-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
Dialects of the DNA Uptake Sequence in
In all sexual organisms, adaptations exist that secure the safe reassortment of homologous alleles and prevent the intrusion of potentially hazardous alien DNA. Some bacteria engage in a simple form of sex known as transformation. In the human pathogen Neisseria meningitidis and in related bacterial species, transformation by exogenous DNA is regulated by the presence of a specific DNA Uptake Sequence (DUS), which is present in thousands of copies in the respective genomes. DUS affects transformation by limiting DNA uptake and recombination in favour of homologous DNA. The specific mechanisms of DUS–dependent genetic transformation have remained elusive. Bioinformatic analyses of family Neisseriaceae genomes reveal eight distinct variants of DUS. These variants are here termed DUS dialects, and their effect on interspecies commutation is demonstrated. Each of the DUS dialects is remarkably conserved within each species and is distributed consistent with a robust Neisseriaceae phylogeny based on core genome sequences. The impact of individual single nucleotide transversions in DUS on meningococcal transformation and on DNA binding and uptake is analysed. The results show that a DUS core 5′-CTG-3′ is required for transformation and that transversions in this core reduce DNA uptake more than two orders of magnitude although the level of DNA binding remains less affected. Distinct DUS dialects are efficient barriers to interspecies recombination in N. meningitidis, N. elongata, Kingella denitrificans, and Eikenella corrodens, despite the presence of the core sequence. The degree of similarity between the DUS dialect of the recipient species and the donor DNA directly correlates with the level of transformation and DNA binding and uptake. Finally, DUS–dependent transformation is documented in the genera Eikenella and Kingella for the first time. The results presented here advance our understanding of the function and evolution of DUS and genetic transformation in bacteria, and define the phylogenetic relationships within the Neisseriaceae family.
Published in the journal: Dialects of the DNA Uptake Sequence in. PLoS Genet 9(4): e32767. doi:10.1371/journal.pgen.1003458
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1003458Summary
In all sexual organisms, adaptations exist that secure the safe reassortment of homologous alleles and prevent the intrusion of potentially hazardous alien DNA. Some bacteria engage in a simple form of sex known as transformation. In the human pathogen Neisseria meningitidis and in related bacterial species, transformation by exogenous DNA is regulated by the presence of a specific DNA Uptake Sequence (DUS), which is present in thousands of copies in the respective genomes. DUS affects transformation by limiting DNA uptake and recombination in favour of homologous DNA. The specific mechanisms of DUS–dependent genetic transformation have remained elusive. Bioinformatic analyses of family Neisseriaceae genomes reveal eight distinct variants of DUS. These variants are here termed DUS dialects, and their effect on interspecies commutation is demonstrated. Each of the DUS dialects is remarkably conserved within each species and is distributed consistent with a robust Neisseriaceae phylogeny based on core genome sequences. The impact of individual single nucleotide transversions in DUS on meningococcal transformation and on DNA binding and uptake is analysed. The results show that a DUS core 5′-CTG-3′ is required for transformation and that transversions in this core reduce DNA uptake more than two orders of magnitude although the level of DNA binding remains less affected. Distinct DUS dialects are efficient barriers to interspecies recombination in N. meningitidis, N. elongata, Kingella denitrificans, and Eikenella corrodens, despite the presence of the core sequence. The degree of similarity between the DUS dialect of the recipient species and the donor DNA directly correlates with the level of transformation and DNA binding and uptake. Finally, DUS–dependent transformation is documented in the genera Eikenella and Kingella for the first time. The results presented here advance our understanding of the function and evolution of DUS and genetic transformation in bacteria, and define the phylogenetic relationships within the Neisseriaceae family.
Introduction
Transformation in bacteria is a complex process involving uptake of naked extracellular DNA followed by homologous recombination (HR). Different reproductive barriers have evolved in diverse transformation-competent bacteria, which distinguish in favour of acquisition and recombination of homologous DNA sequences and discriminate against heterologous and potentially hazardous DNA [1]. In particular, interspecies recombination with heterologous DNA in single cellular organisms could cause gene disruptions and/or disturb sensitive cellular processes, which could in turn have adverse phenotypic consequences. Adaptations that may contribute to sexual isolation and at the same time promote genetic stability include restriction modification systems, fratricide in streptococci and cannibalism in Bacillus subtilis, quorum-sensing, biofilm formation and HR regulation and suppression [2], [3], [4], [5]. Transformation in Neisseria sp. and members of the Pasteurellaceae family is unique in the requirement for short uptake sequences in the transforming DNA, named DNA Uptake Sequences (DUS) and Uptake Signal Sequences (USS), respectively [6], [7]. The genomes of these organisms harbour thousands of DUS and USS, constituting up to 1% of their entire chromosomes [8], [9], [10]. DUS has accumulated in the core genome, i.e. the set of common genes, of N. meningitidis, N. gonorrhoeae and N. lactamica and was found to maintain its sequence identity from frequent recombination [11]. DUS was first identified in N. gonorrhoeae as a 10-mer (5′-GCCGTCTGAA-3′) and has been documented functional in transformation of meningococci and gonococci [7], [12], [13]. Later, a revised 12-mer DUS (5′-AT-GCCGTCTGAA-3′, here named AT-DUS) was shown to elevate transformation further [14], [15]. High level expression of the competence and minor pilin protein ComP has been shown to increase DUS-specific uptake, and a definite association between DUS and ComP was published recently [16], [17], [18]. A linear relationship between the number of DUS and the ability to competitively inhibit the uptake of radio-labelled DNA in N. gonorrhoeae has been documented, suggesting initial surface binding of DUS [19]. An additive effect of DUS has been documented also in transformation experiments in N. meningitidis, although no linear relationship between the number of DUS and transformation frequencies was evident [20]. Importantly, DNA binding and uptake assays do not fully correlate with the outcome of transformation assays, indicating that more than one level of DUS specificity exist [15]. Recently an influence of DUS location relative to homologous and recombinogenic regions of transforming DNA was demonstrated, suggesting that DUS may initiate DNA processing by a yet undefined way [20]. Two versions of USS have been described in Pasteurellaceae: version A (5′-AAGTGCGGT-3′), named Hin-USS, is found in Haemophilus influenzae and Actinobacillus actinomycetemcomitans (now named Aggregatibacter actinomycetemcomitans) and USS version B (5′-ACAAGCGGT-3′), named the Apl-USS subtype, is found in Actinobacillus pleuropneumoniae [21]. DUS-like repeat sequences have been described for N. subflava and N. sicca [22] and recently also in N. elongata [23]. Different variants of DUS are here termed DUS dialects alluding to their role as nucleotide ‘words’ in genetic ‘communication’ and in concordance with the previous use of the term ‘dialects’ in genetic contexts [24].
Even though DUS seems to have disseminated in the genus Neisseria, virtually nothing is known about DUS repeats in the family Neisseriaceae genera Kingella, Eikenella and Simonsiella. To fill this knowledge gap, the work presented here examines DUS specificity and dialects within the family Neisseriaceae [11], [25]. The results reveal the presence of eight DUS dialects in different branches of the robust Neisseriaceae phylogenetic tree. In transformation assays, the DUS sequence divergence negatively influences inter-species transfer of DNA. A DUS core of only three nucleotides is present in all dialects and is strictly required for transformation. Assays with radiolabelled DNA show species specific relevance of DUS dialects for both binding and uptake of DNA. This work supports the idea that DUS specificity is a highly efficient barrier to interspecies transformation, that has great impact on the evolution of the Neisseriaceae.
Results
DUS dialects
Neisseriaceae genomes were obtained from online databases (i.e., the Human Microbiome Project [26] and other initiatives) and searched for overrepresented/highly-repeated sequences. Several very overrepresented 10-mers were identified in different genomes that displayed high degrees of similarity to the canonical DUS sequence first described in N. gonorrhoeae [7], [12], [13]. Eight distinct and abundant variants of DUS were identified and are shown in Figure 1, five of which are potential DUS as they were not previously functionally confirmed. The DUS variants are called dialects in concordance with previous use of the term for describing variants of short DNA motifs that probably are strongly affected by some DNA template-dependent processing proteins [24], and each DUS dialect was given a name according to the nomenclature scheme described in materials and methods. Every DUS dialect was found in exceptionally high numbers in their respective genomes and the exact occurrences are presented in Table S1 together with the number of degenerate DUS in which one nucleotide position were permitted to vary. AT-DUS was found in all available N. gonorrhoeae and N. meningitidis genomes (10-mer: n≈1900). In addition, AT-DUS was found as the most overrepresented repeat in the genomes of N. lactamica (n≈2200), N. cinerea strain ATCC 14685 (n = 943) and N. polysaccharea strain ATCC 43768 (n = 2183). AG-DUS was identified in Neisseria sp. oral taxon 014 (n = 3236), N. subflava strain NJ9703 (n = 2871), N. flavescens (n = 1196 and n = 2767 in strains NRL30031 H210 and SK114, respectively), N. mucosa strain C102 (n = 2964), N. bacilliformis strain ATCC BAA-1200 (n = 4265), N. weaveri strains ATCC 51223 and LMG 5135 (n≈2850), and N. elongata subsp. glycolytica strain ATCC 29315 (n = 3273). AG-mucDUS was the most prevalent repeat in N. mucosa strain ATCC25996 (n = 1543), N. sicca (n = 3770) and N. macacae strain ATCC 33926 (n = 3729) (Table S1).
