-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement
article has not abstract
Published in the journal: Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med 6(7): e32767. doi:10.1371/journal.pmed.1000097
Category: Guidelines and Guidance
doi: https://doi.org/10.1371/journal.pmed.1000097Summary
article has not abstract
Introduction
Systematic reviews and meta-analyses have become increasingly important in health care. Clinicians read them to keep up to date with their field [1],[2], and they are often used as a starting point for developing clinical practice guidelines. Granting agencies may require a systematic review to ensure there is justification for further research [3], and some health care journals are moving in this direction [4]. As with all research, the value of a systematic review depends on what was done, what was found, and the clarity of reporting. As with other publications, the reporting quality of systematic reviews varies, limiting readers' ability to assess the strengths and weaknesses of those reviews.
Several early studies evaluated the quality of review reports. In 1987, Mulrow examined 50 review articles published in four leading medical journals in 1985 and 1986 and found that none met all eight explicit scientific criteria, such as a quality assessment of included studies [5]. In 1987, Sacks and colleagues [6] evaluated the adequacy of reporting of 83 meta-analyses on 23 characteristics in six domains. Reporting was generally poor; between one and 14 characteristics were adequately reported (mean = 7.7; standard deviation = 2.7). A 1996 update of this study found little improvement [7].
In 1996, to address the suboptimal reporting of meta-analyses, an international group developed a guidance called the QUOROM Statement (QUality Of Reporting Of Meta-analyses), which focused on the reporting of meta-analyses of randomized controlled trials [8]. In this article, we summarize a revision of these guidelines, renamed PRISMA (Preferred Reporting Items for Systematic reviews and Meta-Analyses), which have been updated to address several conceptual and practical advances in the science of systematic reviews (Box 1).
Box 1: Conceptual Issues in the Evolution from QUOROM to PRISMA
Completing a Systematic Review Is an Iterative Process
The conduct of a systematic review depends heavily on the scope and quality of included studies: thus systematic reviewers may need to modify their original review protocol during its conduct. Any systematic review reporting guideline should recommend that such changes can be reported and explained without suggesting that they are inappropriate. The PRISMA Statement (Items 5, 11, 16, and 23) acknowledges this iterative process. Aside from Cochrane reviews, all of which should have a protocol, only about 10% of systematic reviewers report working from a protocol [22]. Without a protocol that is publicly accessible, it is difficult to judge between appropriate and inappropriate modifications.
Conduct and Reporting Research Are Distinct Concepts
This distinction is, however, less straightforward for systematic reviews than for assessments of the reporting of an individual study, because the reporting and conduct of systematic reviews are, by nature, closely intertwined. For example, the failure of a systematic review to report the assessment of the risk of bias in included studies may be seen as a marker of poor conduct, given the importance of this activity in the systematic review process [37].
Study-Level Versus Outcome-Level Assessment of Risk of Bias
For studies included in a systematic review, a thorough assessment of the risk of bias requires both a “study-level” assessment (e.g., adequacy of allocation concealment) and, for some features, a newer approach called “outcome-level” assessment. An outcome-level assessment involves evaluating the reliability and validity of the data for each important outcome by determining the methods used to assess them in each individual study [38]. The quality of evidence may differ across outcomes, even within a study, such as between a primary efficacy outcome, which is likely to be very carefully and systematically measured, and the assessment of serious harms [39], which may rely on spontaneous reports by investigators. This information should be reported to allow an explicit assessment of the extent to which an estimate of effect is correct [38].
Importance of Reporting Biases
Different types of reporting biases may hamper the conduct and interpretation of systematic reviews. Selective reporting of complete studies (e.g., publication bias) [28] as well as the more recently empirically demonstrated “outcome reporting bias” within individual studies [40],[41] should be considered by authors when conducting a systematic review and reporting its results. Though the implications of these biases on the conduct and reporting of systematic reviews themselves are unclear, some previous research has identified that selective outcome reporting may occur also in the context of systematic reviews [42].
