-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
Aripiprazole in the Maintenance Treatment of Bipolar Disorder: A Critical Review of the Evidence and Its Dissemination into the Scientific Literature
Background:
Aripiprazole, a second-generation antipsychotic medication, has been
increasingly used in the maintenance treatment of bipolar disorder and
received approval from the U.S. Food and Drug Administration for this
indication in 2005. Given its widespread use, we sought to critically review
the evidence supporting the use of aripiprazole in the maintenance treatment
of bipolar disorder and examine how that evidence has been disseminated inthe scientific literature.
Methods and Findings:
We systematically searched multiple databases to identify double-blind,
randomized controlled trials of aripiprazole for the maintenance treatment
of bipolar disorder while excluding other types of studies, such as
open-label, acute, and adjunctive studies. We then used a citation search to
identify articles that cited these trials and rated the quality of their
citations. Our evidence search protocol identified only two publications,
both describing the results of a single trial conducted by Keck et al.,
which met criteria for inclusion in this review. We describe four issues
that limit the interpretation of that trial as supporting the use ofaripiprazole for bipolar maintenance:
(1) insufficient duration to
demonstrate maintenance efficacy; (2) limited generalizability due to its
enriched sample; (3) possible conflation of iatrogenic adverse effects of
abrupt medication discontinuation with beneficial effects of treatment; and
(4) a low overall completion rate. Our citation search protocol yielded 80
publications that cited the Keck et al. trial in discussing the use of
aripiprazole for bipolar maintenance. Of these, only 24 (30%)
mentioned adverse events reported and four (5%) mentioned studylimitations.
Conclusions:
A single trial by Keck et al. represents the entirety of the literature on
the use of aripiprazole for the maintenance treatment of bipolar disorder.
Although careful review identifies four critical limitations to the
trial's interpretation and overall utility, the trial has beenuncritically cited in the subsequent scientific literature.
:
Please see later in the article for the Editors' Summary
Published in the journal: Aripiprazole in the Maintenance Treatment of Bipolar Disorder: A Critical Review of the Evidence and Its Dissemination into the Scientific Literature. PLoS Med 8(5): e32767. doi:10.1371/journal.pmed.1000434
Category: Research Article
doi: https://doi.org/10.1371/journal.pmed.1000434Summary
Background:
Aripiprazole, a second-generation antipsychotic medication, has been
increasingly used in the maintenance treatment of bipolar disorder and
received approval from the U.S. Food and Drug Administration for this
indication in 2005. Given its widespread use, we sought to critically review
the evidence supporting the use of aripiprazole in the maintenance treatment
of bipolar disorder and examine how that evidence has been disseminated inthe scientific literature.
Methods and Findings:
We systematically searched multiple databases to identify double-blind,
randomized controlled trials of aripiprazole for the maintenance treatment
of bipolar disorder while excluding other types of studies, such as
open-label, acute, and adjunctive studies. We then used a citation search to
identify articles that cited these trials and rated the quality of their
citations. Our evidence search protocol identified only two publications,
both describing the results of a single trial conducted by Keck et al.,
which met criteria for inclusion in this review. We describe four issues
that limit the interpretation of that trial as supporting the use ofaripiprazole for bipolar maintenance:
(1) insufficient duration to
demonstrate maintenance efficacy; (2) limited generalizability due to its
enriched sample; (3) possible conflation of iatrogenic adverse effects of
abrupt medication discontinuation with beneficial effects of treatment; and
(4) a low overall completion rate. Our citation search protocol yielded 80
publications that cited the Keck et al. trial in discussing the use of
aripiprazole for bipolar maintenance. Of these, only 24 (30%)
mentioned adverse events reported and four (5%) mentioned studylimitations.
Conclusions:
A single trial by Keck et al. represents the entirety of the literature on
the use of aripiprazole for the maintenance treatment of bipolar disorder.
Although careful review identifies four critical limitations to the
trial's interpretation and overall utility, the trial has beenuncritically cited in the subsequent scientific literature.
:
Please see later in the article for the Editors' SummaryIntroduction
First-generation antipsychotic medications have been used for many decades in the short-term treatment of acute manic episodes associated with bipolar disorder [1]. Second-generation antipsychotic medications have increasingly gained popularity for this use as well [2]. However, their promotion for the maintenance treatment of bipolar disorder is a more recent phenomenon [3]–[6]. In one recently published nationally representative survey of physicians, mood disorders accounted for the majority of antipsychotic medication prescriptions [7], and a recent shift to prescription of antipsychotic medications was observed in a sample of San Diego county Medicaid beneficiaries with bipolar disorder [8].
Traditionally, the clinical care of patients diagnosed with bipolar disorder has been divided into three phases (borrowed from clinical consensus about the phases of treatment for major depressive disorder [9],[10]): treatment of acute episodes to symptomatic remission, continuation treatment to prevent relapse, and maintenance treatment to prevent recurrence. The 2 mo following recovery from the acute episode is commonly described as acute phase recovery, and the continuation phase of treatment (during which the natural course of the episode is considered still active even though the patient may be asymptomatic) is defined as lasting from months 2 through 6 [11],[12]. The medication used for treatment in the acute phase is often extended for treatment in the continuation and maintenance phases [13],[14] and in this context may include lithium, valproate, lamotrigine, or a second-generation antipsychotic medication such as olanzapine, aripiprazole, quetiapine, risperidone, or ziprasidone [15]. However, although the use of second-generation antipsychotic medications to treat acute mania is supported by a relatively well-established evidence base [16]–[18], efficacy in treatment of acute mania does not necessarily imply efficacy for maintenance or prophylaxis [13],[19],[20]. As Goodwin and Jamison note: “Simply because a drug has anti-manic properties (and if continued, will protect against relapse back into mania in the months after the acute episode), one cannot assume that it will be effective in the prevention of new episodes. While this assumption may be true (to some extent) for lithium, it is not well supported by the data with respect to all the other antimanic agents” (p. 800) [21].
Despite the need for robust evidence on the maintenance and/or long-term prophylactic treatment of bipolar disorder, to date very little has been supplied in this regard [4],[15],[22],[23]. There remains little consensus about recommended courses of maintenance or prophylactic treatment, and consequently overall psychopharmacological treatment patterns vary widely [24]–[28]. Aripiprazole, first approved by the U.S. Food and Drug Administration (FDA) for the treatment of schizophrenia in 2002, is the newest of the second-generation antipsychotic medications to have obtained FDA approval for use in bipolar disorder. In 2004 it was approved for the treatment of acute manic and mixed episodes associated with bipolar disorder, and in 2005 it was granted an additional indication for the maintenance treatment of bipolar disorder [29]. Since its approval, aripiprazole has rapidly become a popular choice among clinicians in the maintenance treatment of bipolar disorder. Total U.S. sales for aripiprazole (across all indications) increased from US$1.5 billion in 2005 to US$4 billion in 2009 [30]. In a recent study in which U.S.-based physicians were queried about their preferred pharmacological treatments for schizophrenia and bipolar disorder, only 3% of psychiatrists and 7% of primary care physicians named aripiprazole as their first choice for treating schizophrenia, whereas 23% of psychiatrists and 16% of primary care physicians named aripiprazole as their first choice for treating bipolar disorder [31]. Consistent with this survey, from 2002–2007, the most common indication for the prescription of aripiprazole in office-based practice settings was for bipolar disorder (International Classification of Diseases, Ninth Revision, Clinical Modification [ICD-9-CM] Diagnosis Code 296.0) [32].
In the setting of chronic illnesses such as bipolar disorder, critical appraisal of long-term treatments has important implications for policy making. Overall medication costs for the chronically ill are driven largely by decisions about the ongoing use of prescription medications, rather than by decisions about whether to initiate their use [33]. Spending on prescription medications is the fastest-growing category of the U.S. health care budget [34], further underscoring the need for a rigorous evidence-based approach regarding their prescription and use. Given the rapid adoption and widespread use of aripiprazole in the maintenance treatment of bipolar disorder, we decided to review the scientific data supporting its use in this setting. A secondary aim of this study was to examine the diffusion of this data into the subsequent scientific literature.
Methods
Primary Evidence Search
We sought to identify double-blind (i.e., where participants and physicians administering medications were blind to treatment assignment), randomized controlled studies of aripiprazole for the maintenance treatment of bipolar disorder, while also avoiding inadvertent inclusion of acute treatment studies or other study designs. Therefore we required studies to have a duration greater than 4 mo in order to be included in our review, and excluded open-label, acute, and adjunctive studies. We searched for published literature as well as unpublished and ongoing clinical trials, with no language restrictions. The following systematic search strategy was employed to search PubMed: “bipolar disorder”[MeSH Terms] OR (“bipolar”[All Fields] AND “disorder”[All Fields]) OR (“bipolar disorder”[All Fields]) AND (“aripiprazole”[Substance Name] OR “aripiprazole”[All Fields]) AND (“maintenance”[MeSH Terms] OR “maintenance”[All Fields]). We also searched Scopus (including Embase and MEDLINE) using the same search terms (“bipolar disorder” OR “bipolar” AND “disorder” AND “aripiprazole” AND “maintenance”). We also searched ClincalTrials.gov, the Cochrane Central Register of Controlled Trials (Issue 3 of 4, July 2010), and the World Health Organization International Clinical Trials Registry Platform Search Portal using the terms “aripiprazole” and “bipolar.” We did not attempt to contact the manufacturer directly to inquire about possibly unpublished trials, but we screened all listings on the Bristol-Myers Squibb Clinical Trials Disclosure Web site under Clinical Trial Results, Psychiatric Disorders [35]. All searches were conducted in July 2010. And finally, we submitted a request under the U.S. Freedom of Information Act [36] for the supplemental New Drug Application (NDA) filed by the study sponsor to obtain additional labeling for the use of aripiprazole as maintenance therapy in bipolar I disorder [29], and we searched it manually for further reference to other published or unpublished studies.
