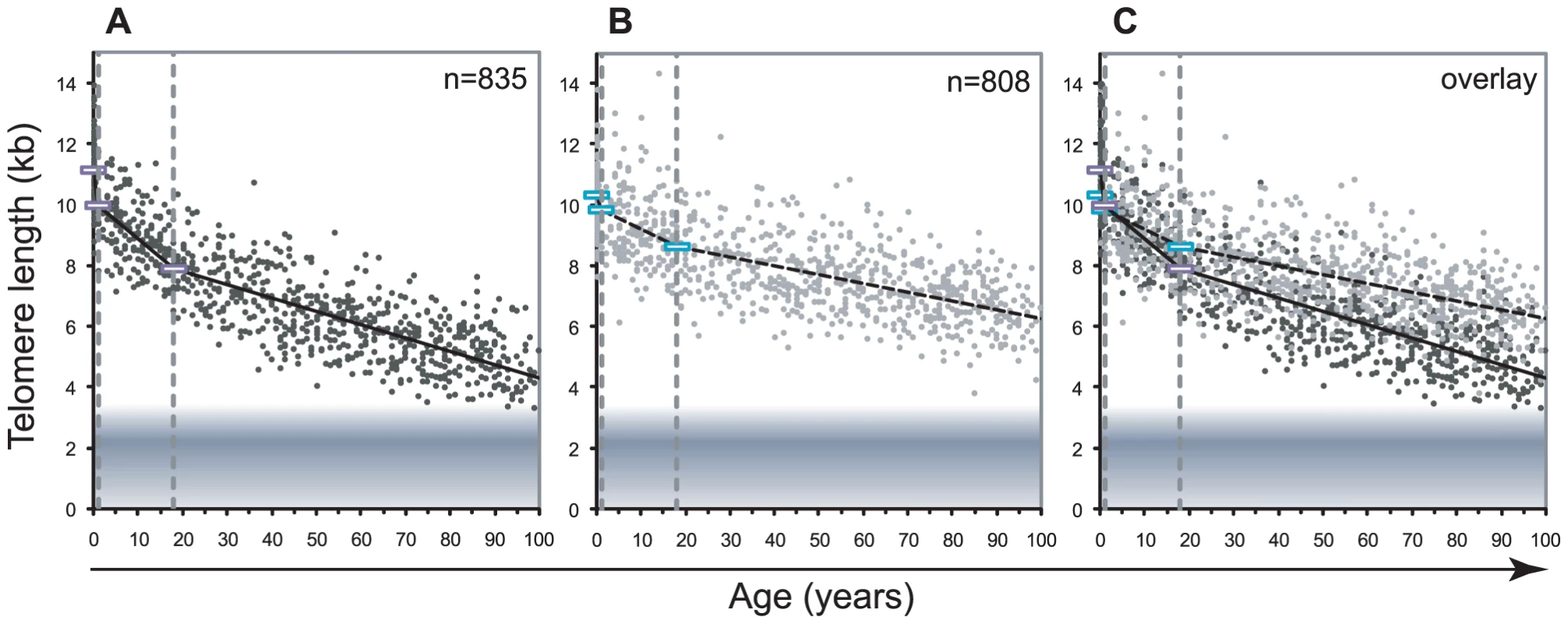
The median telomere length in nucleated blood cells from 835 healthy individuals ranging from birth (umbilical cord blood) to 102 years of age were measured by flow FISH. The results were used to calculate the telomere attrition over time using linear regression in three age segments. A. Median telomere length in lymphocytes (black dots). B. Median telomere length in granulocytes (grey dots). Breakpoints in the piece-wise linear regression lines are marked by rectangles and the three age groups are marked by dotted vertical grey lines at 1 and 18 years. On average 8 individuals were tested per age-year. C. At any given age, a wide range of telomere length was observed and the decline in telomere length with age in lymphocytes was more pronounced than in granulocytes. The shaded area represents the estimated length of subtelomeric DNA. Note that in older individuals, on average only 1–2 kb of telomere repeats were present in lymphocytes. Tab. 1. Telomere length distribution and age-related telomere length decline in healthy individuals. 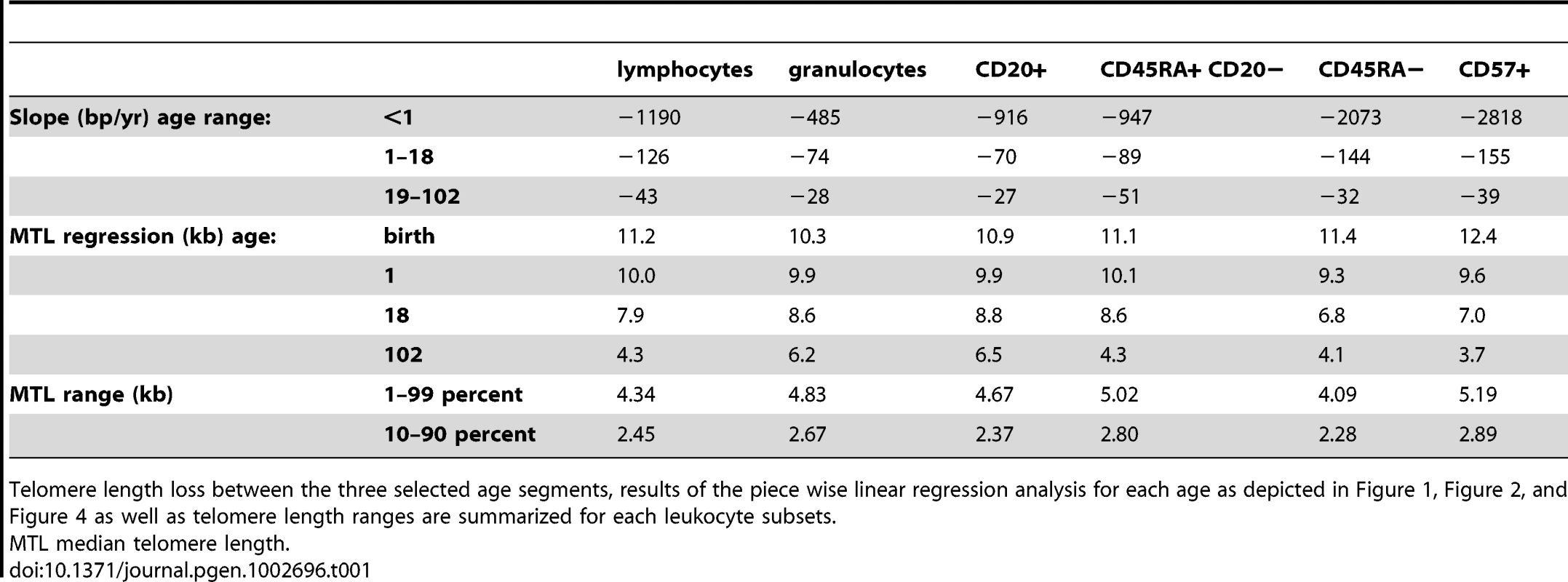
Telomere length loss between the three selected age segments, results of the piece wise linear regression analysis for each age as depicted in Figure 1, Figure 2, and Figure 4 as well as telomere length ranges are summarized for each leukocyte subsets. The decline in telomere length with age varies between leukocyte subpopulations
Telomere length measurements versus age in granulocytes and lymphocyte subpopulations were used to determine the regression lines for telomere attrition in the three selected age ranges (regression estimates shown in Figure 2A; the complete data set can be accessed in Table S1). These regression lines were shifted according to data distribution (from the overall regression estimate) to represent the 99th, 90th, 10th and 1st percentile of the telomere length distribution in each age segment for each blood cell subset in healthy individuals. The rate of telomere length decline varied amongst the different lymphocyte subsets analyzed. The telomere length decline with age in B lymphocyte subset (CD45RA+ CD20+) was comparable to that in granulocytes. Memory T (CD45RA−CD20−) and mature NK/T (CD45RA+CD57+) lymphocyte subsets showed the sharpest decline in telomere length with age, particularly during childhood with slopes of −144 and −155 bp per year respectively. The CD45RA+CD20 − T lymphocyte subset enriched for “naïve” T cells and the CD45RA+CD57+ mature NK/T lymphocyte subset displayed the widest distributions, 2.80 and 2.89 kilobase (kb) respectively between the 10th and 90th percentile of the normal distribution, throughout the age ranges. Unlike other subsets CD45RA+CD20 − T lymphocytes showed only a modest difference in the telomere attrition rate between childhood and adulthood: 89 and 51 bp per year respectively. In contrast, the memory T lymphocyte subset (CD45RA−CD20−) displayed the narrowest range of telomere length distribution (2.28 kb between the 10th and 90th percentile of the normal distribution). Overall, the shortest telomere lengths were measured in memory T and mature NK/T lymphocytes from older individuals.