Fig. 1. Alignment of eight distinct DUS dialects. 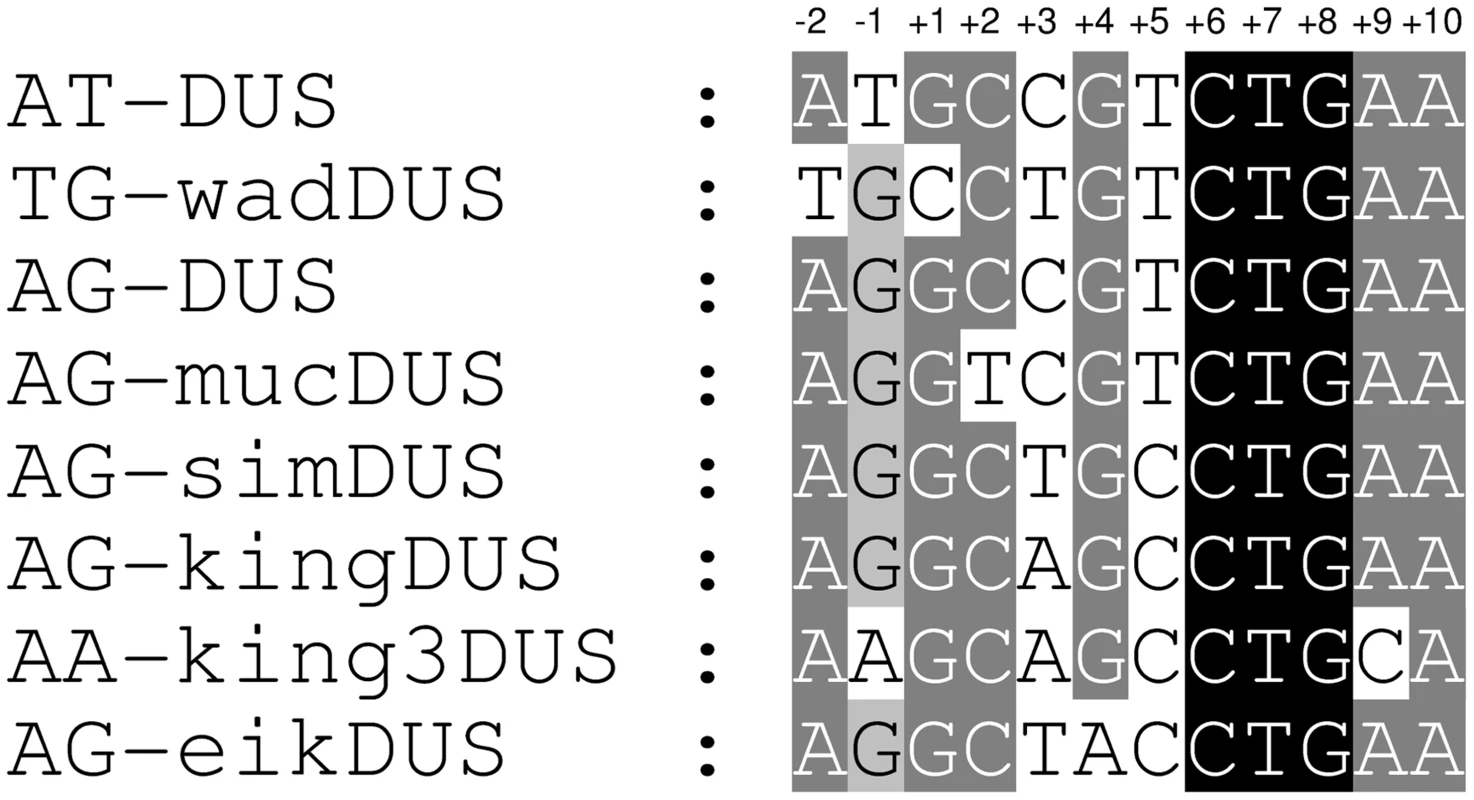
Alignment of the eight different DUS dialects identified in the genomes of Neisseriaceae family members. Two nucleotides at the 5′-end of DUS are included and the numbering is given on the top. Relative shading indicate the level of sequence conservation. A previous study showed that in N. subflava strain ATCC 19243, a 7 kb long sequence harbouring folP (GeneBank AJ581792.1) contained 7 mucDUS and 1 AG-DUS [22], whereas the genome of the N. subflava NJ9703 strain investigated here contained mainly the AG-DUS. The folP fragment is absent in the equivalent position in N. subflava NJ9703 and elsewhere in the genome. A BLAST search of all available Neisseriaceae genomes with the 7 kb fragment showed that the mucDUS positions around folP in N. subflava ATCC 19243 were present in N. sicca.
The genome of N. wadsworthii 9715 displayed a distinct DUS dialect, wadDUS (n = 2426), which is identical to AT-DUS with a T insertion after position +3 (Figure S1A).
In the genome of K. oralis ATCC 51147, yet another new dialect was discovered, the kingDUS (n = 5918). The occurrence of nearly six thousand kingDUS in a single small genome (2.4 Mb) is the highest density of any DUS dialect detected so far. By allowing a single nucleotide divergence in the kingDUS, the number of kingDUS-similar sequences increased by 21% to a total 7153 hits for the K. oralis genome. The completion, closure and annotation of the K. oralis genome may eventually alter the absolute numbers of kingDUS present, but approximately 2,5% of this particular genome will still remain occupied by the kingDUS which is very high compared to the approximate 1% DUS occupancy in N. meningitidis and N. gonorrhoeae genomes.
The genome of S. muelleri ATCC 29453 displayed the simultaneous presence of two DUS dialects. The kingDUS (n = 2257) described above and a new dialect simDUS (n = 2292) were in the S. muelleri genome detected in nearly equal numbers with a total count of 4549. SimDUS differed from the kingDUS by an A/T transversion at position +3 (Figure 1).
The genome sequences of K. kingae ATCC 23330 and K. denitrificans ATCC 33394 also revealed a new dialect, king3DUS, which differed from the kingDUS in an A/C transversion in position +9 (Figure 1) and a G/A transition in position −1 (Figure 1). The king3DUS was present in 2787 and 3603 copies in the genomes of K. kingae ATCC 23330 and K. denitrificans ATCC 33394, respectively.
Finally, the most divergent dialect of DUS relative to the AT-DUS was identified in the genomes of E. corrodens ATCC 23834 (n = 3269) and Neisseria shayeganii 871 (n = 2245), termed eikDUS. Notably, eikDUS was the only DUS with an A in position +4 (Figure 1). All the different dialects of DUS were conserved in positions +6, +7, +8 (CTG) as well as +10 (A) as demonstrated in Figure 1.
Based on the available genome sequences of genus Neisseria, no genome was devoid of any dialect of DUS. In the family Neisseriaceae, however, five genomes were found not to contain an abundant repeat that was an obvious DUS; these were the genomes of Laribacter hongkongensis HLHK9, Lutiella nitroferrum 2002, Pseudogulbenkiania sp. NH8B, Chromobacterium sp. C-61 and Chromobacterium violaceum ATCC12472. Their respective over-represented 10-mers are listed in Table S1D. We noticed, however, that the DUS core sequence 5′-CTG-3′ was found as the reverse complement sequence 5′-CAG-3′ in the most over-represented 10-mer sequences from Laribacter hongkongensis and C. violaceum (Table S1D).
Neisseriaceae phylogeny
Until now, a core genome phylogenetic tree for members of the Neisseriaceae was made only for the human genus Neisseria species [27], [28], [29], for all the available N. meningitidis genomes [25] and for collections of Neisseria strains [30], [31]. Here, a phylogenetic tree encompassing 23 representative members of the family Neisseriaceae was generated based on their common core genome containing 474 coding sequences (Figure 2). The 16SrDNA phylogenetic tree [32], [33] made for the Neisseriaceae differed from the core genome based tree (Figure 2). Notably, the DUS dialect distribution in the two trees differed considerably, and the core genome tree branches reflected the presence of different dialects in a congruent manner. Also a phylogenetic tree based on ComP, a recently reported DUS-specific binding protein [18], displays high degree of congruence with different DUS dialects, although some deviations are apparent (Figure S7). The robust phylogeny finds that N. shayeganii 871 is closely related to E. corrodens ATCC 23834, and S. muelleri ATCC 29453 is located among the three different Kingella species. Neisseria sp. oral taxon 014 is in the 16SrDNA tree wrongly placed close to the cluster containing N. lactamica. N. mucosa C102 is more closely related to N. subflava and N. flavescens than to N. mucosa ATCC 25996. The latter strain is the one in which the mucDUS was first described [28] and is located on the same branch in the 16SrDNA phylogenetic tree as the type strain N. mucosa ATCC 19696, based on the available partial sequence (data not shown). This observation separated N. mucosa C102 from the N. mucosa ATCC 25996 reference strain. C. violaceum served as the outgroup in Figure 2, based on its suitable genomic distance from the other Neisseriaceae members.
Fig. 2. Phylogenetic tree based on the core genome and DUS dialect distribution. 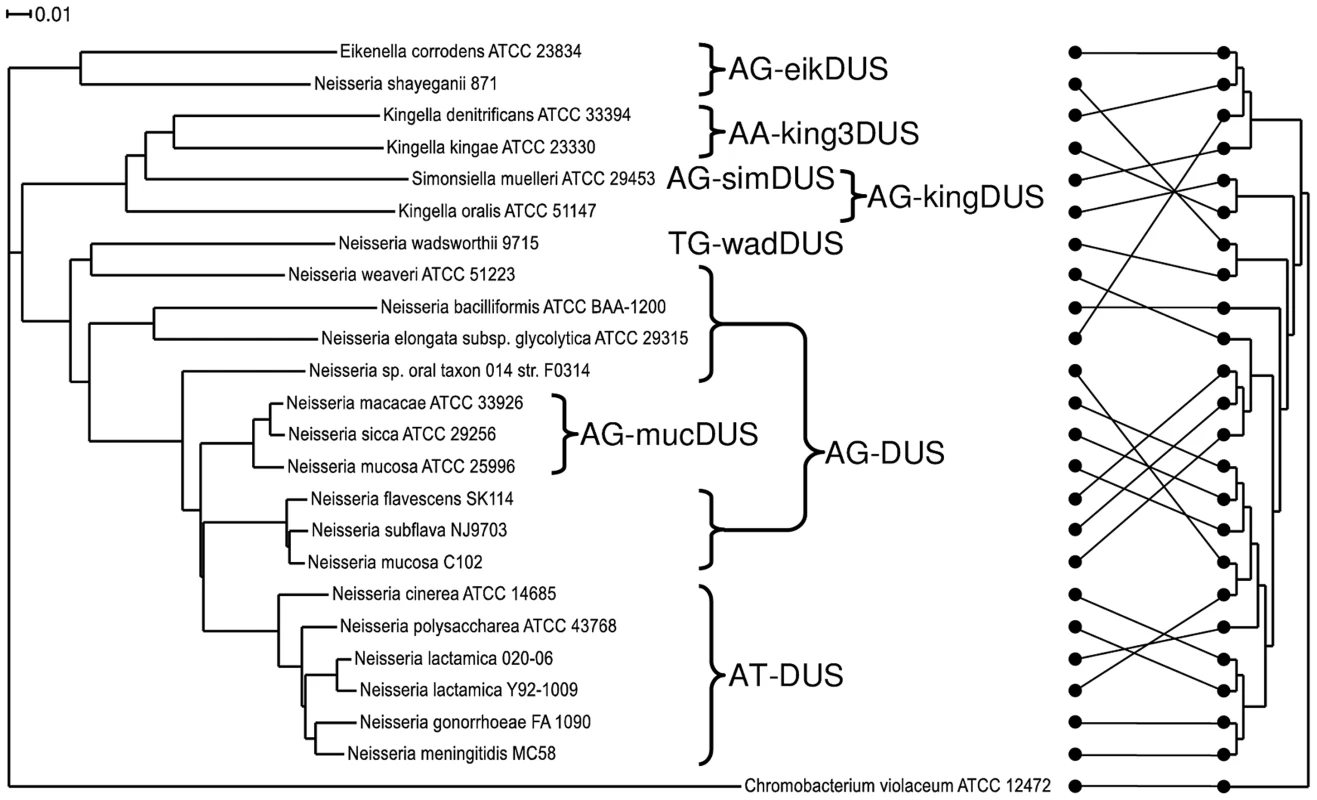
The phylogenetic tree obtained by the EDGAR service based on the core genome sequences of representative bacteria used in this study. The occurrences of the DUS dialects evolved are indicated. The scale bar represents 0.01 substitutions per nucleotide site. Chromobacterium violaceum was used as an outgroup. The core genome for this tree consisted of 474 coding sequences. On the right is the 16SrDNA based cladogram with the connectors of the dots showing the relation to the phylogram on the left. The evolutionary history of DUS in Neisseriaceae may be traced and depicted as follows: The DUS-based transformation system evolved after the split from the shared common ancestor with C. violaceum. Neither could a ComP be indentified by BLAST searches of the genome of C. violaceum. Among the DUS-containing bacteria, the eikDUS-group separated first from the main branch, and is also the only group with an A in position +4 of the DUS. N. shayeganii strain 871 clusters with E. corrodens and may erroneously have been taxonomically assigned to the genus Neisseria. Thereafter, the kingDUS - and king3DUS-groups branched off from the canonical DUS-group and S. muelleri might have been in the process of separating itself from the Kingella group. N. wadsworthii separated from the AT-DUS-group by a change in DUS specificity evident from the insertion of a T in position +3 of AT-DUS. N. macacae, N. sicca and N. mucosa ATCC 25996 were separated from the AT-DUS - and AG-DUS-groups by the C/T transition in position +2.
