-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
Ensuring the Involvement of Children in the Evaluation of New Tuberculosis Treatment Regimens
article has not abstract
Published in the journal: Ensuring the Involvement of Children in the Evaluation of New Tuberculosis Treatment Regimens. PLoS Med 5(8): e176. doi:10.1371/journal.pmed.0050176
Category: Essay
doi: https://doi.org/10.1371/journal.pmed.0050176Summary
article has not abstract
Global tuberculosis control is threatened by dramatic increases in HIV-related tuberculosis and by the emergence of multidrug-resistant strains. Highly lethal outbreaks of extensively drug-resistant tuberculosis among HIV-infected persons in South Africa [1] demonstrate the public health emergency that results when these two forces converge in the same setting. Fortunately, at this time of great need, tuberculosis drug development has been roused from its decades-long slumber. New ways of using existing drugs and the development of new drug classes hold great promise for the treatment of both drug-susceptible [2–6] and drug-resistant tuberculosis [4–8].
Children are a critical part of the global tuberculosis pandemic, with an estimated 900,000 cases and 100,000 deaths per year [9]. In high-burden settings, children make up as much as 20% of incident cases of active tuberculosis [9,10]. Furthermore, young children have an increased risk of severe, rapidly progressive forms of tuberculosis, such as disseminated disease and meningitis (Figure 1) [11,12]. Therefore, it is imperative that children benefit from improvements made in tuberculosis treatment. However, children have only been included in one study of these new agents (a phase III trial of once-weekly rifapentine + isoniazid for latent tuberculosis) [13].
Fig. 1. Chest Radiograph of an Infant with Pulmonary Tuberculosis, Complicated by an Immune Reconstitution Syndrome Event Following the Initiation of Antiretroviral Therapy 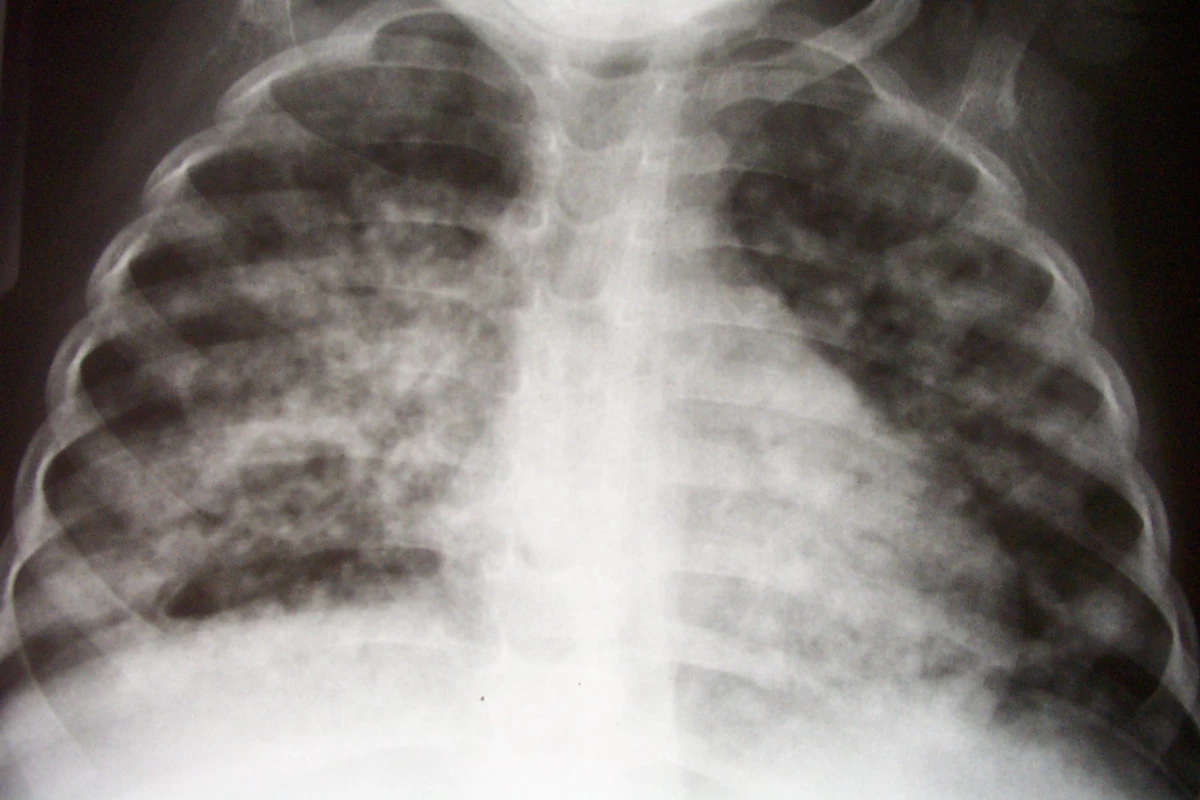
Barriers to Involving Children
Although not well articulated in the published medical literature, a number of barriers to the involvement of children have been raised in discussions of tuberculosis drug development (Box 1). Our concern is that these barriers may, once again, lead the field down the path of least resistance—the exclusion of children from tuberculosis drug development efforts.