Fig. 2. Cell type–specific differences in the range and attrition rate of leukocyte telomere length. 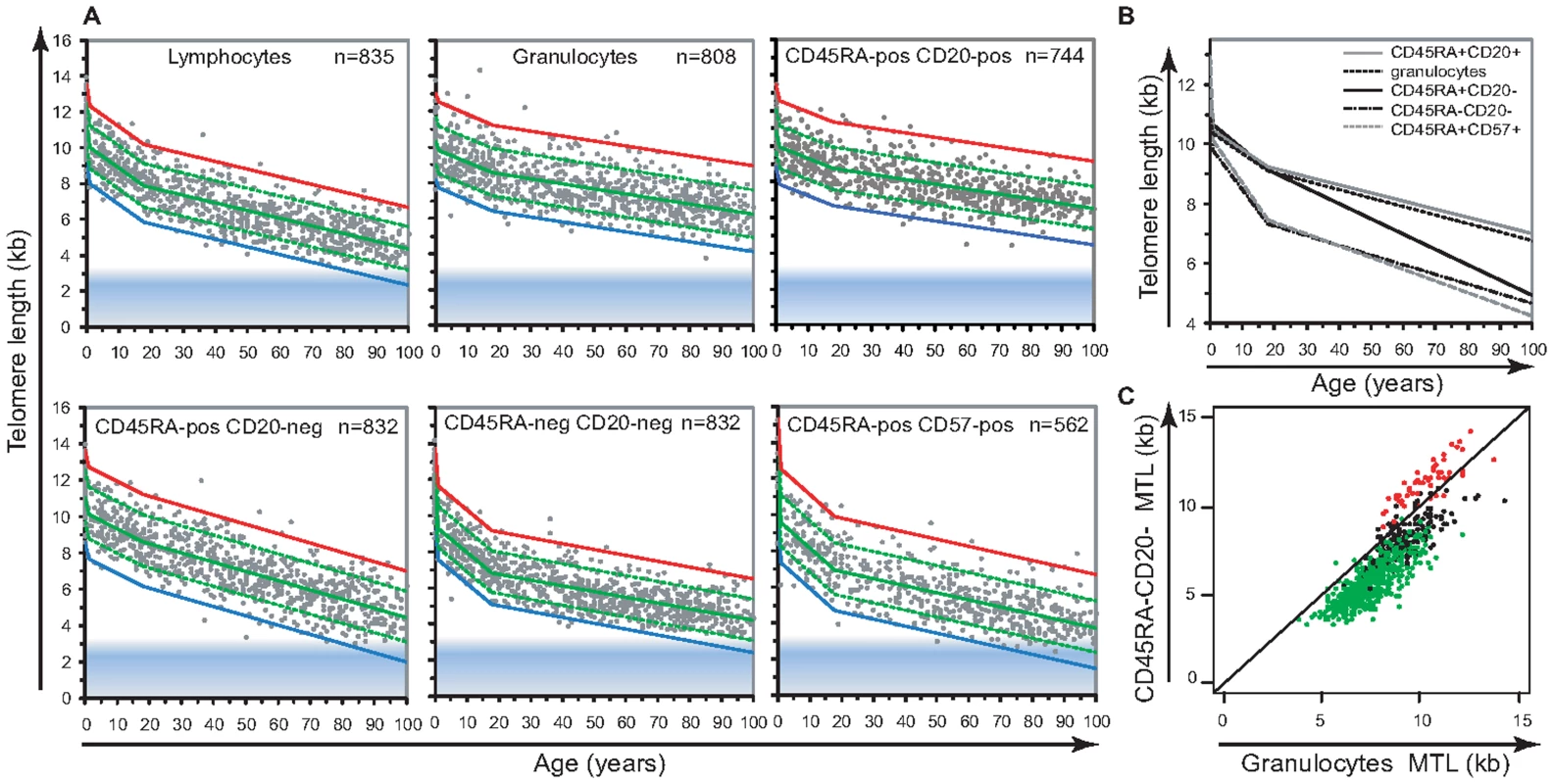
A. Cross-sectional median telomere length in nucleated cell types determined by flow FISH in 835 healthy individuals over the age range from birth to 102 years of age. The following nucleated blood cell subtypes were analyzed : lymphocytes, granulocytes, CD45RA positive CD20 positive B lymphocytes (CD20+), CD45RA positive CD20 negative lymphocytes (CD45RA+pos CD20−) “naïve” T cells, CD45RA negative CD20 negative lymphocytes (CD45RA−) memory T cells and CD45RA positive CD57 positive mature NK/T cells (CD57+). Data were analyzed using a piece-wise linear regression model in the age categories 0, 1 yr; 18 yrs and 102 years and for calculation and representation of the telomere length distribution range at any given age (expressed as a percentile): 99th (red), 90th (dashed green top), 50th (green), 10th (dashed green bottom) and 1st (blue). Some of the healthy subjects (n = 835) did not have sufficient cells for analysis of one or more of the cell subsets. B. Piece-wise linear regression analysis overlay representing the modeled estimate of telomere length per age for: B lymphocytes (grey), granulocytes (black dashed), “naïve” T lymphocytes (black), memory T lymphocytes (black interrupted dashed) and mature NK/T lymphocytes (grey dashed). Regression breakpoints were set at age 1 and age 18 years. C. Paired comparison of telomere length in granulocytes and memory T lymphocytes from the same individuals. Age groups were as follows: cord blood samples and below age 1 (n = 60, red), age 1 to 18 (n = 171, black), 19 and above 19 (n = 604, green). Comparisons of telomere length in different leukocyte subsets
From our cross-sectional data, we determined the average telomere length decline with age for the different leukocyte subpopulations (Figure 2B). During childhood, granulocytes, CD45RA+CD20 − “naïve” T lymphocytes and CD20+ B lymphocytes all showed a very similar decline in telomere length, whereas the rate of decline in memory T cells was much higher. Paired MTL values in different blood cell subsets from the same individual revealed that around one year of age the telomere length values in memory T lymphocytes drop below those of granulocytes (Figure 2C). In contrast, telomere length values in B lymphocytes remained comparable to those in granulocytes over the entire age range. One caveat in our measurement of telomere length in “naïve” (CD45RA+CD20−) T lymphocytes is that terminally differentiated effector lymphocytes re-expressing CD45RA are likely to represent an increased proportion within this cell population in older individuals. As a consequence, measurements within the subset of “naïve” T cells are variably skewed in older individuals (as illustrated by the direct comparison of MTL between “naïve” T lymphocytes and other cell subtypes from the same individual over 4 distinct age groups in Figure S2A and Table S2).