Terminology
The terminology used to describe a systematic review and meta-analysis has evolved over time. One reason for changing the name from QUOROM to PRISMA was the desire to encompass both systematic reviews and meta-analyses. We have adopted the definitions used by the Cochrane Collaboration [9]. A systematic review is a review of a clearly formulated question that uses systematic and explicit methods to identify, select, and critically appraise relevant research, and to collect and analyze data from the studies that are included in the review. Statistical methods (meta-analysis) may or may not be used to analyze and summarize the results of the included studies. Meta-analysis refers to the use of statistical techniques in a systematic review to integrate the results of included studies.
Developing the PRISMA Statement
A three-day meeting was held in Ottawa, Canada, in June 2005 with 29 participants, including review authors, methodologists, clinicians, medical editors, and a consumer. The objective of the Ottawa meeting was to revise and expand the QUOROM checklist and flow diagram, as needed.
The executive committee completed the following tasks, prior to the meeting: a systematic review of studies examining the quality of reporting of systematic reviews, and a comprehensive literature search to identify methodological and other articles that might inform the meeting, especially in relation to modifying checklist items. An international survey of review authors, consumers, and groups commissioning or using systematic reviews and meta-analyses was completed, including the International Network of Agencies for Health Technology Assessment (INAHTA) and the Guidelines International Network (GIN). The survey aimed to ascertain views of QUOROM, including the merits of the existing checklist items. The results of these activities were presented during the meeting and are summarized on the PRISMA Web site (http://www.prisma-statement.org/).
Only items deemed essential were retained or added to the checklist. Some additional items are nevertheless desirable, and review authors should include these, if relevant [10]. For example, it is useful to indicate whether the systematic review is an update [11] of a previous review, and to describe any changes in procedures from those described in the original protocol.
Shortly after the meeting a draft of the PRISMA checklist was circulated to the group, including those invited to the meeting but unable to attend. A disposition file was created containing comments and revisions from each respondent, and the checklist was subsequently revised 11 times. The group approved the checklist, flow diagram, and this summary paper.
Although no direct evidence was found to support retaining or adding some items, evidence from other domains was believed to be relevant. For example, Item 5 asks authors to provide registration information about the systematic review, including a registration number, if available. Although systematic review registration is not yet widely available [12],[13], the participating journals of the International Committee of Medical Journal Editors (ICMJE) [14] now require all clinical trials to be registered in an effort to increase transparency and accountability [15]. Those aspects are also likely to benefit systematic reviewers, possibly reducing the risk of an excessive number of reviews addressing the same question [16],[17] and providing greater transparency when updating systematic reviews.
The PRISMA Statement
The PRISMA Statement consists of a 27-item checklist (Table 1; see also Text S1 for a downloadable Word template for researchers to re-use) and a four-phase flow diagram (Figure 1; see also Figure S1 for a downloadable Word template for researchers to re-use). The aim of the PRISMA Statement is to help authors improve the reporting of systematic reviews and meta-analyses. We have focused on randomized trials, but PRISMA can also be used as a basis for reporting systematic reviews of other types of research, particularly evaluations of interventions. PRISMA may also be useful for critical appraisal of published systematic reviews. However, the PRISMA checklist is not a quality assessment instrument to gauge the quality of a systematic review.