Box 1. Barriers to Including Children in Tuberculosis Drug Development
-
Infrequent transmission of tuberculosis from children to others
-
Difficulty of confirming active tuberculosis among children
-
Existence of effective therapy for drug-susceptible tuberculosis
-
Concerns about pediatric-specific side effects
-
Uncertainties about the appropriate time to involve children in drug development and the optimal trial designs for doing so
-
Regulatory requirements engendered by the inclusion of children
-
Concerns about further subdividing the limited resources available for drug development
What happens when children are not included in drug development.
There is a rich history of clinical trials for tuberculosis treatment, beginning with the landmark streptomycin trial [14] and followed by a remarkable series of trials establishing that multidrug therapy could be curative, that it was possible to do so with ambulatory treatment, and that therapy could be shortened from two years to six months [15]. Children were almost completely left out of this series of clinical trials.
The result? Nearly 40 years after the development of short-course treatment in adults, there are still fundamental uncertainties about age-appropriate dosing of isoniazid, rifampicin, pyrazinamide, and ethambutol [16,17]. Children, particularly very young children, do not achieve adequate serum concentrations of these agents when given weight-based dosing based on pharmacokinetic data from adults. The uncertainties about pediatric dosing reflect the lamentable paucity of pharmacokinetic data for first-line drugs in children. Another consequence of the lack of involvement of children in the initial phase of tuberculosis drug development is that only in recent years has there been a substantial effort to manufacture child-friendly formulations of first-line tuberculosis drugs (crushable mini-pills, granules, oral suspensions). A number of controversies in the treatment of pediatric tuberculosis stem from the lack of clinical trials focused on child-specific questions (e.g., the optimal duration and dosing frequency of tuberculosis treatment, how to ensure safe use of ethambutol in children). There are even greater uncertainties about less common therapeutic questions (e.g., the treatment or prevention of drug-resistant disease).
The history of tuberculosis drug development reflects the lack of involvement of children and the consequences of that omission. Concerted efforts are necessary to avoid repeating this unfortunate experience in this era of renewed interest in tuberculosis drug development.
The example of antiretroviral drug development.
Antiretroviral drugs have been evaluated among children, age-specific pharmacokinetic data obtained, and child-friendly formulations developed and marketed. Throughout this process, the evaluation of antiretroviral drugs for children has not lagged far behind their development and licensure for adults. As a result, age-appropriate regimens are available in a range of formulations for children, and the rates of HIV-related morbidity and mortality decreased in concert for children [18–20] and adults [21]. The keys to this success were advocacy [22], earmarked funding for pediatric research, focused clinical trial designs on questions whose answers could not be extrapolated from research in adults, and incentives for the pharmaceutical industry to include children in drug development.