Gender differences in leukocyte telomere length measurements
Measurements from cord blood samples provided the earliest opportunity to assess the telomere lengths in cells from healthy individuals. Interestingly, of all the cell types measured from cord blood, granulocytes showed the shortest telomeres at birth: differences were significant for granulocytes versus CD45−CD20 − memory T lymphocytes and CD45+CD20 − “naïve” T lymphocytes but not for granulocytes versus B lymphocytes. Comparisons were tested by one-way ANOVA (n = 58): F(5,265) = 5.7; P = 0.0002, Table S3, followed by Tukey's multiple comparison test, see details in Table S4). Interestingly, female newborns appeared to have longer telomeres than males (Figure 3A); however this trend did not reach statistical significance. Further comparisons of telomere length in leukocyte subsets as a function of gender showed highly significant differences between males and females in the CD45RA+CD20 − “naïve” T lymphocyte subset over the entire age range (F(4,825) = 9.05; P = 3.7×10−7, ANOVA test result comparing regression fits; Figure 3B and Table S5). Significant differences were also seen for other leukocyte subsets in each age segment with the exception of granulocytes and memory T lymphocytes, which displayed similar, average telomere lengths after 18 years of age (Figure S3 and Table S5).
Fig. 3. Gender differences in leukocyte telomere length. 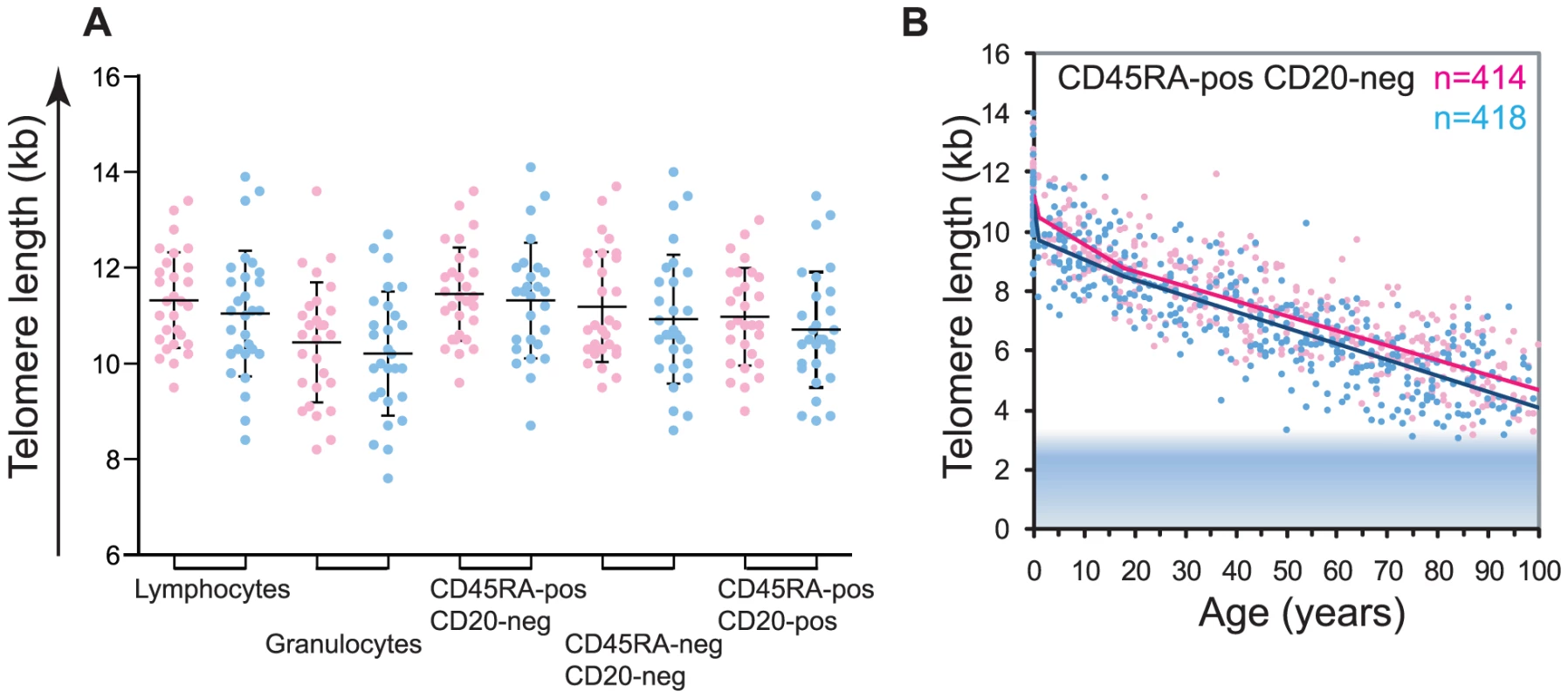
A. Telomere length measurements in leukocyte subsets from female (pink) versus male (blue) healthy newborns (n = 58; females n = 29, males n = 29). The following white blood cell subsets were tested lymphocytes, granulocytes, “naïve” T lymphocytes (CD45RA+CD20−), memory T lymphocytes (CD45RA−CD20−) and B lymphocytes (CD20+). Each dot represents an individual sample, mean (horizontal bar) and standard deviation (vertical bar) for each cell type are shown. All subsets display a trend for longer average telomere lengths in females; however this trend does not reach statistical significance (Student's t test, data not shown). B. Piece-wise linear regression analysis representing the calculated telomere length per age for females (pink) versus males (blue) “naïve” T lymphocytes (CD45RA+CD20−). Breakpoints were at 1 year of age and at 18 years. Analysis of variance for females versus males (statistical model in R) showed significance (F(4,825) = 9.05; P = 3.7×10−7, ANOVA test result comparing regression fits). For data on other subsets and details of statistical analysis, see Figure S3 and Table S5. Telomerase is essential to maintain leukocyte telomere length homeostasis
To study the role of telomerase in hematopoietic cells, we analyzed the telomere length in leukocyte subpopulations of individuals carrying a mutation in either hTERT or hTERC (n = 60) in comparison to non-carrier relatives (n = 37). The results, plotted on the telomere length versus age distribution curves derived from healthy individuals (Figure 2A) are shown in Figure 4 and Table 1. Strikingly, telomerase heterozygous individuals showed very short telomeres (typically below the 1st percentile of the normal distribution) at all ages and for all blood cell subsets tested (ANOVA test P<2.2×10−16, for full details of analyses see Table S5). The shortest telomeres were measured in mature NK/T cells (mean of 3.7±0.7 kb for all telomerase heterozygous individuals not adjusted for age, Figure 4A) and the CD45RA+CD20 − “naive” lymphocyte subset appeared the most severely impacted by telomerase deficiency with an average difference to the normal distribution (adjusted for age) of Δtel: 3.2 kb. Differential analysis of leukocyte telomere lengths in leukocytes from hTERT vs. hTERC heterozygous individuals showed a similar effect on most blood cell subtypes (Figure 4B and Table 2). Exceptions were an increased telomere loss in the CD45RA+CD57+ mature NK/T cells (difference of 0.4 Kb) of hTERT deficient individuals (n = 37) and a slightly increased effect (difference of 0.2 Kb) on the CD45RA+CD20 − “naive” lymphocyte subset for hTERC deficient individuals (n = 23).