Citation Search
We also sought to better understand the influence of the primary evidence on the broader scientific literature. To do this, we used the Web of Science(R) Science Citation Index Expanded to search for articles that cited the primary evidence identified through the evidence search protocol detailed above. Next, we evaluated the articles on how they cited the primary evidence, using criteria similar to those used in a previous study on the quality of news media reports of medication trials [37]. Each of the citing articles was rated on three quality criteria by a single study author (NZR). A 15% random sample of articles (n = 15) was double-coded independently by another study author (ACT), and the Cohen's kappa coefficient was calculated in order to assess the degree of inter-rater agreement [38]. We chose dichotomous quality ratings to provide conservative estimates of citation quality and in order to limit subjective judgments by the rater. First, articles were screened for any mention of the use of aripiprazole specifically for ongoing, maintenance, or prophylactic treatment of bipolar disorder. If the answer to this question was “yes,” then the article was further rated on the three quality criteria: (1) whether the article reported any quantitative data from the primary evidence (e.g., odds ratios, percentages, or p-values); (2) whether the article mentioned any adverse events described in the primary evidence; and (3) whether the article mentioned any limitations of the primary evidence.
Although our citation search protocol was not specifically targeted towards identifying treatment guidelines and review articles on pharmacological treatment strategies in bipolar disorder, we manually highlighted for further discussion those that were identified in the citation search. Our citation search protocol likely underestimates the influence of the primary evidence because we did not also use a database such as Google Scholar that could have also identified guidelines implemented by hospitals, government, or other institutions whose documents in this area have not been published in peer-reviewed journals or indexed in services such as PubMed. However, we chose to highlight treatment guidelines and reviews because they can be particularly influential in shaping prescribing behavior.
Results
Our primary evidence search protocol identified 177 unique citations (Figure 1). Of these 177 citations, only two publications met criteria for inclusion in our review [39],[40]. Searching the clinical trials registries yielded two listings meeting inclusion criteria, but these referred to the two publications already identified (Figure 2) [39],[40]. Further details on the excluded acute and adjunctive studies are provided in Tables S1 and S2. Two unpublished trials initially appeared to meet criteria for inclusion but were ultimately excluded. The first, Otsuka NCT00606177 [41], was a 3-wk placebo-controlled trial of aripiprazole for treatment of acute mania with a 22-wk extension phase, but it was described as currently still recruiting study participants. The second, BMS CN138-135LT [42], was a completed 40-wk extension of a 12-wk randomized lithium - and placebo-controlled trial of aripiprazole for acute mania. Although the 12-wk acute outcomes data from BMS CN138-135 were published in a peer-reviewed journal [43], the outcomes data from the 40-wk extension have not, to our knowledge, been published (and the little data made available in the synopsis posted online by the manufacturer were inadequate for detailed critical assessment). The manufacturer's synopsis indicates that 4.5% of participants on aripiprazole completed the extension phase, compared to 8.1% for those on lithium. A third arm of the study, completed by 8.5% of participants, entailed treatment with placebo for 3 wk followed by crossover to aripiprazole. Finally, the supplemental NDA contained no references to additional studies, published or unpublished, meeting inclusion criteria (Text S1) [29].
Fig. 1. Publications identified for review. 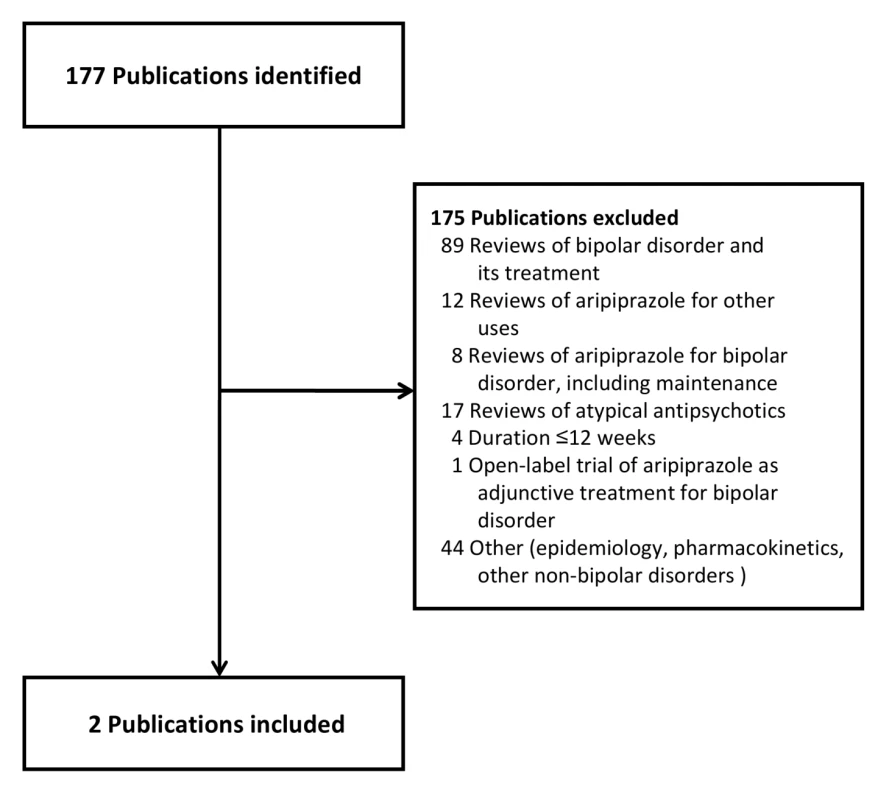
Fig. 2. Clinical trials identified for review. 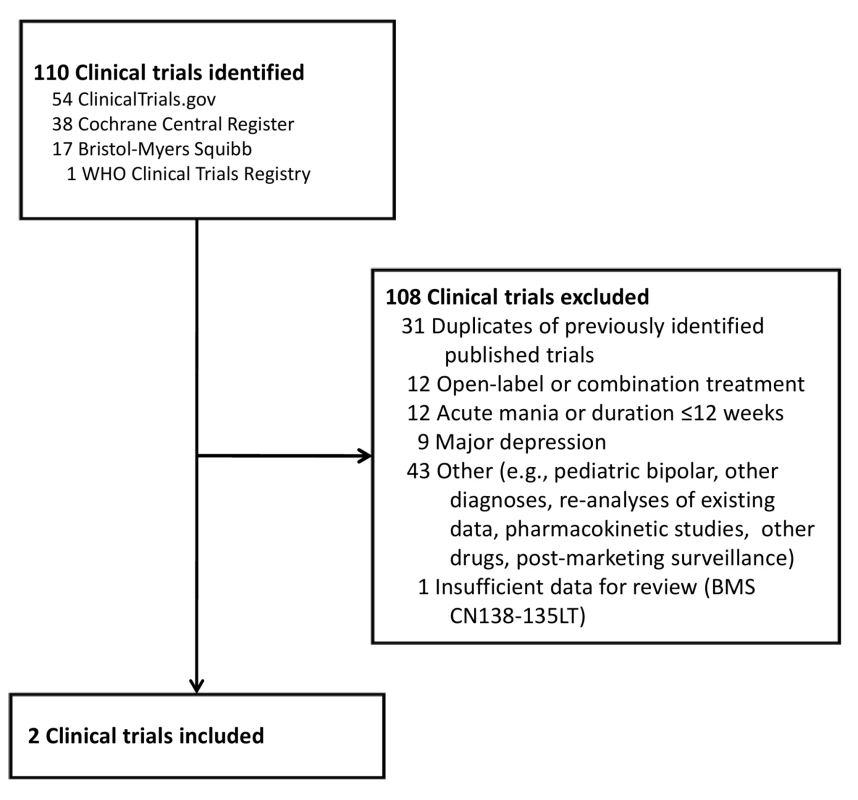
The two peer-reviewed publications included in our review report the results of a single randomized trial (hereafter referred to as “the Keck trial”) implemented under the auspices of the Aripiprazole Study Group and sponsored by the manufacturer of the drug, Bristol-Myers Squibb Co. One publication describes the initial 26-wk double-blind phase [39], and the other its 74-wk extension [40]. We also identified a post hoc subgroup analysis of data from the Keck trial focused on participants diagnosed with the rapid-cycling variant of bipolar disorder [44]. We also identified a separate trial [45], also authored by Keck and colleagues, examining the efficacy of aripiprazole in the treatment of acute manic episodes, with outcomes assessed at 3 wk. Given the paucity of available evidence on aripiprazole for the maintenance treatment of bipolar disorder, we decided to review the Keck trial [39],[40] in detail.