Overcoming the Barriers to the Involvement of Children
The first and perhaps the most important step toward involving children in tuberculosis drug development is to clearly articulate the necessity of doing so. To shine a light on the path of least resistance is to show how clearly unacceptable it is; children have the same right to benefit from research as do adults. Researchers, regulatory agencies, advocates, and government agencies and private foundations that fund drug development must insist that the development pathways for all new agents/regimens include specific plans for when and how children will be involved.
Once agreement has been reached on the necessity of including children in trials of new tuberculosis treatment regimens, the specific barriers to the involvement of children must be identified and then overcome (Table 1). The difficulty of culture confirmation of active tuberculosis among children is well known [17]. Because a positive culture is both an enrollment criterion and the primary endpoint of phase II and III clinical trials for tuberculosis treatment, some observers have concluded that tuberculosis treatment trials cannot be done among children. This limitation is quite real—because of it, pediatric trials are not the setting for the definitive evaluation of the efficacy of a new drug or treatment regimen. However, it does not mean that treatment trials cannot be done among children.
Tab. 1. Summary of Barriers to the Involvement of Children in Tuberculosis Drug Development and Suggested Ways to Overcome Them 
Improved specimen collection techniques (e.g., induced sputum and string test) can provide culture confirmation in a higher percentage of pediatric patients than previously thought possible [23–26]. Furthermore, case definitions for culture-negative pediatric tuberculosis can be used in clinical trials, as criteria both for enrollment and for evaluating efficacy [27,28]. While imperfect, these case definitions can be applied by an events committee blinded to treatment assignment to ensure unbiased assessment of diagnosis and response to treatment.
Although concerns about pediatric-specific side effects have led some to argue against inclusion of children in clinical trials, these concerns fail to recognize that new drugs will be used off-label among children, in the absence of data on pediatric pharmacokinetics and tolerability, as soon as they are approved for adults. Children are indeed vulnerable participants in research because of their inability to provide fully informed consent. However, an overzealous attempt to protect some children from the possible harms of research perversely causes harm, by either denying access to treatment or through exposing children to the risks of inappropriate dosages of new medications.
Effective therapy is available for drug-susceptible tuberculosis. However, the limitations of current first-line tuberculosis treatment should be recognized. Despite the appeal of our current nomenclature of “short-course therapy,” a six-month treatment duration leads to worrisome numbers of patients who do not complete treatment in many programmatic situations [29–32]. The side effects of current regimens are appreciable as well: high rates of bothersome side effects, such as nausea and vomiting, and substantial rates of serious adverse events, such as hepatotoxicity [33]. In spite of the availability of effective therapy for drug-susceptible tuberculosis, new agents should still be evaluated among children; there is much room for improvement in “short-course therapy.”
At what points in tuberculosis drug development should studies be undertaken in children? This crucial question requires discussion among investigators, the pharmaceutical industry, advocates, and regulatory officials. As a starting point for such discussions, we offer initial suggestions in Table 2. If children are to be involved at specific points in drug development, appropriate timelines are needed for initial work on formulations and pharmacokinetic studies among children. It is inappropriate to wait until the drug development plan for a new drug or regimen has been completed in adults before beginning its evaluation in children.