Fig. 4. Telomerase is essential to maintain telomere length in leukocytes. 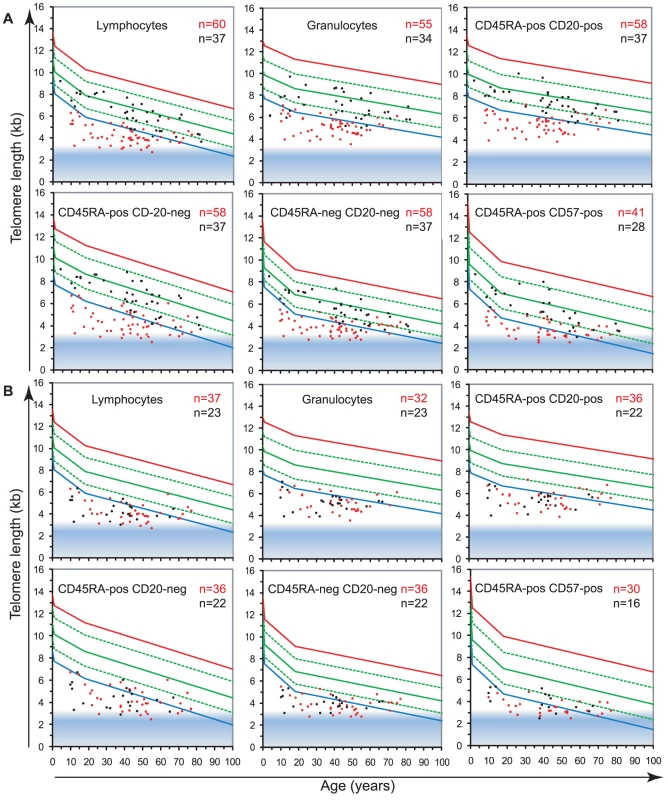
The telomere length distribution in healthy individuals (Figure 2) was used to plot the median telomere length values of leukocyte subsets obtained with: A. leukocytes from 60 telomerase deficient patients (red) and 37 non-carrier relatives (black). B. leukocytes from 37 hTERT mutation heterozygous individuals (red) and 23 hTERC mutation heterozygous carriers (black). Tab. 2. Telomere loss corrected for age (Δtel) in telomerase heterozygous individuals and unaffected relatives. 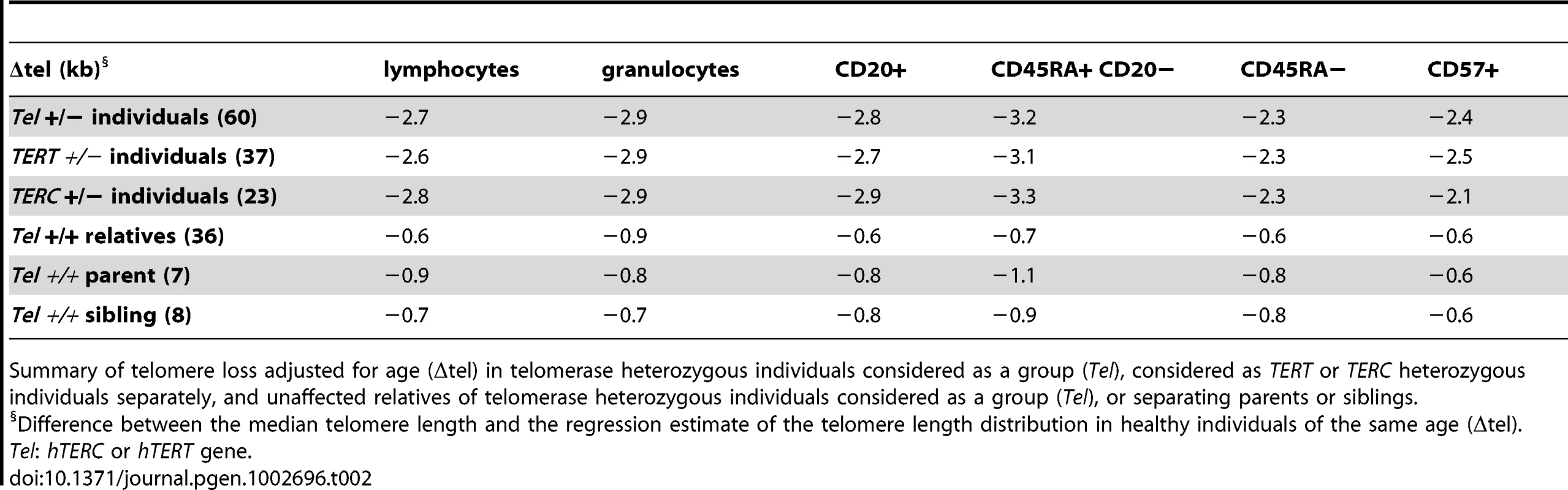
Summary of telomere loss adjusted for age (Δtel) in telomerase heterozygous individuals considered as a group (Tel), considered as TERT or TERC heterozygous individuals separately, and unaffected relatives of telomerase heterozygous individuals considered as a group (Tel), or separating parents or siblings. Non-carrier relatives of telomerase deficient individuals (hTERT and hTERC considered together), despite having intact telomerase genes, also showed somewhat shorter median telomere lengths in all leukocytes compared to the control population. The largest difference was measured in the granulocyte subset of non-carrier relatives considered together, with Δtel: 0.9 kb, which may be indicative of HSC deficit and warrants further investigation (Figure 4A; ANOVA test: F(3,837) = 8.1; P = 2.63×10−5, for full details of analyses see Table S5). Both parents and siblings of heterozygous individuals were found to have slightly shorter telomere lengths for age (Table 2 and Figure S4). Differential analysis of parents (n = 6) and siblings (n = 4) of hTERT deficient individuals was also performed and showed comparable telomere length deficits for all cell subsets tested (Figure S4 and data not shown) in this relatively small group.
Discussion
In this report, we show telomere length data for five distinct leukocyte subpopulations from over 800 healthy individuals, representing a comprehensive and representative cross-sectional analysis of telomere length in leukocyte subpopulations over the entire human life span. The value of this data is illustrated by our analysis of individuals with heritable telomerase deficiencies. Leukocyte telomere length was found to clearly distinguish between relatives with and without mutations in hTERT or hTERC supporting telomere length measurements as a screen for mutations in “telomere maintenance” genes. Our results confirm and extend earlier reports of telomere loss in leukocytes with age [13], [14] and document a crucial role for telomerase in controlling leukocyte telomere length.
Telomerase expression is readily detected in most hematopoietic cells [20]–[22], yet this activity appears unable to prevent the overall loss of telomeric DNA with age or proliferation. Most likely, telomerase is primarily required to directly act on chromosome ends in hematopoietic cells themselves, however secondary, indirect effects of telomerase via cells that support cell proliferation [29] or possible effects of the TERT protein on transcription in stem cells [30] are difficult to exclude.
Heterozygosity for one of the telomerase genes, expected to reduce telomerase levels by half, results in a striking telomere deficit (Figure 4). How can this finding be explained? One possibility is that the primary function of telomerase in somatic cells is the repair [8] or protection [31] of critically short telomeres. Failure to properly “cap” all chromosome ends with telomere repeats results in activation of a DNA damage response [1], [32]. Detrimental consequences for HSCs and lymphocytes could result when DNA damage signals from uncapped telomeres persist or reach a certain threshold and cause apoptosis of such cells. Impaired “capping” of telomeres in cells with reduced telomerase could affect telomere length directly and indirectly. Direct effects on telomere length could result from normal replication of telomeric DNA [8] and damage caused by reactive oxygen species [33]–[35]. Indirect effect on telomere length would result from the additional cell division required to compensate for the increased cell losses. Compensatory cell divisions in cells from telomerase deficient individuals could be particularly taxing as more short telomeres are expected to emerge with each extra cell division. The resulting feed-forward loop could exhaust the stem cell compartment in infants and children explaining the marrow failure typically seen in pediatric telomerase deficient patients. In cases where sufficient stem cells survived till adulthood, the same unproductive feed-forward loop could exhaust cells of the immune system. This possibility is in line with the observation that after puberty telomere attrition in more mature subsets of T and NK cells is notably higher than in granulocytes as a surrogate marker for stem cells (Figure 2). We speculate that the balance between end cells such as granulocytes and macrophages on the one hand and various other immune cell types on the other is perturbed in older telomerase deficient patients. Such an imbalance could result in failure to clear pathogens and immunogens and create pro-fibrotic conditions or result in failure to remove senescent cells [36].