Single nucleotide transversion mutation analysis of AT-DUS in N. meningitidis
The new DUS dialects identified here exhibit several divergent positions (Figure 1) and we became interested in studying the discrete impact of the nucleotides that constitute a functional DUS. In a transversion mutation approach, the contribution of each individual nucleotide of the well-characterized AT-DUS was tested in quantitative transformation of N. meningitidis strain MC58 and the results are shown in Figure 3A. Also the effects of single transversion mutations in all twelve AT-DUS positions on DNA binding and uptake were measured and the results are summarized in Figure 3B. Any alteration of AT-DUS significantly reduced the transformation frequency (paired t-test, seven experiments, p≤0.02), although to a variable extent. The negative control lacking DUS does not transform at all. Our previous finding [14] was confirmed in that the two semi-conserved nucleotides in positions −2 and −1 at the 5′ end of the DUS, constituting the revised 12-mer AT-DUS, positively contribute to transformation efficacy, since both their respective transversions performed less than the complete AT-DUS. Furthermore, the transversions in individual positions of the 10-mer DUS were found to impair transformation performance. When the G in position +1 was transversed, the performance in transformation was reduced to 50% relative to the performance of the complete signal. Alterations in position +2 and +3 reduced the relative performance down to 20% and 28%, respectively. Alterations of position +4 (5%) and +5 (2%) had a more than one log reduction in relative transformation performance. The C, T and G at the 3′ half of the DUS (positions +6, +7 and +8) was shown to be particularly important for the DUS effect, since transformation was nearly abolished when the nucleotides in these positions were altered. A G/C transversion in position +8 gave rise to a total of only 2 CFU in seven experiments emphasizing the near complete loss of DUS-function. This functionally important 5′-CTG-3′ core is conserved in all dialects of DUS (Figure 1). The two adenines at the 3′ end of DUS (position +9 and +10) display minor contributions to the overall effect of DUS, and mutants perform at around 50% of the full AT-DUS. The A in position +10 is also conserved in all DUS dialects but contributes less significantly to the functionality of DUS than the 5′-CTG-3′ core. The distance-dependent gradual influence of the bases around the short 5′-CTG-3′ core sequence may reflect the strength of molecular interactions between DUS and the electropositive stripe on the surface of ComP [18] that warrants further investigations.
Fig. 3. Effects of point mutations in the DUS on quantitative transformation and DNA binding and uptake of N. meningitidis MC58. 
(A) Quantitative transformation of Neisseria meningitidis strain MC58 with plasmid DNA containing a panel of modified DUS sequences. Transformation rates are shown as percentage relative to the AT-DUS. Standard deviations from 7 independent experiments are indicated by bars. (B) DNA binding (gray bars) and uptake (white bars) assay with radiolabelled plasmid DNA. Average values from 4 independent experiments are shown as percentage of total DNA added. T-test results for (A) transformation rates compared to AT-DUS and (B) for DNA binding versus uptake are indicated by stars (p≤0.2 = *, p≤0.05 = **, p≤0.001 = ***). DUS sequence transversions are given as abscissa labels. The sequence logo is based on the 2742 occurrences of DUS, with a single nucleotide divergence allowed, found in the genome of N. meningitidis MC58. The effect of single transversion mutations on DNA binding and uptake in Figure 3B shows that DNA binding is high in N. meningitidis strain MC58 and that only DNA uptake is significantly affected. All the individual alterations of DUS bind better than the negative control lacking DUS. Relative DNA uptake was greatly reduced for the DNA without DUS and DUS with mutations in positions +4 to +8, being approximately 2% of bound DNA for the mutations in the core positions +6 to +8 (Figure 3B). In another strain, N. meningitidis 8013, DNA binding is lower overall and is together with the uptake negatively affected by the alterations of DUS. Again it is the alterations 5′-CTG-3′ that most dramatically affects DNA uptake and/or binding in both strains tested (Figure S4).
DUS–mediated commutation in Neisseriaceae
Potential commutation, defined as the interchange of DUS-linked genetic information, between different Neisseriaceae was first investigated by employing different DUS dialects in quantitative transformation experiments of N. meningitidis strain MC58, and the results are shown in Figure 4A and Table 1. Inversely, K. denitrificans, E. corrodens and N. elongata were tested for their respective DUS dependency by using PCR products of the rpsL gene conferring streptomycin resistance flanked by their own DUS or other DUS dialects. As shown in Table 1, the transformation frequency was always highest for their autologous DUS variant. The AG-DUS differs from AT-DUS in just a single nucleotide in position -1, and has 90% efficacy in N. meningitidis MC58. AG-mucDUS differs from AT-DUS in a T/G transversion in position −1 and a C/T transition in position +2. A 50% difference in transforming abilities of DUS and the 10-mer mucDUS in N. meningitidis was previously shown, although without statistical significance [22]. The 12-mer AG-mucDUS, which occurs 165 times in the genome of N. meningitidis strain MC58, displayed here a 66% reduced transformation efficacy relative to that of AT-DUS (Figure 4A and Table 1). For the more drastic C/A transversion in the second position (+2) in AT-DUS, a DUS-like sequence that occurred only once in the entire MC58 genome, the relative transformation was reduced by about 80% (Figure 3A). The AT-mucDUS was found 19 times, but the AG-mucDUS was found 88 times in the MC58 genome indicating previous interspecies transfer from the AG-mucDUS group. These transformation assays in N. meningitidis MC58 showed inter assay variations (Figure S3) but the Kendall's W test showed a very high concordance of gained orders (Kendall's W = 0.9145, χ2 = 44.8112, df = 7, p<0.0001).
Fig. 4. Quantitative transformation of N. meningitidis and binding and uptake of DNA with DUS from other Neisseriaceae. 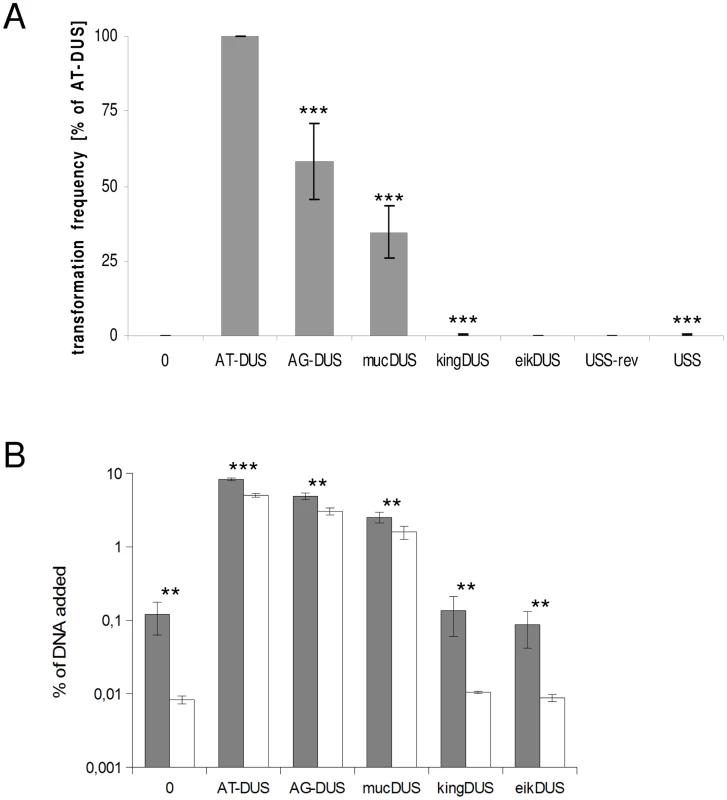
(A) Quantitative transformation of Neisseria meningitidis strain MC58 with plasmid DNA containing different DUS dialects and with the Haemophilus influenzae USS. Transformation rates are shown as percent relative to the AT-DUS. The standard deviations from 6 independent experiments are indicated by bars. (B) DNA binding (gray bars) and uptake (white bars) assay with radiolabelled plasmid DNA. Average values from 3 independent experiments are shown as percentage of total DNA added. Abscissa labels give the DUS variant. Statistic analysis as in Figure 3. Tab. 1. DUS–dependent transformation frequencies. 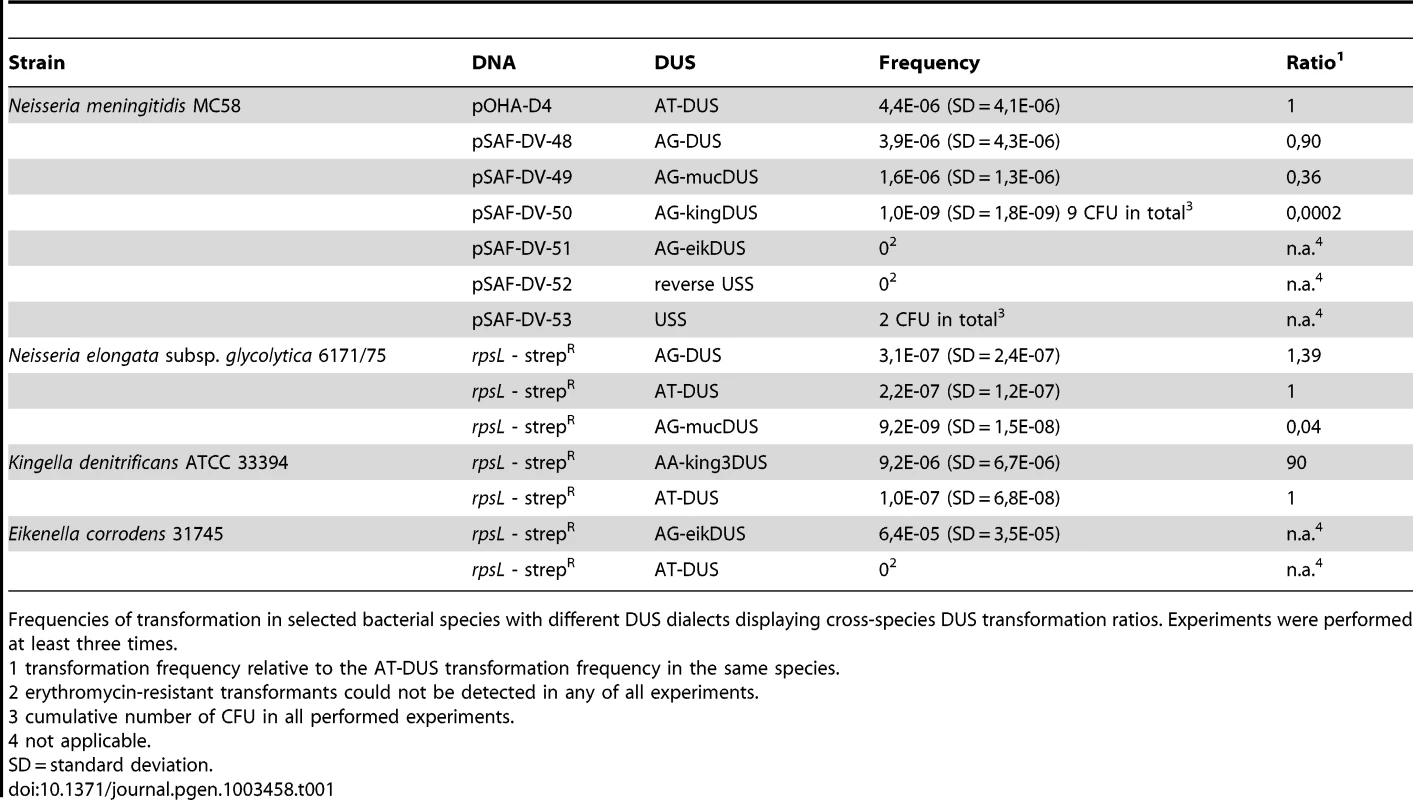
Frequencies of transformation in selected bacterial species with different DUS dialects displaying cross-species DUS transformation ratios. Experiments were performed at least three times. AT-DUS containing DNA was completely unable to transform E. corrodens, the most phylogenetic distant DUS-containing species with the most divergent DUS dialect of the Neisseriaceae. E. corrodens was however readily transformed with its autogenic eikDUS documenting DUS-favoured transformation in the Eikenella genus for the first time. Also K. denitrificans transformed very poorly with AT-DUS and showed significant transformation with the autogenic king3DUS demonstrating DUS-favoured transformation in the Kingella genus for the first time. Low but significant transformation was achieved with AT-DUS in N. elongata harbouring the very similar AG-DUS. No biological transformation data was generated for Neisseria mucosa and N. sicca harbouring AG-mucDUS since the two strains tested, Neisseria mucosa type strain ATCC 19696 and N. sicca ATCC 29259, were not transformable with PCR-generated DNA or isogenic genomic DNA, both conferring streptomycin resistance.
