Strengthening the Informed Consent Process in International Health Research through Community Engagement: The KEMRI-Wellcome Trust Research Programme Experience
article has not abstract
Published in the journal:
Strengthening the Informed Consent Process in International Health Research through Community Engagement: The KEMRI-Wellcome Trust Research Programme Experience. PLoS Med 8(9): e32767. doi:10.1371/journal.pmed.1001089
Category:
Health in Action
doi:
https://doi.org/10.1371/journal.pmed.1001089
Summary
article has not abstract
Summary Points
-
Informed consent is fundamental to ethical health research.
-
Significant challenges are experienced in obtaining consent for research, particularly in poor settings.
-
Consenting processes can be strengthened by taking into account local social, cultural, and economic contexts in the design and administration of consent forms.
-
Institutional wide support is important in ensuring consistency in the consenting process for all studies within a given institution.
The Challenge
Informed consent is fundamental to ethical health research. However, significant challenges are experienced worldwide in ensuring regulatory and practical requirements for informed consent are met [1]–[4]. These challenges are partly attributable to differences in the understanding of research concepts and processes between researchers and research participants, differences that are most acute where there are large gaps between these groups in access to resources, literacy levels, and perceptions of health and illness, and in contexts where access to biomedical health care is severely constrained [1],[5]. This paper describes a programmatic approach to strengthening consent processes in a low resource setting and aims to contribute to global dialogue on practical ways of strengthening informed consent processes for health research.
The Kenya Medical Research Institute (KEMRI)-Wellcome Trust Research Programme (KWTRP) in Kilifi, Kenya, is a collaborative multidisciplinary research programme established in 1979 that focuses on the major causes of ill health in Kenya and sub-Saharan Africa. The Programme faces many of the challenges mentioned above. Studies in the area on community perceptions [1],[6]–[9] and institutional experiences of community engagement in research [10],[11] have highlighted a number of issues that undermine informed consent processes, including:
-
Limited exposure through formal education to research concepts and procedures, and lack of local terms for key elements of research.
-
Rumours about research activities and their purpose, for example on reasons for taking blood samples from well children.
-
Difficulties for potential participants or their guardians in listening to or understanding large volumes of research-related information, especially when children are sick and consenting processes are considered to be delaying initiation of treatment.
-
Perceptions that research procedures are part of standard care (therapeutic misconceptions) [12],[13], or vice versa [14].
-
Lack of in-depth understanding of research or research ethics among those responsible for explaining research activities.
Development of Contextualized Informed Consent Form Templates and Consenting Procedures
KWTRP has a large number of studies going on concurrently, and ensuring research is conducted to the highest scientific and ethical standards is a key Programme goal. To address the challenges identified in relation to consent processes, and to ensure harmonization and careful review of consenting processes, a committee designated “Consent and Communication Committee” (CCC) was constituted in 2005. Members of the CCC are drawn from different departments within the Programme, including laboratory-based staff, clinicians, social scientists, nurses, clinical trial coordinators, and community facilitators. The committee meets on a monthly basis to review consent forms and processes for all new proposed studies.