The Keck Trial
A total of 633 adult participants meeting DSM-IV criteria for bipolar I disorder were enrolled in the Keck trial. A flow chart of the trial is shown in Figure 3. For inclusion, participants must either have completed a prior 3-wk acute mania trial [45], met eligibility criteria for a prior acute mania trial but declined participation in that trial, or experienced a manic or mixed episode within the prior 3 mo. The publication describing the 26-wk double-blind phase [39] indicates that participants were recruited from 76 sites in the U.S., Mexico, and Argentina (but does not specify the numbers of sites within each country or the numbers of patients from each site). Of the original enrollees, 567 entered the “stabilization phase,” which consisted of open-label treatment with aripiprazole for 6–18 wk. Participants remained in this phase until their Young Mania Rating Scale (YMRS) was ≤10 and their Montgomery-Asberg Depression Rating Scale (MADRS) was ≤13 during four consecutive visits over a minimum of 6 wk. 206 participants completed the stabilization phase. Of these, 161 entered the double-blind phase. The supplemental NDA indicates that participants who completed the stabilization phase and entered randomization were derived from 45 sites in the U.S. (n = 124), three sites in Argentina (n = 7), and two sites in Mexico (n = 30). These 161 participants were assigned either to an intervention arm in which they continued taking aripiprazole at the stabilizing dose (n = 77 or 78; both numbers are reported [40]) or to a placebo arm in which aripiprazole was abruptly discontinued and replaced with placebo (n = 83). 39 (50% of the 77 or 78 who entered randomization) in the intervention arm and 28 participants (34%) in the placebo arm completed the 26-wk double-blind phase. Time to relapse was described as longer for participants treated with aripiprazole compared to those who were switched to placebo. Mean times to relapse were not provided, but the hazard ratio for relapse was given as 0.52 (95% confidence interval [CI] 0.30–0.91). When time to relapse was partitioned into manic versus depressive relapse, the difference in overall time to relapse was found to be driven primarily by an effect on manic relapse (23% relapse rate on placebo versus 8% relapse rate on aripiprazole). No differences in time to depressive relapse (13% versus 12%) or to mixed state relapse (6% versus 5%) were noted. Keck and colleagues concluded that aripiprazole “was superior to placebo in maintaining efficacy in patients with bipolar I disorder with a recent manic or mixed episode who were stabilized and maintained on aripiprazole treatment for 6 weeks” (p. 626) [39].
Fig. 3. Keck study participant flow. 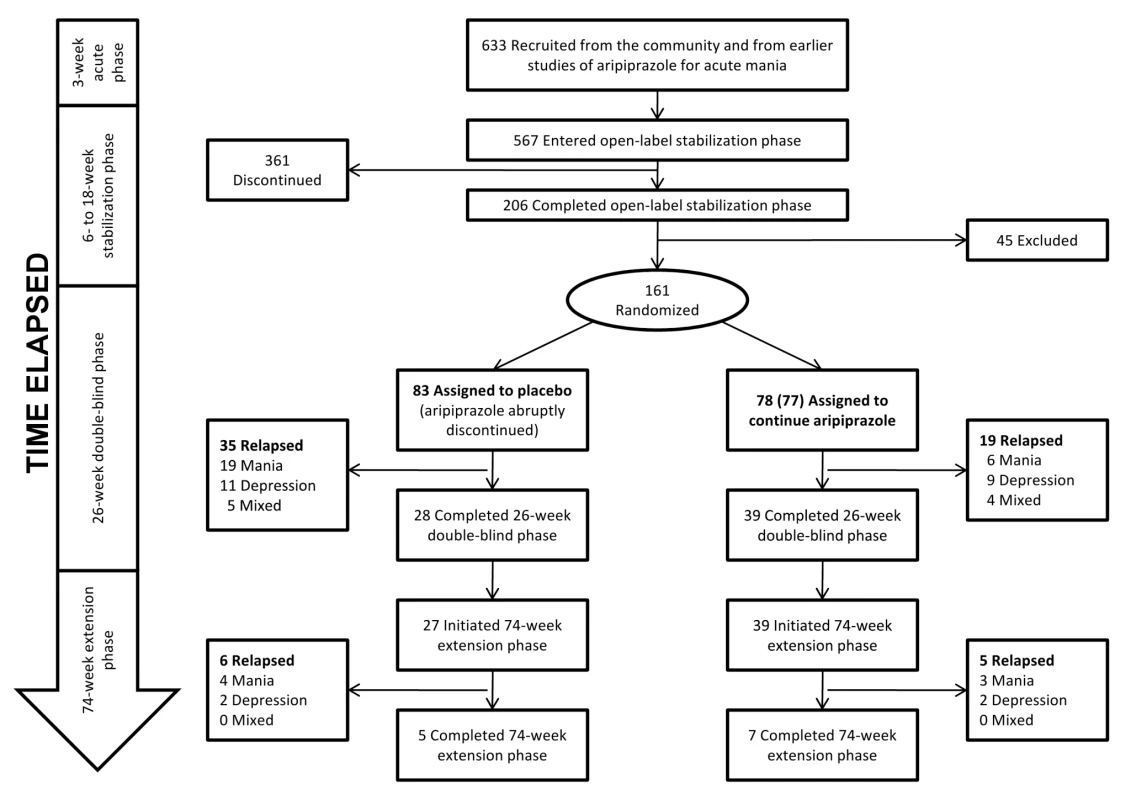
The extension phase of the Keck trial, published as a separate paper [40], followed the remaining participants over the subsequent 74 wk: 27 participants in the placebo group (of the 28 who completed the double-blind phase) and 39 participants in the aripiprazole group. The authors concluded: “Over a 100-week treatment period, aripiprazole monotherapy was effective for relapse prevention in patients who were initially stabilized on aripiprazole for 6 consecutive weeks, and it maintained a good safety and tolerability profile” (p. 1480) [40]. Similar to the data from the first 26 wk, time to manic relapse was reported to be longer for the aripiprazole group (with no difference between groups in time to depressive relapse).
Four Substantive Criticisms of the Keck Trial
Although the Keck trial was the sole basis for aripiprazole receiving an additional FDA-approved indication for the maintenance treatment of bipolar disorder [29], we believe that reading the Keck trial as supporting the use of aripiprazole for this indication is an overinterpretation of the trial's design and the data it generated. First, the duration of the Keck trial was insufficient to demonstrate prophylactic efficacy. Second, the double-blind phase of the Keck trial was based on an enriched sample of patients who had already responded to the medication during the stabilization phase, thereby limiting generalizability of the trial's findings. Third, the randomized discontinuation design of the Keck trial may conflate iatrogenic adverse effects of abrupt medication discontinuation with the beneficial effects of short-term continuation treatment. All of the putative benefit occurred during the double-blind phase of the trial, and little improvement was gained during the extension phase. And finally, the overall completion rate was 1.3%, requiring unrealistic extrapolation to draw meaningful conclusions. Keck et al. [39],[40] mention lack of generalizability as a potential limitation of the enrichment design, but they do not discuss how these other limitations may have compromised the trial's internal validity.
The FDA's review of the Keck trial identified other substantive concerns, including the fact that the p-value for the primary analysis changed from 0.02 to 0.10 when the statistical reviewer excluded data from one of the trial's two Mexican sites (where the relapse rate among participants in the aripiprazole arm was lower than the other trial sites) [46]. While of general concern, this and other issues identified by the FDA are unrelated to our methodological critiques. All of these factors undercut even a cautious interpretation of the Keck trial as supporting the use of aripiprazole for maintenance treatment of bipolar disorder. Below we review each of these criticisms in detail.
Insufficient duration to demonstrate prophylactic efficacy
In the open-label phase of the Keck trial, stability was defined by whether or not a participant maintained YMRS and MADRS scores in the asymptomatic range for at least 6 consecutive weeks. To meet this criterion, on average the trial participants spent 89 d in the stabilization phase. Comparing their own work to other randomized discontinuation studies of maintenance treatment in bipolar disorder that required a shorter duration of stability [47]–[49], Keck et al. describe their stability criterion as “the most stringent criteria to date to define stability” (p. 634) [39]. Intervention-arm participants who had achieved stability on aripiprazole were then assigned to continue with aripiprazole, and placebo-arm participants abruptly switched to placebo, for the following 26 wk.
Contrary to the authors' claims, we argue here that, given the natural history of bipolar disorder, the design of the Keck trial was unsuitable for evaluating the efficacy of aripiprazole in the maintenance treatment of bipolar disorder. The episodic nature of recurrent mania and depression require investigators to randomize, enroll, and retain patients for a duration sufficient to demonstrate maintenance and/or prophylactic efficacy. While there is high interindividual variation, the median length of untreated episodes has been reported to vary from 3–6 mo in clinical trial settings and from 2–3 mo in epidemiological studies [50], with depressive episodes typically lasting longer than manic episodes [21],[51]. Thus, even if one does not accept the other methodological concerns we describe in this paper, the Keck trial, with its stabilization criterion of 6 wk, could at best be used to demonstrate a short-term benefit of continuation treatment in preventing relapse of symptoms attributable to an ongoing acute episode [52]. Demonstration of maintenance efficacy in preventing recurrence of mood episodes would require benefit to be shown at least 6 mo after the acute phase. 6 mo has been traditionally recognized as the point at which continuation treatment becomes maintenance treatment [10],[11],[12],[52]–[54]. Appropriately, the clinical review contained in the supplemental NDA describes a meeting with the study's sponsors in which the FDA's Division of Neuropharmacological Drug Products “expressed that the duration of the open-label stabilization phase defines duration of effect and noted that an optimal study design would include a six month open-label stabilization phase and randomized withdrawal of patient subgroups at specified timepoints” (p. 9) [55]. The leading textbook in the field suggests an even more stringent threshold study duration: “Because the natural history of bipolar disorder is for it to recur, on average, every 16–18 months, true prophylaxis cannot be evaluated in 6 or 12 months” (p. 801) [21]. Although somewhat controversial, the idea that demonstration of true prophylactic efficacy requires a study duration longer than that which has been typically utilized has been supported by other leading researchers as well [11],[52],[56].