Although the transformation efficiency is a measure of the final biological outcome, it is not useful for a quantitative measure of DNA binding to the cell and the DNA uptake. To assess the latter parameters in regard to the DUS dialects, the levels of binding and uptake of radiolabelled DNA was measured in different strains and species. In N. meningitidis MC58, binding of DNA with different DUS dialects was only reduced 1.7-fold and 3.3-fold for AG-DUS and AG-mucDUS, respectively, but about 60-fold for AG-kingDUS and about 95-fold for AG-eikDUS compared to AT-DUS (Figure 4B). DNA uptake was around 60% of bound DNA for AT-DUS, AG-DUS and AG-mucDUS but only around 10% of bound DNA for AG-kingDUS and AG-eikDUS. Another N. meningitidis strain, serogroup C strain 8013, was also tested and showed reduced overall sequence specific and dialect dependent DNA binding but did not show sequence specific DNA uptake (Figure S4 and Figure S5). ComP in these two meningococcal strains are identical, indicating that more factors influence DUS-dependent DNA uptake in these strains. N. mucosa ATCC 25996, N. elongata subsp. glycolytica ATCC 29315, K. oralis ATCC 51147 and E. corrodens ATCC 23834 were also tested for DNA binding and uptake (Figure 5). N. mucosa showed a DNA binding of >2% of added DNA while all other species tested displayed values <0.3%. Only N. elongata showed a DUS dialect-dependent DNA binding with 0.3% for its own AG-DUS and 0.01% for AG-eikDUS (Figure 5B). The binding and uptake performance of individual DUS-dialects in N. elongata mirrors those of N. meningitidis strain 8013 (Figure S5). The counts for K. oralis and E. corrodens were below 100 cpm and differences in performance of the different DNA templates were accordingly small. However, it is noteworthy that DNA binding of the autogenous DUS is significantly (p≤0.05) higher than the negative control in K. oralis. Similarly, the DNA uptake of the autogenous DUS is significantly (p≤0.05) higher than the negative control in E. corrodens. DNA uptake was around 60% of bound DNA for N. elongata, K. oralis and E. corrodens but only around 0.4% for N. mucosa. The results suggest that the investigated strains of these four bacterial species may not, or only to a small extent, carry out DUS-specific uptake of DNA (Figure 5) contrasting the clear transformation data (Table 1).
Fig. 5. Quantification of DNA binding and uptake of Neisseriaceae with different DUS variants. 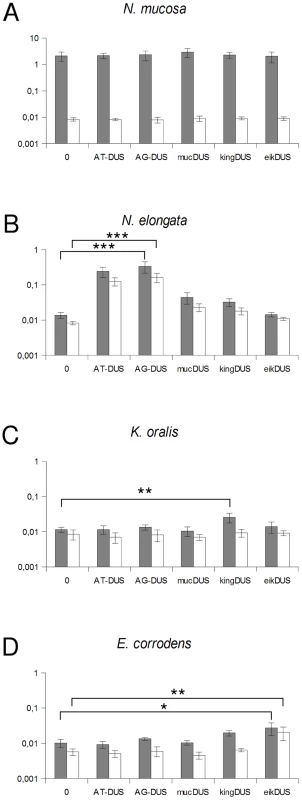
Total DNA binding shown as gray bars and benzonase resistant DNA (uptake) shown as white bars plotted as percentage of DNA added. The species tested are Neisseria mucosa (A), Neisseria elongata subsp. glycolytica (B), Kingella oralis (C) and Eikenella corrodens (C). Abscissa labels give the DUS variant. Represented are the results from 3 (A, D) and 5 (B, C) independent experiments. Student's t-test values are indicated (p≤0.1 = *, p≤0.05 = **, p≤0.001 = ***). Discussion
Genetic transformation in bacteria is a most common event in nature that requires a complex DNA uptake machinery and well-conserved recombination proteins [2], [34], [35], [36], [37]. Transformation distinguishes itself from other modes of horizontal gene transfer (conjugation and transduction) in that the recipient cell is actively taking part in the mobilization and integration of incoming DNA. Transformation in phylogenetically distant bacteria is thus adapted to ensure efficient uptake and recombination of homologous DNA [4]. This process relies on homologous recombination proteins whose processing functions are ubiquitously conserved from single celled bacteria to complex organisms, including humans. As such, transformation may be considered a low-complexity form of sex that is not firmly linked to reproduction but may have evolved to provide a similar selective advantage in breaking up associations among alleles [38]. The bacterial families Neisseriaceae and Pasteurellaceae are most suitable model organisms for the study of transformation and its role as a possible barrier to uptake of heterologous DNA. Here, we report the presence of eight distinct DUS dialects in the Neisseriaceae and correlate their distribution to the robust phylogeny of the family. This association emphasizes the influence of autogenic recombination on evolution and divergence of lineages. DUS-dependent transformation is documented for the first time in the genera Eikenella and Kingella. In a transversion mutant analysis the differential importance of each individual nucleotide that constitutes the AT-DUS was shown in N. meningitidis and these observations were found to relate to a conserved DUS core and the potential for interspecific DUS-mediated transformation.
DUS counts
There are highly overrepresented sequences in the genomes of bacteria in general and the skewed occurrence of di-, tri - and tetra-mers has been particularly well documented [39]. These repeat distributions have proven valuable for classification [40]. The crossover hotspot instigator (Chi) sequence differs between bacterial species and new Chi sequences have been identified by a bioinformatics search for motifs [41]. Although uptake sequences and Chi sequences both are closely linked to homologous recombination, Chi and USS are distinctly different sequences in H. influenzae with no functional overlap [42]. As previously demonstrated [40], the DUS sequence is the most abundant repeat in the genomes of N. meningitidis and N. gonorrhoeae with about 1900 occurrences in the 2.2–2.3 Mb genomes (Table S1). In the Neisseria sp. containing the AG-DUS dialect, the counts were generally higher, around 3000. The most frequent DUS dialect was found for the kingDUS in K. oralis ATCC 51147 with nearly 6000 kingDUS within its 2.4 Mb genome sequence. Despite the high numbers of accurate DUS hits, the numbers of DUS with a single nucleotide divergence were considerably higher in all species (Table S1), revealing a potential for the activation of even more DUS positions.
The difference in total DUS count and ratio of DUS to genome size may reflect an ultimate saturation-state of DUS or indicate that this state has not yet been reached. Also, if DUS specificity was for some reason lost, the DUS could be degenerating slowly but progressively, as observed for pseudogenes. Bacterial genomes with a high number of DUS had a relatively low number of DUS with a single divergence (DUS+1mut) and vice versa. For example, Neisseria bacilliformis ATCC BAA-1200 had 4265 DUS and 4914 DUS+1mut (ratio 1∶1.15) while Neisseria cinerea ATCC 14685 had 943 DUS and 1372 DUS+1mut (ratio 1∶1.45) (Table S1). These differences could reflect differences in DUS dependency, which is known to vary in different N. gonorrhoeae strains, and may therefore also vary between species and their respective dialects [15]. Future studies will seek to address the influence of sequence variation and regulation of ComP and its antagonist PilV [17] in this regard. DUS saturation of the chromosome may also be opposed by factors such as the degree of interference with coding ability for intragenic DUS. Notably, all DUS dialects, except king3DUS, harboured a stop codon (UGA) in one reading frame, which imposes an obvious limitation on the liberty of positioning DUS.