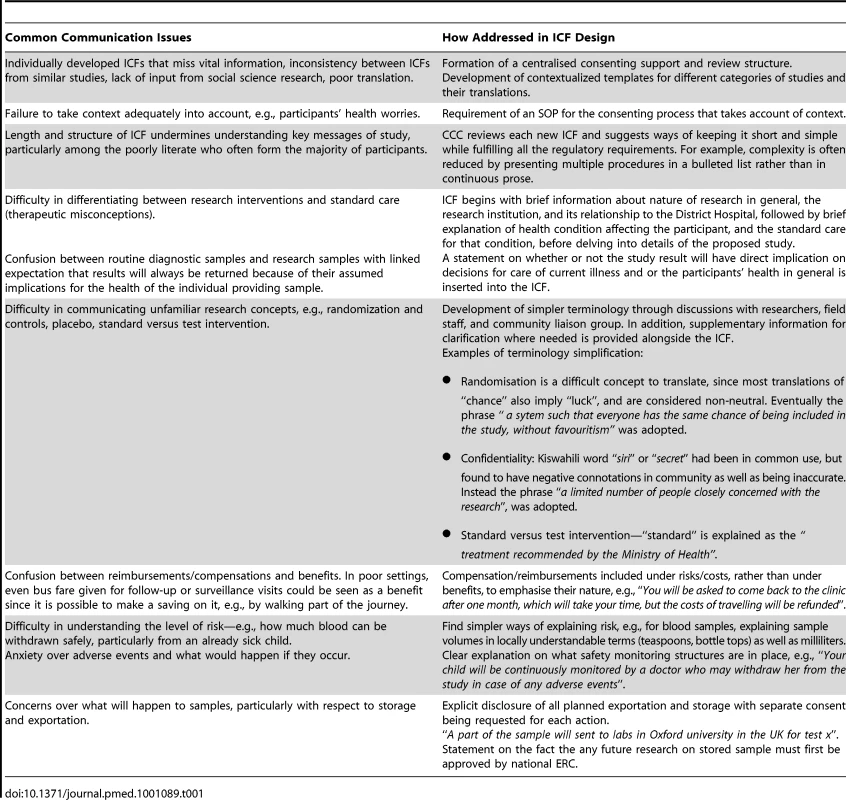
The CCC members were concerned that many consent forms were unnecessarily long, complicated, and, importantly, failed to take into account local priorities and concerns identified through the research and activities described above, while at times missing key elements required nationally or internationally [15]–[17]. In an effort to improve the situation, the committee drew upon internationally available templates [18]–[20] and on local research and insights to develop locally contextualized templates for each of the four main types of research conducted by the Programme: 1) clinical trials; 2) sampling only involving no intervention; 3) observational studies involving no sampling or interventions; and 4) interview only studies. Some issues and concerns might be more relevant to one type of study than another. For example, interventions and sampling studies might raise higher safety concerns than an interview only study, while the latter might raise more salient confidentiality issues as it is harder to disguise a recorded voice than it is to anonymise a sample. Table 1 highlights the key communication issues impacting consent processes in our context, and how these were tackled in the consent templates. Wording of key elements of the consent templates has been tested through ongoing community engagement activities.
Most informed consent processes in Kilifi take place in a local language, usually Kiswahili or Kigiriama. However, informed consent forms (ICFs) are usually developed in English by researchers as part of the proposal development process, and later translated into local languages. The CCC was aware of major limitations of many translations of consent forms and so they adopted a systematic approach to translation of ICF templates to Kiswahili versions. A series of workshops involving community facilitators (who are native speakers of the local languages and Kiswahili), nurses, scientists, and a professional translator (all competent Kiswahili speakers) were held in which each concept covered in the templates was written directly in the local languages, rather than simply translated from the English. The resulting Kiswahili templates were checked for accuracy and meaning by community facilitators not involved in the initial development.
The design of an ICF is only part of a wider consent process [21]. Thus, in addition to enhancing the ICF design, the CCC designed a template for developing a standard operating procedure (SOP) to guide researchers to consider key elements of the wider consenting process for their studies. These elements include training of those who will administer the ICF, considering the implications and flexibility of the timing of the consenting process (for example, whether it will happen before, during, or after admission of patient to the ward), and considering possibilities for re-visiting information over time. The SOP also prompts researchers to consider what supportive information about the illness or about the context might be important for staff conducting the informed consent process to answer participants' questions.
Experience of Using Contextualized ICF Templates and SOP in Kilifi
Box 1 summarises the output of the consent strengthening process. The contextualised ICF templates have been in use for over two years now and were used in the majority of approximately 90 new research proposals that covered all types of studies developed between May 2008 and November 2009. Although more research is needed to systematically assess the impact of this enhanced consent process (including contextualized ICFs), informal responses concerning this programmatic approach to strengthening consenting include:
-
Appreciation of the support that this approach provides for developing consent processes: Practically all researchers now engage the CCC for input in their studies' ICF design and in discussions around any consenting issues anticipated for their studies.
-
A perception among CCC members that availability of contextualised templates and their corresponding translations have simplified ICF development, and contributed to relatively clear and consistent ICFs that are relevant to the local context.