Fig. 1. Flow of information through the different phases of a systematic review. 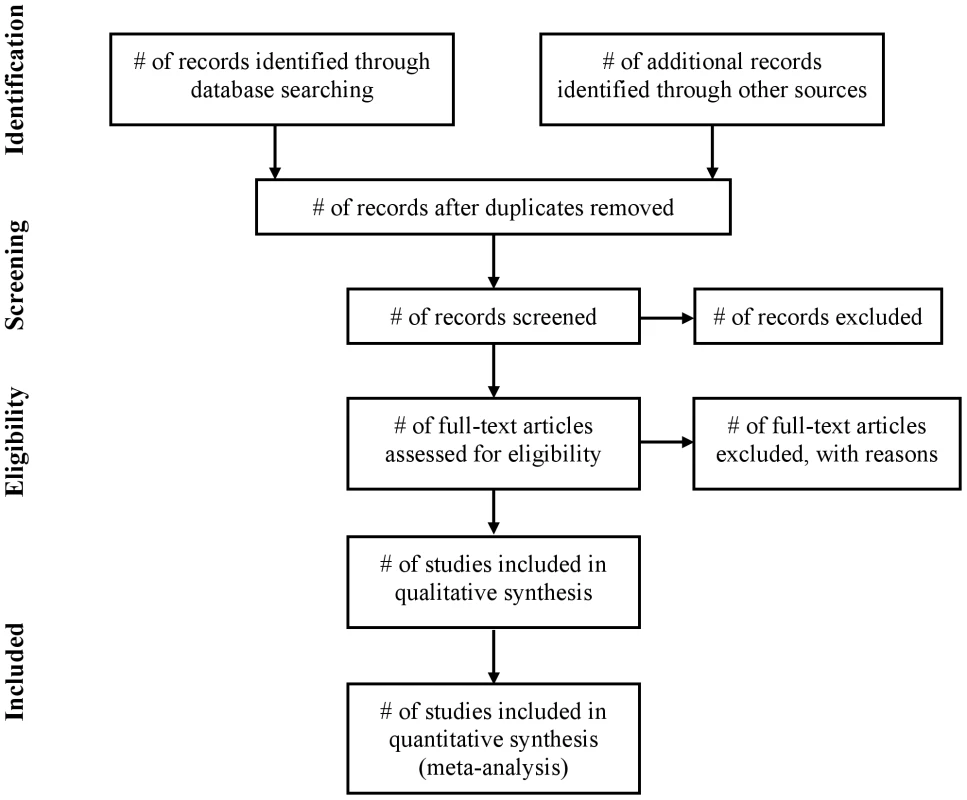
Tab. 1. Checklist of items to include when reporting a systematic review or meta-analysis. 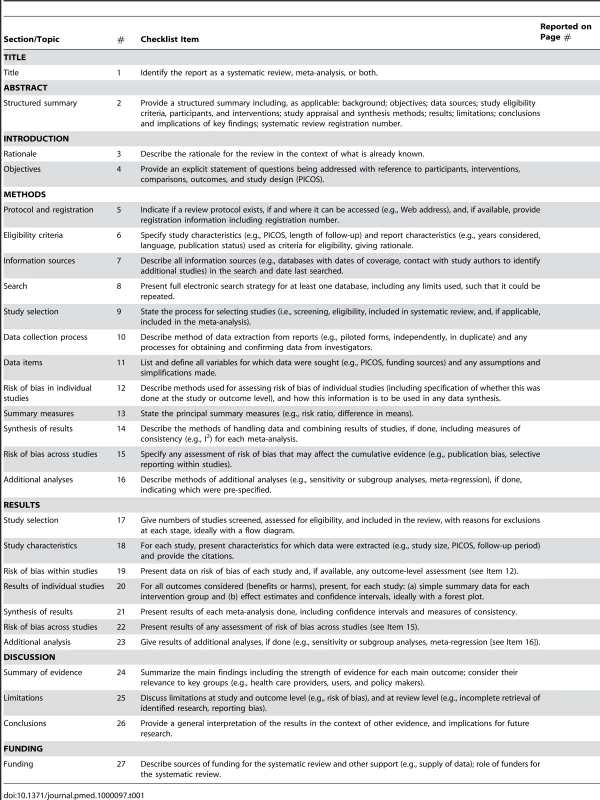
From QUOROM to PRISMA
The new PRISMA checklist differs in several respects from the QUOROM checklist, and the substantive specific changes are highlighted in Table 2. Generally, the PRISMA checklist “decouples” several items present in the QUOROM checklist and, where applicable, several checklist items are linked to improve consistency across the systematic review report.