By exploiting two different DUS dialects simultaneously some flexibility may be achieved in regard to which amino acids that are encoded by intragenic DUS. The genome sequence of S. muelleri harboured both the kingDUS and the simDUS, allowing for the variation of Q↔L and S↔C at the protein level when a DUS is found within a coding sequence. However, other explanations for the co-occurrence of two DUS-dialects in a single genome are high frequency of commutation between species (simDUS and kingDUS differ in a single nucleotide only), or consecutive habitats in different mammalian hosts with access to variant DUS dialects. DUS specificity may be altered by mutations in comP or by the acquisition of alleles encoding a novel DUS dialect, or simply by the presence of two DUS-specific proteins with different affinities, which could have originated from a simple gene duplication. However, only a single copy of comP was identified in the S. muelleri genome. It is also noteworthy that 1294 occurrences of the simDUS and kingDUS in S. muelleri are arranged as overlapping pairs in a dyad symmetry structure (Figure S6), which may indicate a dimer-based mechanism of DUS recognition. No preference for a single reading frame or positioning inside or outside of coding sequences was obvious when using the preliminarily annotated genome sequence from S. muelleri. A similar symmetry is found also in Kingella species where king3DUS pairs with king2DUS. The latter showed positive influence on transformation (data not shown) but is not a commonly found DUS by itself (Figure S6).
DUS have been found to locate in permissive regions of the core genomes of N. meningitidis, N. gonorrhoeae and N. lactamica, and intragenic DUS positions are common allowing them to be transcribed [11]. Intergenic regions, on the other hand, are particularly permissive and DUS sequences have been found to associate with transcriptional terminators by having frequently adopted an inverted paired organization, able to form stem-loop structures on ssDNA [7], [14], [43]. This inverted pair organization was found in high numbers in all genomes harbouring DUS dialects, suggestive of their association to transcriptional terminators (Table S2). In contrast to the simDUS and kingDUS arrangement in the S. muelleri genome, the individual DUS in an inverted pair DUS do not overlap.
Another interesting observation is the occurrence of peregrine DUS, exemplified by the mucDUS in Table S1A. N. meningitidis and N. gonorrhoeae genomes had a very consistent mucDUS count of about 160 and 110, respectively, while Neisseria sp. oral taxon 014 st. F0314 had the highest mucDUS count (467), and the N. weaveri strains had the lowest counts (15) in the canonical DUS group. The N. meningitidis genome for example contained 8% mucDUS in addition to the canonical DUS, while the genomes of the mucDUS-containing group of bacteria harboured between 7% and 12% DUS. These numbers likely reflect recent commutation between these two groups sharing the same ecological niche. The exchange of highly selectable markers between N. meningitidis and the commensal Neisseriae is well established [44], [45], [46], [47], [48], [49]. The amount of mucDUS relative to the canonical DUS was particularly low in the N. weaveri genomes (0.5%, Table S1A), which is interesting since N. weaveri is a canine commensal and only an opportunistic pathogen to humans [31], [50], [51].
In contrast to a previous report on the abundance of mucDUS in N. subflava strain ATCC 19243, we found that AG-DUS is the most abundant dialect in N. subflava NJ9703 [22]. Possibly, N. subflava strain ATCC 19243 acquired the folP sequence fragment from N. sicca and therefore harbours the mucDUS in this region, or alternatively, N. subflava strain ATCC 19243 is more closely related to N. sicca than to N. subflava NJ9703. We identified more canonical DUS in the N. elongata subsp. glycolytica strain ATCC 29315, 3273 as opposed to 2142 than in the former study by Higashi et al. [23], and more mucDUS, 174 as opposed to 117. These differences could possibly be due to recent updates of the genome sequence files available. Comparison of the predominantly mucDUS-containing bacteria is difficult, as Higashi et al. did not specify which strains were analysed, both were, however, reported to contain >3400 copies of the mucDUS. In contrast to this observation, we identified only 1543 mucDUS in N. mucosa strain ATCC 25996 while N. mucosa C102 had only 155 mucDUS and 2964 canonical DUS. These discrepancies indicate that strain C102 may erroneously be assigned N. mucosa, also since both the core genome phylogeny (Figure 2) and that of ComP demonstrated the close genetic relationship between this strain and N. subflava and N. flavescens.
Core genome-based phylogeny
Neisseriaceae are highly recombinogenic yielding a polyphyletic family structure, and resolving the family into distinct species was achieved by including large amounts of sequence data in the analysis. Initially, such analyses were based on sequence divergence of a single gene (16SrDNA) or on a small number of housekeeping genes as in multi locus sequence typing (MLST) [52].
The evolution of distinct DUS dialects in this phylogenetically compact family is a striking example of how preference for homologous DNA in highly transformable bacteria affects evolution. Differences between the dialects are expected to be mirrored in the amino acid sequence(s) of the recently confirmed DUS-specific binding protein ComP, and warrants further functional investigation. The congruence between DUS-dialect and phylogeny and the presence of ComP suggests that those dialects that remain to be confirmed functional DUS are true DUS. This is also further emphasized by the exceptional overrepresentation and conservation of each dialect in their respective genomes. The most plausible hypothesis explaining these observations is DUS-dependent bias in frequent transformation/recombination [11].
Single nucleotide mutation analysis of DUS
The differential influence of each nucleotide in AT-DUS on N. meningitidis transformation and DNA binding/uptake was tested by employing donor DNA harbouring altered DUS. Single nucleotides were altered to be the transverse (purine↔pyrimidine) and the least common nucleotide at that position in the N. meningitidis genome. A similar analysis, based on the uptake of radioactive labelled DNA, has previously been reported in H. influenzae [53], [54]. Here the first steps of transformation were investigated by a DNA binding and uptake assay. The quantitative transformation method employed here measures the outcome of both uptake and recombination of DNA. This gradual analysis is important since it has been documented that DUS may influence multiple steps during transformation [15]. The most significant 5′-CTG-3′ core identified in transformation was conserved in all dialects of DUS (Figure 1). In contrast to the transformation experiments with N. meningitidis MC58, DNA binding did not display differential binding of AT-DUS and mutated AT-DUS versions, but clearly showed that binding discriminated against the DUS-less negative control (Figure 3B). This observation suggests that DNA binding in this strain is not very strict in terms of DUS specificity, and that DUS and single nucleotide mutated DUS can contribute to binding. Also the observation that the DUS dialects most similar to AT-DUS bound better than the more distant dialects emphasizes this point (Figure 4B).
It has been hypothesized that DUS specificity may function at more than one level during transformation [15] and one may speculate that initial binding by ComP [17], [18] could display weak DUS specificity and that the influence of the core 5′-CTG-3′ first become influential during uptake or later during the transformation process. The DNA uptake data from N. meningitidis (Figure 3B and Figure 4B) are corroborating the transformation data in that AT-DUS outperforms the other dialects tested. The differences in relative uptake of the close and distant DUS dialects in N. meningitidis (Figure 4B) suggest that DNA uptake is not only a relative function of binding, but may be influenced by DUS specificity.
DUS–mediated commutation in Neisseriaceae species
The transformation performance of individual DUS-dialects from separate phylogenetic branches (Figure 2) was tested in N. meningitdis, N. elongata, E. corrodens and K. denitirificans (Table 1). The degree of similarity between the DUS-dialect of the recipient species, AT-DUS in N. meningitidis, and that in the donor DNA, AG-DUS, AG-mucDUS, AG-kingDUS and AG-eikDUS, directly correlate with the level of transformation. The potential for high levels of commutation when DUS dialects are similar is reflected in reports describing interchange of DNA between pathogenic and commensal Neisseria in vivo [45], [55].
This correlation is also evident in transformations of the AG-DUS species N. elongata, since AT-DUS outperforms AG-mucDUS. These observations suggest further that a nucleotide change in position −1 of the DUS is less influential than a change in position +2 in concordance with the results in N. meningitidis (Figure 3A). Transformations in the genera Eikenella and Kingella show strict autologous DUS-dependency in transformation indicating that AT-DUS is too divergent to allow transformation. It is well established that general sequence divergence between recipient chromosome and transforming DNA is strongly affecting homologous recombination, the last step in transformation [3]. Based on these observations one may anticipate that the phylogenetic distance correlates with the potential for commutation since DUS dialect distribution is reflected in the orthology of the Neisseriaceae. Furthermore, no significant transformation of N. meningitidis was observed when transforming DNA carried USS, which is the DUS of the Pasteurellaceae. Since H. influenzae and N. meningitidis share the same habitats, and are likely to encounter each other's DNA in e.g. oropharyngeal biofilms, the establishment of a functional barrier to commutation between these species may be important for the preservation of genome integrity. Genetic exchange between N. meningitidis and H. influenzae is rare [56] while the frequent commutation within the Pasteurellaceae is well documented [21], [57].
N. elongata subsp. glycolytica was previously shown to be transformable with a GT-mucDUS, but with an 8-fold reduced efficacy compared to a GT-DUS [23]. In our analysis, using a similar reporter construct, this factor was higher for the AG-mucDUS when compared to the AT-DUS (25-fold) and when compared to the AG-DUS (35-fold) (Table 1). These differences could relate to the employment of the non-ideal GT-DUS in the initial study. The DNA binding and uptake assays show that N. elongata subsp. glycolytica, like N. meningitidis, binds DNA in a DUS-specific manner with preference for the most similar DUS sequence corroborating the transformation results discussed above.
The influence of AG-DUS and AT-DUS in transformation of N. mucosa or N. sicca could not be tested since these strains were incompetent for transformation. The molecular basis for this remains unexplored, but the DNA uptake data show that transformation deficiency can be linked to the reduced ability to take up DNA, suggestive of a malfunction in this initial step of transformation. It is curious that N. mucosa binds DNA exceptionally well in a DUS-independent manner and this observation warrants further investigation. The functionality of AG-mucDUS in transformation has also been verified in other laboratories (N. Weyand, personal communication, [22]) but mucDUS-dependent transformation of species in the mucDUS-group has not yet been demonstrated. Recent observations in our lab confirm that also wadDUS is a true DUS affecting transformation in N. wadsworthii (unpublished data). The simDUS found in S. muelleri and kingDUS found in K. oralis are the only DUS that remains to be functionally verified, but the presence of comP genes in their respective genomes [18], the high overrepresentation of each DUS dialect and intragenomic DUS conservation in addition to their high similarity to the other DUS strongly suggests that they are, or at least have been, genuine DUS.
E. corrodens and K. denitrificans have previously been shown competent for transformation with homospecific DNA [58], [59]. Here, we established that this specificity is DUS-dependent. The DNA binding and uptake results did not reflect the differences observed in transformation since DNA binding was relatively uniform irrespective of the DUS-dialect used. However, it must be noted that binding was very low in both K. oralis and E. corrodens although a weak but statistically significant preference for autologous DUS in DNA binding or uptake, respectively, is evident (Figure 5).
Here, we expanded the number of bacteria that utilize a DUS-dependent mechanism for transformation of homologous DNA. Eight distinct dialects of DUS in the family Neisseriaceae were described, and the ability to overcome transformation barriers was assayed by both transformation and DNA binding and uptake for five of these. Furthermore, the evolution of DUS dialects corresponds with the evolution of the distinct core genomes of each phylogenetic clade. The DUS signal was analyzed by single nucleotide mutational analysis and an essential three nucleotide core sequence was found to be strictly required for transformation. This functional DUS core is conserved in all eight DUS dialects. The level of commutation was found to correlate with the phylogenetic distance and also to the similarity of the DUS sequences themselves. Future studies will explore the evolution of DUS dialects in regard of the recently confirmed association between DUS and ComP.