-
Researchers who have developed study-specific consent SOPs report that they have been a useful tool in training study staff, and in providing an ongoing reference over the course of their studies.
-
The two main ethics committees that review research done in KWTRP, the Kenya National Ethics Review Committee (ERC) and the Oxford Tropical Medicine Ethical Research Committee (OXTREC), have responded supportively to the templates. The Kenya ERC will be making a formal review of these forms with the aim of making these available to other researchers in Kenya. OXTREC welcomed the templates as a practical guidance tool and have requested that these are made available as examples to other researchers.
-
The Global Health Trials Web site (http://ght.globalhealthehub.org/), a Web-based international support platform, has uploaded the templates as part of responding to many requests for examples of consent forms for different types of research.
Box 1. Summary of the Output of the Consent Strengthening Process
-
A committee that provides systematic support for ICF design and for development of consent processes.
-
A set of standardized, locally specific templates for ICFs in Kiswahili and English that cover the types of studies commonly undertaken, and which draw on social science research and community engagement processes within the Programme to address common communication issues.
-
SOP guidelines that prompt researchers to consider the wider context within which informed consent forms will be used, including training and monitoring of consent administrators.
Challenges for Developing a Locally Relevant ICF and Consenting Process
An important challenge for the development of locally specific ICF templates concerns generating and maintaining sufficient understanding of relevant local community and research contexts. This requires developing good community engagement structures and capacity to conduct good quality health research. Similarly, developing locally relevant research terms and their corresponding translations requires a good understanding of research and the ability to draw on a wide range of expertise in different research areas. For example, to address the risks of conflation between research and treatment amongst patients, research participants, and staff in Kilifi, the CCC drew on clinical researchers' expertise to develop an agreed definition of local “standard of care”.
As acknowledged for research ethics review processes in general [22], the work of the CCC relies on a range of conditions, including staff expertise, commitment, and availability alongside normal responsibilities. Although the use of templates has facilitated review processes, there are also communication challenges for an internal review group in supporting their colleagues to develop informed consent processes. For example, SOP templates, which are a crucial component of the strengthened consent process, have not been applied as consistently as have the consent templates. This may be because currently consent SOPs are not included in the wider proposal review process undertaken before any study can begin. The CCC is therefore keen to ensure that SOPs are reviewed in advance. The challenge will be to do this in a way that is seen as supportive of researchers' efforts to oversee strong consent processes, rather than a new bureaucratic hurdle.
The adaptation of the templates cited here in other settings is likely to require similar steps for context-specific development relevant to participant communities and research staff, types, and institutions. Experiences in Kilifi suggest that locally adapted communication processes that combine the development of contextualised ICF templates and ongoing supportive processes for their use are a valuable investment of resources through their potential to strengthen informed consent, particularly in international research. The ICF and SOP ICF templates described in this paper are available on the Programme's Web site (http://www.kemri-wellcome.org/) and the Global Health Trials Web site (http://ght.globalhealthehub.org/).