Tab. 2. Suggested Types of Research Activity among Children by the Stage of Clinical Trial Efforts among Adults for a New Antituberculosis Drug/Regimen 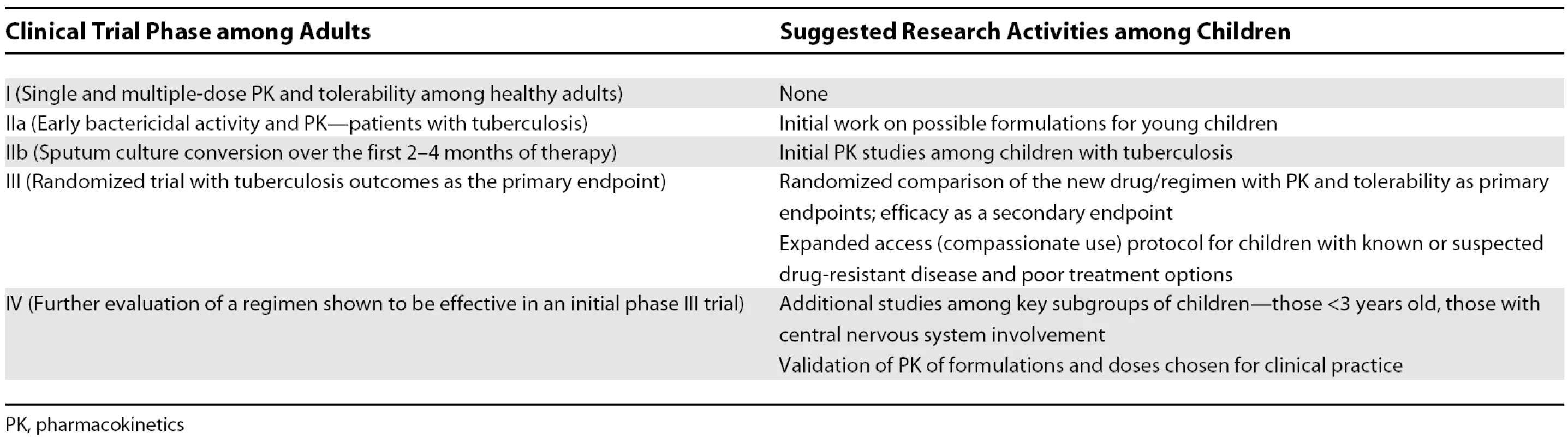
PK, pharmacokinetics What kinds of trials should be undertaken among children? Not all phase III trials in adults need repeating in children; it is highly likely that children will respond well to a new regimen if given a drug formulation and dose that achieves pharmacokinetic parameters comparable to those among adults. Questions that are specific to children, or where answers from adults are unlikely to extrapolate to children, require separate evaluation. Key examples of studies that must be done among children are those assessing the pharmacokinetics and tolerability of new drugs [17].
It can be difficult to obtain blood specimens by venipuncture from very young and very ill children. This challenge should not preclude pharmacokinetic studies of new antituberculosis drugs among children; more efficient study designs and techniques for use of ultra-small quantities of blood can overcome this limitation. Sparse sampling schemes analyzed with Bayesian statistical methods that incorporate pharmacokinetic data from adults, in addition to knowledge about maturation of metabolic pathways in children, facilitate the design and implementation of pharmacokinetic studies in children [34].
An instructive example of optimizing methodologies for pharmacokinetic studies for children comes from malaria research. Severe malaria is predominantly a disease of young children, so it is critical that the pharmacokinetics of antimalarial drugs among infants and young children be well understood. In these studies, very small samples of blood (100 microliters) obtained by finger - or heel-stick are blotted onto filter paper, allowed to dry, stored at room temperature, and later used to determine concentrations of antimalarial drugs [34,35]. Thus, pharmacokinetic studies can be extended to infants and young children, and such samples can be obtained under field conditions where the disease is common. The extension of these techniques to the study of new antituberculosis drugs requires further research, but the challenge of pharmacokinetic sampling among young children calls for this kind of innovation.
There are many regulatory steps between the development of a study protocol and its implementation at study sites. Some have expressed concern that involving children in drug development will slow down the already lengthy timeline for study implementation by triggering more rigorous regulatory review. This concern may be well founded. However, key funding and regulatory agencies have policies and incentives to encourage evaluation of new drugs among children for conditions, like tuberculosis, that are common among that age group (the US National Institutes of Health requires specific justification if children are not included in a study). Some of those incentives, such as extended patent protection for the United States market, are unlikely to encourage tuberculosis drug development efforts for children. However, the US Food and Drug Administration has recently been authorized to take additional steps to promote the inclusion of children in drug development [36], and the European Union now requires that any new drug that could potentially be used in children have a Pediatric Implementation Plan for the drug to be licensed in adults [37]. Other groups are developing alternative incentive structures for diseases of poverty, such as tuberculosis [38].