Apart from cell turnover and telomerase levels, the telomere length in parental chromosomes at fertilization is another probable variable in the disease manifestations of telomerase deficient patients. This variable will determine when critically short telomeres, requiring repair or capping by telomerase, will appear: during development or during adult life. This notion is in line with the age-related onset of symptoms or “anticipation” in multi-generation telomerase deficiency disorders [24], [37] and our observation that telomeres in cells from unaffected children and parents of telomerase heterozygous individuals are somewhat shorter than expected (Figure 4 and Table 2).
As was shown previously for human lymphocytes and granulocytes [14], [38] and confirmed in longitudinal studies of non-human primates [28], leukocyte telomere length shortens most dramatically very early in life. This rapid decline can be explained by steady proliferation of stem cells and immune cells after birth. After one year of age we observed a rapid deceleration in telomere loss most likely reflecting an intrinsic, ontogeny-related change in stem cell turnover and function [39], which has also been observed in postnatal mice [40], [41]. Our observations with human and primate cells suggest that each HSC cell division in these species is “counted” by the loss of telomeric DNA. Why a relative modest decline in telomerase activity in humans results in a wide spectrum of diseases whereas complete loss of telomerase is typically tolerated for several generations in yeast, plants, worms and mice remains incompletely understood [42].
The shortest overall telomere lengths were measured in the mature NK/T cell subsets of older healthy individuals and of hTERT telomerase-deficient individuals. These results suggest that one of the primary consequences of telomere attrition and telomerase deficiencies could be the loss of NK immune function. This notion is compatible with the reported age-related decline in the number and function of these cells [43]. Of note, in some individuals the estimated telomere length in mature NK/T cells was near the predicted minimal telomere length (represented as a shaded area in Figure 1 and Figure 2) meaning that on average each chromosome end in those cells has fewer than 1 kb of telomere repeats.
Despite the finding that at birth telomeres in lymphocytes are longer than in granulocytes and despite the selective expression of telomerase in cells of the lymphoid lineage upon activation [22], [44], T lymphocytes displayed a sharp decline in telomere length with age. The steady decline in the telomere length in T cells likely contributes to compromised adaptive immunity in the elderly [45] and in individuals with telomerase deficiencies [46]. Interestingly, the narrowest telomere length distribution in leukocyte subsets from healthy individuals was observed in the memory T cell compartment, pointing to a possible role of telomere length in shaping the T cell repertoire and immune memory.
Our study of gender specific differences in telomere length confirmed previous observations that telomere lengths on average appear to be somewhat longer in females than in males [47]. The fact that this trend is already seen at birth raises questions that warrant further investigation: do females have fewer HSC at birth? Do female HSC have a higher replicative potential because of longer telomeres? Do stem cells in females have higher telomerase activity, possibly influenced by levels of sex hormones [48] or do other factors explain the longer telomeres in female leukocytes?
Epidemiological studies have been conducted to examine the potential validity of using relative leukocyte telomere length as a disease or aging associated biomarker. Interest in this area has greatly increased following recent reports of associations of shorter leukocyte telomere lengths with morbidity (such as cardiovascular disease or diabetes reviewed in [49]) and in response to external factors such as chronic stress [50]. More data is needed to confirm these findings and establish whether shorter leukocyte telomere lengths are associated with overall increased mortality in older adults [51], [52] and whether the increased risk of infection such as pneumonia in elderly individuals differs significantly in relation to their telomere lengths.
In conclusion, the data presented here contribute valuable base-line information regarding the telomere length in subpopulations of leukocytes during normal human ageing. This information will be a useful reference in studies of a variety of health conditions. Our data show that suppression of half of telomerase levels over a lifetime can severely compromise the telomere homeostasis of granulocytes as a surrogate marker for HSCs, and of immune cells. This likely is a dominant factor in the serious impairment of cell function and proliferative capacity that has been documented in telomerase deficient individuals. It seems possible that more effective short-term inhibition of telomerase could compromise the function of hematopoietic cells more acutely. Most likely, limitations imposed by progressive telomere loss act as a tumor suppressor mechanism in long-lived animals [42]. If so, caution is also needed for strategies that aim to rejuvenate older cells by reactivation of telomerase. The telomere length data described in this paper provide reference data for therapeutic strategies that target telomerase and for further studies on the role of telomeres and telomerase in normal aging and a variety of pathological conditions.
Materials and Methods
Ethical statement
All subjects enrolled in this study in Vancouver signed informed consent forms that were approved by the University of British Columbia (BC) and BC Cancer Agency Research Ethics Board. All samples from patients outside Vancouver were obtained with informed consent and approval of local ethical review boards in accordance with the Declaration of Helsinki.
Healthy donors, telomerase-deficient individuals, and non-carrier relatives
Anonymous cord blood samples were obtained from healthy full term births with parental informed consent. Since no associate information was available for these samples, gender testing was performed by FISH as described below.
Anonymous peripheral blood samples were obtained from 835 healthy individuals between the ages of 6 months to 102 years of age screened for clotting disorders; samples where no clotting disorders were found were made available for study; only gender and age information were provided.
Samples from 60 individuals with confirmed telomerase deficiencies due to heterozygous mutations for either the telomerase reverse transcriptase (hTERT) or the RNA template (hTERC) gene and their 37 (non-carrier) relatives were included in our analysis and were described previously, (mean ages for both groups were 41 and 45 years respectively [25], [53]–[59]; all 97 participants or their guardians provided written informed consent in accordance with the Declaration of Helsinki.
Cord blood gender determination
X and Y chromosome specific FISH was preformed as previously described [60]. Briefly, nucleated cord blood cells were fixed with methanol–acetic acid then dropped onto slides. Slides were fixed with formaldehyde, treated with pepsin, and dehydrated with ethanol. The hybridization mix containing fluorescently labeled peptide nucleic acid (PNA) probes specific for centromere repeats of respectively the X chromosome and the long arm of the Y chromosome were added to the slides. Following denaturation of DNA at 80°C for 3 minutes slides were incubated at room temperature for 30 minutes, washed, counterstained with DAPI and mounted using DABCO anti-fading reagent (Sigma Aldrich). Images were acquired and analyzed as previously described [60].
Mutational analysis
hTERT and hTERC genotyping was performed as described previously [25], [53]–[59].
Telomere length measurements by flow FISH
Telomere length measurements using automated multicolor flow-fluorescence in situ hybridization (flow FISH) was performed as described [61]. Briefly, white blood cells (WBCs) were isolated by osmotic lysis of erythrocytes in whole blood using NH4Cl. The WBCs were then mixed with bovine thymocytes of known telomere length (which serve as an internal control), denatured in formamide at 87°C, and hybridized with a fluorescein-conjugated (CCCTAA)3 peptide nucleic acid (PNA) probe specific for telomere repeats and counterstained with LDS751 DNA dye. The fluorescence intensity in, granulocytes, total lymphocytes and lymphocyte subsets defined by labeled antibodies specific for CD20, CD45RA and CD57 relative to internal control cells and unstained controls was measured on a FACSCalibur instrument (Becton Dickinson) to calculate the median telomere length from duplicate measurements. Further details regarding telomere length measurements and data sets are described in Online Supplementary Material as well as depicted in Figure S1.