Tab. 2. Substantive specific changes between the QUOROM checklist and the PRISMA checklist (a tick indicates the presence of the topic in QUOROM or PRISMA). 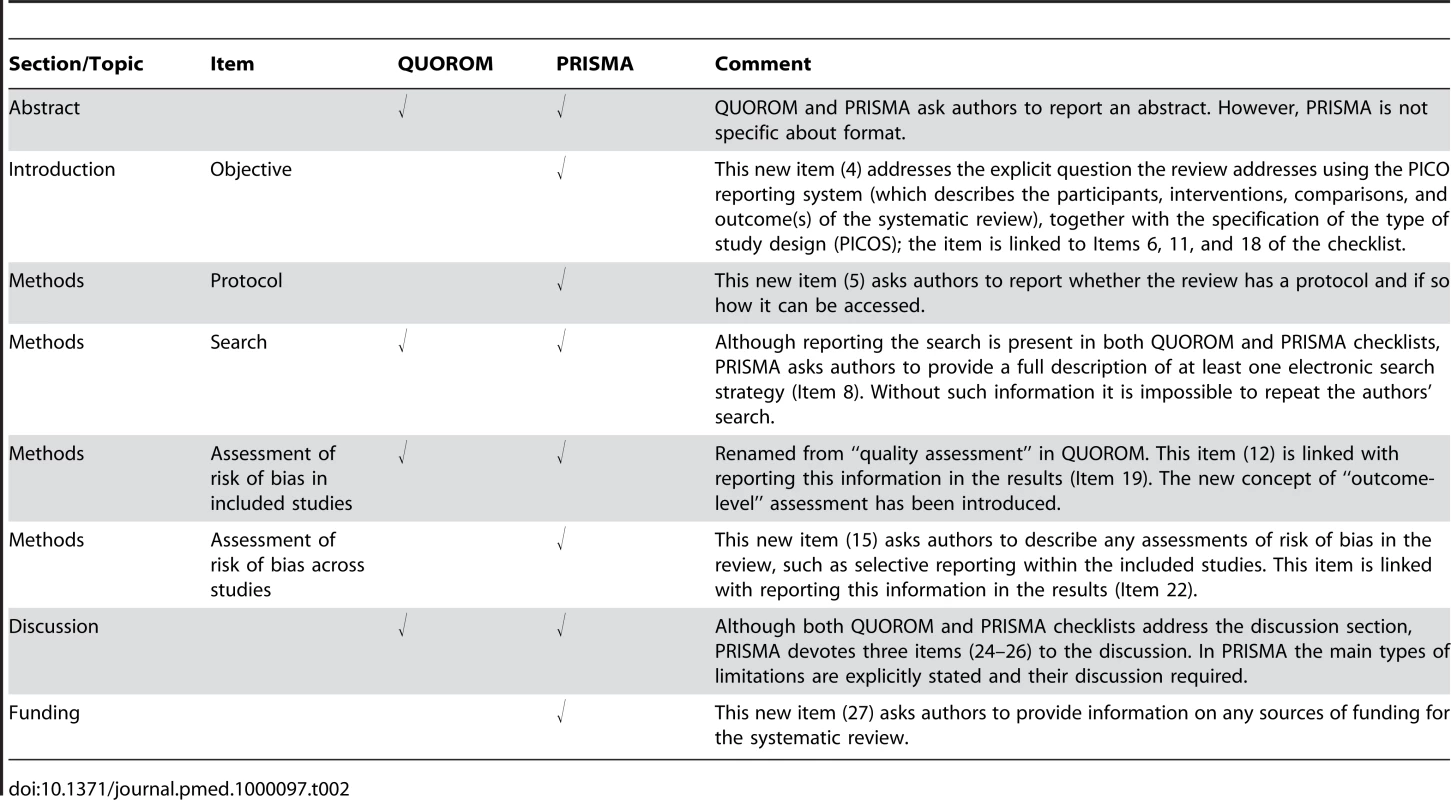
The flow diagram has also been modified. Before including studies and providing reasons for excluding others, the review team must first search the literature. This search results in records. Once these records have been screened and eligibility criteria applied, a smaller number of articles will remain. The number of included articles might be smaller (or larger) than the number of studies, because articles may report on multiple studies and results from a particular study may be published in several articles. To capture this information, the PRISMA flow diagram now requests information on these phases of the review process.