Finally, there is the concern that including children in clinical trials will dilute the already inadequate funding for tuberculosis drug development [39], thus slowing down the pathway to licensure of new drugs. Proponents of this zero-sum argument may be willing to face difficult facts, but risk fostering the continued existence of an unacceptable situation. The expansion of antiretroviral therapy and the treatment of multidrug-resistant tuberculosis in high-burden settings are examples of two interventions which were said to be inadvisable, based on zero-sum arguments, but which have now been shown to be both feasible and critical for disease control [40–42]. Rather than being a detriment to funding for tuberculosis drug development, the inclusion of children may draw funding to the field.
Summary
We are on the threshold of revolutionary improvements in the treatment of tuberculosis. Within five to ten years, it is likely that highly effective three-month regimens will be available to treat both active and latent drug-susceptible tuberculosis. New drug classes that have the potential to dramatically improve the treatment of multidrug-resistant tuberculosis are entering clinical trials. Children have the same right as adults to benefit from research with these new treatments. By making a deliberate choice to avoid the path of least resistance, we can ensure that both adults and children benefit from these advances in tuberculosis treatment.
Zdroje
1. GandhiNRMollASturmAWPawinskiRGovenderT
2006
Extensively drug-resistant tuberculosis as a cause of death in patients co-infected with tuberculosis and HIV in a rural area of South Africa.
Lancet
368
1575
1580
2. RosenthalIMZhangMWilliamsKNPeloquinCATyagiS
2007
Daily dosing of rifapentine cures tuberculosis in three months or less in the murine model.
PLoS Med
4
e344
doi:10.1371/journal.pmed.0040344
3. ChapuisLJiBTruffot-PernotCO'BrienRJRaviglioneMC
1994
Preventive therapy of tuberculosis with rifapentine in immunocompetent and nude mice.
Am J Respir Crit Care Med
150
1355
1362
4. MatsumotoMHashizumeHTomishigeTKawasakiMTsubouchiH
2006
OPC-67683, a nitro-dihydro-imidazooxazole derivative with promising action against tuberculosis in vitro and in mice.
PLoS Med
3
e466
doi:10.1371/journal.pmed.0030466
5. StoverCKWarrenerPVanDevanterDRShermanDRArainTM
2000
A small-molecule nitroimidazopyran drug candidate for the treatment of tuberculosis.
Nature
405
962
966
6. NikonenkoBVProtopopovaMSamalaREinckLNacyCA
2007
Drug therapy of experimental tuberculosis (TB): Improved outcome by combining SQ109, a new diamine antibiotic, with existing TB drugs.
Antimicrob Agents Chemother
51
1563
1565
7. LounisNVezirisNChauffourATruffot-PernotCAndriesK
2006
Combinations of R207910 with drugs used to treat multidrug-resistant tuberculosis have the potential to shorten treatment duration.
Antimicrob Agents Chemother
50
3543
3547
8. RagnoRMarshallGRDi SantoRCostiRMassaS
2000
Antimycobacterial pyrroles: synthesis, anti-Mycobacterium tuberculosis activity and QSAR studies.
Bioorg Med Chem
8
1423
1432
9. NelsonLJWellsCD
2004
Global epidemiology of childhood tuberculosis.
Int J Tuberc Lung Dis
8
636
647
10. MaraisBJGrahamSMCottonMFBeyersN
2007
Diagnostic and management challenges for childhood tuberculosis in the era of HIV.
J Infect Dis
196
Suppl 1
S76
S85
11. MaraisBJGieRPSchaafHSHesselingACEnarsonDA
2006
The spectrum of disease in children treated for tuberculosis in a highly endemic area.
Int J Tuberc Lung Dis
10
732
738
12. MaraisBJGieRPSchaafHSHesselingACObiharaCC
2004
The natural history of childhood intra-thoracic tuberculosis: A critical review of literature from the pre-chemotherapy era.
Int J Tuberc Lung Dis
8
392
402
13. Tuberculosis Trials Consortium
2008
TBTC Study 26: Weekly RFP/INH for 3 mo. vs. daily INH for 9 mo. for the treatment of LTBI.