Some of the total 835 healthy subjects did not have sufficient cells for analyses of one or more of the cell subsets tested: granulocytes, B lymphocytes, or mature NK/T cells. Specific improvements were developed during the 8 year of the healthy donor study allowing for the testing of additional cell subsets (B and mature NK/T [62]) explaining why fewer measurements are reported for these subsets. In addition, a slight modification was made to the cell lysis protocol: from a semi-automated small volume lysis in a 96 well format [63]) to the current larger volume individual sample lysis [61]. The data obtained during these two experimental periods were first analyzed separately to test for differences between the first and second data sets. Briefly, both data sets were found to have comparable telomere length distributions over age, with a small but notable decrease in the calculated granulocyte telomere length together with a decrease in the range of granulocyte telomere length values in the second data set (Figure S1). These differences may be explained by the protocol improvements in the second set that resulted in a better resolution of signal and a reduction in the background fluorescence observed in cell types with large volumes of cytoplasm. Since the ranges lower limits were similar between the two data sets and since the majority of the data for both sets falls within the same 10th to 90th percentile range, the two sets were merged and analyzed together for curve fitting models and reference comparisons.
Statistical analyses
Analyses were performed using Microsoft Excel (Microsoft Office 2007), GraphPad Prism (version 4) and R (version 2.6.1, 2007, The R Foundation for Statistical Computing); t-Tests were two-tailed and performed on data with a normal distribution (KS test). Linear modeling (lm function in the R language) was used to carry out the regression analysis and estimate the piecewise linear curves with breakpoints hinged at 1 and 18 years of age. We found that this gave the best fit with the least mean square error and more consistent error distribution across the age ranges as compared to using a number of polynomial fits (linear, quadratic, cubic or quartic). The 99th, 90th, 10th and 1st percentile curves were obtained by vertical shift of the estimated regression curve to span the desired number of data points in the primary data set of healthy subjects (n = 835) that were representative for this segment of distribution. To compare the telomere lengths of one population against another, the ANOVA function in the R language was employed to test if the data was from the same or 2 different model fits [64].
Online supplementary material
Figure S1 depicts data from two consecutive experimental periods separately for lymphocytes and granulocytes respectively, and displays the previous quadratic curve fitting model (first ∼400 data points) compared to the current three piece-wise linear regression model (for which first and second data sets were combined) used in Figure 2, Figure 3, and Figure 4.
Figure S2 complements Figure 2 and highlights the telomere length skewing specifically seen in CD45RA+ CD20 − lymphocytes of older individuals, where a higher proportion of terminally differentiated lymphocytes are likely present within the cell population with this phenotype. Further, Figure S2 depicts the skewing observed in telomerase heterozygous individuals, comparable to that seen in healthy older individuals (over 75 years of age).
Figure S3 complements Figure 3 and depicts gender segregated telomere length data measured by flow FISH for all leukocyte cell subsets tested, together with the model fit statistical test results from these analyses.
Figure S4 complements Figure 4 and depicts further analysis of leukocyte telomere length data from direct relatives, siblings or parents of telomerase heterozygous individuals. From this relatively small group, although a trend towards shorter MTLs is observed, no statistical difference between the groups and no statistically significant difference compared to healthy individuals was detected (Table S5).
Table S1 displays the complete telomere length data sets. Duplicate leukocyte telomere length data were collected over an eight year period and analyzed on two occasions (first analysis after 391 samples and the present analysis after next 445 samples). For the first set of data, freshly isolated nucleated blood cells were used whereas for the second set, nucleated cells were frozen prior to flow FISH (see Materials and Methods and data set comparisons, Figure S1). No marked differences in the calculated telomere length between the two data sets were observed for lymphocytes or lymphocyte subsets. Although the granulocyte telomere length values were slightly lower and more narrowly distributed in the second data set, the overall results were pooled for the current analysis.
Table S2 displays telomere length cell subset correlations at different age ranges. This table complements Figure 2 and Figure S2. It displays the correlative r values between paired cell population telomere length values. The cell population chosen as a reference has a set value of 1.
Tables S3, S4 and S5 display the complete statistical ANOVA analysis results for comparing telomere lengths of leukocyte subsets in cord blood (at birth), and comparing the two linear models (an estimate of the entire population) and an estimate of where factor “X” (gender for example) was taken into consideration and showed a significant difference.
Supporting Information
Zdroje
1. d'Adda di FagagnaFReaperPMClay-FarraceLFieglerHCarrP 2003 A DNA damage checkpoint response in telomere-initiated senescence. Nature 426 194 198
2. SmogorzewskaAde LangeT 2002 Different telomere damage signaling pathways in human and mouse cells. Embo J 21 4338 4348
3. Britt-ComptonBRowsonJLockeMMackenzieIKiplingD 2006 Structural stability and chromosome-specific telomere length is governed by cis-acting determinants in humans. Hum Mol Genet 15 725 733
4. GreiderCWBlackburnEH 1985 Identification of a specific telomere terminal transferase activity in Tetrahymena extracts. Cell 43 405 413
5. MuntoniANeumannAAHillsMReddelRR 2009 Telomere elongation involves intra-molecular DNA replication in cells utilizing alternative lengthening of telomeres. Hum Mol Genet 18 1017 1027
6. LansdorpPM 2005 Major cutbacks at chromosome ends. Trends Biochem Sci 30 388 395
7. HemannMTStrongMAHaoLYGreiderCW 2001 The shortest telomere, not average telomere length, is critical for cell viability and chromosome stability. Cell 107 67 77
8. AubertGLansdorpPM 2008 Telomeres and aging. Physiol Rev 88 557 579
9. AubertGHillsMLansdorpPM 2011 Telomere length measurement-Caveats and a critical assessment of the available technologies and tools. Mutat Res 730 59 67
10. LansdorpPMVerwoerdNPvan de RijkeFMDragowskaVLittleMT 1996 Heterogeneity in telomere length of human chromosomes. Hum Mol Genet 5 685 691
11. MartensUMZijlmansJMPoonSSDragowskaWYuiJ 1998 Short telomeres on human chromosome 17p. Nat Genet 18 76 80
12. ChangEHarleyCB 1995 Telomere length and replicative aging in human vascular tissues. Proc Natl Acad Sci U S A 92 11190 11194
13. HastieNDDempsterMDunlopMGThompsonAMGreenDK 1990 Telomere reduction in human colorectal carcinoma and with ageing. Nature 346 866 868
14. RuferNBrummendorfTHKolvraaSBischoffCChristensenK 1999 Telomere fluorescence measurements in granulocytes and T lymphocyte subsets point to a high turnover of hematopoietic stem cells and memory T cells in early childhood. J Exp Med 190 157 167