Materials and Methods
Genome sequence bioinformatic analysis
Search for overrepresented repeat sequences
Sequences from completed and unfinished Neisseriaceae genomes were retrieved from NCBI GenBank. For unfinished sequences, the available contigs were concatenated into a single FASTA file. The Perl script repeat_finder.pl [21] was used for the in silico search for overrepresented sequences in these files. Since DUS and USS reportedly are 12 and 9 base pairs long, respectively, this range was used for the search. Highly overrepresented sequences were aligned to find repeated patterns and similarities to known repeats. The program fuzznuc from the EMBOSS package [60] was used to count the number of occurrences of new identified repeated sequences, allowing also for nucleotide variations (see Table S1). An in-house Perl-script was used to extract the repeat sequences including surrounding sequence into a FASTA file, which was used as input for the WebLogo service [61], to generate the sequence logos shown in Figure S1. To clearly distinguish between new DUS dialects and existing terms, a nomenclature scheme was devised. The basic name DUS was applied to 10-mer DNA Uptake Sequences in general and specifically assigned to the canonical 10-mer DUS found in N. meningitidis and individual nucleotides were numbered 1–10 as in previous publications. Capital letters in front of DUS-name depict conserved bases in positions −1 and −2 such as AT in the 12-mer AT-DUS in concordance with previous use [14], [15]. The new DUS dialects were assigned an abbreviated name of the host species, in which the variant was first found, in small letters in front of DUS (Figure 1). The eikDUS was first identified in the genome of Eikenella corrodens, the kingDUS in Kingella oralis, the king3DUS in Kingella denitrificans, the simDUS in Simonsiella muelleri and the wadDUS in N. wadsworthii. The mucDUS, which was previously called “altered DUS” or “DUS1” [22], [23], was first identified in N. mucosa.
Construction of a core genome-based phylogenetic tree
A phylogenetic tree for the family Neisseriaceae was made using the annotated completed genomes and annotated contigs from Genbank. In addition the sequences from Neisseria sp., oral taxon 014 and S. muelleri ATCC 29453 were annotated with GenDB [62]. C. violaceum ATCC 12472 was used as the outgroup in the phylogenetic analysis. With the availability of genome sequences it was now possible to establish a phylogeny based on whole genome alignments. These comprehensive alignments provide information about the pan-genome and the core genome and thereby allow the establishment of a very robust phylogeny. Many bioinformatics tools were designed that can be used for this task [63], [64], [65], [66]. With the increasing number of genome sequences, highly stable core genome phylogenetic trees were generated for different groups of the genus Neisseria [25], [27], [28], [29], [31]. Here, we used the EDGAR service to build the phylogenetic unrooted tree based on 474 coding sequences constituting the core genome of 22 bacteria [67].
Construction and purification of plasmids containing the DUS variants
Plasmids containing the dialects and transversion variants of DUS were based on the plasmid p0-DUS [14]. Oligonucleotides listed in Table S3 were used to amplify the pilG::ermC fragment from p0-DUS by PCR whereby the oligomer OH3 was always used as reverse primer. The PCR products were digested with XhoI and SacII and inserted into the multiple cloning site of the vector pBluescript II SK+ (Stratagene, USA). E. coli strain ER2566 (NEB, USA) was used for cloning and the strain XL-1 Blue (Stratagene, USA) was used for large scale purification of plasmids due to higher yields. Plasmids were purified using the QIAGEN Plasmid Plus Midi Kit (Qiagen, Germany). Plasmids are listed in Table S4. The DNA was diluted to a concentration of 100 ng/µl in 10 mM Tris, pH 8, and stored at −20°C until used.
Bacterial strains and growth conditions
Bacteria used in this study are listed in Table S4. Escherichia coli strains were grown on LB medium. E. coli XL-1 blue was used for the quantitative production of the pDV plasmids. N. meningitidis and N. elongata were grown on blood agar plates, on GC medium plates or in liquid GC medium supplemented with IsoVitaleX. K. denitrificans and E. corrodens were grown on blood or chocolate agar plates or, when in liquid, in brain heart infusion broth. Antibiotics were added to the media when appropriate.
Quantitative transformation
Quantitative transformation was performed as previously described [14] using plasmid or genomic DNA carrying an antibiotic resistance marker. Briefly, for N. meningitidis, cells grown over night at 37°C were suspended in 5% CO2 saturated GC medium containing IsoVitaleX and 7 mM MgCl2. 5 µl of DNA (100 ng/µl) were provided in 15 ml tubes, 500 µl cell suspension was added, shortly mixed by vortexing and incubated at 37°C for 30 min without agitation. Each sample was diluted by adding 4.5 ml GC medium and incubated for 4.5 h at 37°C on a tumbler (60 rpm). The cultures were then mixed and serial dilutions prepared in GC medium. Of each undiluted sample, 50 µl aliquots were spread on blood agar plates containing 8 µg ml−1 erythromycin or 50 µg ml−1 streptomycin and 50 µl of the 10−7 dilution were spread on blood agar plates without antibiotics. At least 2 agar plates were inoculated from each sample and experiments were repeated at least three times. Colonies were counted following over night incubation in a 5% CO2 atmosphere at 37°C. Individual transformation frequencies were calculated as the number of antibiotic-resistant colony forming units (CFU) per total CFU. The absolute transformation frequencies varied between experiments, possibly due to the difficulty in reproducing bacterial suspensions with identical fractions of competent bacteria. This problem was also reported earlier [22], and was resolved here by the use of relative values based on an internal standard. A Kendall's W test showed a very high concordance of gained orders (Kendall's W = 0.9346, χ2 = 85.0506, df = 13, p<0.0001). The absolute transformation frequencies are plotted in Figure S2.
In order to transform N. elongata, K. denitrificans and E. corrodens, small alterations of the protocol were required. The N. elongata subsp. glycolytica strain employed here was the type strain originally isolated by Henriksen et al. [68]. Agglutinating P+ colonies of N. elongata were pre-selected since previous experiments had documented a positive correlation between agglutination and transformation [69]. The type strain of K. denitrificans and E. corrodens strain 31745 were used for quantitative transformation. The E. corrodens strain 31745 is a reference strain that was previously shown to be transformable [58]. To generate donor DNA for the quantitative transformation experiments, spontaneous streptomycin-resistant mutants of N. elongata, K. denitrificans and E. corrodens were isolated and the genomic DNA from these strains used as a template for a PCR of the rpsL gene using primers listed in Table S5. The rpsL genes were sequenced, confirming the Lys to Arg mutation at amino acid position 43 conferring streptomycin resistance [70]. rpsL PCR products were adjusted to 50 ng/µl and 10 µl thereof was used in transformation of 500 µl cell suspension. K. denitrificans was transformed in Brain heart infusion medium supplemented with 7 mM MgCl2 and the second incubation time was extended to 5 hours.
DNA binding and uptake assay with radiolabeled DNA substrates
For radiolabeling the pDV plasmids (Table S4) were linearized by digestion with ScaI, purified using QIAquick columns (Qiagen) and treated with exonuclease III (Fermentas). After heat inactivation 10 µg of the partially single stranded DNA were incubated with 3 µM dNTPs, 20 µCi [α-32P]dCTP and 10 units Klenow fragment (3′→5′ exo–) (NEB) for 90 min at 37°C. The fill-in reaction was finished after increasing the dNTP concentration to 30 µM and additional incubation for 30 min. The products were purified as before and showed specific activities of 4×105 to 2×106 CPM µg−1. The Neisseriaceae were grown in liquid medium to an optical density at 660 nm of approximately 1. MgCl2 was added to 7 mM and one ml aliquots were incubated with DNA with about 5×106 CPM activity and rotated at 37°C for 45 min. The samples were then split into two 500 µl samples and 12.5 units Benzonase (Merck) was added to one of these. After additional incubation for 15 min the cells were washed three times by centrifugation for 3 min at 5000× g and resuspension in liquid medium including 7 mM MgCl2. The final pellets were resuspendet in 3 ml scintillation fluid (Ultima Gold MV, PerkinElmer) and measured twice for 3 min in a Tri-Carb 2900TR (PerkinElmer) using an energy window LL-UL = 50–1700. DNA binding and uptake are reported as the percentage of DNA added and percentage of cell-bound DNA, respectively, and the results are presented as the means of for 3 to 5 replicates.
Supporting Information
Zdroje
1. ThomasCM, NielsenKM (2005) Mechanisms of, and barriers to, horizontal gene transfer between bacteria. Nat Rev Microbiol 3 : 711–721.
2. JohnsborgO, EldholmV, HavarsteinLS (2007) Natural genetic transformation: prevalence, mechanisms and function. Res Microbiol 158 : 767–778.
3. RobertsMS, CohanFM (1993) The effect of DNA sequence divergence on sexual isolation in Bacillus. Genetics 134 : 401–408.
4. MajewskiJ (2001) Sexual isolation in bacteria. FEMS Microbiol Lett 199 : 161–169.
5. TortosaP, DubnauD (1999) Competence for transformation: a matter of taste. Curr Opin Microbiol 2 : 588–592.
6. DannerDB, DeichRA, SiscoKL, SmithHO (1980) An eleven-base-pair sequence determines the specificity of DNA uptake in Haemophilus transformation. Gene 11 : 311–318.
7. GoodmanSD, ScoccaJJ (1988) Identification and arrangement of the DNA sequence recognized in specific transformation of Neisseria gonorrhoeae. Proc Natl Acad Sci U S A 85 : 6982–6986.
8. SmithHO, GwinnML, SalzbergSL (1999) DNA uptake signal sequences in naturally transformable bacteria. Res Microbiol 150 : 603–616.
9. DavidsenT, RodlandEA, LagesenK, SeebergE, RognesT, et al. (2004) Biased distribution of DNA uptake sequences towards genome maintenance genes. Nucleic Acids Res 32 : 1050–1058.
10. TettelinH, SaundersNJ, HeidelbergJ, JeffriesAC, NelsonKE, et al. (2000) Complete genome sequence of Neisseria meningitidis serogroup B strain MC58. Science 287 : 1809–1815.
11. TreangenTJ, AmburOH, TønjumT, RochaEP (2008) The impact of the neisserial DNA uptake sequences on genome evolution and stability. Genome Biol 9: R60.
12. ElkinsC, ThomasCE, SeifertHS, SparlingPF (1991) Species-specific uptake of DNA by gonococci is mediated by a 10-base-pair sequence. J Bacteriol 173 : 3911–3913.
13. GravesJF, BiswasGD, SparlingPF (1982) Sequence-specific DNA uptake in transformation of Neisseria gonorrhoeae. J Bacteriol 152 : 1071–1077.