Available: http://www.clinicaltrials.gov/ct2/show/NCT00023452?term=tuberculosis%2C+rifapentine&rank=5. Accessed 17 July 2008
14. Medical Research Council
1948
Streptomycin treatment of pulmonary tuberculosis.
BMJ
1
769
782
15. FoxWEllardGAMitchisonDA
1999
Studies on the treatment of tuberculosis undertaken by the British Medical Research Council Tuberculosis Units, 1946–1986, with relevant subsequent publications.
Int J Tuberc Lung Dis
3
S231
S279
16. SchaafHSParkinDPSeifartHIWerelyCJHesselingPB
2005
Isoniazid pharmacokinetics in children treated for respiratory tuberculosis.
Arch Dis Child
90
614
618
17. DonaldPR
2007
The assessment of new anti-tuberculosis drugs for a paediatric indication.
Int J Tuberc Lung Dis
11
1162
1165
18. GibbDMDuongTTookeyPASharlandMTudor-WilliamsG
2003
Decline in mortality, AIDS, and hospital admissions in perinatally HIV-1 infected children in the United Kingdom and Ireland.
BMJ
327
1019
19. Bolton-MooreCMubiana-MbeweMCantrellRAChintuNStringerEM
2007
Clinical outcomes and CD4 cell response in children receiving antiretroviral therapy at primary health care facilities in Zambia.
JAMA
298
1888
1899
20. GortmakerSLHughesMCerviaJBradyMJohnsonGM
2001
Effect of combination therapy including protease inhibitors on mortality among children and adolescents infected with HIV-1.
N Engl J Med
345
1522
1528
21. PalellaFJrDelaneyKMoormanALovelessMFuhrerJ
1998
Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection.
N Engl J Med
338
853
860
22. HavensPLGibbDM
2007
Increasing antiretroviral drug access for children with HIV infection.
Pediatrics
119
838
845
23. ZarHJTannenbaumEApollesPRouxPHansloD
2000
Sputum induction for the diagnosis of pulmonary tuberculosis in infants and young children in an urban setting in South Africa.
Arch Dis Child
82
305
308
24. MaraisBJHesselingACGieRPSchaafHSEnarsonDA
2006
The bacteriologic yield in children with intrathoracic tuberculosis.
Clin Infect Dis
42
e69
e71
25. ChowFEspirituNGilmanRHGutierrezRLopezS
2006
La cuerda dulce—A tolerability and acceptability study of a novel approach to specimen collection for diagnosis of paediatric pulmonary tuberculosis.
BMC Infect Dis
6
67
26. VargasDGarciaLGilmanRHEvansCTiconaE
2005
Diagnosis of sputum-scarce HIV-associated pulmonary tuberculosis in Lima, Peru.
Lancet
365
150
152
27. MaraisBJGieRPHesselingACSchaafHSLombardC
2006
A refined symptom-based approach to diagnose pulmonary tuberculosis in children.
Pediatrics
118
e1350
e1359
28. ZarHJCottonMFStraussSKarpakisJHusseyG
2007
Effect of isoniazid prophylaxis on mortality and incidence of tuberculosis in children with HIV: Randomised controlled trial.
BMJ
334
136
29. LindtjornBMadeboT
2001
The outcome of tuberculosis treatment at a rural hospital in southern Ethiopia.
Trop Doct
31
132
135
30. TanguisHGCaylaJAGarcia de OlallaPGJansaJMBrugalMT
2000
Factors predicting non-completion of tuberculosis treatment among HIV-infected patients in Barcelona (1987–1996).
Int J Tuberc Lung Dis
4
55
60
31. Radilla-ChavezPLaniado-LaborinR
2007
Results of directly observed treatment for tuberculosis in Ensenada, Mexico: Not all DOTS programs are created equally.
Int J Tuberc Lung Dis
11
289
292
32. HesselingACSchaafSHWestraAEWerschkullHDonaldPR
2005
Outcome of HIV-infected children with culture-confirmed tuberculosis.
Arch Dis Child
33. YeeDValiquetteCPelletierMParisienIRocherI
2003
Incidence of serious side effects from first-line antituberculosis drugs among patients treated for active tuberculosis.