Limited generalizability due to the enriched sample
Only participants who had responded to aripiprazole in the stabilization phase of the trial were included in the double-blind phase of the trial. Of the 567 participants who entered the stabilization phase, only 206 completed it. Some of the randomized participants received unblinded medication and were therefore discontinued [29], so the double-blind efficacy dataset consisted of 161 participants. This means that 361 of the 567 (74%) participants who entered the stabilization phase but dropped out were excluded from randomization because of adverse events, lack of efficacy, withdrawal of consent, and other reasons as detailed in the publication—leaving behind a selected group of participants who had responded favorably to aripiprazole in the stabilization phase to be subsequently randomized. This design could have the effect of biasing the trial's findings away from the null [57], and, even in the absence of such bias, the results from this enriched sample cannot be generalized to the majority of persons diagnosed with bipolar disorder. This limitation of the randomized discontinuation design has long been recognized by drug trialists [11],[12],[56],[58]–[65] and is not dissimilar to criticisms voiced about the first generation of randomized trials evaluating the use of lithium for maintenance treatment in bipolar disorder, i.e., that those study designs selected preferentially for lithium-responsive variants of the disorder [66],[67].
Possible conflation of iatrogenic effects with beneficial effects
The randomized discontinuation study design could explain the putatively positive findings on preventing relapse even in the absence of a true drug effect. In the Keck trial, the randomization sample was enriched with participants who had already responded to aripiprazole in the stabilization phase and were therefore more likely to experience an iatrogenic relapse of symptoms when aripiprazole was abruptly discontinued in the double-blind phase. Abrupt discontinuation, or even abrupt partial removal, of a drug used for maintenance has long been known to provoke relapse in patients diagnosed with bipolar disorder [68]–[77]. This “bipolar rebound phenomenon” has been most often described for lithium, but it has also been observed in the setting of abruptly withdrawn antiepileptic [78], antipsychotic [3],[12],[48],[56],[78]–[80], and antidepressant medications [59],[75],[78],[81] administered to persons diagnosed with other mood and psychotic disorders. For this reason, Geddes et al. specifically excluded studies with a randomized discontinuation design from their systematic review and meta-analysis of the long-term use of lithium in the treatment of bipolar disorder [82].
Thus, if aripiprazole had no effect on preventing relapse, the Keck trial might still be expected to show a higher relapse rate early in the double-blind phase among participants assigned to the placebo arm (compared to those assigned to the intervention arm), and then similar relapse rates between study arms during the extension phase. This particular design element appears to have substantially influenced the outcome of the Keck trial, as is evident from a comparison of data from the 26-wk double-blind phase with data from the 74-wk extension phase. During the 26-wk double-blind phase, 19 out of 83 participants (23%) in the placebo arm experienced a manic relapse, whereas only four (5%) did so in the subsequent 74-wk extension phase.
When relapse data from the 74-wk extension phase are examined separately from those from the first 26 wk (Figure 3), only four participants in the placebo arm experienced a relapse to mania, compared to 3 participants in the intervention arm (4.8% versus 3.8%). This information is not explicitly presented in either paper and can only be discerned by comparing the papers side by side and calculating the differences by hand. Figure 4 in the 74-wk extension phase publication (p. 1486) [40] shows that 28% of participants in the placebo arm relapsed to mania over 100 wk of follow up. Given n = 83 in the placebo arm, this suggests 23 participants in the placebo arm relapsed to mania over 100 wk of follow up. Because 19 participants in the placebo arm relapsed to mania in the first 26 wk (Figure 5 in the 26-wk double-blind phase publication [p. 531] [39]), this means four participants relapsed to mania during the 74-wk extension phase. We employed similar reasoning to calculate the number of participants who relapsed to mania in the intervention arm, as well as the number of participants who relapsed to depressive and mixed states. Similar patterns are observed for relapse to depression and relapse to mixed state for the placebo and aripiprazole arms. Thus, virtually all of the reported placebo-aripiprazole difference in relapse occurred during the first 26 wk of the trial.
Fig. 4. Publications citing the Keck study. 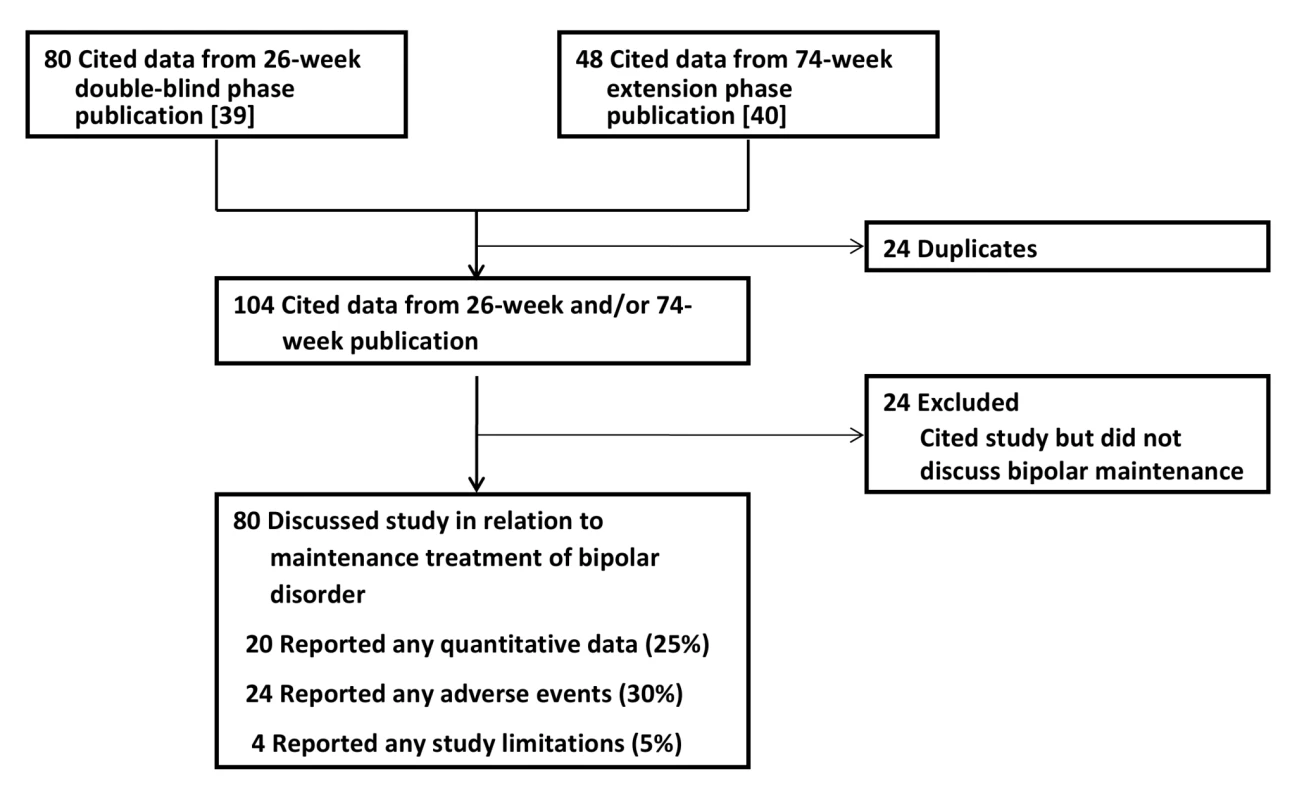
Limitations of the low completion rate
Only seven of 39 (18%) aripiprazole-treated participants and five of 27 (19%) placebo-treated participants completed the 74-wk extension phase. The low completion rate in the treatment arm is especially striking given that only participants who had proven to be responders in the initial stabilization phase were included in the double-blind and extension phases and that the placebo group matched the aripiprazole group in terms of trial completion. Out of the 633 participants who entered the trial, after excluding the 83 who were switched to placebo, only seven aripiprazole-treated participants completed the 100-wk trial, for a completion rate among aripiprazole-treated participants of less than 1.3%. This is not explicitly noted anywhere in the paper [40]. Keck et al. [40] acknowledge that only 12 participants completed the trial, but the smaller denominator used for comparison is the number of participants who entered the 74-wk extension phase rather than all participants who entered the trial.
We argue that drawing meaningful conclusions from a trial with an overall completion rate of less than 1.3% is an inappropriate undertaking. The completion rate substantially limits generalizability of the trial's findings, as trial completers very likely were dissimilar to the enrolled and/or randomized participant pools. The meaningful differences between completers and noncompleters were demonstrated in a randomized trial of divalproex versus lithium for relapse prevention in bipolar disorder [83]: participants who completed the trial had milder symptoms at baseline and a less severe lifetime illness course [84]. Keck et al. identify the low completion rate in the extension phase as a potential limitation but appeal to the low completion rates observed in other maintenance trials [47],[48] to support the generalizability of their results. In contrast, we view the similar completion rates observed in other long-term studies as similarly raising concerns about how to draw inferences from these trials to inform routine clinical practice. Still, we observe that other investigators have successfully implemented long-term studies in this patient population with greater rates of completion: for example, earlier studies of lithium in the treatment of affective disorders demonstrated completion rates of 92.9% (26/28) [85] and 73.2% (74/101) [86] among lithium-treated participants.