Zdroje
1. MolyneuxCSPeshuNMarshK 2004 Understanding of informed consent in a low-income setting: three case studies from the Kenyan Coast. Soc Sci Med 59 2547 2559
2. FloryJEmanuelE 2004 Interventions to improve research participants' understanding in informed consent for research: a systematic review. JAMA 292 1593 1601
3. MarshallPAAdebamowoCAAdeyemoAAOgundiranTOVekichM 2006 Voluntary participation and informed consent to international genetic research. Am J Public Health 96 1989 1995
4. CorneliALBentleyMESorensonJRHendersonGEvan der HorstC 2006 Using formative research to develop a context-specific approach to informed consent for clinical trials. J Empir Res Hum Res Ethics 1 45 60
5. MarshallPA 2008 “Cultural competence” and informed consent in international health research. Camb Q Healthc Ethics 17 206 215
6. MolyneuxCSPeshuNMarshK 2005 Trust and informed consent: insights from community members on the Kenyan coast. Soc Sci Med 61 1463 1473
7. MolyneuxCSWassenaarDRPeshuNMarshK 2005 ‘Even if they ask you to stand by a tree all day, you will have to do it (laughter)…!’: community voices on the notion and practice of informed consent for biomedical research in developing countries. Soc Sci Med 61 443 454
8. GikonyoCBejonPMarshVMolyneuxS 2008 Taking social relationships seriously: lessons learned from the informed consent practices of a vaccine trial on the Kenyan Coast. Soc Sci Med 67 708 720
9. MolyneuxCGoudgeJRussellSChumaJTebogaG 2009 Conducting heatlh-related social science research in low income settings: Ethical dilemas faced in Kenyan and South Africa. Journal of International Development 21 309 326
10. MarshVKamuyaDMlambaAWilliamsTNMolyneuxS 2010 Experiences with community engagement and informed consent in a genetic cohort study of severe childhood diseases in Kenya. BMC Med Ethics 11 13
11. MarshVKamuyaDRowaYGikonyoCMolyneuxS 2008 Beginning community engagement at a busy biomedical research programme: experiences from the KEMRI CGMRC-Wellcome Trust Research Programme, Kilifi, Kenya. Soc Sci Med 67 721 733
12. LidzCWAppelbaumPS 2002 The therapeutic misconception: problems and solutions. Med Care 40 V55 V63
13. AppelbaumPSRothLHLidzCWBensonPWinsladeW 1987 False hopes and best data: consent to research and the therapeutic misconception. Hastings Cent Rep 17 20 24
14. MarshVMKamuyaDKParkerMJMolyneuxCS 2011 Working with concepts: the role of community in international collaborative biomedical research. Public Health Ethics 4 26 39
15. NCST 2005 Guidelines for ethical conduct of biomedical research involving human subjects in Kenya Nairobi National Council for Science and Technology
16. CIOMS 2002 International ethical guidelines for biomedical research involving human subjects. Available: http://www.cioms.ch/publications/guidelines/guidelines_nov_2002_blurb.htm Accessed 10 August 2011
17. GCP 2010 International conference on harmonisation of technical requirements for registration of pharmaceuticals for human use: good clinical practice. Available: http://ichgcp.net/. Accessed 4 August 2011
18. CTN Clinical Trials Network Best Practices, Duke Clinical Research Institute. Informed consent document templates & instructions. Available: https://www.ctnbestpractices.org/resources/study-patient-management/informed-consent/templates-forms/. Accessed 4 August 2011
19. National Health Service 2002 Information sheets and consent forms: guidance for researchers and reviewers London National Patient Safety Agency – National Research Ethics Service, National Health Service Available: http://www.nres.npsa.nhs.uk/applications/guidance/consent-guidance-and-forms/. Accessed 4 August 2011
20. Office of Human Subject Research 2006 Guidelines for writing informed consent documents Bethesda Office of Human Subject Research, NIH Available: http://ohsr.od.nih.gov/info/info.html. Accessed 4 August 2011
21. WHO 2010 Informed consent form templates Geneva WHO Available: http://www.who.int/rpc/research_ethics/informed_consent/en/. Accessed 4 August 2011
22. MilfordCWassenaarDSlackC 2006 Resource and needs of research ethics committees in Africa: preparations for HIV vaccine trials. IRB 28 1 9
Štítky
Interné lekárstvoČlánok vyšiel v časopise
PLOS Medicine
2011 Číslo 9
- Parazitičtí červi v terapii Crohnovy choroby a dalších zánětlivých autoimunitních onemocnění
- Statinová intolerance
- Vztah mezi statiny a rizikem vzniku nádorových onemocnění − metaanalýza
- Pleiotropní účinky statinů na kardiovaskulární systém
- Hydroresponzivní krytí v epitelizační fázi hojení rány
Najčítanejšie v tomto čísle
- Living Alone and Alcohol-Related Mortality: A Population-Based Cohort Study from Finland
- Cardiovascular Risk with Non-Steroidal Anti-Inflammatory Drugs: Systematic Review of Population-Based Controlled Observational Studies
- , , and Variants Additively Predict Response to Therapy in Chronic Hepatitis C Virus Infection in a European Cohort: A Cross-Sectional Study
- Towards Improved Measurement of Financial Protection in Health