15. SlagboomPEDroogSBoomsmaDI 1994 Genetic determination of telomere size in humans: a twin study of three age groups. Am J Hum Genet 55 876 882
16. HarleyCBFutcherABGreiderCW 1990 Telomeres shorten during ageing of human fibroblasts. Nature 345 458 460
17. BaerlocherGMMakJRothARiceKSLansdorpPM 2003 Telomere shortening in leukocyte subpopulations from baboons. J Leukoc Biol 73 289 296
18. HillsMLuckeKChavezEAEavesCJLansdorpPM 2009 Probing the mitotic history and developmental stage of hematopoietic cells using single telomere length analysis (STELA). Blood 113 5765 5775
19. VaziriHDragowskaWAllsoppRCThomasTEHarleyCB 1994 Evidence for a mitotic clock in human hematopoietic stem cells: loss of telomeric DNA with age. Proc Natl Acad Sci U S A 91 9857 9860
20. BroccoliDYoungJWde LangeT 1995 Telomerase activity in normal and malignant hematopoietic cells. Proc Natl Acad Sci U S A 92 9082 9086
21. ChiuCPDragowskaWKimNWVaziriHYuiJ 1996 Differential expression of telomerase activity in hematopoietic progenitors from adult human bone marrow. Stem Cells 14 239 248
22. WengNPPalmerLDLevineBLLaneHCJuneCH 1997 Tales of tails: regulation of telomere length and telomerase activity during lymphocyte development, differentiation, activation, and aging. Immunol Rev 160 43 54
23. ShepherdBEGuttorpPLansdorpPMAbkowitzJL 2004 Estimating human hematopoietic stem cell kinetics using granulocyte telomere lengths. Exp Hematol 32 1040 1050
24. CaladoRTYoungNS 2009 Telomere diseases. N Engl J Med 361 2353 2365
25. AlterBPBaerlocherGMSavageSAChanockSJWekslerBB 2007 Very short telomere length by flow fluorescence in situ hybridization identifies patients with dyskeratosis congenita. Blood 110 1439 1447
26. KirwanMDokalI 2008 Dyskeratosis congenita: a genetic disorder of many faces. Clin Genet 73 103 112
27. ArmaniosMYChenJJCoganJDAlderJKIngersollRG 2007 Telomerase mutations in families with idiopathic pulmonary fibrosis. N Engl J Med 356 1317 1326
28. BaerlocherGMRiceKVultoILansdorpPM 2007 Longitudinal data on telomere length in leukocytes from newborn baboons support a marked drop in stem cell turnover around 1 year of age. Aging Cell 6 121 123
29. JuZJiangHJaworskiMRathinamCGompfA 2007 Telomere dysfunction induces environmental alterations limiting hematopoietic stem cell function and engraftment. Nat Med 13 742 747
30. ParkJIVenteicherASHongJYChoiJJunS 2009 Telomerase modulates Wnt signalling by association with target gene chromatin. Nature 460 66 72
31. XuLBlackburnEH 2007 Human cancer cells harbor T-stumps, a distinct class of extremely short telomeres. Mol Cell 28 315 327
32. TakaiHSmogorzewskaAde LangeT 2003 DNA damage foci at dysfunctional telomeres. Curr Biol 13 1549 1556
33. LansdorpPM 2000 Repair of telomeric DNA prior to replicative senescence. Mech Ageing Dev 118 23 34
34. RheeDBGhoshALuJBohrVALiuY 2011 Factors that influence telomeric oxidative base damage and repair by DNA glycosylase OGG1. DNA Repair (Amst) 10 34 44
35. SahinECollaSLiesaMMoslehiJMullerFL 2011 Telomere dysfunction induces metabolic and mitochondrial compromise. Nature 470 359 365
36. BakerDJWijshakeTTchkoniaTLeBrasseurNKChildsBG 2011 Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature 479 232 236
37. VulliamyTJDokalI 2008 Dyskeratosis congenita: the diverse clinical presentation of mutations in the telomerase complex. Biochimie 90 122 130
38. FrenckRWJrBlackburnEHShannonKM 1998 The rate of telomere sequence loss in human leukocytes varies with age. Proc Natl Acad Sci U S A 95 5607 5610
39. LansdorpPMDragowskaWMayaniH 1993 Ontogeny-related changes in proliferative potential of human hematopoietic cells. J Exp Med 178 787 791
40. BowieMBKentDGDykstraBMcKnightKDMcCaffreyL 2007 Identification of a new intrinsically timed developmental checkpoint that reprograms key hematopoietic stem cell properties. Proc Natl Acad Sci U S A 104 5878 5882
41. KimISaundersTLMorrisonSJ 2007 Sox17 dependence distinguishes the transcriptional regulation of fetal from adult hematopoietic stem cells. Cell 130 470 483
42. LansdorpPM 2009 Telomeres and disease. EMBO J 28 2532 2540
43. MocchegianiEMalavoltaM 2004 NK and NKT cell functions in immunosenescence. Aging Cell 3 177 184
44. BuchkovichKJGreiderCW 1996 Telomerase regulation during entry into the cell cycle in normal human T cells. Mol Biol Cell 7 1443 1454
45. GeigerHRudolphKL 2009 Aging in the lympho-hematopoietic stem cell compartment. Trends Immunol 30 360 365
46. KnudsonMKulkarniSBallasZKBesslerMGoldmanF 2005 Association of immune abnormalities with telomere shortening in autosomal-dominant dyskeratosis congenita. Blood 105 682 688
47. BenetosAOkudaKLajemiMKimuraMThomasF 2001 Telomere length as an indicator of biological aging: The gender effect and relation with pulse pressure and pulse wave velocity. Hypertension 37 381 385
48. CaladoRTYewdellWTWilkersonKLRegalJAKajigayaS 2009 Sex hormones, acting on the TERT gene, increase telomerase activity in human primary hematopoietic cells. Blood 114 2236 2243
49. SalpeaKDHumphriesSE 2010 Telomere length in atherosclerosis and diabetes. Atherosclerosis 209 35 38
50. EpelESBlackburnEHLinJDhabharFSAdlerNE 2004 Accelerated telomere shortening in response to life stress. Proc Natl Acad Sci U S A 101 17312 17315
51. CawthonRMSmithKRO'BrienESivatchenkoAKerberRA 2003 Association between telomere length in blood and mortality in people aged 60 years or older. Lancet 361 393 395
52. NjajouOTHsuehWCBlackburnEHNewmanABWuSH 2009 Association between telomere length, specific causes of death, and years of healthy life in health, aging, and body composition, a population-based cohort study. J Gerontol A Biol Sci Med Sci 64 860 864
53. AlderJKChenJJLancasterLDanoffSSuSC 2008 Short telomeres are a risk factor for idiopathic pulmonary fibrosis. Proc Natl Acad Sci U S A 105 13051 13056
54. CaladoRTRegalJAHillsMYewdellWTDalmazzoLF 2009 Constitutional hypomorphic telomerase mutations in patients with acute myeloid leukemia. Proc Natl Acad Sci U S A 106 1187 1192
55. CaladoRTRegalJAKajigayaSYoungNS 2009 Erosion of telomeric single-stranded overhang in patients with aplastic anaemia carrying telomerase complex mutations. Eur J Clin Invest 39 1025 1032
56. GoldmanFDAubertGKlingelhutzAJHillsMCooperSR 2008 Characterization of primitive hematopoietic cells from patients with dyskeratosis congenita. Blood 111 4523 4531
57. LyHSchertzerMJastaniahWDavisJYongSL 2005 Identification and functional characterization of two variant alleles of the telomerase RNA template gene (TERC) in a patient with Dyskeratosis Congenita. Blood 106 1246 1252
58. YamaguchiHBaerlocherGMLansdorpPMChanockSJNunezO 2003 Mutations of the human telomerase RNA gene (TERC) in aplastic anemia and myelodysplastic syndrome. Blood 102 916 918
59. YamaguchiHCaladoRTLyHKajigayaSBaerlocherGM 2005 Mutations in TERT, the gene for telomerase reverse transcriptase, in aplastic anemia. N Engl J Med 352 1413 1424
60. TanejaKLChavezEACoullJLansdorpPM 2001 Multicolor fluorescence in situ hybridization with peptide nucleic acid probes for enumeration of specific chromosomes in human cells. Genes Chromosomes Cancer 30 57 63
61. BaerlocherGMVultoIde JongGLansdorpPM 2006 Flow cytometry and FISH to measure the average length of telomeres (flow FISH). Nat Protoc 1 2365 2376
62. Lopez-VergesSMilushJMPandeySYorkVAArakawa-HoytJ 2010 CD57 defines a functionally distinct population of mature NK cells in the human CD56dimCD16+ NK-cell subset. Blood 116 3865 3874