Impact of the Keck Trial on the Literature
Our citation search protocol identified 80 articles that cited the results from the 26-wk double-blind phase [39] and 48 articles that cited the results of the 74-wk extension phase [40]. After eliminating duplicates, the two publications from the Keck trial garnered 104 subsequent citations in total. Of these citing articles, 24 did not contain any mention of the Keck trial in relation to long-term or maintenance treatment of bipolar disorder and were excluded from further analysis (Figure 4). Double-coding revealed a high degree of inter-rater agreement on the quality assessment measures. There was 100% agreement on whether the publications were classified as mentioning aripiprazole for maintenance treatment. Among the double-coded publications mentioning maintenance treatment, there was 100% agreement on whether quantitative data and limitations were mentioned. There was one disagreement about whether adverse events were mentioned, yielding a kappa coefficient of 0.75 (95% CI 0.05–0.95). The overall kappa coefficient was 0.95 (95% CI 0.73–0.99).
Of the 80 articles that cited the Keck trial in reference to maintenance treatment of bipolar disorder, only 20 (25%) presented any quantitative data from the Keck trial; the remainder reported qualitative statements only (e.g., “Aripiprazole significantly delayed the time to relapse into a new mood episode in patients with bipolar I disorder over both 26 and 100 weeks of treatment.” [87]). 24 publications (30%) mentioned any of the adverse events reported in the trial. Only four (5%) made any mention of study limitations.
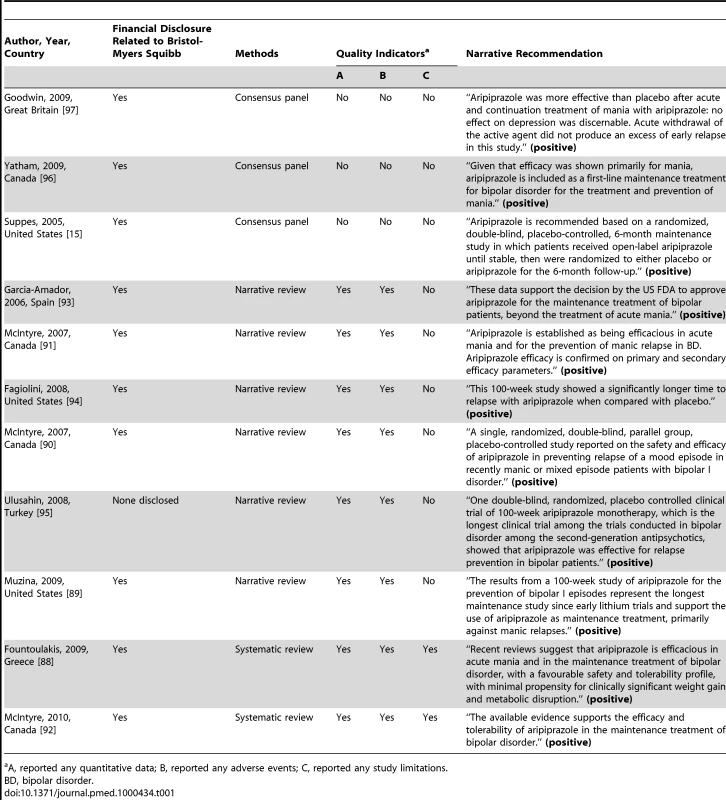
Among the articles identified through our citation search protocol were eight literature reviews [88]–[95] and three bipolar treatment guidelines [15],[96],[97] that specifically discussed the use of aripiprazole in the treatment of bipolar disorder. Because review articles and treatment guidelines can be particularly influential in shaping policy and prescribing behavior, we chose to highlight these in our discussion (Table 1). The evidence summaries employed the methodologies of consensus panel (n = 3), narrative review (n = 6), or systematic review (n = 2). Ten of the 11 reviews and treatment guidelines contained a financial disclosure related to Bristol-Myers Squibb.
Overall, the eight reviews were favorable in their assessment as to the putative efficacy of aripiprazole in the maintenance treatment of bipolar disorder. Solely on the basis of the results of the Keck trial, the Texas Medication Algorithm Project update listed aripiprazole as having “level 2” evidence (out of five levels of quality, with level 1 being the highest-quality) for maintenance treatment of bipolar disorder [15]. The Canadian Network for Mood and Anxiety Treatments and International Society for Bipolar Disorders recommended aripiprazole as first-line maintenance treatment of bipolar disorder, although it is noted that this is “mainly for preventing mania” (p. 235) [96]. This treatment recommendation was based on the Keck trial, along with a 30-wk pediatric bipolar trial that has only been published in abstract form [98]. The British Association for Psychopharmacology (BAP) based its positive endorsement of aripiprazole for relapse prevention solely on the Keck trial [97]. Contrary to the criticisms we described earlier, the BAP guidelines note, “Acute withdrawal of the active agent did not produce an excess of early relapse in this study” (p. 26).
Discussion
Our evaluation of the evidence base supporting the use of aripiprazole for the maintenance treatment of bipolar disorder reveals that the justification for this practice relies on the results from a single trial by Keck and colleagues published in two peer-reviewed journal articles [39],[40]. The methodology and reporting of the Keck trial are such that the results cannot be generalized to inform the treatment of most patients with bipolar disorder. Published interpretations of the data notwithstanding, in our opinion the Keck trial does not provide data to support the use of aripiprazole in the maintenance treatment of bipolar disorder. This lack of robust evidence of benefit should be weighed against the potential for long-term harms that have been described with other antipsychotic medications [3] and adverse events related to aripiprazole use, including tremor, akathisia, and significant weight gain [39]. Our concern about the critical imitations in this trial is further accentuated by the apparent widespread use of aripiprazole as a first-line agent for the maintenance treatment of bipolar disorder [31],[32].
Although we appreciate that the unique clinical features of bipolar disorder make controlled study extremely difficult [67],[84],[99]–[103], many of the weaknesses we document stem from the use of the randomized discontinuation design. Further study is needed in order to determine whether the problems described in this particular case are also more widely applicable to other continuation or maintenance treatment studies in bipolar disorder. We find unpersuasive the argument that a randomized discontinuation study such as this is valuable because it reflects common clinical practice [104],[105]. The two-arm, parallel randomized controlled trial may yield information that is more clinically useful than the discontinuation design. Under the parallel design, data from all participants (not just those who demonstrated an acute response) would contribute to our understanding of the drug's short - and long-term efficacy: one of two medications (or placebo) would be given to participants in the acute phase, and they would be followed throughout the continuation and maintenance phases (and beyond) to document response to treatment. (This study design, as well as the other study designs we describe, clearly could be used to study nonpharmacological treatments, including evidence-based psychotherapies. However, because this paper has emphasized discussion about pharmacologic treatments, we use the phrase “medication” for simplicity.) A two-arm, parallel randomized controlled trial of sufficient duration would directly answer the substantive research question, “Does aripiprazole treat symptoms to remission and prevent recurrent episodes when given to patients diagnosed with bipolar disorder presenting in a manic or mixed state?” This is clearly different from the question answered by the discontinuation design, “Among patients diagnosed with bipolar presenting in a manic or mixed state who have achieved a reasonable symptomatic improvement after being given aripiprazole, should aripiprazole be continued to maintain the initial improvement?” [106].
The primary disadvantage of the parallel design is that a greater proportion of (acutely ill) study participants would be subjected to placebo for the full duration of the trial, exactly the ethical issue that the discontinuation design was intended to address [107],[108]. Keck et al. [39] stated that they sought to minimize the extent to which stabilized participants were administered placebo. Yet their study could have been modified to diminish its exposure to the weaknesses that we have described above. First, the duration of stability required prior to randomization could be lengthened. One likely cost of this design modification is that the proportion of participants actually randomized would decrease further [109]. A second modification would be to gradually taper the discontinuation of medication among participants randomized to receive placebo. In previously published randomized discontinuation studies, medications administered during the open-label phase were tapered over a period of 2 to 3 wk rather than abruptly discontinued [47],[83]. Greenhouse et al. [59] suggest implementing the taper over several months.
Aside from these modifications to the parallel design, other alternatives have been suggested. Greenhouse et al. [59] proposed an alternative randomization scheme in which study participants are randomized to one of six treatment strategies. In the acute phase of treatment, study participants would receive one of two medications. In the maintenance phase of treatment, study participants would either remain on the medication initiated during the acute phase, be switched (gradually) to the alternative medication, or be switched (gradually) to placebo. This innovative study design would address the substantive research question, “Which treatment strategy is better in controlling and preventing the recurrence of depression?” (p. 318) [59]. A pure prophylactic design has also been recommended [10],[12],[100], in which patients previously diagnosed with a recurrent mood disorder would be enrolled during a medication-free remission period. Then, while participants are in remission, they would be offered one of two medications (or placebo). All participants would be followed in the study for a prespecified duration, and the treatment arms would be compared in terms of time to recurrence of a mood episode. This design would avoid the previously described error of possibly conflating beneficial treatment effects with iatrogenic adverse effects of abrupt medication discontinuation. However, as noted by Goodwin, Whitman, and Ghaemi [12], the failure of the divalproex study by Bowden et al. [83] was partly attributed to its enrollment of participants with low severity of illness [84]—and it was the last study to utilize the lithium-era prophylaxis design.