14. AmburOH, FryeSA, TønjumT (2007) New functional identity for the DNA uptake sequence in transformation and its presence in transcriptional terminators. J Bacteriol 189 : 2077–2085.
15. DuffinPM, SeifertHS (2010) DNA uptake sequence-mediated enhancement of transformation in Neisseria gonorrhoeae is strain dependent. J Bacteriol 192 : 4436–4444.
16. AasFE, LovoldC, KoomeyM (2002) An inhibitor of DNA binding and uptake events dictates the proficiency of genetic transformation in Neisseria gonorrhoeae: mechanism of action and links to Type IV pilus expression. Mol Microbiol 46 : 1441–1450.
17. AasFE, WolfgangM, FryeS, DunhamS, LovoldC, et al. (2002) Competence for natural transformation in Neisseria gonorrhoeae: components of DNA binding and uptake linked to type IV pilus expression. Mol Microbiol 46 : 749–760.
18. CehovinA, SimpsonPJ, McDowellMA, BrownDR, NoscheseR, et al. (2013) Specific DNA recognition mediated by a type IV pilin. Proc Natl Acad Sci U S A
19. GoodmanSD, ScoccaJJ (1991) Factors influencing the specific interaction of Neisseria gonorrhoeae with transforming DNA. J Bacteriol 173 : 5921–5923.
20. AmburOH, FryeSA, NilsenM, HovlandE, TonjumT (2012) Restriction and sequence alterations affect DNA uptake sequence-dependent transformation in Neisseria meningitidis. PLoS ONE 7: e39742 doi:10.1371/journal.pone.0039742.
21. RedfieldRJ, FindlayWA, BosseJ, KrollJS, CameronAD, et al. (2006) Evolution of competence and DNA uptake specificity in the Pasteurellaceae. BMC Evol Biol 6 : 82.
22. QvarnstromY, SwedbergG (2006) Variations in gene organization and DNA uptake signal sequence in the folP region between commensal and pathogenic Neisseria species. BMC Microbiol 6 : 11.
23. HigashiDL, BiaisN, WeyandNJ, AgellonA, SiskoJL, et al. (2011) N. elongata produces type IV pili that mediate interspecies gene transfer with N. gonorrhoeae. PLoS ONE 6: e21373 doi:10.1371/journal.pone.0021373.
24. PazA, KirzhnerV, NevoE, KorolA (2006) Coevolution of DNA-interacting proteins and genome “dialect”. Mol Biol Evol 23 : 56–64.
25. BudroniS, SienaE, Dunning HotoppJC, SeibKL, SerrutoD, et al. (2011) Neisseria meningitidis is structured in clades associated with restriction modification systems that modulate homologous recombination. Proc Natl Acad Sci U S A 108 : 4494–4499.
26. TurnbaughPJ, LeyRE, HamadyM, Fraser-LiggettCM, KnightR, et al. (2007) The human microbiome project. Nature 449 : 804–810.
27. SchoenC, BlomJ, ClausH, Schramm-GluckA, BrandtP, et al. (2008) Whole-genome comparison of disease and carriage strains provides insights into virulence evolution in Neisseria meningitidis. Proc Natl Acad Sci U S A 105 : 3473–3478.
28. MarriPR, PaniscusM, WeyandNJ, RendonMA, CaltonCM, et al. (2010) Genome sequencing reveals widespread virulence gene exchange among human Neisseria species. PLoS ONE 5: e11835 doi:10.1371/journal.pone.0011835.
29. HaoW, MaJH, WarrenK, TsangRS, LowDE, et al. (2011) Extensive genomic variation within clonal complexes of Neisseria meningitidis. Genome Biol Evol 3 : 1406–1418.
30. BennettJS, JolleyKA, EarleSG, CortonC, BentleySD, et al. (2012) A genomic approach to bacterial taxonomy: an examination and proposed reclassification of species within the genus Neisseria. Microbiology 158 : 1570–1580.
31. YiH, ChoYJ, YoonSH, ParkSC, ChunJ (2011) Comparative genomics of Neisseria weaveri clarifies the taxonomy of this species and identifies genetic determinants that may be associated with virulence. FEMS Microbiol Lett
32. Fitz-GibbonST, HouseCH (1999) Whole genome-based phylogenetic analysis of free-living microorganisms. Nucleic Acids Res 27 : 4218–4222.
33. SnelB, BorkP, HuynenMA (1999) Genome phylogeny based on gene content. Nat Genet 21 : 108–110.
34. KovacsAT, SmitsWK, MironczukAM, KuipersOP (2009) Ubiquitous late competence genes in Bacillus species indicate the presence of functional DNA uptake machineries. Environ Microbiol 11 : 1911–1922.
35. DubnauD (1999) DNA uptake in bacteria. Annu Rev Microbiol 53 : 217–244.
36. LorenzMG, WackernagelW (1994) Bacterial gene transfer by natural genetic transformation in the environment. Microbiol Rev 58 : 563–602.
37. ClaverysJP, MartinB (2003) Bacterial “competence” genes: signatures of active transformation, or only remnants? Trends Microbiol 11 : 161–165.
38. VosM (2009) Why do bacteria engage in homologous recombination? Trends Microbiol 17 : 226–232.
39. BurgeC, CampbellAM, KarlinS (1992) Over - and under-representation of short oligonucleotides in DNA sequences. Proc Natl Acad Sci U S A 89 : 1358–1362.
40. CoenyeT, VandammeP (2004) Use of the genomic signature in bacterial classification and identification. Syst Appl Microbiol 27 : 175–185.
41. HalpernD, ChiapelloH, SchbathS, RobinS, Hennequet-AntierC, et al. (2007) Identification of DNA motifs implicated in maintenance of bacterial core genomes by predictive modeling. PLoS Genet 3: e153 doi:10.1371/journal.pgen.0030153.
42. SouriceS, BiaudetV, El KarouiM, EhrlichSD, GrussA (1998) Identification of the Chi site of Haemophilus influenzae as several sequences related to the Escherichia coli Chi site. Mol Microbiol 27 : 1021–1029.
43. KingsfordCL, AyanbuleK, SalzbergSL (2007) Rapid, accurate, computational discovery of Rho-independent transcription terminators illuminates their relationship to DNA uptake. Genome Biol 8: R22.
44. SprattBG, BowlerLD, ZhangQY, ZhouJ, SmithJM (1992) Role of interspecies transfer of chromosomal genes in the evolution of penicillin resistance in pathogenic and commensal Neisseria species. J Mol Evol 34 : 115–125.
45. SprattBG, ZhangQY, JonesDM, HutchisonA, BranniganJA, et al. (1989) Recruitment of a penicillin-binding protein gene from Neisseria flavescens during the emergence of penicillin resistance in Neisseria meningitidis. Proc Natl Acad Sci U S A 86 : 8988–8992.
46. ZhouJ, BowlerLD, SprattBG (1997) Interspecies recombination, and phylogenetic distortions, within the glutamine synthetase and shikimate dehydrogenase genes of Neisseria meningitidis and commensal Neisseria species. Mol Microbiol 23 : 799–812.
47. SmithNH, HolmesEC, DonovanGM, CarpenterGA, SprattBG (1999) Networks and groups within the genus Neisseria: analysis of argF, recA, rho, and 16S rRNA sequences from human Neisseria species. Mol Biol Evol 16 : 773–783.
48. FeilEJ, MaidenMC, AchtmanM, SprattBG (1999) The relative contributions of recombination and mutation to the divergence of clones of Neisseria meningitidis. Mol Biol Evol 16 : 1496–1502.
49. LinzB, SchenkerM, ZhuP, AchtmanM (2000) Frequent interspecific genetic exchange between commensal Neisseriae and Neisseria meningitidis. Mol Microbiol 36 : 1049–1058.
50. HolmesB, CostasM, OnSL, VandammeP, FalsenE, et al. (1993) Neisseria weaveri sp. nov. (formerly CDC group M-5), from dog bite wounds of humans. Int J Syst Bacteriol 43 : 687–693.
51. AndersenBM, SteigerwaltAG, O'ConnorSP, HollisDG, WeyantRS, et al. (1993) Neisseria weaveri sp. nov., formerly CDC group M-5, a gram-negative bacterium associated with dog bite wounds. J Clin Microbiol 31 : 2456–2466.
52. HanageWP, FraserC, SprattBG (2005) Fuzzy species among recombinogenic bacteria. BMC Biol 3 : 6.
53. BakkaliM (2007) Genome dynamics of short oligonucleotides: the example of bacterial DNA uptake enhancing sequences. PLoS ONE 2: e741 doi:10.1371/journal.pone.0000741.
54. MaughanH, WilsonLA, RedfieldRJ (2010) Bacterial DNA uptake sequences can accumulate by molecular drive alone. Genetics 186 : 613–627.
55. BowlerLD, ZhangQY, RiouJY, SprattBG (1994) Interspecies recombination between the penA genes of Neisseria meningitidis and commensal Neisseria species during the emergence of penicillin resistance in N. meningitidis: natural events and laboratory simulation. J Bacteriol 176 : 333–337.
56. KrollJS, WilksKE, FarrantJL, LangfordPR (1998) Natural genetic exchange between Haemophilus and Neisseria: intergeneric transfer of chromosomal genes between major human pathogens. Proc Natl Acad Sci U S A 95 : 12381–12385.
57. AlbrittonWL, SetlowJK, ThomasML, SottnekFO (1986) Relatedness within the Family Pasteurellaceae as Determined by Genetic Transformation. International Journal of Systematic Bacteriology 36 : 103–106.
58. TønjumT, HagenN, BøvreK (1985) Identification of Eikenella corrodens and Cardiobacterium hominis by genetic transformation. Acta Pathol Microbiol Immunol Scand B 93 : 389–394.
59. BøvreK (1964) Studies on Transformation in Moraxella and Organisms Assumed to Be Related to Moraxella. 1. a Method for Quantitative Transformation in Moraxella and Neisseria, with Streplomycin Resistance as the Genetic Marker. Acta Pathol Microbiol Scand 61 : 457–473.
60. RiceP, LongdenI, BleasbyA (2000) EMBOSS: the European Molecular Biology Open Software Suite. Trends Genet 16 : 276–277.
61. CrooksGE, HonG, ChandoniaJ-M, BrennerSE (2004) WebLogo: A Sequence Logo Generator. Genome Res 14 : 1188–1190.
62. MeyerF, GoesmannA, McHardyAC, BartelsD, BekelT, et al. (2003) GenDB–an open source genome annotation system for prokaryote genomes. Nucleic Acids Res 31 : 2187–2195.
63. LaingC, BuchananC, TaboadaEN, ZhangY, KropinskiA, et al. (2010) Pan-genome sequence analysis using Panseq: an online tool for the rapid analysis of core and accessory genomic regions. BMC Bioinformatics 11 : 461.
64. KorbelJO, SnelB, HuynenMA, BorkP (2002) SHOT: a web server for the construction of genome phylogenies. Trends Genet 18 : 158–162.