63. BaerlocherGMLansdorpPM 2003 Telomere length measurements in leukocyte subsets by automated multicolor flow-FISH. Cytometry A 55 1 6
64. ChambersJMHastieTJ 1992 Statistical Models in S. Pacific Grove, CA: Wadsworth & Brooks/Cole
Štítky
Genetika Reprodukčná medicína
Článek Functional Centromeres Determine the Activation Time of Pericentric Origins of DNA Replication inČlánek Dynamic Deposition of Histone Variant H3.3 Accompanies Developmental Remodeling of the TranscriptomeČlánek Integrin α PAT-2/CDC-42 Signaling Is Required for Muscle-Mediated Clearance of Apoptotic Cells inČlánek Prdm5 Regulates Collagen Gene Transcription by Association with RNA Polymerase II in Developing BoneČlánek Acquisition Order of Ras and p53 Gene Alterations Defines Distinct Adrenocortical Tumor Phenotypes
Článok vyšiel v časopisePLOS Genetics
Najčítanejšie tento týždeň
2012 Číslo 5- Gynekologové a odborníci na reprodukční medicínu se sejdou na prvním virtuálním summitu
- Je „freeze-all“ pro všechny? Odborníci na fertilitu diskutovali na virtuálním summitu
-
Všetky články tohto čísla
- Slowing Replication in Preparation for Reduction
- Chromosome Pairing: A Hidden Treasure No More
- Loss of Imprinting Differentially Affects REM/NREM Sleep and Cognition in Mice
- Six Novel Susceptibility Loci for Early-Onset Androgenetic Alopecia and Their Unexpected Association with Common Diseases
- Regulation by the Noncoding RNA
- UDP-Galactose 4′-Epimerase Activities toward UDP-Gal and UDP-GalNAc Play Different Roles in the Development of
- Deletion of PTH Rescues Skeletal Abnormalities and High Osteopontin Levels in Mice
- Karyotypic Determinants of Chromosome Instability in Aneuploid Budding Yeast
- Genome-Wide Copy Number Analysis Uncovers a New HSCR Gene:
- MicroRNA-277 Modulates the Neurodegeneration Caused by Fragile X Premutation rCGG Repeats
- Functional Centromeres Determine the Activation Time of Pericentric Origins of DNA Replication in
- Dynamic Deposition of Histone Variant H3.3 Accompanies Developmental Remodeling of the Transcriptome
- Scientist Citizen: An Interview with Bruce Alberts
- YY1 Regulates Melanocyte Development and Function by Cooperating with MITF
- Congenital Heart Disease–Causing Gata4 Mutation Displays Functional Deficits
- Recombination Drives Vertebrate Genome Contraction
- KATNAL1 Regulation of Sertoli Cell Microtubule Dynamics Is Essential for Spermiogenesis and Male Fertility
- Re-Patterning Sleep Architecture in through Gustatory Perception and Nutritional Quality
- Using Whole-Genome Sequence Data to Predict Quantitative Trait Phenotypes in
- Genome-Wide Analysis of GLD-1–Mediated mRNA Regulation Suggests a Role in mRNA Storage
- Meiotic Chromosome Pairing Is Promoted by Telomere-Led Chromosome Movements Independent of Bouquet Formation
- LINT, a Novel dL(3)mbt-Containing Complex, Represses Malignant Brain Tumour Signature Genes
- The H3K27 Demethylase UTX-1 Is Essential for Normal Development, Independent of Its Enzymatic Activity
- Suppresses Senescence Programs and Thereby Accelerates and Maintains Mutant -Induced Lung Tumorigenesis
- Genome-Wide Association of Pericardial Fat Identifies a Unique Locus for Ectopic Fat
- An Essential Role for Katanin p80 and Microtubule Severing in Male Gamete Production
- Identification of Genes That Promote or Antagonize Somatic Homolog Pairing Using a High-Throughput FISH–Based Screen
- Principles of Carbon Catabolite Repression in the Rice Blast Fungus: Tps1, Nmr1-3, and a MATE–Family Pump Regulate Glucose Metabolism during Infection
- Integrin α PAT-2/CDC-42 Signaling Is Required for Muscle-Mediated Clearance of Apoptotic Cells in
- Histone H3 Localizes to the Centromeric DNA in Budding Yeast
- Collapse of Telomere Homeostasis in Hematopoietic Cells Caused by Heterozygous Mutations in Telomerase Genes
- Hypersensitive to Red and Blue 1 and Its Modification by Protein Phosphatase 7 Are Implicated in the Control of Arabidopsis Stomatal Aperture
- Extent, Causes, and Consequences of Small RNA Expression Variation in Human Adipose Tissue
- TBC-8, a Putative RAB-2 GAP, Regulates Dense Core Vesicle Maturation in
- Regulating Repression: Roles for the Sir4 N-Terminus in Linker DNA Protection and Stabilization of Epigenetic States
- Common Genetic Determinants of Intraocular Pressure and Primary Open-Angle Glaucoma
- Prdm5 Regulates Collagen Gene Transcription by Association with RNA Polymerase II in Developing Bone
- Fitness Landscape Transformation through a Single Amino Acid Change in the Rho Terminator
- Repeated, Selection-Driven Genome Reduction of Accessory Genes in Experimental Populations
- Allelic Variation and Differential Expression of the mSIN3A Histone Deacetylase Complex Gene Promote Mammary Tumor Growth and Metastasis
- DNA Demethylation and USF Regulate the Meiosis-Specific Expression of the Mouse
- Knowledge-Driven Analysis Identifies a Gene–Gene Interaction Affecting High-Density Lipoprotein Cholesterol Levels in Multi-Ethnic Populations
- A Duplication CNV That Conveys Traits Reciprocal to Metabolic Syndrome and Protects against Diet-Induced Obesity in Mice and Men
- EMT Inducers Catalyze Malignant Transformation of Mammary Epithelial Cells and Drive Tumorigenesis towards Claudin-Low Tumors in Transgenic Mice
- Inactivation of a Novel FGF23 Regulator, FAM20C, Leads to Hypophosphatemic Rickets in Mice
- Genome-Wide Association for Abdominal Subcutaneous and Visceral Adipose Reveals a Novel Locus for Visceral Fat in Women
- Stratifying Type 2 Diabetes Cases by BMI Identifies Genetic Risk Variants in and Enrichment for Risk Variants in Lean Compared to Obese Cases
- New Insight into the History of Domesticated Apple: Secondary Contribution of the European Wild Apple to the Genome of Cultivated Varieties
- Activated Cdc42 Kinase Has an Anti-Apoptotic Function
- The Region Is Critical for Birth Defects and Electrocardiographic Dysfunctions Observed in a Down Syndrome Mouse Model
- COP9 Signalosome Integrity Plays Major Roles for Hyphal Growth, Conidial Development, and Circadian Function
- Bmps and Id2a Act Upstream of Twist1 To Restrict Ectomesenchyme Potential of the Cranial Neural Crest
- Psip1/Ledgf p52 Binds Methylated Histone H3K36 and Splicing Factors and Contributes to the Regulation of Alternative Splicing
- The Number of X Chromosomes Causes Sex Differences in Adiposity in Mice
- Target Gene Analysis by Microarrays and Chromatin Immunoprecipitation Identifies HEY Proteins as Highly Redundant bHLH Repressors
- Acquisition Order of Ras and p53 Gene Alterations Defines Distinct Adrenocortical Tumor Phenotypes
- ELK1 Uses Different DNA Binding Modes to Regulate Functionally Distinct Classes of Target Genes
- Histone H1 Depletion Impairs Embryonic Stem Cell Differentiation
- IDN2 and Its Paralogs Form a Complex Required for RNA–Directed DNA Methylation
- Separation of DNA Replication from the Assembly of Break-Competent Meiotic Chromosomes
- Genomic Hypomethylation in the Human Germline Associates with Selective Structural Mutability in the Human Genome
- PLOS Genetics
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- Inactivation of a Novel FGF23 Regulator, FAM20C, Leads to Hypophosphatemic Rickets in Mice
- Genome-Wide Association of Pericardial Fat Identifies a Unique Locus for Ectopic Fat
- Slowing Replication in Preparation for Reduction
- An Essential Role for Katanin p80 and Microtubule Severing in Male Gamete Production
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