We recognize that the proposed study designs will be regarded by some as too costly or infeasible. Although some have suggested that a study with selected limitations may be useful in guiding clinical practice [104], we would disagree with this argument. The current “anti-Hippocratic” state of psychopharmacological practice described by Ghaemi [56],[110] raises questions about the extent to which research with substantive limitations is appropriately interpreted with conservative sensibilities. These concerns are borne out in our data. Thomson Reuters Essential Science Indicators(SM) places the 26-wk double-blind phase publication in the top 1%, and the 74-wk extension phase publication in the top 1%–2%, of papers published in the fields of psychiatry and psychology. Thus, by our conservative estimates, the Keck trial could be regarded as relatively influential. More importantly, we found that the Keck trial was cited uncritically by a subsequent generation of authors, through treatment guidelines, reviews, and other publications. All failed to note the consequences of abrupt and premature discontinuation of antimanic medication, especially during the vulnerable continuation period. The uncritical manner in which the Keck trial has been cited is reminiscent of the “echo chamber” effect described by Carey et al. [111] in their assessment of the now-discredited use of gabapentin in the treatment of bipolar disorder. Although the analogy is somewhat limited as there were no reportedly positive double-blind trials examining the use of gabapentin for this indication, we document a similar pattern of uncritical citations of the primary evidence regarding aripiprazole in the maintenance treatment of bipolar disorder.
Of further concern regarding the uncritical citation of the Keck trial's claims is that ten of the 11 treatment guidelines and review articles in our sample contained a financial disclosure related to the drug's manufacturer, Bristol-Myers Squibb Co. Financial conflicts of interest are highly prevalent across a wide range of medical subfields [112], and while they are known to be associated with recommendations in review articles [113], there is no systematic research documenting their influence on clinical practice guideline recommendations [114]. However, financial conflicts of interest have been found to be associated with biased reporting of outcomes in randomized trials [115],[116], which serve as the evidence upon which treatment guideline recommendations are based.
In summary, we provide here a critical appraisal of the available evidence regarding the use of aripiprazole for the maintenance treatment of bipolar disorder. The available evidence consists of a single trial by Keck et al. [39],[40], which is subject to several substantive methodological limitations but has nonetheless been cited uncritically in the ensuing scientific literature. Several alternative modifications or study designs may improve the probability of generating more useful data from studies in this vulnerable patient population to inform the treatment of similar patients in the future. We are concerned that the publication and apparently uncritical acceptance of this trial may be diverting patients away from more effective treatments.
Supporting Information
Zdroje
1. Tohen
M
Jacobs
TG
Feldman
PD
2000
Onset of action of antipsychotics in the treatment of
mania.
Bipolar Disord
2
261
268
2. Wolfsperger
M
Greil
W
Rossler
W
Grohmann
R
2007
Pharmacological treatment of acute mania in psychiatric
in-patients between 1994 and 2004.
J Affect Disord
99
9
17
3. Healy
D
2006
The latest mania: selling bipolar disorder.
PLoS Med
3
e185
doi:10.1371/journal.pmed.0030185
4. Malhi
GS
Adams
D
Berk
M
2009
Medicating mood with maintenance in mind: bipolar depression
pharmacotherapy.
Bipolar Disord
11
Suppl 2
55
76
5. Spielmans
GI
Parry
PI
2010
From evidence-based medicine to marketing-based medicine:
evidence from internal industry documents.
J Bioeth Inquiry
7
13
29
6. Healy
D
2010
From mania to bipolar disorder.
Yatham
LN
Maj
M
Bipolar disorder: clinical and neurobiological foundations
Oxford
John Wiley & Sons, Ltd
1
7
7. Mark
TL
2010
For what diagnoses are psychotropic medications being
prescribed?: a nationally representative survey of
physicians.
CNS Drugs
24
319
326
8. Depp
C
Ojeda
VD
Mastin
W
Unutzer
J
Gilmer
TP
2008
Trends in use of antipsychotics and mood stabilizers among
Medicaid beneficiaries with bipolar disorder, 2001-2004.
Psychiatr Serv
59
1169
1174
9. Frank
E
Prien
RF
Jarrett
RB
Keller
MB
Kupfer
DJ
1991
Conceptualization and rationale for consensus definitions of
terms in major depressive disorder. Remission, recovery, relapse, and
recurrence.
Arch Gen Psychiatry
48
851
855
10. Storosum
JG
van Zwieten
BJ
Vermeulen
HD
Wohlfarth
T
van den Brink
W
2001
Relapse and recurrence prevention in major depression: a critical
review of placebo-controlled efficacy studies with special emphasis on
methodological issues.
Eur Psychiatry
16
327
335
11. Ghaemi
SN
Pardo
TB
Hsu
DJ
2004
Strategies for preventing the recurrence of bipolar
disorder.
J Clin Psychiatry
65
Suppl 10
16
23
12. Goodwin
FK
Whitham
EA
Ghaemi
SN
2011
Relapse prevention in bipolar disorder: have any neuroleptics
been shown to be ‘mood stabilizers’?
CNS Drugs
In press
13. Sachs
GS
1996
Bipolar mood disorder: practical strategies for acute and
maintenance phase treatment.
J Clin Psychopharmacol
16
32S
47S
14. Sachs
GS
Printz
DJ
Kahn
DA
Carpenter
D
Docherty
JP
2000
The expert consensus guideline series: medication treatment of
bipolar disorder 2000.
Postgrad Med Spec No
1
104
15. Suppes
T
Dennehy
EB
Hirschfeld
RM
Altshuler
LL
Bowden
CL
2005
The Texas implementation of medication algorithms: update to the
algorithms for treatment of bipolar I disorder.
J Clin Psychiatry
66
870
886
16. Perlis
RH
Welge
JA
Vornik
LA
Hirschfeld
RM
Keck PE Jr.
2006
Atypical antipsychotics in the treatment of mania: a
meta-analysis of randomized, placebo-controlled trials.
J Clin Psychiatry
67
509
516
17. Scherk
H
Pajonk
FG
Leucht
S
2007
Second-generation antipsychotic agents in the treatment of acute
mania: a systematic review and meta-analysis of randomized controlled
trials.
Arch Gen Psychiatry
64
442
455
18. Vieta
E
Sanchez-Moreno
J
2008
Acute and long-term treatment of mania.
Dialogues Clin Neurosci
10
165
179
19. Bauer
MS
Mitchner
L
2004
What is a “mood stabilizer”? An evidence-based
response.
Am J Psychiatry
161
3
18
20. Ghaemi
SN
2001
On defining ‘mood stabilizer’.
Bipolar Disord
3
154
158
21. Goodwin
FK
Jamison
KR
2007
Manic-depressive illness: bipolar disorders and recurrent
depression.
New York
Oxford University Press
22. Suppes
T
Vieta
E
Liu
S
Brecher
M
Paulsson
B
2009
Maintenance treatment for patients with bipolar I disorder:
results from a north american study of quetiapine in combination with
lithium or divalproex (trial 127).
Am J Psychiatry
166
476
488
23. Geddes
JR
Goodwin
GM
Rendell
J
Azorin
JM
Cipriani
A
2010
Lithium plus valproate combination therapy versus monotherapy for
relapse prevention in bipolar I disorder (BALANCE): a randomised open-label
trial.
Lancet
375
385
395
24. Ghaemi
SN
Hsu
DJ
Thase
ME
Wisniewski
SR
Nierenberg
AA
2006
Pharmacological treatment patterns at study entry for the first
500 STEP-BD participants.
Psychiatr Serv
57
660
665
25. Lim
PZ
Tunis
SL
Edell
WS
Jensik
SE
Tohen
M
2001
Medication prescribing patterns for patients with bipolar I
disorder in hospital settings: adherence to published practice
guidelines.
Bipolar Disord
3
165
173
26. Frangou
S
Raymont
V
Bettany
D
2002
The Maudsley bipolar disorder project. A survey of psychotropic
prescribing patterns in bipolar I disorder.
Bipolar Disord
4
378
385
27. Blanco
C
Laje
G
Olfson
M
Marcus
SC
Pincus
HA
2002
Trends in the treatment of bipolar disorder by outpatient
psychiatrists.
Am J Psychiatry
159
1005
1010
28. Tohen
M
Baraibar
G
Kando
JC
Mirin
J
Zarate CA Jr.
1995
Prescribing trends of antidepressants in bipolar
depression.
J Clin Psychiatry
56
260
264
29. U.S. Food and Drug Administration
2005
Approval package for 21-436/S-005 & S-008 &
21-713/S-003.
Rockville (Maryland)
U.S. Food and Drug Administration
30. IMS Health
2010
Top 15 U.S. pharmaceutical products by sales. IMS National Sales
Perspectives(TM).
Norwalk
IMS Health
31. Chow
ST
von Loesecke
A
Hohenberg
KW
Epstein
M
Buurma
AK
2008
Brands and strategies: atypical antipsychotics.
Waltham (Massachusetts)
Decision Resources, Inc
32. Diak
I
Mehta
H
2008
New Molecular Entity review follow-up:
aripiprazole.
Rockville (Maryland)
U.S. Food and Drug Administration
33. Cuttler
L
Silvers
JB
Singh
J
Tsai
AC
Radcliffe
D
2005
Physician decisions to discontinue long-term medications using a
two-stage framework: the case of growth hormone therapy.
Med Care
43
1185
1193
34. Levit
K
Smith
C
Cowan
C
Lazenby
H
Sensenig
A
2003
Trends in U.S. health care spending, 2001.