Endorsement
The PRISMA Statement should replace the QUOROM Statement for those journals that have endorsed QUOROM. We hope that other journals will support PRISMA; they can do so by registering on the PRISMA Web site. To underscore to authors, and others, the importance of transparent reporting of systematic reviews, we encourage supporting journals to reference the PRISMA Statement and include the PRISMA Web address in their Instructions to Authors. We also invite editorial organizations to consider endorsing PRISMA and encourage authors to adhere to its principles.
The PRISMA Explanation and Elaboration Paper
In addition to the PRISMA Statement, a supporting Explanation and Elaboration document has been produced [18] following the style used for other reporting guidelines [19]–[21]. The process of completing this document included developing a large database of exemplars to highlight how best to report each checklist item, and identifying a comprehensive evidence base to support the inclusion of each checklist item. The Explanation and Elaboration document was completed after several face to face meetings and numerous iterations among several meeting participants, after which it was shared with the whole group for additional revisions and final approval. Finally, the group formed a dissemination subcommittee to help disseminate and implement PRISMA.
Discussion
The quality of reporting of systematic reviews is still not optimal [22]–[27]. In a recent review of 300 systematic reviews, few authors reported assessing possible publication bias [22], even though there is overwhelming evidence both for its existence [28] and its impact on the results of systematic reviews [29]. Even when the possibility of publication bias is assessed, there is no guarantee that systematic reviewers have assessed or interpreted it appropriately [30]. Although the absence of reporting such an assessment does not necessarily indicate that it was not done, reporting an assessment of possible publication bias is likely to be a marker of the thoroughness of the conduct of the systematic review.
Several approaches have been developed to conduct systematic reviews on a broader array of questions. For example, systematic reviews are now conducted to investigate cost-effectiveness [31], diagnostic [32] or prognostic questions [33], genetic associations [34], and policy making [35]. The general concepts and topics covered by PRISMA are all relevant to any systematic review, not just those whose objective is to summarize the benefits and harms of a health care intervention. However, some modifications of the checklist items or flow diagram will be necessary in particular circumstances. For example, assessing the risk of bias is a key concept, but the items used to assess this in a diagnostic review are likely to focus on issues such as the spectrum of patients and the verification of disease status, which differ from reviews of interventions. The flow diagram will also need adjustments when reporting individual patient data meta-analysis [36].
We have developed an explanatory document [18] to increase the usefulness of PRISMA. For each checklist item, this document contains an example of good reporting, a rationale for its inclusion, and supporting evidence, including references, whenever possible. We believe this document will also serve as a useful resource for those teaching systematic review methodology. We encourage journals to include reference to the explanatory document in their Instructions to Authors.
Like any evidence-based endeavor, PRISMA is a living document. To this end we invite readers to comment on the revised version, particularly the new checklist and flow diagram, through the PRISMA Web site. We will use such information to inform PRISMA's continued development.
Supporting Information
Zdroje
1. OxmanAD
CookDJ
GuyattGH
1994 Users' guides to the medical literature. VI. How to use an overview. Evidence-Based Medicine Working Group. JAMA 272 1367 1371
2. SwinglerGH
VolminkJ
IoannidisJP
2003 Number of published systematic reviews and global burden of disease: Database analysis. BMJ 327 1083 1084
3. Canadian Institutes of Health Research 2006 Randomized controlled trials registration/application checklist (12/2006). Available: http://www.cihr-irsc.gc.ca/e/documents/rct_reg_e.pdf. Accessed 19 May 2009
4. YoungC
HortonR
2005 Putting clinical trials into context. Lancet 366 107
5. MulrowCD
1987 The medical review article: State of the science. Ann Intern Med 106 485 488
6. SacksHS
BerrierJ
ReitmanD
Ancona-BerkVA
ChalmersTC
1987 Meta-analysis of randomized controlled trials. New Engl J Med 316 450 455
7. SacksHS
ReitmanD
PaganoD
KupelnickB
1996 Meta-analysis: An update. Mt Sinai J Med 63 216 224
8. MoherD
CookDJ
EastwoodS
OlkinI
RennieD
1994 Improving the quality of reporting of meta-analysis of randomized controlled trials: The QUOROM statement. Lancet 354 1896 1900
9. GreenS
HigginsJ
2005 Glossary. Cochrane handbook for systematic reviews of interventions 4.2.5. The Cochrane Collaboration. Available: http://www.cochrane.org/resources/glossary.htm. Accessed 19 May 2009
10. StrechD
TilburtJ
2008 Value judgments in the analysis and synthesis of evidence. J Clin Epidemiol 61 521 524
11. MoherD
TsertsvadzeA
2006 Systematic reviews: When is an update an update? Lancet 367 881 883
12. University of York 2009 Centre for Reviews and Dissemination. Available: http://www.york.ac.uk/inst/crd/. Accessed 19 May 2009
13. The Joanna Briggs Institute 2008 Protocols & work in progress. Available: http://www.joannabriggs.edu.au/pubs/systematic_reviews_prot.php. Accessed 19 May 2009
14. De AngelisC
DrazanJM
FrizelleFA
HaugC
HoeyJ
2004 Clinical trial registration: A statement from the International Committee of Medical Journal Editors. CMAJ 171 606 607
15. WhittingtonCJ
KendallT
FonagyP
CottrellD
CotgroveA
2004 Selective serotonin reuptake inhibitors in childhood depression: Systematic review of published versus unpublished data. Lancet 363 1341 1345
16. BagshawSM
McAlisterFA
MannsBJ
GhaliWA
2006 Acetylcysteine in the prevention of contrast-induced nephropathy: A case study of the pitfalls in the evolution of evidence. Arch Intern Med 166 161 166
17. Biondi-ZoccaiGG
LotrionteM
AbbateA
TestaL
RemigiE
2006 Compliance with QUOROM and quality of reporting of overlapping meta-analyses on the role of acetylcysteine in the prevention of contrast associated nephropathy: Case study. BMJ 332 202 209
18. LiberatiA
AltmanDG
TetzlaffJ
MulrowC
GøtzscheP
2009 The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. PLoS Med 6 e1000100 doi:10.1371/journal.pmed.1000100
19. AltmanDG
SchulzKR
MoherD
EggerM
DavidoffF
2001 The revised CONSORT statement for reporting randomized trials: Explanation and elaboration. Ann Intern Med 134 663 694
20. BossuytPM
ReitsmaJB
BrunsDE
GatsonisCA
GlasziouPP
2003 Towards complete and accurate reporting of studies of diagnostic accuracy: The STARD explanation and elaboration. Ann Intern Med 138 W1 W12
21. VandenbrouckeJP
von ElmE
AltmanDG
GøtzschePC
MulrowCD
2007 Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): Explanation and elaboration. Ann Intern Med 147 W163 W194
22. MoherD
TetzlaffJ
TriccoAC
SampsonM
AltmanDG
2007 Epidemiology and reporting characteristics of systematic reviews. PLoS Med 4 e78 doi:10.1371/journal.pmed.0040078
23. BhandariM
MorrowF
KulkarniAV
TornettaP
2001 Meta-analyses in orthopaedic surgery: A systematic review of their methodologies. J Bone Joint Surg Am 83-A 15 24
24. KellyKD
TraversA
DorganM
SlaterL
RoweBH
2001 Evaluating the quality of systematic reviews in the emergency medicine literature. Ann Emerg Med 38 518 526
25. RichardsD
2004 The quality of systematic reviews in dentistry. Evid Based Dent 5 17
26. ChoiPT
HalpernSH
MalikN
JadadAR
TramerMR
2001 Examining the evidence in anesthesia literature: A critical appraisal of systematic reviews. Anesth Analg 92 700 709
27. DelaneyA
BagshawSM
FerlandA
MannsB
LauplandKB
2005 A systematic evaluation of the quality of meta-analyses in the critical care literature. Crit Care 9 R575 R582
28. DickersinK
2005 Publication bias: Recognizing the problem, understanding its origins and scope, and preventing harm.
RothsteinHR
SuttonAJ
BorensteinM
Publication bias in meta-analysis-Prevention, assessment and adjustments Chichester (UK) John Wiley & Sons 11 33