65. DarlingAE, MauB, PernaNT (2010) progressiveMauve: multiple genome alignment with gene gain, loss and rearrangement. PLoS ONE 5: e11147 doi:10.1371/journal.pone.0011147.
66. TreangenTJ, MesseguerX (2006) M-GCAT: interactively and efficiently constructing large-scale multiple genome comparison frameworks in closely related species. BMC Bioinformatics 7 : 433.
67. BlomJ, AlbaumSP, DoppmeierD, PuhlerA, VorholterFJ, et al. (2009) EDGAR: a software framework for the comparative analysis of prokaryotic genomes. BMC Bioinformatics 10 : 154.
68. HenriksenSD, HoltenE (1976) Neisseria elongata subsp. glycolytica subsp. nov. International Journal of Systematic Bacteriology October 1976 : 478–481.
69. BøvreK, FrøholmLO, HenriksenSD, HoltenE (1977) Relationship of Neisseria elongata subsp. glycolytica to other members of the family Neisseriaceae. Acta Pathol Microbiol Scand B 85B: 18–26.
70. ToriiN, NozakiT, MasutaniM, NakagamaH, SugiyamaT, et al. (2003) Spontaneous mutations in the Helicobacter pylori rpsL gene. Mutat Res 535 : 141–145.
71. AltschulSF, GishW, MillerW, MyersEW, LipmanDJ (1990) Basic local alignment search tool. J Mol Biol 215 : 403–410.
Štítky
Genetika Reprodukčná medicína
Článek The G4 GenomeČlánek Mondo/ChREBP-Mlx-Regulated Transcriptional Network Is Essential for Dietary Sugar Tolerance inČlánek RpoS Plays a Central Role in the SOS Induction by Sub-Lethal Aminoglycoside Concentrations inČlánek Tissue Homeostasis in the Wing Disc of : Immediate Response to Massive Damage during DevelopmentČlánek Disruption of TTDA Results in Complete Nucleotide Excision Repair Deficiency and Embryonic LethalityČlánek DJ-1 Decreases Neural Sensitivity to Stress by Negatively Regulating Daxx-Like Protein through dFOXO
Článok vyšiel v časopisePLOS Genetics
Najčítanejšie tento týždeň
2013 Číslo 4- Gynekologové a odborníci na reprodukční medicínu se sejdou na prvním virtuálním summitu
- Je „freeze-all“ pro všechny? Odborníci na fertilitu diskutovali na virtuálním summitu
-
Všetky články tohto čísla
- Epigenetic Upregulation of lncRNAs at 13q14.3 in Leukemia Is Linked to the Downregulation of a Gene Cluster That Targets NF-kB
- A Big Catch for Germ Cell Tumour Research
- The Quest for the Identification of Genetic Variants in Unexplained Cardiac Arrest and Idiopathic Ventricular Fibrillation
- A Nonsynonymous Polymorphism in as a Risk Factor for Human Unexplained Cardiac Arrest with Documented Ventricular Fibrillation
- The Hourglass and the Early Conservation Models—Co-Existing Patterns of Developmental Constraints in Vertebrates
- Smaug/SAMD4A Restores Translational Activity of CUGBP1 and Suppresses CUG-Induced Myopathy
- Balancing Selection on a Regulatory Region Exhibiting Ancient Variation That Predates Human–Neandertal Divergence
- The G4 Genome
- Extensive Natural Epigenetic Variation at a Originated Gene
- Mouse Oocyte Methylomes at Base Resolution Reveal Genome-Wide Accumulation of Non-CpG Methylation and Role of DNA Methyltransferases
- The Environment Affects Epistatic Interactions to Alter the Topology of an Empirical Fitness Landscape
- TIP48/Reptin and H2A.Z Requirement for Initiating Chromatin Remodeling in Estrogen-Activated Transcription
- Aconitase Causes Iron Toxicity in Mutants
- Tbx2 Terminates Shh/Fgf Signaling in the Developing Mouse Limb Bud by Direct Repression of
- Mondo/ChREBP-Mlx-Regulated Transcriptional Network Is Essential for Dietary Sugar Tolerance in
- Sex-Differential Selection and the Evolution of X Inactivation Strategies
- Identification of a Tissue-Selective Heat Shock Response Regulatory Network
- Phosphorylation-Coupled Proteolysis of the Transcription Factor MYC2 Is Important for Jasmonate-Signaled Plant Immunity
- RpoS Plays a Central Role in the SOS Induction by Sub-Lethal Aminoglycoside Concentrations in
- Six Homeoproteins Directly Activate Expression in the Gene Regulatory Networks That Control Early Myogenesis
- Rtt109 Prevents Hyper-Amplification of Ribosomal RNA Genes through Histone Modification in Budding Yeast
- ATP-Dependent Chromatin Remodeling by Cockayne Syndrome Protein B and NAP1-Like Histone Chaperones Is Required for Efficient Transcription-Coupled DNA Repair
- Iron-Responsive miR-485-3p Regulates Cellular Iron Homeostasis by Targeting Ferroportin
- Mutations in Predispose Zebrafish and Humans to Seminomas
- Cytotoxic Chromosomal Targeting by CRISPR/Cas Systems Can Reshape Bacterial Genomes and Expel or Remodel Pathogenicity Islands
- Tissue Homeostasis in the Wing Disc of : Immediate Response to Massive Damage during Development
- All SNPs Are Not Created Equal: Genome-Wide Association Studies Reveal a Consistent Pattern of Enrichment among Functionally Annotated SNPs
- Functional 358Ala Allele Impairs Classical IL-6 Receptor Signaling and Influences Risk of Diverse Inflammatory Diseases
- The Tissue-Specific RNA Binding Protein T-STAR Controls Regional Splicing Patterns of Pre-mRNAs in the Brain
- Neutral Genomic Microevolution of a Recently Emerged Pathogen, Serovar Agona
- Genetic Requirements for Signaling from an Autoactive Plant NB-LRR Intracellular Innate Immune Receptor
- SNF5 Is an Essential Executor of Epigenetic Regulation during Differentiation
- Dialects of the DNA Uptake Sequence in
- Reference-Free Population Genomics from Next-Generation Transcriptome Data and the Vertebrate–Invertebrate Gap
- Senataxin Plays an Essential Role with DNA Damage Response Proteins in Meiotic Recombination and Gene Silencing
- High-Resolution Mapping of Spontaneous Mitotic Recombination Hotspots on the 1.1 Mb Arm of Yeast Chromosome IV
- Rod Monochromacy and the Coevolution of Cetacean Retinal Opsins
- Evolution after Introduction of a Novel Metabolic Pathway Consistently Leads to Restoration of Wild-Type Physiology
- Disruption of TTDA Results in Complete Nucleotide Excision Repair Deficiency and Embryonic Lethality
- Insulators Target Active Genes to Transcription Factories and Polycomb-Repressed Genes to Polycomb Bodies
- Signatures of Diversifying Selection in European Pig Breeds
- The Chromosomal Passenger Protein Birc5b Organizes Microfilaments and Germ Plasm in the Zebrafish Embryo
- The Histone Demethylase Jarid1b Ensures Faithful Mouse Development by Protecting Developmental Genes from Aberrant H3K4me3
- Regulates Synaptic Development and Endocytosis by Suppressing Filamentous Actin Assembly
- Sensory Neuron-Derived Eph Regulates Glomerular Arbors and Modulatory Function of a Central Serotonergic Neuron
- Analysis of Rare, Exonic Variation amongst Subjects with Autism Spectrum Disorders and Population Controls
- Scavenger Receptors Mediate the Role of SUMO and Ftz-f1 in Steroidogenesis
- DNA Double-Strand Breaks Coupled with PARP1 and HNRNPA2B1 Binding Sites Flank Coordinately Expressed Domains in Human Chromosomes
- High-Resolution Mapping of H1 Linker Histone Variants in Embryonic Stem Cells
- Comparative Genomics of and the Bacterial Species Concept
- Genetic and Biochemical Assays Reveal a Key Role for Replication Restart Proteins in Group II Intron Retrohoming
- Genome-Wide Association Studies Identify Two Novel Mutations Responsible for an Atypical Hyperprolificacy Phenotype in Sheep
- The Genetic Correlation between Height and IQ: Shared Genes or Assortative Mating?
- Comprehensive Assignment of Roles for Typhimurium Genes in Intestinal Colonization of Food-Producing Animals
- An Essential Role for Zygotic Expression in the Pre-Cellular Drosophila Embryo
- The Genome Organization of Reflects Its Lifestyle
- Coordinated Cell Type–Specific Epigenetic Remodeling in Prefrontal Cortex Begins before Birth and Continues into Early Adulthood
- Improved Detection of Common Variants Associated with Schizophrenia and Bipolar Disorder Using Pleiotropy-Informed Conditional False Discovery Rate
- Site-Specific Phosphorylation of the DNA Damage Response Mediator Rad9 by Cyclin-Dependent Kinases Regulates Activation of Checkpoint Kinase 1
- Npc1 Acting in Neurons and Glia Is Essential for the Formation and Maintenance of CNS Myelin
- Identification of , a Retrotransposon-Derived Imprinted Gene, as a Novel Driver of Hepatocarcinogenesis
- Aag DNA Glycosylase Promotes Alkylation-Induced Tissue Damage Mediated by Parp1
- DJ-1 Decreases Neural Sensitivity to Stress by Negatively Regulating Daxx-Like Protein through dFOXO
- Asynchronous Replication, Mono-Allelic Expression, and Long Range -Effects of
- Differential Association of the Conserved SUMO Ligase Zip3 with Meiotic Double-Strand Break Sites Reveals Regional Variations in the Outcome of Meiotic Recombination
- Focusing In on the Complex Genetics of Myopia
- Continent-Wide Decoupling of Y-Chromosomal Genetic Variation from Language and Geography in Native South Americans
- Breakpoint Analysis of Transcriptional and Genomic Profiles Uncovers Novel Gene Fusions Spanning Multiple Human Cancer Types
- Intrinsic Epigenetic Regulation of the D4Z4 Macrosatellite Repeat in a Transgenic Mouse Model for FSHD
- Bisphenol A Exposure Disrupts Genomic Imprinting in the Mouse
- Genetic and Genomic Architecture of the Evolution of Resistance to Antifungal Drug Combinations
- Transposable Elements Are Major Contributors to the Origin, Diversification, and Regulation of Vertebrate Long Noncoding RNAs
- Functional Dissection of the Condensin Subunit Cap-G Reveals Its Exclusive Association with Condensin I
- PLOS Genetics
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- The G4 Genome
- Neutral Genomic Microevolution of a Recently Emerged Pathogen, Serovar Agona
- The Histone Demethylase Jarid1b Ensures Faithful Mouse Development by Protecting Developmental Genes from Aberrant H3K4me3
- The Tissue-Specific RNA Binding Protein T-STAR Controls Regional Splicing Patterns of Pre-mRNAs in the Brain
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