Health Aff (Millwood)
22
154
164
35. Bristol-Myers Squibb Company
2010
Clinical trials registry.
New York
Bristol Myers Squibb Co
36. Gidiere
S
2006
The federal information manual.
Chicago
American Bar Association
37. Moynihan
R
Bero
L
Ross-Degnan
D
Henry
D
Lee
K
2000
Coverage by the news media of the benefits and risks of
medications.
N Engl J Med
342
1645
1650
38. Fleiss
J
1981
Statistical methods for rates and proportions. 2nd
edition.
New York
Wiley
39. Keck
PE
Jr
Calabrese
JR
McQuade
RD
Carson
WH
Carlson
BX
2006
A randomized, double-blind, placebo-controlled 26-week trial of
aripiprazole in recently manic patients with bipolar I
disorder.
J Clin Psychiatry
67
626
637
40. Keck
PE
Jr
Calabrese
JR
McIntyre
RS
McQuade
RD
Carson
WH
2007
Aripiprazole monotherapy for maintenance therapy in bipolar I
disorder: a 100-week, double-blind study versus placebo.
J Clin Psychiatry
68
1480
1491
41. Otsuka Pharmaceutical Co., Ltd.
2010
A multicenter, placebo-controlled, double-blind investigative
extension trial of the safety and efficacy of aripiprazole in the treatment
of patients with bipolar disorder experiencing a manic or mixed episode
(NCT00606177).
Tokyo
Otsuka Pharmaceutical Co., Ltd
42. Bristol-Myers Squibb Company
2008
A multicenter, randomized, double-blind, placebo-controlled study
of aripiprazole monotherapy in the treatment of acutely manic patients with
bipolar I disorder (CN138-135LT).
New York
Bristol-Myers Squibb Co
43. Keck
PE
Orsulak
PJ
Cutler
AJ
Sanchez
R
Torbeyns
A
2009
Aripiprazole monotherapy in the treatment of acute bipolar I
mania: a randomized, double-blind, placebo - and lithium-controlled
study.
J Affect Disord
112
36
49
44. Muzina
DJ
Momah
C
Eudicone
JM
Pikalov
A
McQuade
RD
2008
Aripiprazole monotherapy in patients with rapid-cycling bipolar I
disorder: an analysis from a long-term, double-blind, placebo-controlled
study.
Int J Clin Pract
62
679
687
45. Keck
PE
Jr
Marcus
R
Tourkodimitris
S
Ali
M
Liebeskind
A
2003
A placebo-controlled, double-blind study of the efficacy and
safety of aripiprazole in patients with acute bipolar mania.
Am J Psychiatry
160
1651
1658
46. He
K
2004
Statistical review and evaluation. Center for Drug Evaluation and
Research approval package for 21-436/S-005 & S-008 &
21-713/S-003.
Rockville (Maryland)
U.S. Food and Drug Administration
47. Bowden
CL
Calabrese
JR
Sachs
G
Yatham
LN
Asghar
SA
2003
A placebo-controlled 18-month trial of lamotrigine and lithium
maintenance treatment in recently manic or hypomanic patients with bipolar I
disorder.
Arch Gen Psychiatry
60
392
400
48. Tohen
M
Calabrese
JR
Sachs
GS
Banov
MD
Detke
HC
2006
Randomized, placebo-controlled trial of olanzapine as maintenance
therapy in patients with bipolar I disorder responding to acute treatment
with olanzapine.
Am J Psychiatry
163
247
256
49. Calabrese
JR
Bowden
CL
Sachs
G
Yatham
LN
Behnke
K
2003
A placebo-controlled 18-month trial of lamotrigine and lithium
maintenance treatment in recently depressed patients with bipolar I
disorder.
J Clin Psychiatry
64
1013
1024
50. Angst
J
Sellaro
R
2000
Historical perspectives and natural history of bipolar
disorder.
Biol Psychiatry
48
445
457
51. Tohen
M
Frank
E
Bowden
CL
Colom
F
Ghaemi
SN
2009
The International Society for Bipolar Disorders (ISBD) Task Force
report on the nomenclature of course and outcome in bipolar
disorders.
Bipolar Disord
11
453
473
52. Ghaemi
SN
2010
From BALANCE to DSM-5: taking lithium seriously.
Bipolar Disord
12
673
677
53. Quitkin
FM
Rabkin
JG
1981
Methodological problems in studies of depressive disorder:
utility of the discontinuation design.
J Clin Psychopharmacol
1
283
288
54. Quitkin
F
Rifkin
A
Klein
DF
1976
Prophylaxis of affective disorders. Current status of
knowledge.
Arch Gen Psychiatry
33
337
341
55. Prodruchny
TA
2004
Clinical review. Center for Drug Evaluation and Research approval
package for 21-436/S-005 & S-008 & 21-713/S-003.
Rockville (Maryland)
U.S. Food and Drug Administration
56. Ghaemi
SN
2006
Hippocratic psychopharmacology for bipolar disorder: an
expert's opinion.
Psychiatry (Edgmont)
3
30
39
57. Deshauer
D
Fergusson
D
Duffy
A
Albuquerque
J
Grof
P
2005
Re-evaluation of randomized control trials of lithium
monotherapy: a cohort effect.
Bipolar Disord
7
382
387
58. Smith
LA
Cornelius
V
Warnock
A
Bell
A
Young
AH
2007
Effectiveness of mood stabilizers and antipsychotics in the
maintenance phase of bipolar disorder: a systematic review of randomized
controlled trials.
Bipolar Disord
9
394
412
59. Greenhouse
JB
Stangl
D
Kupfer
DJ
Prien
RF
1991
Methodologic issues in maintenance therapy clinical
trials.
Arch Gen Psychiatry
48
313
318
60. Wehr
TA
1990
Unnecessary confusion about opposite findings in two studies of
tricyclic treatment of bipolar illness.
Arch Gen Psychiatry
47
787
788
61. Sachs
GS
Thase
ME
2000
Bipolar disorder therapeutics: maintenance
treatment.
Biol Psychiatry
48
573
581
62. Shapiro
DR
Quitkin
FM
Fleiss
JL
1990
Unnecessary confusion about opposite findings in two studies of
tricyclic treatment of bipolar illness. Reply.
Arch Gen Psychiatry
47
788
63. Deshauer
D
Moher
D
Fergusson
D
Moher
E
Sampson
M
2008
Selective serotonin reuptake inhibitors for unipolar depression:
a systematic review of classic long-term randomized controlled
trials.
CMAJ
178
1293
1301
64. Levenson
M
2008
Antiepileptic drugs and suicidality.
Rockville (Maryland)
U.S. Food and Drug Administration
65. Stone
M
Laughren
T
Jones
ML
Levenson
M
Holland
PC
2009
Risk of suicidality in clinical trials of antidepressants in
adults: analysis of proprietary data submitted to US Food and Drug
Administration.
BMJ
339
b2880
66. Calabrese
JR
Rapport
DJ
Shelton
MD
Kimmel
SE
2001
Evolving methodologies in bipolar disorder maintenance
research.
Br J Psychiatry
Suppl 41
s157
163
67. Calabrese
JR
Rapport
DJ
1999
Mood stabilizers and the evolution of maintenance study designs
in bipolar I disorder.
J Clin Psychiatry
60
Suppl 5
5
13; discussion 14-15
68. Lapierre
YD
Gagnon
A
Kokkinidis
L
1980
Rapid recurrence of mania following lithium
withdrawal.
Biol Psychiatry
15
859
864
69. Mander
AJ
Loudon
JB
1988
Rapid recurrence of mania following abrupt discontinuation of
lithium.
Lancet
2
15
17
70. Baldessarini
RJ
Suppes
T
Tondo
L
1996
Lithium withdrawal in bipolar disorder: implications for clinical
practice and experimental therapeutics research.
Am J Ther
3
492
496
71. Suppes
T
Baldessarini
RJ
Faedda
GL
Tondo
L
Tohen
M
1993
Discontinuation of maintenance treatment in bipolar disorder:
risks and implications.
Harv Rev Psychiatry
1
131
144
72. Faedda
GL
Tondo
L
Baldessarini
RJ
Suppes
T
Tohen
M
1993
Outcome after rapid vs gradual discontinuation of lithium
treatment in bipolar disorders.
Arch Gen Psychiatry
50
448
455
73. Baldessarini
RJ
1995
Risks and implications of interrupting maintenance psychotropic
drug therapy.
Psychother Psychosom
63
137
141
74. Baldessarini
RJ
Tondo
L
Faedda
GL
Suppes
TR
Floris
G
1996
Effects of the rate of discontinuing lithium maintenance
treatment in bipolar disorders.
J Clin Psychiatry
57
441
448
75. Baldessarini
RJ
Tondo
L
Ghiani
C
Lepri
B
2010
Illness risk following rapid versus gradual discontinuation of
antidepressants.
Am J Psychiatry
167
934
941
76. Suppes
T
Baldessarini
RJ
Faedda
GL
Tohen
M
1991
Risk of recurrence following discontinuation of lithium treatment
in bipolar disorder.
Arch Gen Psychiatry
48
1082
1088
77. Goodwin
GM
1994
Recurrence of mania after lithium withdrawal. Implications for
the use of lithium in the treatment of bipolar affective
disorder.
Br J Psychiatry
164
149
152
78. Franks
MA
Macritchie
KA
Mahmood
T
Young
AH
2008
Bouncing back: is the bipolar rebound phenomenon peculiar to
lithium? A retrospective naturalistic study.