29. SuttonAJ
2005 Evidence concerning the consequences of publication and related biases.
RothsteinHR
SuttonAJ
BorensteinM
Publication bias in meta-analysis-Prevention, assessment and adjustments Chichester (UK) John Wiley & Sons 175 192
30. LauJ
IoannidisJP
TerrinN
SchmidCH
OlkinI
2006 The case of the misleading funnel plot. BMJ 333 597 600
31. LadabaumU
ChopraCL
HuangG
ScheimanJM
ChernewME
2001 Aspirin as an adjunct to screening for prevention of sporadic colorectal cancer: A cost-effectiveness analysis. Ann Intern Med 135 769 781
32. DeeksJJ
2001 Systematic reviews in health care: Systematic reviews of evaluations of diagnostic and screening tests. BMJ 323 157 162
33. AltmanDG
2001 Systematic reviews of evaluations of prognostic variables. BMJ 323 224 228
34. IoannidisJP
NtzaniEE
TrikalinosTA
Contopoulos-IoannidisDG
2001 Replication validity of genetic association studies. Nat Genet 29 306 309
35. LavisJ
DaviesH
OxmanA
DenisJ
Golden-BiddleK
2005 Towards systematic reviews that inform health care management and policy-making. J Health Serv Res Policy 10 35 48
36. StewartLA
ClarkeMJ
1995 Practical methodology of meta-analyses (overviews) using updated individual patient data. Cochrane Working Group. Stat Med 14 2057 2079
37. MojaLP
TelaroE
D'AmicoR
MoschettiI
CoeL
2005 Assessment of methodological quality of primary studies by systematic reviews: Results of the metaquality cross sectional study. BMJ 330 1053 1055
38. GuyattGH
OxmanAD
VistGE
KunzR
Falck-YtterY
2008 GRADE: An emerging consensus on rating quality of evidence and strength of recommendations. BMJ 336 924 926
39. SchunemannHJ
JaeschkeR
CookDJ
BriaWF
El-SolhAA
2006 An official ATS statement: Grading the quality of evidence and strength of recommendations in ATS guidelines and recommendations. Am J Respir Crit Care Med 174 605 614
40. ChanAW
HrobjartssonA
HaahrMT
GøtzschePC
AltmanDG
2004 Empirical evidence for selective reporting of outcomes in randomized trials: Comparison of protocols to published articles. JAMA 291 2457 2465
41. ChanAW
Krleza-JericK
SchmidI
AltmanDG
2004 Outcome reporting bias in randomized trials funded by the Canadian Institutes of Health Research. CMAJ 171 735 740
42. SilagyCA
MiddletonP
HopewellS
2002 Publishing protocols of systematic reviews: Comparing what was done to what was planned. JAMA 287 2831 2834
Štítky
Interné lekárstvo
Článok vyšiel v časopisePLOS Medicine
Najčítanejšie tento týždeň
2009 Číslo 7- Parazitičtí červi v terapii Crohnovy choroby a dalších zánětlivých autoimunitních onemocnění
- Nech brouka žít… Ať žije astma!
- Intermitentní hladovění v prevenci a léčbě chorob
- Co dělat při intoleranci statinů?
- Monoklonální protilátky v léčbě hyperlipidemií
-
Všetky články tohto čísla
- The PRISMA Statement for Reporting Systematic Reviews and Meta-Analyses of Studies That Evaluate Health Care Interventions: Explanation and Elaboration
- Unraveling the Impact of Malaria Exposure Before Birth
- Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement
- The US Food and Drug Administration Provides a Pathway for Licensing Vaccines for Global Diseases
- Compromise or Capitulation? US Food and Drug Administration Jurisdiction Over Tobacco Products
- Ethics Without Borders
- Research Ethics Review in Humanitarian Contexts: The Experience of the Independent Ethics Review Board of Médecins Sans Frontières
- Can the Relationship between Doctors and Drug Companies Ever Be a Healthy One?
- PLOS Medicine
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement
- The PRISMA Statement for Reporting Systematic Reviews and Meta-Analyses of Studies That Evaluate Health Care Interventions: Explanation and Elaboration
- The US Food and Drug Administration Provides a Pathway for Licensing Vaccines for Global Diseases
- Ethics Without Borders
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