Am J Respir Crit Care Med
167
1472
1477
34. MeibohmBLaerSPanettaJCBarrettJS
2005
Population pharmacokinetic studies in pediatrics: Issues in design and analysis.
AAPS J
7
E475
E487
35. BarnesKILindegardhNOgundahunsiOOlliaroPPloweCV
2007
World Antimalarial Resistance Network (WARN) IV: Clinical pharmacology.
Malar J
6
122
36. United States Congress
2007
Food and Drug Administration Amendments Act of 2007.
HR 3580. 110th Cong. Available: http://www.fda.gov/oc/initiatives/HR3580.pdf. Accessed 17 July 2008
37. European Medicines Agency
2007
The EU Paediatric Regulation.
Available: http://www.emea.europa.eu/htms/human/paediatrics/regulation.htm. Accessed 17 July 2008
38. World Health Organization
2006
Intergovernmental Working Group on Public health, Innovation and Intellectual Property.
Available: http://www.who.int/mediacentre/events/2006/intellectual.property.meeting/en/index.html. Accessed 17 July 2008
39. FeuerC
2007
Tuberculosis research and development: A critical analysis of funding trends, 2005–2006.
Treatment Action Group. Available: http://www.aidsinfonyc.org/tag/tbhiv/tbrandd/2007tbranddreport.pdf. Accessed 17 July 2008
40. MitnickCBayonaJPalaciosEShinSFurinJ
2003
Community-based therapy for multidrug-resistant tuberculosis in Lima, Peru.
N Engl J Med
348
119
128
41. BadriMMaartensGMandaliaSBekkerLGPenrodJR
2006
Cost-effectiveness of highly active antiretroviral therapy in South Africa.
PLoS Med
3
e4
doi:10.1371/journal.pmed.0030004
42. FairallLRBachmannMOLouwagieGMvan VuurenCChikobvuP
2008
Effectiveness of antiretroviral treatment in a South African program: A cohort study.
Arch Intern Med
168
86
93
Štítky
Interné lekárstvo
Článok vyšiel v časopisePLOS Medicine
Najčítanejšie tento týždeň
2008 Číslo 8- Parazitičtí červi v terapii Crohnovy choroby a dalších zánětlivých autoimunitních onemocnění
- Intermitentní hladovění v prevenci a léčbě chorob
- Statinová intolerance
- Co dělat při intoleranci statinů?
- Monoklonální protilátky v léčbě hyperlipidemií
-
Všetky články tohto čísla
- Predicting Outcome after Traumatic Brain Injury: Development and International Validation of Prognostic Scores Based on Admission Characteristics
- Greater Response to Placebo in Children Than in Adults: A Systematic Review and Meta-Analysis in Drug-Resistant Partial Epilepsy
- Can a Topical Microbicide Prevent Rectal HIV Transmission?
- Developing a Prognostic Model for Traumatic Brain Injury—A Missed Opportunity?
- Assessing Antimalarial Efficacy in a Time of Change to Artemisinin-Based Combination Therapies: The Role of Médecins Sans Frontières
- Children Are Not Just Small Adults: The Urgent Need for High-Quality Trial Evidence in Children
- The Use of Nonhuman Primate Models in HIV Vaccine Development
- More Evidence Against a Causal Association between C-Reactive Protein and Diabetes
- Strategies to Reduce Mortality from Bacterial Sepsis in Adults in Developing Countries
- Ensuring the Involvement of Children in the Evaluation of New Tuberculosis Treatment Regimens
- An Acute Evolving Flaccid Quadriparesis in an Elderly Woman
- PLOS Medicine
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- Ensuring the Involvement of Children in the Evaluation of New Tuberculosis Treatment Regimens
- Predicting Outcome after Traumatic Brain Injury: Development and International Validation of Prognostic Scores Based on Admission Characteristics
- The Use of Nonhuman Primate Models in HIV Vaccine Development
- Strategies to Reduce Mortality from Bacterial Sepsis in Adults in Developing Countries
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