J Psychopharmacol
22
452
456
79. Viguera
AC
Baldessarini
RJ
Hegarty
JD
van Kammen
DP
Tohen
M
1997
Clinical risk following abrupt and gradual withdrawal of
maintenance neuroleptic treatment.
Arch Gen Psychiatry
54
49
55
80. Baldessarini
RJ
Viguera
AC
1998
Medication removal and research in psychotic
disorders.
Arch Gen Psychiatry
55
281
283
81. Healy
D
2010
Treatment-induced stress syndromes.
Med Hypotheses
74
764
768
82. Geddes
JR
Burgess
S
Hawton
K
Jamison
K
Goodwin
GM
2004
Long-term lithium therapy for bipolar disorder: systematic review
and meta-analysis of randomized controlled trials.
Am J Psychiatry
161
217
222
83. Bowden
CL
Calabrese
JR
McElroy
SL
Gyulai
L
Wassef
A
2000
A randomized, placebo-controlled 12-month trial of divalproex and
lithium in treatment of outpatients with bipolar I disorder. Divalproex
Maintenance Study Group.
Arch Gen Psychiatry
57
481
489
84. Bowden
CL
Swann
AC
Calabrese
JR
McElroy
SL
Morris
D
1997
Maintenance clinical trials in bipolar disorder: design
implications of the divalproex-lithium-placebo study.
Psychopharmacol Bull
33
693
699
85. Coppen
A
Noguera
R
Bailey
J
Burns
BH
Swani
MS
1971
Prophylactic lithium in affective disorders. Controlled
trial.
Lancet
2
275
279
86. Prien
RF
Caffey EMJr., Klett CJ
1973
Prophylactic efficacy of lithium carbonate in manic-depressive
illness. Report of the Veterans Administration and National Institute of
Mental Health collaborative study group.
Arch Gen Psychiatry
28
337
341
87. Kemp
DE
Canan
F
Goldstein
BI
McIntyre
RS
2009
Long-acting risperidone: a review of its role in the treatment of
bipolar disorder.
Adv Ther
26
588
599
88. Fountoulakis
KN
Vieta
E
2009
Efficacy and safety of aripiprazole in the treatment of bipolar
disorder: a systematic review.
Ann Gen Psychiatry
8
16
89. Muzina
DJ
2009
Treatment and prevention of mania in bipolar I disorder: focus on
aripiprazole.
Neuropsychiatr Dis Treat
5
279
288
90. McIntyre
RS
Woldeyohannes
HO
Yasgur
BS
Soczynska
JK
Miranda
A
2007
Maintenance treatment in bipolar disorder: a focus on
aripiprazole.
Expert Rev Neurother
7
919
925
91. McIntyre
RS
Soczynska
JK
Woldeyohannes
HO
Miranda
A
Konarski
JZ
2007
Aripiprazole: pharmacology and evidence in bipolar
disorder.
Expert Opin Pharmacother
8
1001
1009
92. McIntyre
RS
2010
Aripiprazole for the maintenance treatment of bipolar I disorder:
a review.
Clin Ther
32
Suppl 1
S32
38
93. Garcia-Amador
M
Pacchiarotti
I
Valenti
M
Sanchez
RF
Goikolea
JM
2006
Role of aripiprazole in treating mood disorders.
Expert Rev Neurother
6
1777
1783
94. Fagiolini
A
2008
Practical guidance for prescribing with aripiprazole in bipolar
disorder.
Curr Med Res Opin
24
2691
2702
95. Ulusahin
A
2008
Ikiuclu bozuklukta aripiprazol [Aripiprazole for the
treatment of bipolar disorder].
Klinik Psikofarmakoloji Bulteni [Bulletin of Clinical
Psychopharmacology]
18
S27
S34
96. Yatham
LN
Kennedy
SH
Schaffer
A
Parikh
SV
Beaulieu
S
2009
Canadian Network for Mood and Anxiety Treatments (CANMAT) and
International Society for Bipolar Disorders (ISBD) collaborative update of
CANMAT guidelines for the management of patients with bipolar disorder:
update 2009.
Bipolar Disord
11
225
255
97. Goodwin
GM
2009
Evidence-based guidelines for treating bipolar disorder: revised
second edition—recommendations from the British Association for
Psychopharmacology.
J Psychopharmacol
23
346
388
98. Forbes
A
Findling
RL
Nyilas
M
Forbes
RA
Aurang
C
2008
Long-term efficacy of aripiprazole in pediatric patients with
bipolar I disorder.
New Research Abstracts; May 3-8 American Psychiatric
Association
297
99. Baldessarini
RJ
Tohen
M
Tondo
L
2000
Maintenance treatment in bipolar disorder.
Arch Gen Psychiatry
57
490
492
100. Goodwin
GM
Malhi
GS
2007
What is a mood stabilizer?
Psychol Med
37
609
614
101. Bowden
CL
Calabrese
JR
Wallin
BA
Swann
AC
McElroy
SL
1995
Illness characteristics of patients in clinical drug studies of
mania.
Psychopharmacol Bull
31
103
109
102. Post
RM
Keck
P
Jr
Rush
AJ
2002
New designs for studies of the prophylaxis of bipolar
disorder.
J Clin Psychopharmacol
22
1
3
103. Prien
RF
Kupfer
DJ
1989
Outside analysis of a multicenter collaborative
study.
Arch Gen Psychiatry
46
462
464
104. Tohen
M
Lin
D
2006
Maintenance treatment in bipolar disorder.
Psychiatry (Edgmont)
3
43
45
105. Swann
AC
2006
Treating bipolar disorder: for the patient or against the
illness?
Psychiatry (Edgmont)
3
40
42
106. Mallinckrodt
C
Chuang-Stein
Štítky
Interné lekárstvo
Článek The Transit Phase of Migration: Circulation of Malaria and Its Multidrug-Resistant Forms in AfricaČlánek If You Could Only Choose Five Psychotropic Medicines: Updating the Interagency Emergency Health Kit
Článok vyšiel v časopisePLOS Medicine
Najčítanejšie tento týždeň
2011 Číslo 5- Parazitičtí červi v terapii Crohnovy choroby a dalších zánětlivých autoimunitních onemocnění
- Statinová intolerance
- Genetický podklad a screening familiární hypercholesterolémie
- Metabolit živočišné stravy produkovaný střevní mikroflórou zvyšuje riziko závažných kardiovaskulárních příhod
- DESATORO PRE PRAX: Aktuálne odporúčanie ESPEN pre nutričný manažment u pacientov s COVID-19
-
Všetky články tohto čísla
- Primary Prevention of Gestational Diabetes Mellitus and Large-for-Gestational-Age Newborns by Lifestyle Counseling: A Cluster-Randomized Controlled Trial
- Meta-analyses of Adverse Effects Data Derived from Randomised Controlled Trials as Compared to Observational Studies: Methodological Overview
- Effectiveness of Early Antiretroviral Therapy Initiation to Improve Survival among HIV-Infected Adults with Tuberculosis: A Retrospective Cohort Study
- Characterizing the Epidemiology of the 2009 Influenza A/H1N1 Pandemic in Mexico
- The Joint Action and Learning Initiative: Towards a Global Agreement on National and Global Responsibilities for Health
- Let's Be Straight Up about the Alcohol Industry
- Advancing Cervical Cancer Prevention Initiatives in Resource-Constrained Settings: Insights from the Cervical Cancer Prevention Program in Zambia
- The Transit Phase of Migration: Circulation of Malaria and Its Multidrug-Resistant Forms in Africa
- Health Aspects of the Pre-Departure Phase of Migration
- Aripiprazole in the Maintenance Treatment of Bipolar Disorder: A Critical Review of the Evidence and Its Dissemination into the Scientific Literature
- Threshold Haemoglobin Levels and the Prognosis of Stable Coronary Disease: Two New Cohorts and a Systematic Review and Meta-Analysis
- If You Could Only Choose Five Psychotropic Medicines: Updating the Interagency Emergency Health Kit
- Migration and Health: A Framework for 21st Century Policy-Making
- Maternal Influenza Immunization and Reduced Likelihood of Prematurity and Small for Gestational Age Births: A Retrospective Cohort Study
- The Impact of Retail-Sector Delivery of Artemether–Lumefantrine on Malaria Treatment of Children under Five in Kenya: A Cluster Randomized Controlled Trial
- Medical Students' Exposure to and Attitudes about the Pharmaceutical Industry: A Systematic Review
- Estimates of Outcomes Up to Ten Years after Stroke: Analysis from the Prospective South London Stroke Register
- Low-Dose Adrenaline, Promethazine, and Hydrocortisone in the Prevention of Acute Adverse Reactions to Antivenom following Snakebite: A Randomised, Double-Blind, Placebo-Controlled Trial
- PLOS Medicine
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- Low-Dose Adrenaline, Promethazine, and Hydrocortisone in the Prevention of Acute Adverse Reactions to Antivenom following Snakebite: A Randomised, Double-Blind, Placebo-Controlled Trial
- Effectiveness of Early Antiretroviral Therapy Initiation to Improve Survival among HIV-Infected Adults with Tuberculosis: A Retrospective Cohort Study
- Medical Students' Exposure to and Attitudes about the Pharmaceutical Industry: A Systematic Review
- Estimates of Outcomes Up to Ten Years after Stroke: Analysis from the Prospective South London Stroke Register
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy